Abstract
In the current study, we have characterized the global miRNA expression profile in mouse pancreatic acinar cells and during acute pancreatitis using next-generation RNA sequencing. We identified 324 known and six novel miRNAs that are expressed in mouse pancreatic acinar cells. In the basal state, miR-148a-3p, miR-375-3p, miR-217-5p, and miR-200a-3p were among the most abundantly expressed, whereas miR-24-5p and miR-421-3p were the least abundant. Treatment of acinar cells with caerulein (100 nM) and taurolithocholic acid 3-sulfate [TLC-S (250 μM)] induced numerous changes in miRNA expression profile. In particular, we found significant overexpression of miR-21-3p in acini treated with caerulein and TLC-S. We further looked at the expression of miR-21-3p in caerulein, l-arginine, and caerulein + LPS-induced acute pancreatitis mouse models and found 12-, 21-, and 50-fold increased expression in the pancreas, respectively. In summary, this is the first comprehensive analysis of global miRNA expression profile of mouse pancreatic acinar cells in normal and disease conditions. Our analysis shows that miR-21-3p expression level correlates with the severity of the disease.
micrornas (mirna) are small noncoding, single-stranded RNAs that bind to the 3′UTR region of protein coding mRNAs, leading to mRNA cleavage or translational repression of their respective targets (18). Single miRNA can target multiple genes, thereby regulating several signaling molecules or pathways. Thus, aberrant expressions of miRNAs maintain the disease state through the regulation of multiple genes. Dysregulation of miRNA has been reported in various inflammatory diseases like arthritis (20), multiple sclerosis (5), and allergic diseases (21).
Recently, few studies have focused on the use of miRNA as a biomarker in acute pancreatitis, and it has been reported that miR-216 is specifically expressed in pancreas and plasma concentration of miR-216a and miR-216b significantly increased in a rat model of l-arginine-induced acute pancreatitis (7, 11). Using a rat model of bile acid-induced acute pancreatitis, Blenkiron et al. (2) observed increased expression of miRNAs miR-375, miR-217, miR-148a, miR-216a, miR-122, miR-214, and miR-138, in mesenteric lymph fluid (2). Despite their emerging pathophysiological role in various diseases, there is no comprehensive information available about miRNA dysregulation during acute pancreatitis. One of the challenges in studying miRNA in pancreatitis is the nonavailability of acinar cell-specific global expression profile of miRNA during normal and the disease condition.
In this study, we characterized the global miRNA expression profile of mouse acinar cells in basal and hyperstimulated states using next-generation RNA sequencing. Levels of significantly differentially expressed miRNAs were validated using quantitative RT-PCR (qRT-PCR). While various treatments induced different overall miRNA expression profiles, the most significantly upregulated miRNA, miR-21-3p, was the same across all of the treatments. We further examined the expression levels and correlation of miR-21-3p with disease severity in various in vivo models of acute pancreatitis.
MATERIALS AND METHODS
Experimental Animals
All experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Minnesota. Male C57BL/6 mice at age 7–8 wk were purchased from Charles River Laboratories (Wilmington, MA) or Jackson Laboratories (Bar Harbor, ME). All animals were housed in standard autoclaved shoebox cages in a climate-controlled room with an ambient temperature of 23 ± 2°C and a 12:12-h light-dark cycle. Animals were fed standard laboratory chow, given water ad libitum, and randomly assigned to control or experimental groups.
Acinar Cell Preparation, Treatment, and RNA Isolation
Mouse pancreatic acinar cells were prepared, according to our previously reported protocol (22). In brief, the pancreas was harvested from healthy C57BL/6 mice and treated with collagenase IV in HEPES buffer pH 7.4 (10 mM HEPES, 130 mM NaCl, 5 mM KCl, 11 mM glucose, 9 mM sodium pyruvate, 10 mM MgCl2, and 1 mM CaCl2) containing 0.1% BSA, for 30 min at 37°C with gentle shaking in a water bath incubator. After 30 min, released cells were purified by passing twice through 4% BSA in HEPES buffer, followed by washing twice with HEPES buffer. Viability of cells was tested using trypan blue dye. Pancreatic acinar cells were treated with various concentrations of caerulein or bile acid (TLC-S) for 1 h. Total RNA was isolated from these samples using a mirVana (Life Technologies, Carlsbad, CA) kit, according to the manufacturer's instructions. Human pancreatic acinar cells were obtained from the islet transplant research program at the University of Minnesota transplant facility. University of Minnesota Institutional Review Board approved the protocol for acquisition and use of all human pancreatic samples.
Small RNA Library Preparation and Sequencing
High-resolution quantification of miRNA expression was performed using small RNA sequencing from 1 μg of each pool of total RNA from three independent acinar preparations. Small RNA libraries were sequenced (15 samples per lane, average of 9 million reads per sample) on an Illumina HiSeq 2000, PEln51 (San Diego, CA) at the Medical Genome Facility, Mayo Clinic, Rochester, New York.
Small RNA Analysis and Identification of Novel miRNA
Raw sequence FASTQ files were quality checked using FastQC before and after adapter trimming with Cutadapt (16). These were then aligned to the Mouse Dec. 2011 (GRCm38/mm10) Assembly genome using the Bowtie algorithm (13). Sequenced RNAs were analyzed using miRDeep2 to identify candidate novel miRNAs (6). Proposed secondary structures and free energies of candidate novel miRNAs were determined using mfold version 3 (17), and chromosomal positions in the mm10 genome were determined using the BLAT tool in the UCSC Genome Browser (9). The aligned reads were then quantified against the miRBase 21-transcript annotations for both precursor and mature miRNAs (12). Within each sample, reads from miRNA genes were normalized and scaled to counts per million. The mature miRNA that were found on multiple precursors were merged, and their counts were summed. Statistical analysis and clustering were performed using the Partek Genomics Suite software package (Partek, St. Louis, MO). Candidate novel miRNA sequences will be submitted to the miRBase Registry, and final names will be assigned upon acceptance of this article.
The data presented in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO series accession no. GSE81260 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=ynyxkgievzipvuh&acc=GSE81260).
miRNA qPCR
cDNA was prepared using the miRScript II RT kit (Qiagen, Valencia, CA), according to manufacturer's instructions using 100 ng of total RNA. qRT-PCR was performed using miRNA sequence-specific forward primers (Qiagen) and the universal reverse primer (Qiagen), using a LightCycler 480 detection system (Roche Diagnostics, Minneapolis, MN) with fast SYBR Green dye (Life Technologies). SnoU6 was used as the internal control. Fold change was calculated by the 2−ΔΔCT method.
Mouse Models of Acute Pancreatitis
Caerulein model.
Acute pancreatitis was induced in male C57BL/6 mice by seven hourly intraperitoneal injections of caerulein (50 μg/kg). Animals were killed 1 h after the last caerulein injection.
Caerulein + LPS model.
Acute pancreatitis was induced in male C57BL/6 mice by six hourly intraperitoneal injections of caerulein (50 μg/kg) followed by a single intraperitoneal injection of LPS (10 mg/kg). Animals were killed 1 h after LPS injection.
l-Arginine model.
Acute pancreatitis was induced in male C57BL/6 mice by two hourly intraperitoneal injections of 4.5 g/kg of filter-sterilized l-arginine solution (pH 7.0) in saline followed by three hourly subcutaneous injections 1.5 ml of saline. Animals were killed 72 h after the first injection of l-arginine.
Statistical Analysis
Data are represented as means ± SD. The significance of changes was evaluated using Student's t-test when comparing two groups, and ANOVA when comparing three or more groups. P values of less than 0.05 were considered to be significant.
RESULTS
Expression of Small RNAs and miRNAs in Mouse Pancreatic Acinar Cells
To obtain a comprehensive view of the small RNA profiles in mouse pancreatic acinar cells, we performed next-generation RNA sequencing with small RNA libraries generated from three independently prepared acinar samples. On average, 7.5 million reads were subjected to aligner, and we were able to align ∼56% of reads to the mm10 mouse genome. Approximately 68% of the aligned reads mapped to a mature miRNA, and we found on average 324 known miRNAs with ≥5× coverage. miRNAs (known and predicted) comprised the majority (68.2%) of the total small RNA population, followed by mitochondrial rRNA/tRNA (16.4%) and linc RNA (9.3%). snRNA and snoRNA constituted only a small (<1%) fraction of the total small RNA population.
We detected total of 324 miRNAs expressed in mouse pancreatic acinar cells (Supplemental Table S1). miR-148a-3p, miR-375-3p, miR-217-5p, and miR-200-3p were among the most abundant miRNAs in mouse pancreatic acinar cells (Table 1). miR-24-5p, miR-29a-5p, and miR-421-5p were among the minimally expressed miRNAs detected (Table 1).
Table 1.
Top 8 highly abundant and top 8 lowest expressed miRNA in mouse pancreatic acinar cells at the basal state
| miRNA | Total Read⃥9 Million |
|---|---|
| Most Expressed | |
| mmu-miR-148a-3p | 289545 |
| mmu-miR-375-3p | 94844 |
| mmu-miR-217-5p | 83001 |
| mmu-miR-200a-3p | 46778 |
| mmu-miR-200b-3p | 37233 |
| mmu-let-7i-5p | 33801 |
| mmu-miR-30a-5p | 31886 |
| mmu-let-7 g-5p | 24899 |
| Least Expressed | |
| mmu-let-7e-3p | 5.6 |
| mmu-miR-27a-5p | 5.5 |
| mmu-miR-15a-3p | 5.2 |
| mmu-miR-335-5p | 4.9 |
| mmu-miR-93-3p | 4.8 |
| mmu-miR-29a-5p | 4.8 |
| mmu-miR-421-3p | 4.1 |
| mmu-miR-24-1-5p | 3.9 |
Effect of Caerulein Treatment on Mouse Pancreatic Acinar Cells
Stimulation of acinar cells with supramaximal dose of caerulein (100 nM) initiates a series of pathological events resulting in pancreatitis. We hypothesized that these molecular changes are controlled, in part, by altered miRNA expression when acinar cells were stimulated using supramaximal dose of caerulein (100 nM). RNA-Seq analysis showed a change in expression profile of miRNA (Fig. 1A), and significant upregulation of miR-21-3p, miR-29a-5p, and miR-6240 was observed when compared with untreated acinar cells and downregulation of miR-450a-5p, miR-30b-3p, miR-126a-3p, miR-214-5p, miR-129-3p, and miR-126a-5p (Table 2). A complete list is provided as Supplemental Table S2. We validated expression level changes using qRT-PCR for miR-21-3p, miR-29a-5p, and miR-129-3p (Fig. 1B). These miRNAs remain unchanged at maximal stimulatory dose of caerulein (100 pM) (Fig. 1C). Moreover, maximal stimulatory dose of caerulein did not provoke the same differential miRNA expression (Table 2), indicating a potential relationship between dysregulation of these miRNAs and acute pancreatitis.
Fig. 1.
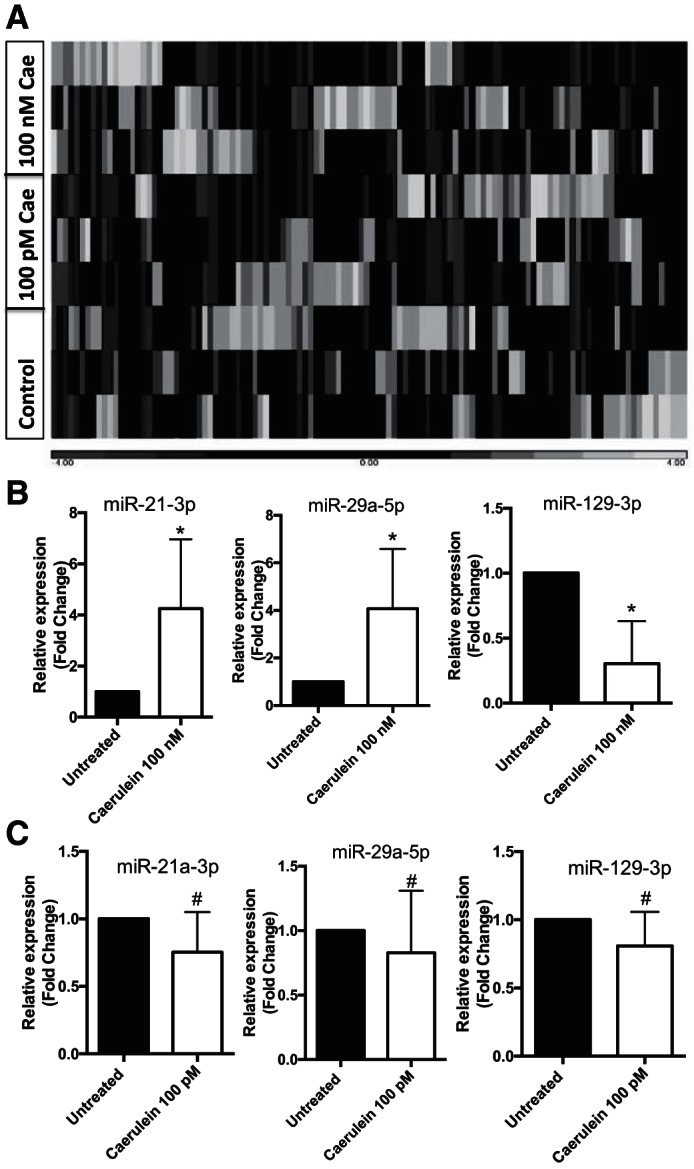
Change in miRNA landscape upon caerulein stimulation. A: heat map diagram and hierarchical clustering of differentially expressed miRNA between untreated control, 100 pM caeruelin (100 pM Cae), and 100 nM caerulein (100 nM Cae). Each column represents a sample either from untreated control, caerulein 100 pM, or caerulein 100 nM doses. B: quantitative PCR validation of selected miRNA, which changed in response to in vitro caerulein (100 nM) stimulation of mouse pancreatic acinar cells. C: expression levels of miR-21-3p, miR-29a-5p, and miR-129-3p after treatment with maximal stimulatory dose (100 pM) of caerulein for 1 h (n = 3; *P < 0.05, #P > 0.05). SnoU6 was used as an internal control. Data are represented as means ± SD.
Table 2.
Lists of top altered mouse miRNA after in vitro caerulein treatment and top altered mouse miRNA in response to in vitro caerulein stimulation
| miRNA | Fold Change | P Value |
|---|---|---|
| Caerulein treatment (100 nM) | ||
| mmu-miR-21-3p | 5.00 | 3.64E-02 |
| mmu-miR-29a-5p | 3.03 | 1.92E-02 |
| mmu-miR-6240 | 2.72 | 4.92E-02 |
| mmu-miR-3968 | 2.07 | 6.01E-04 |
| mmu-miR-450a-5p | −1.52 | 4.58E-02 |
| mmu-miR-126a-3p | −1.55 | 3.25E-02 |
| mmu-miR-30b-3p | −1.68 | 1.67E-02 |
| mmu-miR-214-5p | −2.15 | 4.52E-02 |
| mmu-miR-129-1-3p | −2.90 | 3.82E-02 |
| mmu-miR-126a-5p | −1.78 | 4.74E-03 |
| Caerulein stimulation (100 pM) | ||
| mmu-miR-450a-5p | −1.54 | 4.01E-02 |
| mmu-miR-24-2-5p | −1.55 | 1.29E-02 |
| mmu-miR-181c-3p | −1.58 | 2.24E-02 |
| mmu-miR-126a-5p | −1.61 | 1.09E-02 |
| mmu-miR-30b-3p | −1.70 | 1.55E-02 |
| mmu-miR-455-5p | −1.74 | 2.69E-02 |
| mmu-miR-132-3p | −1.76 | 3.06E-02 |
| mmu-miR-143-5p | −1.81 | 2.20E-02 |
| mmu-miR-17-3p | −2.81 | 4.33E-02 |
| mmu-miR-204-5p | −3.03 | 2.86E-02 |
Effect of Bile Acid (TLC-S) on miRNA Expression in Mouse Pancreatic Acinar Cells
We additionally profiled miRNA expression changes in response to treatment with the natural bile acid TLC-S. We used 250 μM TLC-S, which has previously been shown to induce pancreatic acinar cell injury and pancreatitis (3, 19). TLC-S treatment showed a change in expression profile of miRNA (Fig. 2A). Treatment with TLC-S (250 μM) resulted in significant upregulation of miR-21-3p, miR-27a-5p, and miR-486a/b-5p compared with untreated acinar cells and downregulation of miR-351a-5p and let-7b-3p, according to RNA-seq (Table 3). A lower dose of TLC-S (62.5 μM) did not induce the same differential miRNA expression (Table 3) as a higher dose (250 μM). A complete list is provided as Supplemental Table S3. We validated the deep sequencing data by looking the expression of miR-351-5p and let-7b-3p, which correlated with sequencing data (Fig. 2B). We further verified the expression of miR-21-3p by treating the acinar cells with different concentrations of TLC-S, and we observed a dose-dependent expression of miR-21-3p (Fig. 2C).
Fig. 2.
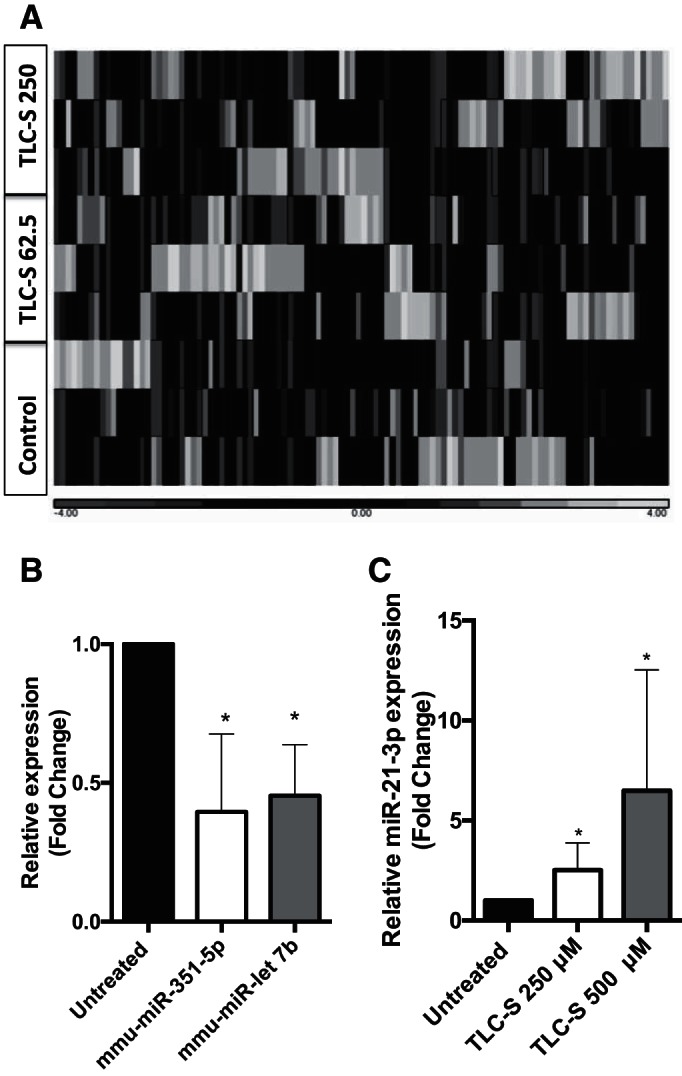
Change in miRNA landscape upon TLC-S stimulation. A: heat map diagram and hierarchical clustering of differentially expressed miRNA between untreated control, TLC-S 62.5 μM (TLC-S 62.5), and 250 μM (TLC-S 250). Each column represents a sample either from untreated control, TLC-S 62.5 μM, or TLC-S 250 μM. B: qPCR validation of selected miRNA in vitro TLC-S treatment. Mouse pancreatic acinar cells were treated with 250 μM of TLC-S for 1 h and total RNA was isolated. qPCR was done using miRNA-specific primer and SYBR Green dye (n = 3–4). C: expression levels of miR-21-3p, after treatment with different doses of TLC-S for 1 h (n = 4–7). SnoU6 was used as an internal control. *P < 0.05. Data are represented as means ± SD.
Table 3.
Lists of top altered mouse miRNA after in vitro 250 μM TLC-S treatment and top altered mouse miRNA in response to in vitro treatment with TLC-S
| miRNA | Fold Change | P Value |
|---|---|---|
| TLC-S treatment, 250 μM | ||
| mmu-miR-21-3p | 3.38 | 0.197 |
| mmu-miR-27b-5p | 2.10 | 7.97E-02 |
| mmu-miR-27a-5p | 1.97 | 4.48E-02 |
| mmu-miR-350-3p | 1.73 | 8.43E-02 |
| mmu-miR-486b-5p | 1.65 | 1.93E-02 |
| mmu-miR-486a-5p | 1.63 | 4.71E-02 |
| mmu-miR-300-3p | 1.62 | 8.32E-02 |
| mmu-let-7b-3p | −1.61 | 7.84E-02 |
| mmu-miR-351-5p | −2.27 | 4.81E-02 |
| TLC-S treatment, 62.5 μM | ||
| mmu-miR-335-5p | 5.26 | 1.22E-05 |
| mmu-miR-542-3p | 2.70 | 1.38E-03 |
| mmu-miR-708-5p | 2.59 | 1.63E-02 |
| mmu-miR-872-3p | 2.54 | 1.77E-02 |
| mmu-miR-1a-3p | 2.42 | 4.90E-02 |
| mmu-miR-184-3p | 2.16 | 1.11E-02 |
| mmu-miR-31-5p | 2.01 | 2.35E-02 |
| mmu-miR-30b-3p | −1.77 | 1.14E-02 |
| mmu-miR-216a-3p | −1.78 | 2.60E-02 |
| mmu-miR-30b-5p | −2.33 | 1.26E-02 |
Expression of miR-21-3p in Human Pancreatic Acinar Cells in Response to Hyperstimulation
miR-21-3p was significantly upregulated in both in vitro models of acute pancreatitis. Next, we examined whether these results could be replicated in acinar cells prepared from healthy human donor pancreas. Treatment with TLC-S (500 μM) or carbachol (1 mM) for 1 h was sufficient to stimulate more than a twofold increase in miR-21-3p expression (Fig. 3).
Fig. 3.
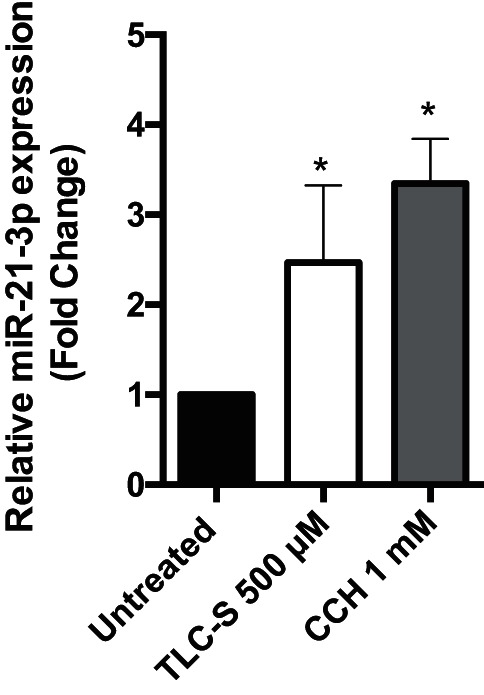
Expression of miR-21-3p in human pancreatic acinar cells. Human acinar cells were treated in vitro with 500 μM TLC-S or 1 mM carbachol (CCH) for 1 h, total RNA was isolated, and qPCR was done using miRNA-specific primer and SYBR Green dye (n = 3) (*P < 0.05). SnoU6 was used as an internal control. Data are represented as means ± SD.
Expression of miR-21-3p in Mouse Model of Acute Pancreatitis
We quantified miR-21-3p expression levels in mouse models of both mild and severe acute pancreatitis using qRT-PCR. We found more than a twofold and >10-fold increase in miR-21-3p expression in pancreata isolated from mice injected with either a single dose or seven hourly injections of caerulein (50 μg·kg−1·h−1), respectively (Fig. 4A, bars 2 and 3). In severe models of acute pancreatitis such as l-arginine or caerulein +LPS, miR-21-3P levels were >20 fold higher compared to the control group, suggesting a correlation between miR-21-3p levels and severity of the disease (Fig. 4, B and C).
Fig. 4.
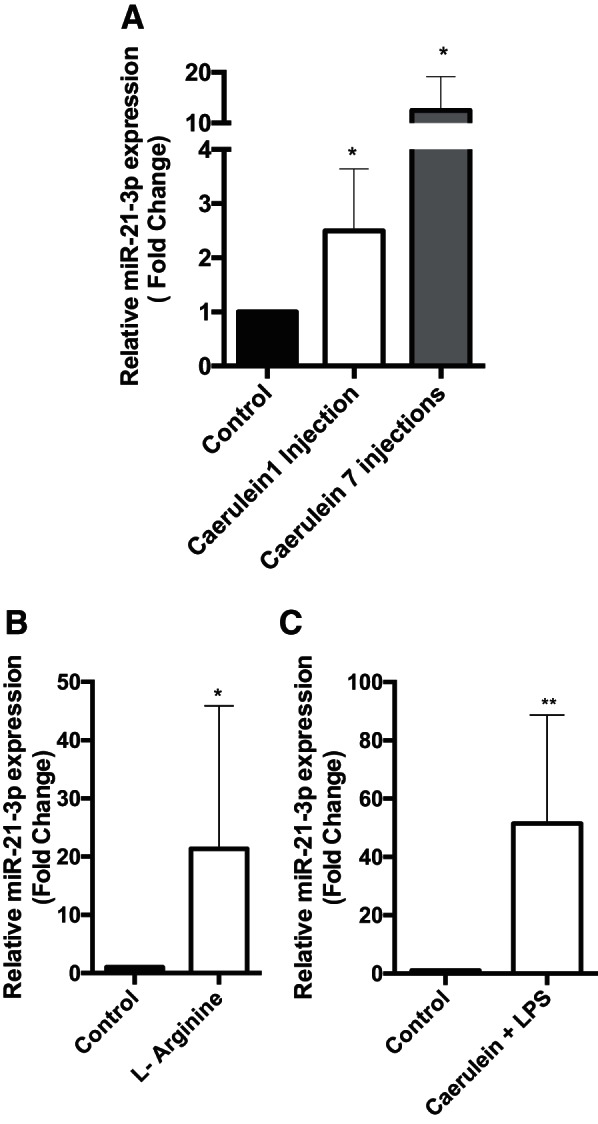
Change in expression level of miR-21-3p in acute pancreatitis mouse models. A: expression levels of miR-21-3p in 1 h or seven hourly injected caerulein mice (n = 4 in each group). SnoU6 was used as an internal control. B: expression levels of miR-21-3p in l-arginine model of acute pancreatitis (n = 5 in each group). C: expression levels of miR-21-3p in caerulein + LPS mice model (n = 5 in each group) (**P < 0.01, *P < 0.05). Data are represented as means ± SD.
Identification of Novel miRNAs
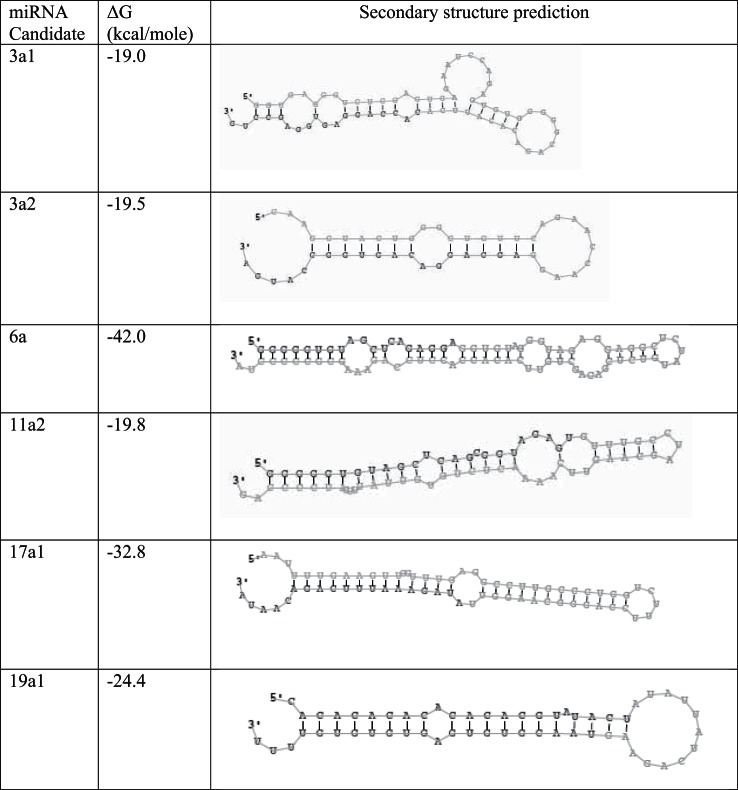
RNA sequences derived from untreated mouse pancreatic acinar cells that did not map to known mouse miRNAs were analyzed to identify candidate novel miRNAs based on length, secondary structure, and free energy (−ΔG). On the basis of this analysis, six novel candidate mature miRNAs and their putative precursor forms were identified (Table 4 and Fig. 5). Two of these newly discovered miRNAs, miR-3a2 and miR-6a1, are significantly upregulated in acinar cells treated with a supramaximal stimulatory dose of caerulein (100 nM), whereas miR-3a1, miR-11a2, miR-17a, and miR-19a1 were downregulated (Fig. 6A). Similar to miR-21-3p, expression levels of these novel miRNAs did not change significantly in acinar cells treated with maximal stimulatory dose of caerulein (100 pM) (Fig. 6B).
Table 4.
List of candidate novel miRNAs in mouse pancreatic acinar cells
| Identity | Sequence | Location | Gene Location | |
|---|---|---|---|---|
| Candidate 1 | 3a1 | caccaggaguggagccug | 3qE1 | Arhgef26, intron (–) |
| Candidate 2 | 3a2 | agcaggacaguggccauga | 3qH2 | Intergenic |
| Candidate 3 | 6a1 | ggggguguagcucagagga | 6qB1 | Intergenic |
| Candidate 4 | 11a2 | aaaacucuguguuauuugauccccag | 11qD | Grn, exon (–) |
| Candidate 5 | 17a1 | aauuuugaaguugguuugagg | 17qE11 | Intergenic |
| Candidate 6 | 19a1 | cacacacacacacacguauacu | 19qD2 | Gpam, intron, (–) |
Names were assigned on the basis of the chromosomal number on which they are located and the region.
Fig. 5.
Predicated secondary structure for novel candidate miRNAs.
Fig. 6.
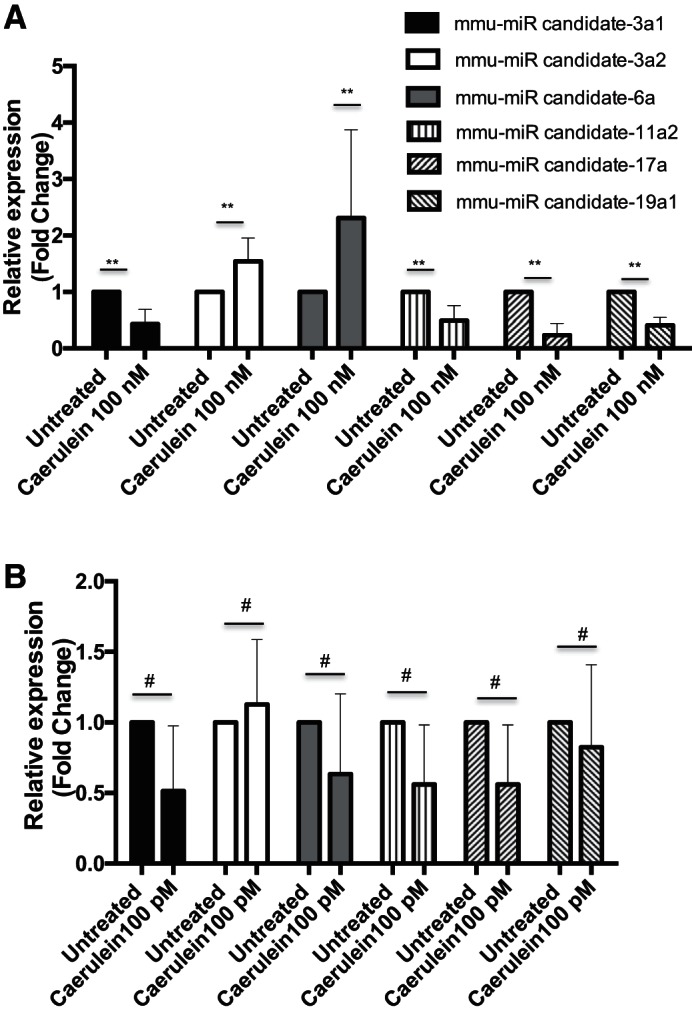
Expression of novel candidate miRNA in mouse pancreatic acinar cells. A: qPCR validation of novel candidate miRNA in in vitro caerulein (100 nM) treatment. (n = 3–6 in each group). B: expression levels of novel candidate miRNA after treatment with maximal stimulatory dose (100 pM) of caerulein for 1 h (n = 3 or 4 in each group). Data are represented as means ± SD (**P < 0.01, #P > 0.05).
DISCUSSION
Most of the previous work has primarily focused on the dysregulation of gene transcription during pancreatitis; however, the role of miRNAs, which can modulate gene expression in pancreatitis, has not yet been studied in detail. A growing body of evidence has established that miRNAs play critical regulatory roles in signaling pathways. Small changes in miRNA expression (twofold or threefold) have been found to be sufficient to induce biologically relevant changes (4). Only a few studies to date have focused on exploring the roles of miRNA in pancreatitis. In this study, we have provided a comprehensive analysis of miRNA expression in pancreatic acinar cells and how this expression signature changes in response to acute pancreatitis.
In our analysis, we found that miR-148a-3p, miR-216-5p, miR-217-5p, and miR-375-3p were among the most highly abundant miRNAs in pancreatic acinar cells. It has previously been reported that these miRNAs are downregulated during pancreatic carcinoma (23). In our study, expression levels of these miRNAs did not change significantly in acinar cells treated with supramaximal dose of caerulein; these miRNAs are, therefore, more likely to have an important role in normal acinar function and maintenance.
Caerulein treatment caused changes in overall miRNA profile, and miR-21a-3p was highly overexpressed followed by miR-29a-5p. We also performed Ingenuity Pathway Analysis functional analysis of their target. The screened miRNAs target genes were found to be associated with organ injury and abnormality, immunological disease, gastrointestinal disease, inflammatory disease, and inflammatory response.
TLC-S treatment had a different overall expression signature compared with caerulein treatment; however, miR-21-3p was common in both caerulein and TLC-S treatments. The higher P value observed in miR-21-3p in RNA-Seq in TLC-S treatment may be due to high variability among samples. We also performed a functional analysis of their target. The screened miRNAs target genes were found to be associated with gastrointestinal disease, organ injury and abnormality, infectious disease, and cellular development, and cancer, among others.
Importantly, these alterations appear to be specific to the acute pancreatitis condition, as these alterations in miRNA population were only observed when acinar cells were stimulated with pathological (supramaximal) dose of caerulein, but not with nonpathological (maximal-stimulatory) dose. Similar observations were made when acinar cells were treated with a higher dose (250 μM) vs. a lower dose (62.5 μM) of TLC-S.
The immature stem-loop form of miR-21, like all miRNA precursors, is processed into two separate mature miRNAs: miR-21-3p and miR-21-5p. Initially, it was thought that the 5p mature miRNA is the “active” miRNA, while the 3p mature miRNA (sometimes referred to as the *miRNA) was quickly degraded and had few functional roles. Recent studies have clearly demonstrated that both mature miRNAs can be functionally active, and even though they are coexpressed, their gene targets and functions may be very different (8, 10). Previous reports on miR-21 have focused on miR-21-5p (including early reports that did not specify 5p/3p, but the 5p mature miRNA was implied), including recent work that demonstrated that miR-21-5p promotes necrosis in acute pancreatitis (14). In our study, we did not observe a significant change in miR-21-5p expression when acinar cells were treated in vitro with caerulein for 1 h. However, we did observe upregulation of miR-21-5p at later time points in the caerulein-induced in vivo mouse model of acute pancreatitis (data not shown), consistent with its role in promoting necrosis at a later stage of disease. However, it is not very clear whether the increased miR-21-5p expression is in pancreatic acinar cells or infiltrating immune cells in in vivo acute pancreatitis models. It is interesting to speculate whether other mature miRNA pairs have complementary functional roles in disease, such as pancreatitis that may have been overlooked due to a bias toward studying the 5p strand.
A recent study showed that miR-21-3p target LAMP-2 mRNA (1) and deletion of LAMP-2 has been associated with pancreatitis (15). LAMP-2 knockout mice develop spontaneous pancreatitis. When we correlated the levels of miR-21-3p and LAMP-2 in mouse pancreatitis samples, we found a negative correlation (data not shown), suggesting miR-21-3p might be involved in pathogenesis via downregulating LAMP-2.
Our study presents first global miRNA expression signature of normal pancreatic acinar cells. It also reports the first characterization of how this miRNA expression profile changes upon induction of acute pancreatitis using both in vitro and in vivo models of the disease. miR-21-3p upregulation was consistently observed in all mouse models of acute pancreatitis. Upregulation of miR-21-3p was replicated in hyperstimulated human acinar cells. Future functional characterization of miR-21-3p and the other dysregulated miRNAs identified in this study will hopefully lead to the identification of novel therapeutic targets and treatment strategies for acute pancreatitis. This study will not only serve as a valuable resource for those interested in the roles of specific miRNAs in normal acinar physiology, it will also provide a reference for the study of miRNA-mediated abnormalities in acute pancreatitis.
GRANTS
This work was supported, in whole or in part, by the National Institutes of Health Grants RO1 DK-058694, RO1 DK-092145, and RO1 DK-093047 to A. K. Saluja.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K.D. conception and design of research; A.K.D. performed experiments; A.K.D., A.E.S., J.G., R.D., and S.S. analyzed data; A.K.D., Z.Y., J.G., U.B., H.C., A.S., S.B., V.D., R.D., S.S., and A.K.S. interpreted results of experiments; A.K.D. prepared figures; A.K.D. drafted manuscript; A.K.D. edited and revised manuscript; V.D. and A.K.S. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Prof. Masato Yamamoto, University of Minnesota and Prof. Sundaram Ramakrishnan, University of Miami, for their critical suggestions during the study.
This work was started at the Department of Surgery, University of Minnesota, 420 Delaware St., SE, Minneapolis MN 55455 USA and was concluded at the University of Miami.
REFERENCES
- 1.Alvarez-Erviti L, Seow Y, Schapira AH, Rodriguez-Oroz MC, Obeso JA, Cooper JM. Influence of microRNA deregulation on chaperone-mediated autophagy and α-synuclein pathology in Parkinson's disease. Cell Death Dis 4: 1–7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blenkiron C, Askelund KJ, Shanbhag ST, Chakraborty M, Petrov MS, Delahunt B, Windsor JA, Phillips AR. MicroRNAs in mesenteric lymph and plasma during acute pancreatitis. Ann Surg 260: 341–347, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Booth DM, Murphy JA, Mukherjee R, Awais M, Neoptolemos JP, Gerasimenko OV, Tepikin AV, Petersen OH, Sutton R, Criddle DN. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology 140: 2166–2125, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Calin G, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 6: 857–866, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Faria OD Jr, Moore CS, Kennedy TE, A J, Bar-Or A, Dhaunchak AS. MicroRNA dysregulation in multiple sclerosis. Front Genet 3: 311, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedlander MR, Mackowiak SD, Li N, Chen W, Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res 40: 37–52, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin D, Rosenzweig B, Zhang J, Xu L, Stewart S, Thompson K, Rouse R. Evaluation of miR-216a and miR-217 as potential biomarkers of acute pancreatic injury in rats and mice. Biomarkers 19: 517–529, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Huang CJ, Nguyen PNN, Choo KB, Sugii S, Wee K, Cheong SK, Kamarul T. Frequent co-expression of miRNA-5p and -3p species and cross-targeting in induced pluripotent stem cells. Int J Med Sci 11: 824–833, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kent WJ. BLAT-The BLAST-like alignment tool. Genome Res 12: 47–56, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choo KB, Soon YL, Nguyen PN, Hiew MS, Huang C-J. MicroRNA-5p and -3p co-expression and cross-targeting in colon cancer cells. J Biomed Sci 21: 95, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong XY, Du YQ, Li L, Liu JQ, Wang GK, Zhu JQ, Man XH, Gong YF, Xiao LN, Zheng YZ, Deng SX, Gu JJ, Li ZS. Plasma miR-216a as a potential marker of pancreatic injury in a rat model of acute pancreatitis. World J Gastroenterol 16: 4599, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozomara A, Griffiths-Jones S.. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 42: D68–D73, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X, Conklin DJ, Li F, Dai Z, Hua X, Li Y, Xu-Monette ZY, Young KH, Xiong W, Wysoczynski M, Sithu SD, Srivastava S, Bhatnagar A, Li Y. The oncogenic microRNA miR-21 promotes regulated necrosis in mice. Nature Commun 6: 7151, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mareninova OA, Sendler M, Malla SR, Yakubov I, French SW, Tokhtaeva E, Vagin O, Oorschot V, Lulülmann-Rauch R, Blanz J, Dawson D, Klumperman J, Lerch MM, Mayerle J, Gukovsky I, Gukovskaya AS. Lysosome-associated membrane proteins (LAMP) maintain pancreatic acinar cell homeostasis: LAMP-2-deficient mice develop pancreatitis. Cell Mol Gastroenterol Hepatol 6: 678–694, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetjournal 17: 2011. [Google Scholar]
- 17.Mathews DH, Sabina J, Zuker M, Turner DH. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol 288: 911–940, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell 148: 1172–1187, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muili KA, Wang D, Orabi AI, Sarwar S, Luo Y, Javed TA, Eisses JF, Mahmood SM, Jin S, Singh VP, Ananthanaravanan M, Perides G, Williams JA, Molkentin JD, Husain SZ. Bile acids induce pancreatic acinar cell injury and pancreatitis by activating calcineurin. J Biol Chem 288: 570–580, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakasa T, Nagata Y, Yamasaki K, Ochi M. A mini-review: microRNA in arthritis. Physiol Genomics 43: 566–570, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Rebane A, Akdis CA. MicroRNAs in allergy and asthma. Curr Allergy Asthma Rep 14: 424, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Singh VP, Bhagat L, Navina S, Sharif R, Dawra RK, Saluja AK. Protease-activated receptor-2 protects against pancreatitis by stimulating exocrine secretion. Gut 56: 958–964, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szafranska AE, Davison TS, John J, Cannon T, Sipos B, Maghnouj A, Labourier E, Hahn SA. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene 26: 4442–4452, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.