Epidermal growth factor receptor (EGFR) and its ligands have been implicated in liver fibrosis. We show that the genetic ablation of EGFR or ERBB3, particularly in combination, results in reduced liver fibrosis upon chronic CCl4 administration. Our results suggest that EGFR-ERBB3 heterodimeric signaling may play a more important role in liver fibrosis than the EGFR-EGFR signaling. We also show in this model increased tyrosine phosphorylation EGFR and ERBB3 in hepatocytes as well as nonparenchymal cells.
Keywords: carbon tetrachloride, epidermal growth factor receptor, ERBB3, fibrosis, liver
Abstract
Epidermal growth factor receptor (EGFR) and its ligands have been implicated in liver fibrosis. However, it has not been directly shown that hepatocellular genetic ablation of either this receptor tyrosine kinase or ERBB3, its interactive signaling partner, affects hepatic fibrosis. Carbon tetrachloride (CCl4)-induced liver fibrosis in hepatocyte-specific (HS) mouse models of EGFR and ERBB3 ablation was evaluated in both single gene knockouts and an HS-EGFR-ERBB3 double knockout (DKO). Loss of hepatocellular EGFR or ERBB3 did not impact cytochrome P450-2E1 expression, the extent of centrilobular injury, or the initial regenerative response, but it did diminish liver fibrosis induced by chronic intraperitoneal administration of CCl4. The reduction of liver fibrosis correlated with reduced α-smooth muscle actin expression. Maximal impact to fibrogenesis occurred in the ERBB3 and EGFR-ERBB3 DKO models, suggesting that EGFR-ERBB3 heterodimeric signaling in damaged hepatocytes may play a more important role in liver fibrosis than EGFR-EGFR homodimeric signaling. Immunohistochemical analyses of phospho-EGFR and phospho-ERBB3 isoforms revealed clear staining in hepatocytes, activated stellate cells, and macrophages. Our results support a role for the hepatocellular ERBB tyrosine kinases in fibrogenesis and suggest that pharmacologic inhibition of EGFR-ERBB3 signaling may reverse or retard hepatic fibrosis.
NEW & NOTEWORTHY
Epidermal growth factor receptor (EGFR) and its ligands have been implicated in liver fibrosis. We show that the genetic ablation of EGFR or ERBB3, particularly in combination, results in reduced liver fibrosis upon chronic CCl4 administration. Our results suggest that EGFR-ERBB3 heterodimeric signaling may play a more important role in liver fibrosis than the EGFR-EGFR signaling. We also show in this model increased tyrosine phosphorylation EGFR and ERBB3 in hepatocytes as well as nonparenchymal cells.
chronic liver injury results in a repetitive wound-healing response that eventually leads to replacement of hepatic tissue by fibrous scar tissue. This, not only diminishes liver function, but also creates a cellular environment that can lead to cirrhosis and even hepatocarcinogenesis. Remodeling of the extracellular matrix (ECM) during fibrosis occurs when cell populations, such as hepatic stellate cells and portal fibroblasts, synthesize fibrillar collagens and elastin in excess of normal ECM. Growth factors, such as platelet-derived factor (PDGF), connective tissue growth factor (CTGF), and transforming growth factor β (TGF-β), and their receptors modulate the proliferation, survival, and function of the fibrogenic cells (46). Fibrogenesis is a complex process involving hepatocellular injury, apoptosis, inflammation, hepatocellular regeneration, stellate cell “activation,” matrix production, and matrix stabilization, and it is now recognized that the damaged hepatocyte plays an active role in fibrogenesis and is not merely a passive bystander (44). Understanding the pathogenesis of liver fibrosis will accelerate the development of novel therapeutic approaches to prevent or reverse it (26, 43).
The ERBB family of receptor tyrosine kinases (RTK) consists of four interactive RTKs that bind to two different classes of ligands, the epidermal growth factor (EGF)-like ligands and heregulins (HRG). The mouse liver expresses EGFR, ERBB2, and ERBB3; however, the expression of ERBB2 decreases substantially after weaning (10) (38). Although previously thought to lack intrinsic kinase activity, ERBB3 is now known to have weak kinase activity, activated by interaction with other ERBB molecules (41). Activated ERBB3 monomers can dissociate from heterodimeric pairings and subsequently form HRG-activated ERBB3 homodimers. Radioligand binding studies indicate that each hepatocyte of the adult male rodent liver expresses about 600,000 EGF receptors (2) (36) but only 20,000 ERBB3 receptors (9). An EGFR monomer can form active signaling homodimers or active signaling heterodimers within other ERBB family members, including ERBB3. Acute hepatocellular phosphorylation of ERBB3 in the liver of the adult mouse by its ligand, HRG, requires EGFR, based on studies using EGFR knockout (KO) mice (38). However, the signaling outcomes of an EGFR-EGFR homodimer compared with an EGFR-ERBB3 heterodimer are distinct, in part attributable to the six phosphatidylinositol 3 kinase (PI3K) binding sites in the intracellular ERBB3 regulatory domain that accentuate the production of 3-phosphorylated phosphoinositides, leading to the surface membrane recruitment and activation of AKT (27).
The role of the ERBB receptor tyrosine kinases in liver fibrosis is not clear. Various global KOs of ERBB ligands, such amphiregulin (AREG) and heparin-binding-EGF (HBEGF), have been used to assess these roles, but the results are conflicting. Both peptides are expressed by fibrogenic cells in the liver after carbon tetrachloride (CCl4) treatment (11, 14) but appear to have divergent effects on fibrogenesis. For example, loss of AREG, which binds and signals through EGFR, decreases liver fibrosis generated by chronic administration of CCl4, a centrilobular hepatotoxin (31). In contrast, loss of HBEGF (17, 22, 42), which can bind and signal, not only through EGFR, but also ERBB3 and ERBB4, promotes it. Diminished fibrosis associated with the genetic ablation of AREG was attributed to a direct effect on ECM-producing cells (31). Recently, CTGF has been shown to be a novel ligand for EGFR. Little is known about the interaction between CTGF and EGFR, but the carboxyl-terminal module (IV) of CTGF shows a clear interaction with the extracellular binding domain and is capable of stimulating EGFR tyrosine phosphorylation of renal epithelial tubular cells in vivo (33). Long-term pharmacological inhibition of EGFR signaling employing erlotinib, a small-molecule EGFR kinase inhibitor, decreased fibrosis in the rat liver after chronic CCl4 administration or bile duct ligation (13). However, it is not known whether this inhibitory effect was due primarily to EGFR inhibition in hepatocytes, fibrogenic cells, or even to an indirect global effect arising from a nonhepatic organ.
In this study, we used a genetic approach to ablate EGFR or ERBB3 in hepatocytes (HS-EGFR-KO or HS-ERBB3-KO) so as to assess their importance in the progression of liver fibrosis caused by CCl4. We also generated a hepatocyte-specific EGFR-ERBB3 double KO (HS-EGFR-ERBB3 DKO). Individual or dual disruption of EGFR and ERBB3 had no effect on the initial injury or regeneration after CCl4, but it did diminish fibrosis induced by chronic CCl4 administration. We also show that tyrosine phosphorylation of EGFR and ERBB3 occurs in hepatocytes and macrophages adjacent to fibrous septa as well as in activated stellate cells within these septa.
MATERIALS AND METHODS
Mice and genetic crosses.
Genetic mouse models B6.129S6-Egfrtm1Dwt (Egfrf) (25), B6.129S6-Erbb3tm1Dwt (Erbb3f) (24), and B6.Cg-Tg(Alb-cre)21Mgn/J (Alb-Cre) (The Jackson Laboratory) were generated as previously described. Egfrf has loxP sites flanking exon 3 of the Egfr. Cre recombinase-mediated deletion of exon 3 causes a frameshift with two stop codons in exon 4 and early termination of translation. To generate hepatocyte-specific EGFR-KO, Alb-Cre mice were crossed with Egfrf/f to generate Alb-Cre, Egfrf/f, or their littermate controls Egfrf/f (38, 39). A similar approach was used to establish Alb-Cre, Erbb3f/f mice (39) or DKO mice. Mice were fed Purina Mills Lab Diet and water ad libitum under specific pathogen-free conditions in a facility approved by the American Association for the Accreditation of Lab Animal Care. Mice were raised under conditions of regulated lighting (lights on 0600–1800), temperature, and humidity. The Vanderbilt Institutional Animal Care and Use Committee approved all experimental approaches.
Genotyping.
DNA was extracted from ear punches or tail biopsies for genotyping by incubating at 95°C in 100 μl of 25 mM NaOH/0.2 mM EDTA for 20 min and then neutralizing with 100 μl of 40 mM Tris·HCl, pH 5.0. Subsequent genotyping reactions were carried out as previously described (39).
Collection of livers and other organ samples.
Mice were anesthetized with 3% isoflurane before being subjected to a thoracotomy and cardiac puncture to obtain blood. Organs were rapidly dissected from each euthanized animal, and the wet weights were recorded. Portions of the liver were either frozen in liquid nitrogen for protein and RNA analyses or fixed in phosphate-buffered 4% paraformaldehyde for subsequent paraffin embedding and various histological analyses.
CCl4 model of liver injury and regeneration.
CCl4 (0.6 μl/g body wt) in olive oil (200 μl) was injected into the peritoneum (ip) of adult male mice, and they were then euthanized at 24, 36, 48, 72, 96, or 120 h after injections. All injections were performed during the middle of the light phase to minimize circadian differences in toxicity (34). There were five or more mice per time point and no postinjection mortality. Because deletion of floxed DNA in hepatocytes is age dependent, we used mice that were at least 8 wk old in regeneration experiments.
CCl4 model of liver injury and fibrosis:.
CCl4 (0.6 μl/g body wt) in olive oil (200 μl) was injected into the peritoneum (ip) of adult male mice (between age-matched controls that were over 2 mo of age). Injections were given every 3–4 days over a 4-wk period. Four days after the final injection the livers were removed and weighed, and pieces were either frozen for biochemical or molecular analyses or fixed in 4% buffered paraformaldehyde for histochemical or immunohistochemical analyses.
Immunohistochemistry.
Livers were fixed in PBS-buffered 4% paraformaldehyde, and immunohistochemistry was carried out as previously described. Hepatic sections were immunostained with pY1197-ERBB3 (CS56E4), pY1068-EGFR (CS2234) (Cell Signaling Technology), anti-cyclin A2 (CCNA2) (sc-596, Santa Cruz Biotechnology), F4/80 (BM8 monoclonal), and LY6G (eBiosciences). Histological images were photographed on an Olympus Vanex AHBT3 microscope using a NIKON E5000 connected by a PTEM 257009 camera to an objective adaptor. In the CCl4 experiments, sections were stained with a total RNA stain to highlight and facilitate quantification of the necrotic areas. These images were photographed and uploaded to a computer, and the percentage of necrotic area was determined by use of the ImageJ program and the Universal Desktop Ruler (AVPSsoft) (40).
Western blotting.
Pieces of liver (about 100 mg) were weighed and then homogenized on ice using a 2-ml Wheaton glass tissue homogenizer in TGH buffer (20 mM HEPES, 1% Triton X-100, 10% glycerol, 50 mM NaCl). This buffer included protease inhibitors (1 mM PMSF, 10 μg/ml aprotinin, and 1 μg/ml leupeptin) as well as phosphatase inhibitors (10 mM sodium molybdate, 1 mM sodium orthovanadate, and 10 mM β-glycerol phosphate). Lysates were immunoblotted as previously described (35). We used the following affinity-purified antibodies from Santa Cruz Biotechnology: sc-634 for BCL2L1 (Bcl-xl), sc-596 for cyclin A2, sc-03 for EGFR, sc-285 for ERBB3, sc-283 for ERBB4, sc-15335 for α-actinin (ACTN), and sc-7150 for poly(ADP-ribose) polymerase (PARP). Antibodies from Cell Signaling Technology included pThr202/pTyr204 (44/42)-MAPK antibody (CS4370), β-catenin (CS8480), pY705-STAT3 (CS9131), pY1197-ERBB3 (CS56E4), pY1068-EGFR (CS2234), MET (CS4560), pThr197-PKA (CS4781), and pSer9-GSK3B (CS9336). For glutamine synthetase (GLUL), we used G2781 from Sigma-Aldrich. For heme oxygenase-1 (HO-1, HMOX1), we used SPA-895 from Stressgen. For cytochrome P450-2E1 (CYP2E1), we used AB28146 from Abcam. For albumin (ALB), we used 25A-2 from ICL Laboratories. For desmin (DES), we used A611 from Dako. For AREG, we used an antibody (Ab-2) from Thermo Fisher Scientific.
After each transfer, we confirmed equal protein loading and transfer by Ponceau S staining of immunoblots, scanning the image for future reference. Sample cohorts were analyzed on a single blot to ensure reliable comparison. Invariant proteins such as ALB, β-catenin, ACTN, and histone H3 served as loading controls. Immunoreactive signal was detected using the enhanced chemiluminescence method using either the Supersignal West Pico Chemiluminescent Substrate or the Supersignal West Femto Maximum Sensitivity Substrate (Thermo Scientific). The decision to use one or the other depended on the quality of the antibody as well as the abundance of the protein. We performed densitometry using an Epson scanner and the ImageJ program (40).
Statistical analysis.
Data are expressed as means ± SE. Statistical analysis was performed using an unpaired, two-tailed Student's t-test assuming equal variances between compared groups. A P value of <0.05 was determined to be statistically significant.
RESULTS
EGFR and ERBB3 expression and function are effectively reduced in the HS-EGFR-ERBB3-DKO.
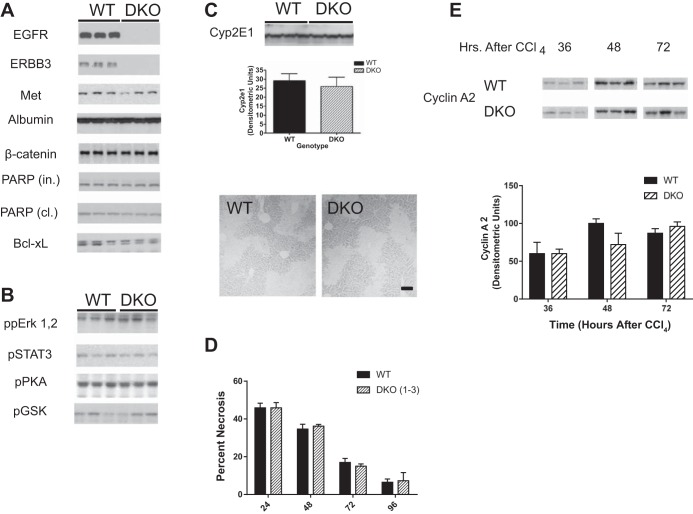
Previously we successfully disrupted EGFR or ERBB3 expression in hepatocytes to create HS-EGFR-KO and HS-ERBB3-KO mouse lines. These lines, which rely on Alb-cre recombinase to excise floxed Egfr or Erbb3 genes, showed reduced expression of EGFR or ERBB3 after weaning (38, 39). In this study, we took a similar approach to ablate the expression of both receptor tyrosine kinases in hepatocytes of the same mouse. To validate that we created a DKO mouse line, homogenates were prepared from the livers of male and female adult mice and immunoblotted for EGFR and ERBB3 as well as several other proteins. Both EGFR and ERBB3 were knocked down although some residual ERBB3 was detected and thought to be derived from nonparenchymal or bile duct cells (Fig. 1A). Somewhat surprisingly, the loss of two ERBB receptors did not lead to changes in the development, size, or histological appearance of the liver. Counter to our expectations, the DKO compared with the wild-type (WT) did not change the expression level or activation status in a number of important proteins. The expression levels of MET, ALB, β-catenin, the intact and cleaved forms of PARP, and Bcl-xL (Fig. 1A) or the phosphorylated isoforms of proteins such as ERK1, 2, STAT3, PKA, or GSK (Fig. 1B) did not change in the DKO compared with WT liver. This indicates that the loss of EGFR-EGFR homodimers or EGFR-ERBB3 heterodimers does not have a widespread effect on protein synthesis or activation.
Fig. 1.
Creation of a hepatocyte-specific epidermal growth factor receptor-ERBB3 double knockout (HS-EGFR-ERBB3 DKO). A and B: EGFR and ERBB3 were genetically ablated in mice and evaluated by immunoblot for the expression of a number of proteins (A) or phosphorylated proteins (B). Of the proteins examined, the only proteins that changed in the livers of adult male mice were EGFR and ERBB3. PARP, poly(ADP-ribose) polymerase; in, intact; cl, cleaved. C: expression of cytochrome P450 2E1 (Cyp2E1) was analyzed in immunoblots of liver lysates from wild-type (WT) and DKO mice. As shown in the immunoblot and densitometric analysis, no difference was seen in the overall expression of this enzyme, which is required for CCl4-induced liver damage. The photomicrograph shows a representative section of the centrilobular damage caused by CCl4. The lighter areas around central veins show the loss of hepatocytes attributable to necrosis and apoptosis. Bar = 50 μm. D: mice were injected with CCl4 at 0 time and monitored for liver damage at 24, 48, 72, and 96 h after injection. No differences were observed in the necrotic area at 24 h, indicative that the initial necrotic areas were comparable in WT and DKO mice. The rate of “wound shrinkage or healing” was also comparable over the next 96 h. E: lysates from the livers of WT and DKO mice injected with CCl4 were monitored by immunoblots (left) for cyclin A2, a marker of DNA synthesis. This marker was not detected in control mice at zero time (data not shown). No differences were seen between WT and DKO mice in quantitative densitometry of cyclin A2 in DKO or WT mice (right).
Loss of ERBB3 and EGFR does not increase liver injury or retard regeneration following the injection of CCl4.
We recently showed that the injection of CCl4 had little or no effect on the extent of centrilobular injury or the regenerative process in HS-EGFR-KO and HS-ERBB3-KO mice (38, 39). The creation of a HS-EGFR-MET (met proto-oncogene)-DKO did have a more severe phenotype than the single gene HS-MET-KO mouse (38). We speculated that the HS-EGFR-ERBB3-DKO would have a more severe phenotype as well. We generated this mouse line and analyzed the changes in liver injury and repair at 24, 48, 72, and 96 h. The loss of EGFR and ERBB3 did not affect the expression of CYP2E1 (Fig. 1C), which metabolizes CCl4 to reactive intermediates capable of causing centrilobular necrosis and inducing liver regeneration. The liver of the DKO did not feature more centrilobular necrosis or a delayed regeneration (Fig. 1, C and D). As we have previously shown for the single-gene EGFR or ERBB3 KOs, when we evaluated cell proliferation after CCl4 injury in these mice using cyclin A2 (CCNA2) expression, we found no differences in the timing or expression levels between DKO and WT mice (Fig. 1E).
Loss of EGFR and ERBB3 reduce CCl4-induced liver fibrosis.
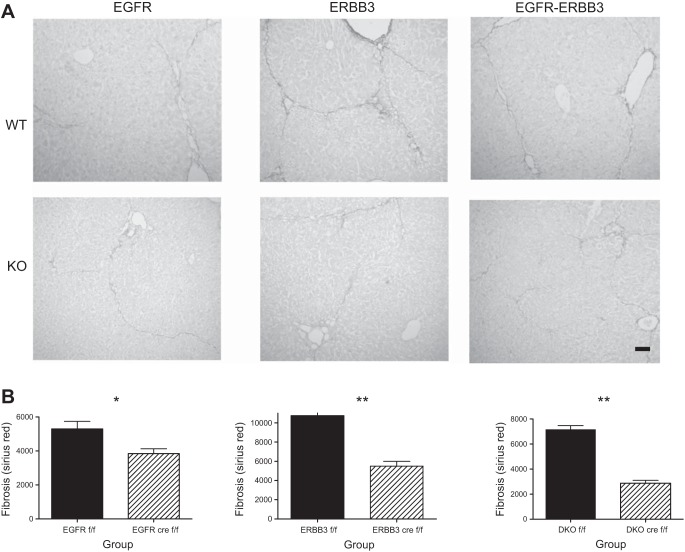
We injected mice with CCl4 every 3–4 days for 4 wk. This period of time is sufficient to induce fibrosis with minimal inflammation. At the end of this period, we euthanized the mice and harvested liver tissue for subsequent immunological or histological analyses. We stained liver sections from the single gene and double-gene KO as well as controls (WT) with Sirius red, a dye that binds fibrillar collagen (Fig. 2A). Sirius red normally stains the portal tracts, but, in the fibrotic liver, additional strands of fibrillar collagen become apparent, eventually bridging between major veins. We were able to quantify the decreased fibrosis to be about 25% in the CCl4-treated HS-EGFR-KO mouse liver (Fig. 2B, left). We then determined whether loss of ERBB3, the primary heterodimeric signaling partner for EGFR in the adult mouse liver, affected fibrosis. Hepatocytes possess 20 times as many EGFR as ERBB3 receptors, and the effect on fibrosis could involve activation of EGFR-ERBB3 heterodimers as opposed to EGFR homodimers. ERBB3 ablation alone decreased fibrosis by nearly 50% (Fig. 2B, middle). When we examined the effect of dual EGFR-ERBB3 genetic disruption, we found that the fibrosis was reduced even further and at a higher statistical significance (Fig. 2B, right).
Fig. 2.
Analysis of fibrosis in WT and DKO mice subjected to repeated CCl4 injection over a 4-wk period. A: we injected HS-EGFR, HS-ERBB3, HS-EGFR-ERBB3 knockout mice, and their respective WT controls with CCl4 biweekly over 4-wk period. This resulted in fibrosis, as shown by staining liver sections with Sirius red to detect fibrillar collagen. Olive oil-injected controls showed staining mainly in the portal triads (data not shown). In contrast, both WT and KO livers showed fibrous strands, which sometimes bridged between central veins. These strands were decreased in all ERBB KO groups, particularly in the DKO mice. Bar = 100 μm. B: we quantified the fibrous area (excluding the normal areas of fibrosis around the portal triads) and confirmed the histologic impression of a significant reduction in fibrosis in the KO mice compared with their WT counterparts, particularly the DKO mice. All bars in graphs represent means + SE. *P < 0.01; **P < 0.0001.
Expression of fibrosis-modulated proteins in the WT and HS-EGFR-ERBB3-DKO mice.
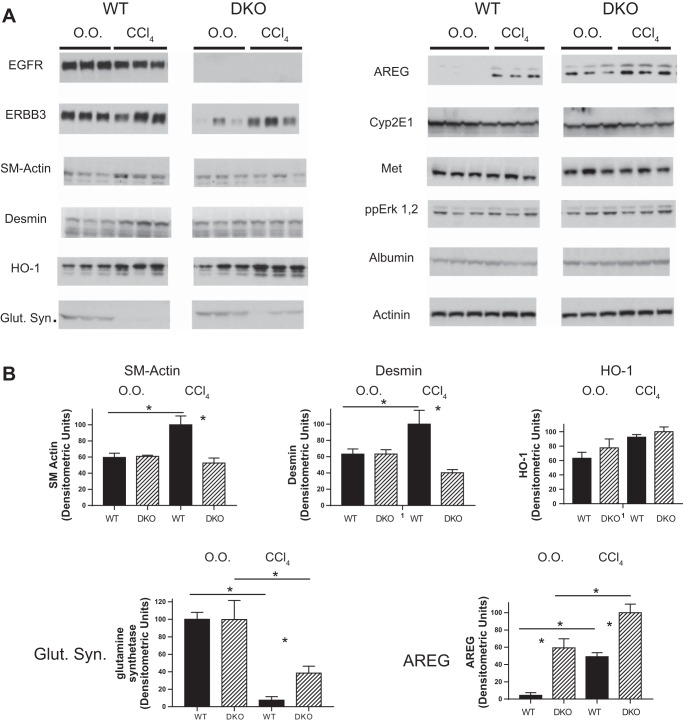
We examined the expression of several fibrosis-modulated proteins in the livers of WT or HS-EGFR-ERBB3-DKO mice after chronic injection of CCl4. Hepatic expression of either EGFR or ERBB3 decreased in the DKO control mice; however, the DKO mice chronically injected with CCl4 showed increased ERBB3 hepatic expression (Fig. 3). Two markers of fibrosis, smooth muscle actin and DES, were decreased in the DKO compared with the WT, consistent with the decrease in fibrosis in the former. HO-1 (HMOX1) acutely increases in the liver of mice injected with CCl4 and confers protection to hepatocytes (30). We hypothesized that the levels are higher in the hepatocytes of HS-EGFR-ERBB3-DKO mice compared with WT mice, thereby reducing injury after chronic CCl4 administration. Although HO-1 increased in both CCl4-treated WT and KO mice, the increase was not statistically significant at 4 days after the last CCl4 injection. GLUL expression decreases after chronic CCl4 injection (15). Interestingly, the levels of GLUL decreased less in the DKO mice compared with the WT mice. AREG increases in the CCl4-injured liver (31). We confirmed this and noted that AREG was actually higher in the livers of the DKO mice, suggesting that EGFR serves as a clearance receptor for AREG. Other proteins such as CYP2E1, the receptor tyrosine kinase, MET, the dually phosphorylated MAPK, ALB, and α-actinin showed identical expression levels in WT and the DKO mice in both control and CCl4-injected mice.
Fig. 3.
Evaluation of proteins or specific phosphoproteins in WT or DKO mice injected with CCl4 biweekly over a 4-wk period. A: we evaluated by immunoblot a number of molecules in lysates prepared from normal or fibrotic livers of WT or DKO mice. Contiguous lanes are represented in each subpanel. Albumin and actinin served as loading controls. SM, smooth muscle; HO-1, heme oxygenase-1; Glut. Syn., glutamine synthetase; AREG, amphiregulin; O.O., olive oil. B: all bars in graphs represent means + SE. Asterisks between bars represent a statistically significant comparison between WT and DKO groups. Asterisks above a line represent a statistically significant comparison between WT/DKO treated with O.O. compared with WT/DKO treated with CCl4. *P < 0.05.
Presence of inflammatory infiltrate.
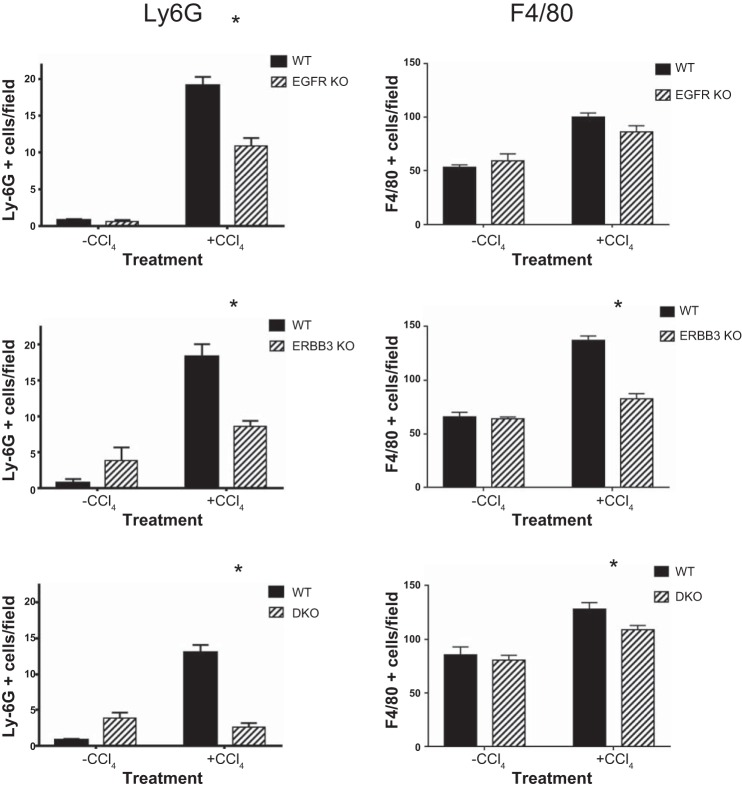
Liver injury in this model is accompanied by an inflammatory reaction involved in development as well as resolution of fibrosis. Although the amount of inflammation is limited at 4 wk after the initial CCl4 injection, we evaluated inflammatory change by looking at the expression of two inflammation markers, LY6G and F4/80 (Fig. 4). The former is a marker for granulocytes and infiltrating monocytes (20). The latter is a marker for resident Kupffer macrophages but also labels infiltrating monocytes. The expression of both increased after chronic CCl4 injection relative to the controls (Fig. 4). The change was most notable for LY6G because very few cells in the control mice expressed this marker, and the immunopositive cells were localized proximal to fibrotic septa (data not shown). In contrast, the normal liver contains many F4/80 Kupffer cells. Nearly all of the LY6G-positive cells appeared to be large monocytes, and only an isolated granulocyte was recognized (data not shown). When we compared the expression of these markers in the EGFR, ERBB3, and DKO mice compared with their respective WT mice, we noted that KO mice had fewer immunopositive cells. This decrease was most pronounced for the LY6G marker, suggesting that there was a reduction in the initial inflammatory reaction to repeated injections of CCl4 in the DKO compared with the WT liver.
Fig. 4.
Decreased inflammation in ERBB KO mice. To evaluate the amount of inflammation in this model, we quantified the number of cells that were positive for two different markers, Ly6G and F4/80. The former is a marker for granulocytes and activated monocytes, and the latter a marker for the resident hepatic macrophages or Kupffer cells. Left: number of Ly6G-positive cells in the various KO mice and their WT counterparts. Very few cells were identified in the olive oil-treated control mice; however, numerous cells, nearly all of which had the histological appearance of infiltrating monocytes, were detected proximal to or within the fibrous septa of the livers from mice injected with CCl4. There was a statistically significant increase of these cells in the various WT mice compared with their single- or double-gene KOs. *P < 0.05. Right: number of F4/80-positive cells in the various KO mice and their WT counterparts. Statistically significant increases were observed in all WT livers relative to KO livers, except for EGFR. *P < 0.05.
Immunohistochemical localization of the activated forms of EGFR and ERBB3.
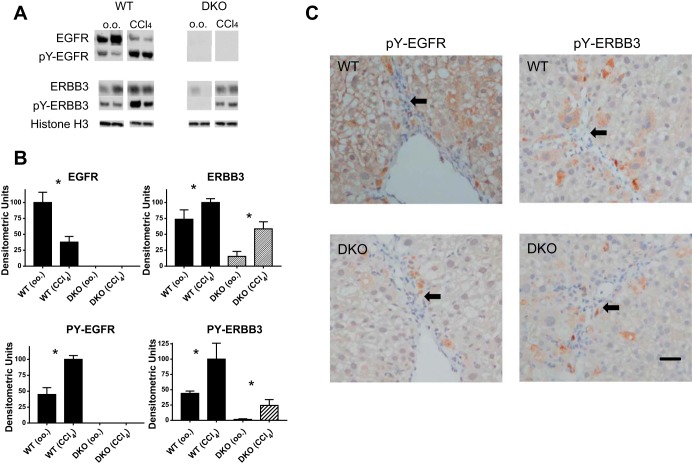
When we immunoblotted liver lysates from WT and DKO mice that had been injected repeatedly with CCl4, we noted that the WT mice relative to the olive oil-treated mice showed a reduction in the total EGFR levels but an increase in EGFR phosphorylation of tyrosine 1068 (PY-1068) (Fig. 5, A and B). Phosphorylation at this site increases upon EGF ligand binding and allows EGFR to dock to the GRB2 adaptor protein. A reduction of the total EGFR combined with increased tyrosine phosphorylation signifies increased ligand-mediated receptor activation because phosphorylation results in EGFR internalization and lysosomal degradation. We have reported a similar change in the ratio of total:phosphorylated EGFR in the liver of a mutant mouse that has a gain-of-function mutation in the EGFR kinase domain (37). When we immunoblotted for ERBB3, we did not see a reduction in total ERBB3 in the WT mice, but we did see increased tyrosine phosphorylation on tyrosine residue 1197 (PY-1197). Phosphorylation at this site increases upon HRG binding and signifies that ERBB3 has heterodimerized with an ERBB-kinase competent monomer, such as EGFR. Ligand-induced endocytosis is less common for ERBB3. Interestingly, we also saw increased ERBB3 and phospho-ERBB3 in the HS-DKO as well as the WT mice. This suggests that ERBB3 as well as EGFR may be hyperphosphorylated in this model of liver injury, not only in hepatocytes, but also in other liver cell types, such as macrophages and activated stellate cells.
Fig. 5.
Immunoblotting and immunohistochemistry for EGFR and ERBB3 in WT and DKO livers. A: we immunoblotted liver sections from mice that had been chronically injected for 4 wk biweekly with olive oil or CCl4 for EGFR, pY-1068 EGFR, ERBB3, and pY-1197 ERBB3. Tyrosine phosphorylation increased for both EGFR and ERBB3; however, the absolute level of EGFR decreased, presumably attributable to ligand-mediated degradation. Equal amounts of protein were loaded in each lane, as confirmed by Ponceau S staining and loading control Histone H3. B: we performed densitometry on bands from the groups represented in A (n = 5). All bars in graphs represent means + SE. *P < 0.05. C: we carried out immunohistochemistry using phospho-specific antibodies for EGFR or ERBB3 on liver sections in mice injected repetitively with CCl4. We noted that all of the antibodies prominently stained cells (brown diaminobenzidine) within the fibrous septa that have the histological appearance consistent with activated stellate cells or macrophages. Hepatocytes near the septa also stained, and this was notable for pY01197 ERBB3. The arrows identify areas of fibrotic septa that include more darkly stained, smaller nuclei. Bar = 35 μm.
We employed immunohistochemistry to define the cellular localization of the activated ERBB proteins, using two antibodies used for immunoblotting (Fig. 5C). We have previously shown that these antibodies show markedly increased immunostaining of EGFR or ERBB3 in mice injected with EGF or HRG, respectively, but little or no staining in the normal liver of mice injected with the PBS carrier (38). Sections from HS-EGFR KO or HS-ERBB3 KO mice injected with these ligands showed no hepatocellular staining, confirming specificity. When we immunostained sections from CCl4-injected mice for phospho-EGFR, we noted intense hepatocellular and nonparenchymal staining within or near the fibrotic septa (marked by arrows), even without exogenous HRG or EGF injection. Staining in fibrogenic cells and macrophages was easily resolved in sections from the HS-DKO mice because these mice showed little or no hepatocellular staining and the immunostained cells were easily recognized. When we immunostained for phospho-ERBB3, we noted a similar pattern of staining; however, the hepatocellular phospho-ERBB3 signal was much lower in sections from the WT livers compared with phospho-EGFR. This is consistent with the observation that hepatocytes have 20 times as many EGFR receptors compared with ERBB3 and are easier to detect by immunohistochemistry. Within fibrotic areas, as for phospho-EGFR, antibody prominently stained macrophages and activated stellate cells (Fig. 5C). These cells presumably account for the increased expression of ERBB3 and phospho-ERBB3 on immunoblot in the chronically injected HS-ERBB3-KO mice. In general, hepatocyte staining for phospho-ERBB3 in WT livers appeared to be more pronounced in cells adjacent to the fibrous septa than further away. Although the staining for EGFR was greater in the CCl4-injected than the olive oil-injected mice, we were not able to appreciate increased staining proximate to the fibrous septa. Phospho-EGFR staining appeared to be more uniform between the fibrous septa in the CCl4-injected mice (even though there is a clear periportal to centrilobular EGFR phosphorylation gradient following a single intraperitoneal injection of EGF in normal mice).
DISCUSSION
In this paper, we evaluated the role played by EGFR and ERBB3 in a model of chronic liver injury caused by repeated administration of the hepatotoxin CCl4. A DKO mouse was generated to study this question (Fig. 1). We originally hypothesized that genetic disruption of these molecules independently or together would increase hepatocyte injury or apoptosis, leading to increased fibrosis, as has been shown for the tyrosine kinase MET, the receptor for hepatic growth factor. Loss of hepatocellular MET is profibrogenic in this model (18, 28a), as well as in a model of chronic cholestasis caused by bile duct ligation (16). However, genetic disruption of either EGFR or ERBB3 in hepatocytes unexpectedly reduced fibrogenesis in CCl4-injected mice (Fig. 2). The loss of ERBB3 reduced fibrosis to a greater extent than EGFR alone, and the dual loss of these highly interactive receptor tyrosine kinases resulted in an even greater reduction in fibrosis. Concomitantly, there was a reduction in the recruitment of inflammatory monocytes (Fig. 4). Others have recently carried out studies showing that damaged hepatocytes contribute to progressive fibrosis by direct modification of the surrounding ECM, as well as through cytokine signaling to effector cells, such as immune cells or activated stellate cells (44). These findings indicate that hepatocytes are not passive bystanders but play active roles in fibrosis. In view of our results, these roles may involve hepatocellular EGFR-ERBB3 signaling.
Other studies implicate a positive regulatory role for ERBB receptors and their ligands in fibrogenesis in other organs (5, 29). A dominant-negative ERBB3, which blocked ERBB3-ERBB2 signaling, attenuated lung fibrosis after bleomycin-induced injury (12). Gefitinib, a small kinase inhibitor of EGFR signaling, also decreased lung damage induced by bleomycin (19). In transgenic mice, respiratory epithelial overexpression of TGF-α, another EGF-type ligand, induced lung fibrosis (32). In the pancreas, overexpression of HBEGF stimulated stromal fibrosis and the replacement of parenchymal tissue. This could be reversed by crossing this mouse to one bearing a hypomorphic allele of Egfr, the Egfrwa2 mutation, which attenuates EGFR kinase activity by 80–95% (5, 29). In each of these cases, the increased fibrosis was attributed to stimulation of pancreatic stellate cells, but the hypomorphic allele was in all pancreatic cells that expressed EGFR. Although we have focused in this paper on hepatocellular ERBB signaling, immunoblot and immunohistochemical studies revealed increased expression and phosphorylation of EGFR and ERBB3 in activated stellate cells and macrophages within or proximal to the fibrogenic septa (Fig. 5C). In subsequent studies, it will be of interest to determine how the loss of ERBB proteins in various nonparenchymal cells alters hepatic fibrogenesis.
The specific mechanisms by which the loss of ERBB signaling in hepatocytes diminishes fibrosis remain to be defined. Both ERBB signaling and fibrogenesis are complex. ERBB signaling involves multiple ligands, including EGF-like and HRG ligands and interactions between members of the ERBB family and other tyrosine kinases (6). Fibrogenesis involves many signaling molecules, some of which are still being discovered. Fibrogenesis involves oxidative stress, hepatocellular injury and apoptosis, regeneration, an inflammatory response involving distinct macrophage populations, and the proliferation and activation of stellate cells. It is a dynamic and reversible process in its early stages. Decreased fibrosis or even increased fibrolysis can modulate the extent of fibrosis (43, 46). In rats, repeated administration of erlotinib, a small-molecule EGFR kinase inhibitor, has been used to show that EGFR kinase inhibition over a several-month period suppressed fibrosis caused by diethylnitrosamine or CCl4 (13). This kinase inhibitor also reduced the fibrosis that ensues after bile duct ligation. Whether EGFR kinase inhibition mainly affected fibrogenic cells or possibly hepatocytes was not determined. Our work extends this observation to the mouse but emphasizes the importance of ERBB3 in EGFR-initiated fibrosis, specifically in hepatocytes.
We initially speculated that the loss of hepatocellular ERBB signaling might result in a decreased production of AREG, a mitogen for fibrogenic cells. It is well known that signaling through EGFR in vitro leads to cascading production of EGF-like ligands, such as AREG (1, 23). Liver injury is also associated with increased production of EGF-like ligands, such as AREG, by hepatocytes as well as matrix-producing cells (3). A global Areg-KO mouse showed half as much fibrosis at 4 wk of biweekly CCl4 injections, comparable to that reported in our HS-EGFR-ERBB3-DKO (31). EGFR signaling causes hepatocytes in vitro to synthesize EGFR ligands. However, immunoblot analysis did not support this hypothesis (Fig. 3, A and B). In fact, AREG was actually increased to a greater extent in the DKO control and experimental mice, consistent with the demonstrated role for hepatocyte EGFR as a clearance receptor for AREG and other EGF-like ligands (7, 8).
ERBB signaling has been shown to increase the expression of CTGF, a key mediator of fibrogenesis in the liver. EGF ligands were shown to increase CTGF in hepatocarcinoma cell lines through a Yes-associated protein transcription factor signaling (45). HRG also induces CTGF expression in hypertrophic scarring fibroblasts through activation of the PI3K- or Src-mediated pathways (21). Surprisingly, the COOH-terminal module of CTGF (IV) has recently been shown to be a novel tyrosine kinase-activating ligand for EGFR (33), and one wonders whether ERBB-stimulated production of CTGF arising from activated stellate cells coordinates the ERBB signaling network in hepatocytes and activated stellate cells. Very little is known about the role of the interaction of CTGF with EGFR or other ERBB family members during liver fibrosis; however, the potential for a direct interaction between CTGF and EGFR in hepatocytes or nonparenchymal cells is intriguing. CTGF is made in vivo by stellate cells. It is a mosaic protein that has different modules allowing it to interact with distinct receptors. Loss of CTGF-induced EGFR-ERBB3 heterodimeric signaling in the hepatocyte injury phenotype could disrupt this potential hepatocyte-stellate cell communication (Fig. 6).
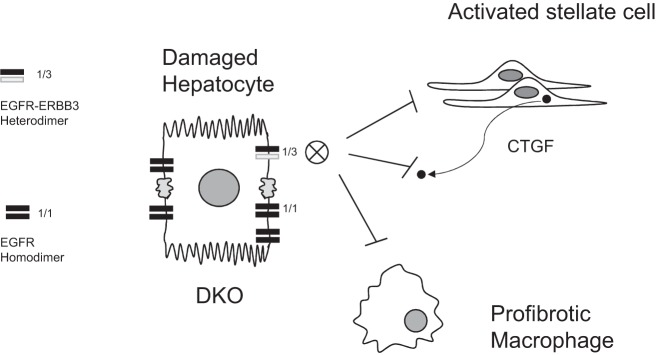
Fig. 6.
Schematic summarizing the major finding of this study. ERBB signaling has been shown to stimulate the proliferation and migration of stellate and fibrogenic cells in vitro; however, we show that loss of EGFR or ERBB3 in hepatocytes diminishes fibrosis. We hypothesize that, in the damaged liver, hepatocellular EGFR-ERBB3 heterodimeric signaling (as opposed to the more numerous EGFR homodimers) blocks inflammation and fibrogenesis. Because connective tissue growth factor (CTGF) is a ligand for EGFR, the DKO hepatocyte would also not be responsive to stellate cell-generated CTGF through EGFR binding and signal transduction (although CTGF is a mosaic ligand capable of binding to several different receptors through alternative domains). Paracrine release of CTGF by activated stellate cells may be a mechanism for stellate cell cross talk with damaged hepatocytes as well as EGFR-bearing macrophages in fibrotic areas.
Limitations of this study include the sampling density after the last injection of CCl4 and the overall duration of treatment. In the fibrosis studies, mice were euthanized at 4 days after the last injection to avoid the acute effects of CCl4. Future experiments in which the liver is monitored at earlier and later time points after the last injection, possibly with a high-throughput analysis of gene expression, may suggest mechanisms as to how ERBB signaling promotes fibrosis. Increasing the overall length of the experiment from 4 wk will also increase the level of inflammatory change and fibrosis. Increasing sampling density and duration of treatment will allow us to better evaluate other inflammatory and fibrotic markers, such as inflammatory cytokines, CTGF, TGF-β, or NADPH oxidases (28). Given the differential localization and activation of EGFR (periportal) and ERBB3 (centrilobular) along the hepatocyte lobule as well as physiological downregulation of EGFR but not ERBB3 in obesity and type II diabetes, it will also be of interest to evaluate how loss of either molecule alters other fibrotic conditions, including those following bile duct ligation or nonalcoholic steatosis (4). We hypothesize that the reduction in EGFR-ERBB3 heterodimer formation in damaged hepatocytes of CCl4-treated mice suppresses a proinflammatory or profibrogenic communication between the hepatocyte and stellate cells or inflammatory cells, such as macrophages (Fig. 6).
GRANTS
This work was supported by R01DK53804 and R21 AA021443 (to W. Russell) and R01CA092479 (to D. Threadgill).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.A.S., D.W.T., and W.E.R. conception and design of research; L.A.S. and X.Z. performed experiments; L.A.S. and X.Z. analyzed data; L.A.S. and X.Z. interpreted results of experiments; L.A.S. prepared figures; L.A.S. drafted manuscript; L.A.S., X.Z., D.W.T., and W.E.R. edited and revised manuscript; L.A.S. and W.E.R. approved final version of manuscript.
REFERENCES
- 1.Barnard JA, Graves-Deal R, Pittelkow MR, DuBois R, Cook P, Ramsey GW, Bishop PR, Damstrup L, Coffey RJ. Auto- and cross-induction within the mammalian epidermal growth factor-related peptide family. J Biol Chem 269: 22817–22822, 1994. [PubMed] [Google Scholar]
- 2.Benveniste R, Danoff TM, Ilekis J, Craig HR. Epidermal growth factor receptor numbers in male and female mouse primary hepatocyte cultures. Cell Biochem Funct 6: 231–235, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Berasain C, Garcia-Trevijano ER, Castillo J, Erroba E, Santamaria M, Lee DC, Prieto J, Avila MA. Novel role for amphiregulin in protection from liver injury. J Biol Chem 280: 19012–19020, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bettaieb A, Jiang JX, Sasaki Y, Chao TI, Kiss Z, Chen X, Tian J, Katsuyama M, Yabe-Nishimura C, Xi Y, Szyndralewiez C, Schroder K, Shah A, Brandes RP, Haj FG, Torok NJ. Hepatocyte nicotinamide adenine dinucleotide phosphate reduced oxidase 4 regulates stress signaling, fibrosis, and insulin sensitivity during development of steatohepatitis in mice. Gastroenterology 149: 468–480; e410, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaine SA, Ray KC, Branch KM, Robinson PS, Whitehead RH, Means AL. Epidermal growth factor receptor regulates pancreatic fibrosis. Am J Physiol Gastrointest Liver Physiol 297: G434–G441, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess AW, Henis YI, Hynes NE, Jovin T, Levitzki A, Pinkas-Kramarski R, Yarden Y. EGF receptor family: Twisting targets for improved cancer therapies. Growth Factors 32: 74–81, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Burwen SJ, Barker ME, Goldman IS, Hradek GT, Raper SE, Jones AL. Transport of epidermal growth factor by rat liver: Evidence for a nonlysosomal pathway. J Cell Biol 99: 1259–1265, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burwen SJ, Barker ME, Goldman IS, Jones AL. The effect of concentration on hepatic transport of exogenous epidermal growth factor. Hepatology 5: 211–214, 1985. [DOI] [PubMed] [Google Scholar]
- 9.Carver RS, Sliwkowski MX, Sitaric S, Russell WE. Insulin regulates heregulin binding and ErbB3 expression in rat hepatocytes. J Biol Chem 271: 13491–13496, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Carver RS, Stevenson MC, Scheving LA, Russell WE. Diverse expression of ErbB receptor proteins during rat liver development and regeneration. Gastroenterology 123: 2017–2027, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Charlotte F, Win KM, Preaux AM, Mavier P, Dhumeaux D, Zafrani ES, Rosenbaum J. Immunolocalization of heparin-binding growth factors (HBGF) types 1 and 2 in rat liver. Selective hyperexpression of HBGF-2 in carbon tetrachloride-induced fibrosis. J Pathol 169: 471–476, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Faress JA, Nethery DE, Kern EF, Eisenberg R, Jacono FJ, Allen CL, Kern JA. Bleomycin-induced pulmonary fibrosis is attenuated by a monoclonal antibody targeting HER2. J Appl Physiol 103: 2077–2083, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, McGinn CM, DePeralta DK, Chen X, Kuroda T, Lanuti M, Schmitt AD, Gupta S, Crenshaw A, Onofrio R, Taylor B, Winckler W, Bardeesy N, Caravan P, Golub TR, Tanabe KK. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology 59: 1577–1590, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii T, Fuchs BC, Yamada S, Lauwers GY, Kulu Y, Goodwin JM, Lanuti M, Tanabe KK. Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol 10: 79, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebhardt R, Reichen J. Changes in distribution and activity of glutamine synthetase in carbon tetrachloride-induced cirrhosis in the rat: Potential role in hyperammonemia. Hepatology 20: 684–691, 1994. [PubMed] [Google Scholar]
- 16.Giebeler A, Boekschoten MV, Klein C, Borowiak M, Birchmeier C, Gassler N, Wasmuth HE, Muller M, Trautwein C, Streetz KL. c-Met confers protection against chronic liver tissue damage and fibrosis progression after bile duct ligation in mice. Gastroenterology 137: 297–308; e291-294, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Huang G, Besner GE, Brigstock DR. Heparin-binding epidermal growth factor-like growth factor suppresses experimental liver fibrosis in mice. Lab Invest 92: 703–712, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA 101: 4477–4482, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii Y, Fujimoto S, Fukuda T. Gefitinib prevents bleomycin-induced lung fibrosis in mice. Am J Respir Crit Care Med 174: 550–556, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50: 261–274, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Kim JS, Choi IG, Lee BC, Park JB, Kim JH, Jeong JH, Jeong JH, Seo CH. Neuregulin induces CTGF expression in hypertrophic scarring fibroblasts. Mol Cell Biochem 365: 181–189, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Kiso S, Kawata S, Tamura S, Ito N, Tsushima H, Yamada A, Higashiyama S, Taniguchi N, Matsuzawa Y. Expression of heparin-binding EGF-like growth factor in rat liver injured by carbon tetrachloride or D-galactosamine. Biochem Biophys Res Commun 220: 285–288, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Komurasaki T, Toyoda H, Uchida D, Nemoto N. Mechanism of growth promoting activity of epiregulin in primary cultures of rat hepatocytes. Growth Factors 20: 61–69, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Lee D, Yu M, Lee E, Kim H, Yang Y, Kim K, Pannicia C, Kurie JM, Threadgill DW. Tumor-specific apoptosis caused by deletion of the ERBB3 pseudo-kinase in mouse intestinal epithelium. J Clin Invest 119: 2702–2713, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee TC, Threadgill DW. Generation and validation of mice carrying a conditional allele of the epidermal growth factor receptor. Genesis 47: 85–92, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: A translational success story. Gut 64: 830–841, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemmon MA, Schlessinger J, Ferguson KM. The EGFR family: Not so prototypical receptor tyrosine kinases. Cold Spring Harb Perspect Biol 6: a020768, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang S, Kisseleva T, Brenner DA. The role of NADPH oxidases (NOXs) in liver fibrosis and the activation of myofibroblasts. Front Physiol 7: 17, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Marquardt JU, Seo D, Gomez-Quiroz LE, Uchida K, Gillen MC, Kitade M, Kaposi-Novak P, Conner EA, Factor VM, Thorgeirsson SS. Loss of c-Met accelerates development of liver fibrosis in response to CCl4 exposure through deregulation of multiple molecular pathways. Biochim Biophys Acta 1822: 942– 951, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Means AL, Ray KC, Singh AB, Washington MK, Whitehead RH, Harris RC Jr, Wright CV, Coffey RJ Jr, Leach SD. Overexpression of heparin-binding EGF-like growth factor in mouse pancreas results in fibrosis and epithelial metaplasia. Gastroenterology 124: 1020–1036, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Nakahira K, Takahashi T, Shimizu H, Maeshima K, Uehara K, Fujii H, Nakatsuka H, Yokoyama M, Akagi R, Morita K. Protective role of heme oxygenase-1 induction in carbon tetrachloride-induced hepatotoxicity. Biochem Pharmacol 66: 1091–1105, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Perugorria MJ, Latasa MU, Nicou A, Cartagena-Lirola H, Castillo J, Goni S, Vespasiani-Gentilucci U, Zagami MG, Lotersztajn S, Prieto J, Berasain C, Avila MA. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology 48: 1251–1261, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Pillow JJ, Korfhagen TR, Ikegami M, Sly PD. Overexpression of TGF-alpha increases lung tissue hysteresivity in transgenic mice. J Appl Physiol 91: 2730–2734, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Rayego-Mateos S, Rodrigues-Diez R, Morgado-Pascual JL, Rodrigues Diez RR, Mas S, Lavoz C, Alique M, Pato J, Keri G, Ortiz A, Egido J, Ruiz-Ortega M. Connective tissue growth factor is a new ligand of epidermal growth factor receptor. J Mol Cell Biol 5: 323–335, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Scheving LA. Biological clocks and the digestive system. Gastroenterology 119: 536–549, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Scheving LA, Buchanan R, Krause MA, Zhang X, Stevenson MC, Russell WE. Dexamethasone modulates ErbB tyrosine kinase expression and signaling through multiple and redundant mechanisms in cultured rat hepatocytes. Am J Physiol Gastrointest Liver Physiol 293: G552–G559, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Scheving LA, Tsai TH, Cornett LE, Feuers RJ, Scheving LE. Circadian variation of epidermal growth factor receptor in mouse liver. Anat Rec 224: 459–465, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Scheving LA, Zhang X, Garcia OA, Wang RF, Stevenson MC, Threadgill DW, Russell WE. Epidermal growth factor receptor plays a role in the regulation of liver and plasma lipid levels in adult male mice. Am J Physiol Gastrointest Liver Physiol 306: G370–G381, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheving LA, Zhang X, Stevenson MC, Threadgill DW, Russell WE. Loss of hepatocyte EGFR has no effect alone but exacerbates carbon tetrachloride-induced liver injury and impairs regeneration in hepatocyte Met-deficient mice. Am J Physiol Gastrointest Liver Physiol 308: G364–G377, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheving LA, Zhang X, Stevenson MC, Weintraub MA, Abbasi A, Clarke AM, Threadgill DW, Russell WE. Loss of hepatocyte ERBB3 but not EGFR impairs hepatocarcinogenesis. Am J Physiol Gastrointest Liver Physiol 309: G942–G954, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinkamp MP, Low-Nam ST, Yang S, Lidke KA, Lidke DS, Wilson BS. ErbB3 is an active tyrosine kinase capable of homo- and heterointeractions. Mol Cell Biol 34: 965–977, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takemura T, Yoshida Y, Kiso S, Saji Y, Ezaki H, Hamano M, Kizu T, Egawa M, Chatani N, Furuta K, Kamada Y, Iwamoto R, Mekada E, Higashiyama S, Hayashi N, Takehara T. Conditional knockout of heparin-binding epidermal growth factor-like growth factor in the liver accelerates carbon tetrachloride-induced liver injury in mice. Hepatol Res 43: 384–393, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: Concept to treatment. J Hepatol 62: S15–S24, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Tu T, Calabro SR, Lee A, Maczurek AE, Budzinska MA, Warner FJ, McLennan SV, Shackel NA. Hepatocytes in liver injury: Victim, bystander, or accomplice in progressive fibrosis? J Gastroenterol Hepatol 30: 1696–1704, 2015. [DOI] [PubMed] [Google Scholar]
- 45.Urtasun R, Latasa MU, Demartis MI, Balzani S, Goni S, Garcia-Irigoyen O, Elizalde M, Azcona M, Pascale RM, Feo F, Bioulac-Sage P, Balabaud C, Muntane J, Prieto J, Berasain C, Avila MA. Connective tissue growth factor autocriny in human hepatocellular carcinoma: Oncogenic role and regulation by epidermal growth factor receptor/Yes-associated protein-mediated activation. Hepatology 54: 2149–2158, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Wallace MC, Friedman SL, Mann DA. Emerging and disease-specific mechanisms of hepatic stellate cell activation. Semin Liver Dis 35: 107–118, 2015. [DOI] [PubMed] [Google Scholar]