Abstract
A comprehensive genomic and proteomic, computational, and physiological approach was employed to examine the (previously unexplored) role of microRNAs (miRNAs) as regulators of internal anal sphincter (IAS) smooth muscle contractile phenotype and basal tone. miRNA profiling, genome-wide expression, validation, and network analyses were employed to assess changes in mRNA and miRNA expression in IAS smooth muscles from young vs. aging rats. Multiple miRNAs, including rno-miR-1, rno-miR-340-5p, rno-miR-185, rno-miR-199a-3p, rno-miR-200c, rno-miR-200b, rno-miR-31, rno-miR-133a, and rno-miR-206, were found to be upregulated in aging IAS. qPCR confirmed the upregulated expression of these miRNAs and downregulation of multiple, predicted targets (Eln, Col3a1, Col1a1, Zeb2, Myocd, Srf, Smad1, Smad2, Rhoa/Rock2, Fn1, Tagln v2, Klf4, and Acta2) involved in regulation of smooth muscle contractility. Subsequent studies demonstrated an aging-associated increase in the expression of miR-133a, corresponding decreases in RhoA, ROCK2, MYOCD, SRF, and SM22α protein expression, RhoA-signaling, and a decrease in basal and agonist [U-46619 (thromboxane A2 analog)]-induced increase in the IAS tone. Moreover, in vitro transfection of miR-133a caused a dose-dependent increase of IAS tone in strips, which was reversed by anti-miR-133a. Last, in vivo perianal injection of anti-miR-133a reversed the loss of IAS tone associated with age. This work establishes the important regulatory effect of miRNA-133a on basal and agonist-stimulated IAS tone. Moreover, reversal of age-associated loss of tone via anti-miR delivery strongly implicates miR dysregulation as a causal factor in the aging-associated decrease in IAS tone and suggests that miR-133a is a feasible therapeutic target in aging-associated rectoanal incontinence.
Keywords: aging-associated changes, rectoanal incontinence, RhoA/ROCK downregulation, microRNA-133a
the basal tone in the internal anal sphincter (IAS) smooth muscle (SM) plays a critical role in the rectoanal incontinence (RI) (5, 22), whereas intrinsic and extrinsic nerves in the IAS play an important role in the rectoanal inhibitory reflex-induced IAS relaxation and modulation of the basal tone (6). In addition, there are significant data to associate a decrease in the IAS tone with the increase in incidence of RI that occurs with aging in humans (2, 45). Attributable in part to the lack of adequate knowledge of molecular mechanisms mediating basal IAS tone, there is no satisfactory therapeutic management of RI or of motility disorders associated with IAS SM dysfunction (5, 34).
Recent studies from our laboratory have focused extensively on uncovering mechanisms regulating basal IAS tone (28, 32) and have ascribed an important role to the RhoA/ROCK pathway in regulating both animal and human IAS basal tone (30, 31). RhoA/ROCK expression and related signal transduction cascade in the IAS SM cells (SMCs) are higher compared with that in the adjoining nontonic SM (28, 31, 36). Activation of RhoA/ROCK causes phosphorylation of myosin-binding subunit of myosin light-chain phosphatase (p-MYPT1), leading to an increase in phosphorylation of regulatory myosin light-chain (p-MLC20) (3, 23, 29), which in turn maintains smooth muscle tone.
In addition to the role of RhoA/ROCK in regulating basal tone, a compromise in the fibroelastic properties (FEP) of the IAS may also play a critical role in the incidence of RI during aging (16, 35, 40). Presently, there are no data on the pathophysiological mechanisms that regulate RhoA/ROCK and related signal transduction or the FEP of the IAS during aging. It is conceivable that the basal tone and the FEP of the IAS may be regulated by changes in the extracellular matrix (ECM), including collagen and elastin. Recent literature suggests that regulatory mechanisms affecting ECM expression, in multiple systems, are regulated by microRNAs (12, 27).
We hypothesized that dysregulation of microRNAs in aging IAS plays a major role in the decrease in the RhoA/ROCK-mediated IAS tone and compromise in the FEP of the IAS. To test this, we performed a genome-wide expression profile of miRNAs, computationally analyzed their target gene networks, and assessed the roles of different miRNAs in regulating contractility in the IAS from younger vs. older rats. We found that aging significantly upregulates specific miRNAs in IAS, resulting in downregulation of target genes critical to the basal tone and FEP of the IAS. We identified a significant correlation between the expression pattern of the highly expressed miR-199a-3p, miR-31-5p, miR-133a, miR-185-5p, miR-200b-3p, and miR-340-5p, known to target genes associated with fibrosis in older vs. young rats (13,602, 13,723, 13,724, 13,608, 13,725, 13,197). Gain-of-function analysis of miR-133a in SM strips isolated from rat IAS further suggests that miR-133a negatively regulates contractile protein expression. Finally, we demonstrate that targeting miR-133a with anti-miRs in rat IAS reverses the age-associated loss of IAS tone. In conclusion, our findings identify miR-133a as an important determinant in the mechanistic regulation of IAS tone and that its dysregulation contributes to the decrease in the IAS tone that occurs with aging-associated RI.
MATERIALS AND METHODS
Gene Expression Microarrays
mRNA microarrays.
All studies were performed using IAS from Fischer rats (F344 of 6-, 18-, and 26-mo-old age groups provided by the National Institutes of Aging). The experimental protocols were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University. Microarray analysis was performed as described previously (47). Briefly, mRNA and miRNA fractions were isolated from the purified SMCs from the circular smooth muscle (CSM) layer of the IAS as previously described (36), by using miRVana miRNA isolation kit following the manufacturer's protocol (8). These studies were performed at the Functional Genome Centre of Thomas Jefferson University.
Amplification of cDNA was performed from 50 ng of RNA using the Ovation Pico WTA-system V2 RNA amplification system according to NuGen protocol (NuGen Technologies). cDNAs (5 μg) were fragmented and chemically labeled with biotin to generate biotinylated cDNA using FL-Ovation cDNA biotin module (NuGen Technologies).
Affymetrix Gene Chips, rat gene 1.0 and 2.0 ST arrays, were hybridized with 5 μg fragmented and biotin-labeled cDNA in 220 μl of hybridization cocktail. Target denaturation was performed at 99°C for 2 min and then 45°C for 5 min, followed by hybridization for 18 h. Arrays were then washed and stained using Gene Chip Fluidic Station 450 using Affymetrix GeneChip hybridization wash and stain kit. Chips were scanned on an Affymetrix Gene Chip Scanner 3000, using Command Console Software.
Data were analyzed using GeneSpring software 11.5 (Agilent Technologies). Heat maps were generated from a differentially expressed gene list, which was loaded into Ingenuity Pathway Analysis (IPA) 8.0 software (http://www.ingenuity.com) for biological network and functional analyses.
miRNA microarrays.
miRNA microarray studies were performed as described previously (33, 43). Briefly, Affymetrix GeneChip miRNA Arrays were hybridized with Flash Tag biotin-labeled total RNA (500 ng) from experimental and control samples in 100 μl hybridization cocktail. Target denaturation was performed at 99°C for 5 min and then 45°C for 5 min followed by hybridization at 48°C for 18 h. Arrays were washed and stained using Fluidic Station 450, and hybridization signals were amplified using antibody amplification with goat IgG and anti-streptavidin biotinylated antibody, followed by scanning as stated above. miRNA data were analyzed by Affymetrix miRNA QC tool and Genespring V 11.5 software using Robust Multichip Average.
Biological function and pathway analysis.
To identify pathways and functions of differentially expressed mRNAs and miRNAs, expression data files were analyzed using IPA software (Ingenuity Systems). Ingenuity functional analysis and canonical pathway analysis were carried out by performing IPA core analysis of log2 fold change in the rat IAS SMCs from young and old groups.
Identification of miRNA target datasets and interactome analysis.
We uploaded miRNA and mRNA data in IPA and applied the miRNA-mRNA interaction filter. Ingenuity Systems combines TargetScan (http://www.targetscan.org) and TarBase (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=site/index) (1, 46). We matched and paired all possible downregulated targets to the upregulated miRNAs in the data from microarrays. These targets sites were based on all experimentally validated miRNA-mRNA interactions reported in the literature and predicted target sites on untranslated regions of mRNAs.
To further explore the impact of expressed miRNA and mRNAs on different pathways and interacting molecules and their role in aging IAS SM, we performed Interactomes analysis using IPA. The miRNA-mRNA interacting molecules were imported into the separate IPA software spread sheet, and Interactomes pathways were generated. Networks generated by this program are scored based on the number of network-eligible molecules. It predicts the cross talk among the downregulated targets of miRNAs by validated interactions among different proteins reported in the literature and facilitates identification of upstream and downstream molecules that may have direct and indirect impact on the pathophysiology of IAS SM during aging. These direct and indirect interactions among different genes and the role of upregulated miRNA during aging were represented in an oval dendrogram.
Validation of Differentially Regulated miRNA and mRNA from Genome-Wide Microarray
qPCR analysis.
We performed quantitative real-time PCR (qPCR) by using cDNA synthesis kit, SYBR Green RT-PCR Kit (Qiagen), and gene-specific primers (Table 1) synthesized by Integrated DNA technology. miRNA RT-PCR was performed using miR Universal cDNA Synthesis Kit (Promega) using miR SYBR Green master mix RT-PCR Kit (Promega) and specific primers to rat miRNAs (Qiagen).
Table 1.
Primers used in this study (listed in alphabetical order)
|
Primer Sequences |
|||
|---|---|---|---|
| Accession No. | Gene Symbol | Forward | Reverse |
| NM_053304.1 | Col1a1 | GAGCGGAGAGTACTGGATCG | GCAGGGACTTCTTGAGGTTG |
| NM_032085.1 | Col3a1 | GAAAAAACCCTGCTCGGAATT | GGATCAACCCAGTATTCTCC |
| NM_012722.1 | Eln | GTGGCTTTCCTGGCTATGGT | CCCTGCTCCTCCAAGATCAC |
| NM_019143.2 | Fn1 | TCGAGGAGGAAATTCCAATG | CTCTTCATGACGCTTGTGGA |
| NM_053713.1 | Klf4 | CGACTAACCGTTGGCGAGAG | CGGGACTCAGTGTAGGGGTA |
| NM_017343 | Mlc20 | AAGAGGCCTTCAACATGATCGACCAG | CTCATCCACTTCCTCATCTGTGAAGC |
| NM_182667.1 | Myocd | CGCCTGTACGGATGAGAGTC | CCCAATGGGGCTGTGAGAAT |
| NM_053890 | Mypt1 | GCATCTCGAATCGAGTCTCTGGAG | ACGGCTTCTTCCTATTGTCTTTTCGGC |
| NM_016802 | Rhoa | CAGCAAGGACCAGTTCCCAGA | TGCCATATACTGCCTTCTTCAGG |
| NM_013022 | Rock2 | TAGAAGAACACCTTAGCAGTGAGGTACAAG | GCTGTCCTCTTCTCCAGCTCTACTTTTG |
| NM_013130.2 | Smad1 | TTTCAGATGCCAGCCGACAC | ACACCTCTCCTCCGACGTAA |
| NM_019191.1 | Smad2 | GGGTGGAGACACCAGTCTTG | CTCCACTGCTGACGGACTTT |
| NM_003186.3 | Tagln, transcript variant 2 | GAAGCCTTCTTTCCCCAGACA | ATCACGCCATTCTTCAGCCA |
| NM_001033701.1 | Zeb2 | GGGACAGATCAGCACCAAAT | GACCCAGAATGAGAGAAGCG |
| NM_017008.4 | Gapdh | GTTACCAGGGCTGCCTTCTC | CTCGTGGTTCACACCCATCA |
Immunoblot analysis.
Immunoblot analysis was performed as described previously (37). Briefly, total protein from each sample was separated by SDS-PAGE and transferred to polyvinylidene difluoride membrane (Millipore). The membranes were subjected to immunoblot analysis using antibodies from Santa Cruz Biotechnology, and immunoreactive proteins relative to GAPDH were visualized as described previously (32, 37).
Immunocytochemical analysis.
With the use of SMCs from 6- and 26-mo-old rats, high-resolution laser scanning fluorescence microscopy was performed using a confocal microscope (Carl Zeiss LSM 510 UV META) and Plan-Apo ×40 oil immersion, numerical aperture 1.0 lens. The images were captured as single acquisitions using Zeiss AIM 4.2 SP1 software (Bioimaging Facility, Kimmel Cancer Center of Thomas Jefferson University) and analyzed by MetaMorph v7.65. The nuclei were stained with 4′,6′-diamidino-2-phenylindole. Images taken at the same magnification and intensity for 6- and 26-mo-old SMCs were imported into ImageJ (National Institutes of Health) using LOCI Bio-Formats for quantitation. Calculations of florescence intensity per unit area were made via ImageJ, by randomly selecting four different diagonally opposite points across the cell. An area of 5 μm2 was selected at each position, and intensity per unit area was calculated by dividing average intensity by the area. Cells (15–20) were studied in each group. Texas red-conjugated IgGs from mouse, goat, and rabbit were used as background fluorescence intensity controls.
IAS SM strip preparation and transfection of miR-133a.
The IAS SM strips (1 × 10 mm) from the CSM layer of 6-mo-old rats prepared as described previously (35), pinned flat on sylgard-coated 33-mm plates containing 2 ml of F12 media were transfected with miRNA-133a and antimiR-133a (0 to 60 nM) using INTERFERin (Polyplus) transfection reagent following the manufacturer's instructions. After 48 h, changes in the basal tone and effect of contractile agonist U46619 (that works via RhoA/ROCK activation) were recorded as follows.
Force measurement.
The IAS SM strips were transferred into 2-ml muscle baths in which force was recorded using force transducers (FORT10, WPI). The strips were continuously perfused with oxygenated Krebs physiological solution (KPS). Initially, 1.0 g of tension was applied, and strips were allowed to equilibrate for 60 min, with repeated washing with fresh KPS every 20 min. All force data were monitored using Chart 4.1.2 via a PowerLab/8SPdata-acquisition system (ADInstruments) (35). The spontaneously developed basal IAS tone and its maximal increase and decrease were recorded in response to 1 μM U46619 and 0 Ca2+, respectively, in the beginning and at the end of each experiment. Concentration-response curves (CRC) for U46619 (0.1 nM to 10.0 μM) were examined in the SM strips pretreated for 48 h with scrambled miRNA (control) and miRNA-133a before and after anti-miR-133a.
In vivo studies: Recording of intraluminal IAS pressures and effect of perianal injection of anti-microRNA.
The intraluminal IAS pressure (IASP) in 6- and 26-mo-old rats was measured before and 48, 72, and 96 h after perianal injections of scrambled (control) vs. in vivo ready miRNA-133a inhibitor (miRCURY LNA Power microRNA inhibitor; Exiqon; 7.5 mg/kg of tissue mass). The IASP was measured using high-fidelity intraluminal manometry catheter assembly via PowerLab/8SP recorder and analyzed via the software Chart 4 PowerLab (ADInstruments). The catheter assembly was initially introduced into the rectum and then gradually pulled out in a precise step-wise manner via a motorized device till the highest and steady pressures (IASP) were recorded in the high-pressure zone of the IAS (7–8 mm from the anal verge). The IASP consisted of rhythmic fluctuations superimposed on the steady tone.
Details of adapted procedure for the perianal injections have been described previously (13). For these injections, we used a microneedle (31 gauge) attached to 300-μl insulin syringe.
Both intraluminal manometry and perianal injection procedures were carried out under isoflurane inhalation anesthesia (initially with 5% isoflurane and then maintained with 1% isoflurane throughout the length of the experiment).
Statistical analyses.
miRNA microarray data were verified by a close correlation between qPCR and microarray via linear regression analysis. qPCR data for mRNA and miRNA were replicated further on four to six rats in different experiments. Genes showing gradient of expression in 6- vs. 26-mo-old old rats were selected for miRNA target analysis. Comparison between two groups was analyzed using the two-tailed Student's t-test, and comparison between multiple groups was made using one-way ANOVA and Newman-Keuls posttest using GraphPad Prism 5.0. Data are presented as the means ± SE.
RESULTS
Differential Gene Expression During Aging in IAS SM
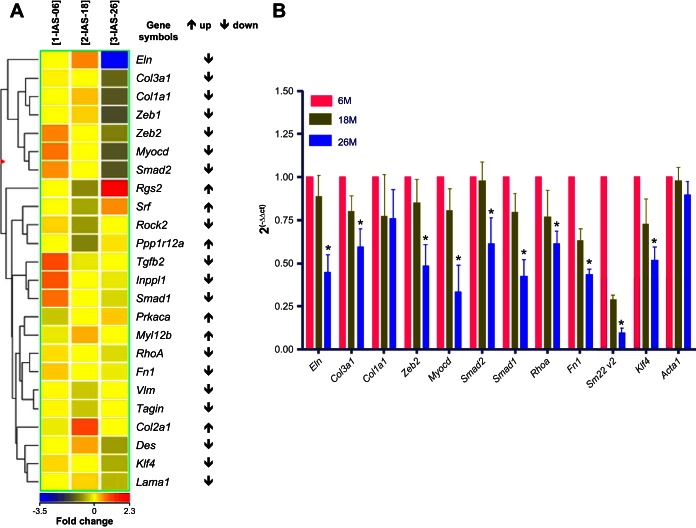
Genome-wide expression profiles via microarray on the total RNA of the IAS SMCs from three groups of rats, 6, 18, and 26 mo old, employed Affymetrix rat Gene Chips 1.0 and 2.0. These chips probed for 27,342 and 29,489 sets of genes, respectively. Six-month-old rats served as control or reference point to determine up- and downregulation of gene transcripts. All unknown transcripts (without known gene name and ID) and transcripts with weak signal intensity (below 50) were ignored. Only the transcripts with noticeable gradient for up- or downregulation between 6-, 18-, and 26-mo-old groups were considered. These transcripts were clustered into groups by biological function. We applied hierarchical clustering (Pearson correlation) to the expression profiles of differentially expressed transcripts in the IAS SMCs from younger vs. older rats to determine patterns in the data. These results are presented as a heat map and dendrogram (Fig. 1A). A striking finding was a significant downregulation of genes known to be involved in SM contractile phenotype (Myocd) and those in the basal tone of IAS (Rhoa), in the IAS of older vs. younger rats.
Fig. 1.
Genome-wide expression profile of mRNA in internal anal sphincter (IAS) smooth muscle (SM) from younger and older rats. A: hierarchical clustering. Heat map shows differentially (↑ for increase, and ↓ for decrease) expressed mRNA detected in the IAS SM cells (SMCs) from 26-mo-old vs. 6-mo-old and 18-mo-old rats. The dendrogram illustrates the clustering tree resulting from hierarchical clustering of gene expression values (involved in SM phenotype and in contractile biomechanics). B: qPCR data for the selected transcripts validating downregulation of important SM markers (initially observed in the mRNA microarray profile) in aging IAS. These data reveal significant decreases in the expression levels in 26- vs. 6-mo-old group (*P < 0.05; n = 4; Student's t-test) but not in 18-mo-old vs. 6-mo-old group (P > 0.05).
Relevant Functions and Pathways in IAS SM of Aged Rat
IPA (25) identified a number of significantly differentially expressed genes in IAS SM during aging. One of the pathways that showed the highest differential gene expression was found to be RhoA/ROCK signaling pathway. Significant IPA canonical pathways and the associated molecules are presented in Supplemental Fig. S1; supplemental material for this article is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website. These include Rho GTPase, Gα12/13, and Gαq/Rho signaling in the IAS SMCs of 26-mo-old rats.
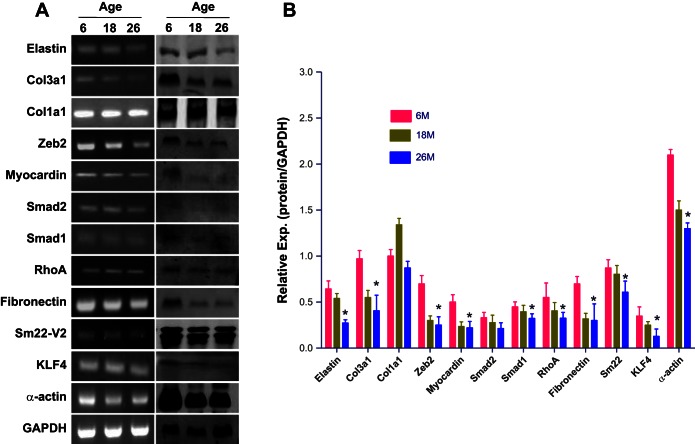
We validated the microarray results using qPCR, immunoblot, and immunofluorescence analyses for 12 selected genes. Data revealed that Eln, Col3a1, Col1a1, Fn1, Zeb2, Klf4, Myocd, Tagln v2 (Sm22-V2), Smad1, Smad2, Rhoa, and Act2 mRNA were downregulated in 26-mo-old IAS SMC samples. The relative expression of these genes from these samples is shown in Fig. 1B (mRNA) and Fig. 2B (protein). PCR and Western blot bands are given in Fig. 2A.
Fig. 2.
Aging downregulates SM contractile and regulatory protein expression in rat IAS. A: RNA and protein were extracted from 6-, 18-, and 26-mo-old rat IAS SMCs and subjected to RT-PCR (left) and immunoblot (right) analysis for the indicated proteins. Representative blots from 4 independent experiments are presented. B: graph showing significant decrease in the relative expression (*P < 0.05; n = 4; Student's t-test) of different proteins (corresponding to the mRNAs described in Fig. 1) in 26-mo-old vs. 6-mo-old rats compared with younger rats.
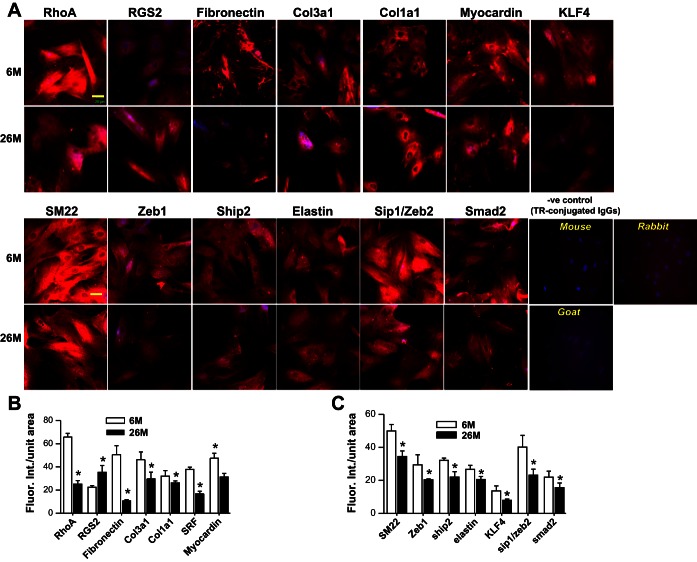
Differential downregulation of these genes plus upregulation of Rgs2 in IAS SMCs of 26-mo-old vs. the younger rats were further confirmed by immunofluorescence studies (Fig. 3, A–C). Because initial comparison of mRNA and miRNA arrays and validation studies between 6-, 18-, and 26-mo-old age groups revealed the most significant and consistent differences in the 26-mo-old vs. 6-mo-old groups, immunofluorescence and other detailed studies were performed in these age groups only.
Fig. 3.
Decreased expression of SM contractile and regulatory protein in rat IAS. SMCs isolated from 6-mo-old vs. 26-mo-old rat IAS SMCs (A) were stained with the indicated antibodies. The images (taken on a Carl Zeiss LSM 510 UV META inverted confocal microscope) (A) compare the expression of the proteins between 6- and 26-mo-old rats (bar = 20 μm). B and C: significant decrease in immunofluorescence intensities for different proteins examined (*P < 0.05; n = 4).
Differential miRNA Expression During Aging in IAS SM
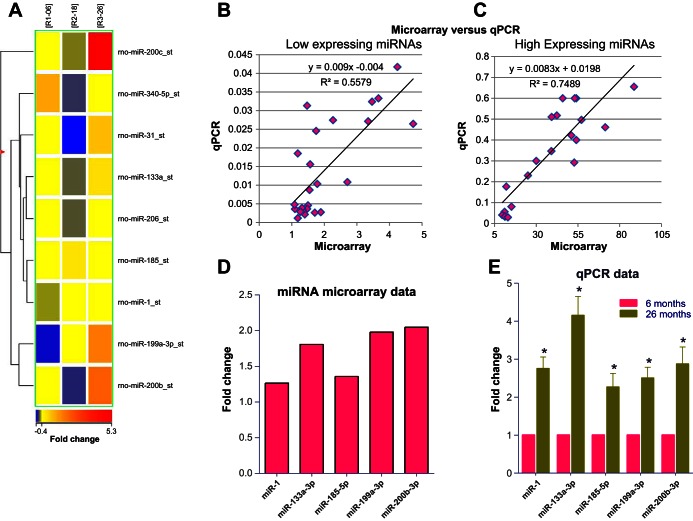
Affymetrix miRNA Expression Profiling Assay system in IAS from 6-, 18-, and 26-mo-old rats identified marked differential expression of a number of miRNAs as shown as a heat map in Fig. 4A. All mRNA and miRNA microarray data were deposited in the Gene Expression Omnibus (GEO) database and can be accessed at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE79350.
Fig. 4.
Genome-wide expression profile of miRNA in IAS from younger and older rats. A: heat map showing differentially expressed miRNAs detected in IAS SMCs from 6-, 18-, and 26-mo-old rats. Analysis was carried out using a 2-color miRNA microarray. Each column represents results from an independent experiment (6-, 18-, and 26-mo-old rats). Each row corresponds to a single miRNA probe. The dendrogram illustrates the clustering tree resulting from hierarchical clustering of gene expression values. B and C: significant correlation between the signal intensity of miRNA expression from the microarray vs. relative expression of qPCR values, examining low-expressing and high-expressing miRNAs, respectively. D: expression levels of selected miRNA (in numerical order) shown as average fold change of miRNA in IAS SMCs of 26- vs. 6-mo-old rat. E: qPCR data showing significant (*P < 0.05; n = 4) increase in the values of selected miRs validating the microarray data for the selected miRNAs in 26-mo-old vs. 6-mo-old rat IAS SMCs. Data were normalized to U6 RNA, and experiments were performed in triplicate.
The correlation between miRNA microarray and qPCR data was confirmed by regression analysis of low- and high-expressing miRNAs (Fig. 4, B and C, respectively).
On the basis of this analysis, we selected miRNAs targeting Rhoa directly and other signaling molecules regulating RhoA expression indirectly (Fig. 4D).
qPCR analysis confirmed that miR-1, miR-133a-3p, miR-185-5p, miR-199a, and miR-200b-3p were significantly upregulated in IAS SMCs from 26-mo-old rats (n = 4; P < 0.05; Fig. 4E).
Following IPA, we identified that multiple miRNAs may target single mRNA. Predicted and experimentally observed targets from the literature are given in Supplemental Fig. S2. The analysis indicates that Smad2, Klf4, Myocd, Rhoa, and Tagln mRNAs are predicted targets of multiple miRNAs.
Predicted Targets of Altered miRNAs and Network Construction
To assess the interaction between miRNAs and genes, the miRNA gene network was built using IPA. By multiple interactions, miR-133a may lead to downregulation of multiple genes in the aging IAS SM. Another important microRNA, miR-199a-3p, is predicted to affect multiple targets. Interactomes of these miRNAs and genes involved are given in supplemental data (Supplemental Figs. S1 and S2). The networks were built based on these differentially upregulated miRNAs in the IAS SMCs from 26-mo-old rat.
miR-133a Overexpression and Effect on RhoA/ROCK Pathway
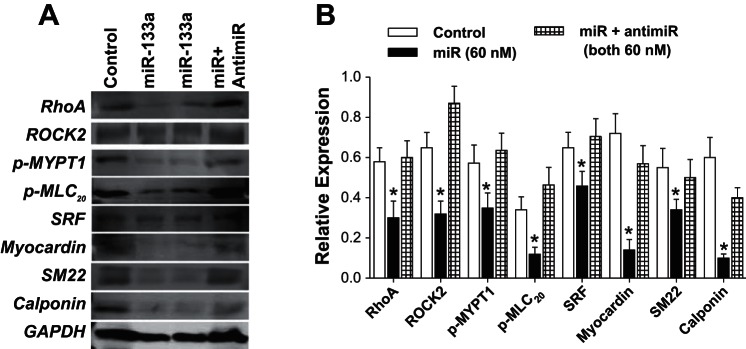
Transfection (for 72 h) of primary SMCs from 6-mo-old rats with miR-133a-3p oligonucleotide caused a significant decrease in the expression levels of RhoA, ROCK2, MLC20, p-MLC20, MYOCD, SRF, and SM22 as determined via immunoblot analyses (Fig. 5, A and B) (n = 4; P < 0.05). These results suggest the involvement of miR-133a in inhibiting regulatory molecules important in IAS tone.
Fig. 5.
A and B: effect of miRNA-133a overexpression of contractile and regulatory proteins in primary IAS SMCs. Immunoblot analysis (A) and quantitative data (B) show a significant decrease (*P < 0.05; n = 4) in the expression levels of RhoA/ROCKII, MYPT1, p-MYPT1, p-MLC20, SRF, myocardin, SM22α, and calponin, following pretreatment of the cells with miRNA-133a and anti-miR-133a. The anti-mR blocks the inhibitory effects of miR-133a to the levels not significantly different from controls.
Effect of miRNA-133a on the Basal Tone of IAS
Given that our earlier studies have shown that Rho kinase is the primary determinant of the basal IAS SM tone (31, 32), we investigated whether the decreased expression of RhoA by miRNA-133a is responsible for the changes in the basal tone. We measured the basal IAS tone in the SM strips pretreated with scrambled or miRNA-133a (both for 48 h).
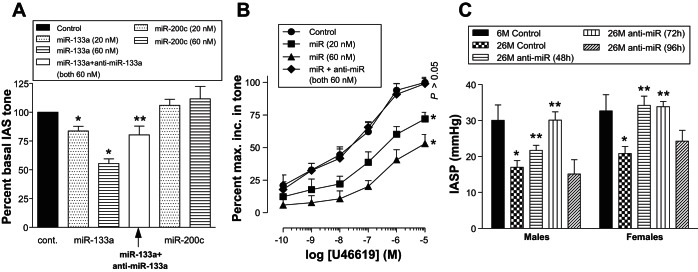
miRNA-133a produced a concentration-dependent and significant decrease in the basal IAS tone (Fig. 6A). The percentage of maximal basal IAS tone following the pretreatment of the strips with 60 nM of miRNA-133a was 55.5 ± 4.1% (*P < 0.05; n = 4). This represents a 45% decrease in the basal IAS tone. The absolute values of basal IAS tone in these experiments in the presence of scrambled vs. 60 nM miRNA-133a-transfected SM strips were 268 ± 32 mg and 149 ± 11 mg, respectively. The decrease in IAS tone caused by miRNA-133a was significantly (**P < 0.05; n = 4; Fig. 6A) blocked by its antagomir. Conversely, miRNA-200c had no significant effect on the basal tone.
Fig. 6.
Effect of miRNA-133a overexpression on basal (A), agonist-induced increase in IAS tone (B), and reversal of the decreased intraluminal pressures of IAS (IASP) by anti-miR-133a (C). A: miR-133a produces significant and concentration-dependent (20 and 60 nM) decreases in the IAS tone (*P < 0.05; n = 4) compared with scrambled miR (control) or another miR-200c. Decrease in IAS tone by 60 nM miR-133a is significantly blocked by 60 nM antagomirs (**P < 0.05; n = 4). B: miR-133a significantly shifts U46619 concentration-response curve (CRC) of increase in the IAS tone toward the right (*P < 0.05; n = 6–8). The latter is blocked by the antagomirs pretreatment so that U46619 CRC in the presence of 60 nM of miR-133a + antimiR is not significantly different from control (P > 0.05; n = 6–8). C: perianal injection of anti-miR-133a significantly (**P < 0.05) rescues the decreased (*P < 0.05) IASP during aging in 26-mo-old rats compared with their corresponding 6-mo-old rats, both in males and females (n = 6 animals, 3 males and 3 females used for each animal group). The rescuing effect was found to be sustained for up to 72 h following the injection of the anti-miR, both in males and females.
Effect of miRNA-133a on Agonist-Induced Contraction in IAS
To investigate the effect of miRNA-133a overexpression on agonist-mediated increase in the IAS tone, we compared the effect of scrambled vs. miRNA-133a on the contractile effect of RhoA/ROCK activator U46619 (15, 41).
U46619 increased IAS tone in a concentration-dependent manner. The pretreatment of muscle strips with miRNA-133a (20 and 60 nM) for 48 h caused a significant and concentration-dependent rightward shift in the U46619 CRC (*P < 0.05; n = 6–8). These concentrations of miRNA-133a were based on the previous smooth muscle studies by Torella et al. (44) and our optimization for the maximal transfection efficiency in rat IAS SMCs and SM strips using fluorescent-tagged scrambled siRNA (10 to 60 nM) from Qiagen. This shift in the U46619 CRC with miR-133a was attenuated by anti-miR-133a, and CRC obtained during the presence of combined use of the miR + anti-miR (both 60 nM) was not significantly (P > 0.05) different from that obtained during scrambled RNA-treated (control) SM strips. These results demonstrate that miRNA-133a reduces the IAS tone, not only in the basal state, but also in the stimulated state, both involving RhoA/ROCK pathway (Fig. 6B).
Rescuing Effect of Anti-miRNA Inhibitor on Aging-Associated Decease in IASP
In agreement with the above in vitro data, in vivo studies revealed significantly (*P < 0.0; n = 3; Fig. 6C) reduced IASP in aging rats. To further implicate miR-133a in the age-associated decrease in IASP, studies in Fig. 6C demonstrate that perianal injections of anti-miR-133a into IAS significantly rescued the loss of IASP 48 h and 72 h after injection both in males and females (**P < 0.05; n = 3 animals of each age and sex group). These recusant effects as determined at 72 h were found to be statistically significant and similar both in male and female rats.
DISCUSSION
In the present study, we performed a high-throughput screening involving microarray and the genome-wide transcriptome analysis to gain insight into miRNA-dependent regulatory mechanisms that influence age-related differences in IAS tone. We identified 22 genes that are downregulated in IAS SM from older rats, including Myocd, Srf, Rhoa, and Rock2, which are known important regulators of SM differentiation and contractility. In parallel, a comprehensive screening identified 11 microRNAs that are significantly upregulated in the aging IAS SM. Subsequent bioinformatics analysis combined with pathway analysis and predicted targets led to the conclusion that miR-133a downregulates RhoA/ROCK2, myocardin (MYOCD), and serum response factor (SRF).
The present study also identified significant changes related to age in the SM signaling transduction molecules (Rgs2, Prkaca, Rhoa/Rock2, PP1r12a, Ship2), intermediate filaments (Vim, Des), growth and transcription factors (Tgfb2, Smads/Zebs, Klf4, Sip1/Zeb2), regulators of SMC differentiation (Srf, Myocd), certain components of ECM (Eln, Col13a1, Co1a1, Fn1), and early markers of SMCs (Tagln, Acta1). It is noteworthy that not all ECM genes were downregulated during aging, as a significant number of other ECM genes were either upregulated or unaffected (data not shown). These data support our previous studies asserting an important role of the RhoA/ROCK pathway in the aging-associated decrease in the IAS tone (35) and also suggest age-related changes in IAS SM phenotype and FEP.
The above conclusions were based on the changes in genetic and miRNA profiles, as found during microarray studies, using SMCs from rats of three different age groups, young (6 mo old), adult (18 mo old), and aging (26 mo old) rats. The reason for using SMCs was twofold. 1) Previous studies have shown that the majority of the basal tone in the IAS in humans and animals is via the specialized myogenic properties of the SMCs (28, 29, 36). 2) This approach avoids contamination with other phenotypic cells in this area, which would have made interpretation of the data difficult. These changes in the genomic and miRNA profiles as a function of aging were further validated via qPCR, immunoblot, immunocytochemistry analyses, and finally by functional studies, especially focusing on 6- vs. 26-mo-old rat IAS SMCs.
miRNAs are well-known small noncoding RNAs that can act as “master switches” of the genome to regulate diverse cellular pathways involved in the pathophysiology of SM function (13,762, 13,728, 13,763, 13,726, 13,649). Therefore, miRNAs may serve as diagnostic and prognostic markers and provide important insights into the pathophysiology and therapeutic targeting of SM dysfunctions. Several miRNAs are ubiquitously expressed, whereas others are tissue specific and are enriched in specific tissues. For example, miR-1, miR-133a, miR-133b, miR-206, miR-208, miR-208b, miR-486, and miR-499 are muscle-enriched miRNAs (4, 12, 24). In the present study, using mRNA and miRNA microarrays followed by bioinformatics analyses, we identified differentially expressed mRNA and miRNAs in the rat IAS SM.
Earlier studies attempting to understand the regulation of basal IAS tone and its decrease during aging have shown the critical role of RhoA/ROCK pathway in animals and humans (29, 30, 32, 35). A number of genomic, proteomic, and functional studies have documented that miR-133a can target RhoA (13,197, 13,756, 13,649), a major upstream trigger for the downstream signal transduction cascade for the sustained SM tone (23, 29, 32). To determine the effect of miR-133a on SM contractile protein expression, we overexpressed miRNA-133a in rat IAS SMC. This overexpression decreased the endogenous protein levels of two markers of myogenic determination, myocardin and SRF, whereas inhibition of the endogenous miR-133a by antagomir-133a resulted in the upregulation of these proteins (data not shown). This is in agreement with previous studies in which manipulating miR-133a levels in other tissue types showed a significant reduction in contractile protein expression (44), implicating miR-133 as a regulator of muscle differentiation (50).
Myocardin is a master regulator of SMC differentiation and contractile phenotype, and its negative regulation by miR-143/145 and miR-204/211 induces SMC synthetic phenotype differentiation (7, 12, 52). The development of SM is a well-coordinated process of cell proliferation, differentiation, and migration that is regulated by evolutionarily conserved networks of myogenic transcription factors (48, 52). The maintenance of a SM contractile phenotype requires a fine balance between the expression and the repression of many genes (19).
The present study demonstrates for the first time that miR-133a is upregulated in aging IAS SM and that its overexpression inhibits the basal tone and agonist-induced contraction. Previous studies have demonstrated that aging reduces the basal IAS tone (35) that has been associated with the downregulation of RhoA/ROCK2. Although the mechanism of downregulation of RhoA and ROCK2 in IAS SM during aging is not fully understood, present data suggest that miRNA-133a is an important factor in that regard.
Present data demonstrate a direct relationship between the changes in miRNA and changes in mRNA expression in the IAS SM from different age groups, especially in 26-mo-old vs. 6-mo-old rats. In addition, miRNA-133a is upregulated in the IAS from aged rat, and the overexpression of miRNA-133a mimics the aging-associated decrease in the IAS tone in basal as well as stimulated state by RhoA/ROCK activator U46619 (15, 41). These findings have significant relevance in the pathophysiology and potential reversal of aging-associated IAS dysfunction, via miRNA intervention. We speculate that aging-associated RI (especially characterized by the hypotensive IAS) is in part associated with changes in expression profile of miRs, which may be reversed by the respective antagomirs or miRs. Conversely, downregulation of miR-133a may lead to anorectal motility disorders characterized by hypertensive IAS (e.g., recurrent anal fissures and hemorrhoids), potentially reversible by overexpression of miR-133a using appropriate oliogmiR. Our data clearly show that reduced IASP observed in aging rats is reversible by anti-miR-133a both in male and female rats.
The present data contribute significantly to our understanding of the role of miRNAs in the regulation of SM contractility and differentiation (26, 38). However, the effects of aging on the expression of miRNA-133a and the associated functional consequences appear to be dependent on system and cell type. For example, studies have implicated decreases in the levels of miRNA-133a in the arterial SMCs from patients with arteriosclerosis obliterans, a common occurrence in the aging population (17). In contrast, aging has been shown to have the opposite effects, i.e., an increase in the levels of miRNA-133a in human skeletal muscle (11). Regardless, it is well known that miRNA-133a negatively regulates RhoA; downregulation of miR-133a promotes the proliferation, migration, and contraction, whereas its upregulation has the opposite effect, by targeting Rhoa (9, 17).
In summary, this work identifies the role of miRNAs in the regulation of IAS SM basal tone and agonist-induced contraction. Data reveal a significant impairment in myogenic factor and contractile protein expression attributable to miRNA/mRNA interaction with aging. These data provide strong evidence for dysregulated miRNA as a key factor in the compromise of SM plasticity with age and in pathogenic mechanisms associated with RI. This constitutes the first study to demonstrate that miRNA-133a and its gene targets are crucial to RhoA signaling pathway in relation to contractility and IAS SM phenotype in the aging. We speculate that the basal tone and the FEP of the IAS are regulated by changes in the transduction molecules, growth and transcription factors, regulators of SMC differentiation, ECM components, and early markers of SMCs via microRNA-targeting diverse signaling pathways. Collectively, the present data identify miR-133a as a potential target for therapeutic application in aging-associated RI with compromise in the IAS basal tone and biomechanics.
GRANTS
The work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grants RO1DK035385 (S. Rattan) and DK100483 (E. Boopathi), William F. Keck Foundation grant (I. Rigoutsos), grant HL58506 (R. Penn), and an institutional grant from Thomas Jefferson University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.S. and S.A. performed experiments, analyzed data, and prepared figures; J.S., E.B., S.A., B.P., I.R., R.B.P., and S.R. interpreted results, drafted, edited, revised, and approved final version of manuscript; R.B.P. and S.R. conception and design of research.
Supplementary Material
REFERENCES
- 1.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife 4: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akervall S, Nordgren S, Fasth S, Oresland T, Pettersson K, Hulten L. The effects of age, gender, and parity on rectoanal functions in adults. Scand J Gastroenterol 25: 1247–1256, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67: 545–554, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoi W, Ichikawa H, Mune K, Tanimura Y, Mizushima K, Naito Y, Yoshikawa T. Muscle-enriched microRNA miR-486 decreases in circulation in response to exercise in young men. Front Physiol 4: 80, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharucha AE, Dunivan G, Goode PS, Lukacz ES, Markland AD, Matthews CA, Mott L, Rogers RG, Zinsmeister AR, Whitehead WE, Rao SS, Hamilton FA. Epidemiology, pathophysiology, and classification of fecal incontinence: State of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol 110: 127–136, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharucha AE, Rao SS. An update on anorectal disorders for gastroenterologists. Gastroenterology 146: 37–45, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bockmeyer CL, Maegel L, Janciaufigspecskiene S, Rische J, Lehmann U, Maus UA, Nickel N, Haverich A, Hoeper MM, Golpon HA, Kreipe H, Laenger F, Jonigk D. Plexiform vasculopathy of severe pulmonary arterial hypertension and microRNA expression. J Heart Lung Transplant 31: 764–772, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol 28: 495–503, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba Y, Tanabe M, Goto K, Sakai H, Misawa M. Down-regulation of miR-133a contributes to up-regulation of RhoA in bronchial smooth muscle cells. Am J Respir Cell Mol Biol 180: 713–719, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Deng L, Blanco FJ, Stevens H, Lu R, Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, Stenmark K, White K, Seto AG, Morrell NW, Bradshaw AC, MacLean MR, Baker AH. MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res 117: 870–883, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drummond MJ, McCarthy JJ, Fry CS, Esser KA, Rasmussen BB. Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am J Physiol Endocrinol Metab 295: E1333–E1340, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi SR, Comer BS, McLendon JM, Gerthoffer WT. MicroRNA regulation of smooth muscle phenotype. Mol Cell Pharmacol 4: 1–16, 2012. [PMC free article] [PubMed] [Google Scholar]
- 13.Jun H, Han MR, Kang NG, Park JH, Park JH. Use of hollow microneedles for targeted delivery of phenylephrine to treat fecal incontinence. J Control Release 207: 1–6, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Kato M, Chen X, Inukai S, Zhao H, Slack FJ. Age-associated changes in expression of small, noncoding RNAs, including microRNAs, in C. elegans. RNA 17: 1804–1820, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MR, Jeon ES, Kim YM, Lee JS, Kim JH. Thromboxane a(2) induces differentiation of human mesenchymal stem cells to smooth muscle-like cells. Stem Cells 27: 191–199, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Krishna CV, Singh J, Kumar S, Rattan S. Heme oxygenase-1 upregulation modulates tone and fibroelastic properties of internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 307: G595–G601, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Ouyang M, Shan Z, Ma J, Li J, Yao C, Zhu Z, Zhang L, Chen L, Chang G, Wang S, Wang W. Involvement of microRNA-133a in the development of arteriosclerosis obliterans of the lower extremities via RhoA targeting. J Atheroscler Thromb 22: 424–432, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Lang N, Chen X, Tang Q, Liu S, Huang J, Zheng Y, Bi F. miR-185 targets RhoA and Cdc42 expression and inhibits the proliferation potential of human colorectal cells. Cancer Lett 301: 151–160, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Liu ZP, Wang Z, Yanagisawa H, Olson EN. Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin. Dev Cell 9: 261–270, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Maegdefessel L, Rayner KJ, Leeper NJ. MicroRNA regulation of vascular smooth muscle function and phenotype: early career committee contribution. Arterioscler Thromb Vasc Biol 35: 2–6, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Mahavadi S, Sriwai W, Kumar DP, Zhou R, Grider JR, Murthy KS. Down-regulation of microRNA-133a due to oxidative stress mediates up-regulation of RhoA expression and increase in Rho kinase activity and gastric muscle contraction in diabetes. Gastroenterology 142: S105, 2012. [Google Scholar]
- 22.Mandaliya R, DiMarino AJ, Moleski S, Rattan S, Cohen S. Survey of anal sphincter dysfunction using anal manometry in patients with fecal incontinence: A possible guide to therapy. Ann Gastroenterol 28: 469–474, 2015. [PMC free article] [PubMed] [Google Scholar]
- 23.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Nachtigall PG, Dias MC, Pinhal D. Evolution and genomic organization of muscle microRNAs in fish genomes. BMC Evol Biol 14: 196, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nazarov PV, Reinsbach SE, Muller A, Nicot N, Philippidou D, Vallar L, Kreis S. Interplay of microRNAs, transcription factors and target genes: Linking dynamic expression changes to function. Nucleic Acids Res 41: 2817–2831, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan F, Xu J, Zhang Q, Qiu X, Yu W, Xia J, Chen T, Pan L, Chen Y, Dai Y. Identification and characterization of the MicroRNA profile in aging rats with erectile dysfunction. J Sex Med 11: 1646–1656, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Park C, Hennig GW, Sanders KM, Cho JH, Hatton WJ, Redelman D, Park JK, Ward SM, Miano JM, Yan W, Ro S. Serum response factor-dependent MicroRNAs regulate gastrointestinal smooth muscle cell phenotypes. Gastroenterology 141: 164–175, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel CA, Rattan S. Cellular regulation of basal tone in internal anal sphincter smooth muscle by RhoA/ROCK. Am J Physiol Gastrointest Liver Physiol 292: G1747–G1756, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Rattan S, Benjamin P, Maxwell PJ 4th. RhoA/ROCK-kinase: Pathophysiologic and therapeutic implications in gastrointestinal smooth muscle tone and relaxation. Gastroenterology 138: 13–18, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rattan S, De Godoy MA, Patel CA. Rho kinase as a novel molecular therapeutic target for hypertensive internal anal sphincter. Gastroenterology 131: 108–116, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Rattan S, Singh J. RhoA/ROCK pathway is the major molecular determinant of basal tone in intact human internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 302: G664–G675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rattan S, Singh J, Kumar S, Phillips B. Nature of extracellular signal that triggers RhoA/ROCK activation for the basal internal anal sphincter tone in humans. Am J Physiol Gastrointest Liver Physiol 308: G924–G933, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sengupta JN, Poschiraju S, Kannampalli P, Bruckett M, Addya S, Yadav P, Miranda A, Shaker R, Banerjee B. MicroRNA-mediated GABAAα-1 receptor subunit down-regulation in adult spinal cord following neonatal cystitis-induced chronic visceral pain in rats. Pain 154: 59–70, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siemionow M. Novel approach to treat fecal incontinence with muscle stem cell-based therapy. Tech Coloproctol 1: 669–670, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Singh J, Kumar S, Krishna CV, Rattan S. Aging-associated oxidative stress leads to decrease in IAS tone via RhoA/ROCK downregulation. Am J Physiol Gastrointest Liver Physiol 306: G983–G991, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh J, Rattan S. Bioengineered human IAS reconstructs with functional and molecular properties similar to intact IAS. Am J Physiol Gastrointest Liver Physiol 303: G713–G722, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh J, Rattan S. Role of PKC and RhoA/ROCK pathways in the spontaneous phasic activity in the rectal smooth muscle. Am J Physiol Gastrointest Liver Physiol 304: G723–G731, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation 121: 1022–1032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith-Vikos T, Slack FJ. MicroRNAs and their roles in aging. J Cell Sci 125: 7–17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Speakman CT, Hoyle CH, Kamm MA, Swash M, Henry MM, Nicholls RJ, Burnstock G. Abnormal internal anal sphincter fibrosis and elasticity in fecal incontinence. Dis Colon Rectum 38: 407–410, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson AS, Matthew JD, Eto M, Luo S, Somlyo AP, Somlyo AV. Uncoupling of GPCR and RhoA-induced Ca2+-sensitization of chicken amnion smooth muscle lacking CPI-17. FEBS Lett 578: 73–79, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Chen D, Cao L, Zhang R, Zhou J, Chen H, Li Y, Li M, Cao J, Wang Z. MiR-490-3p modulates the proliferation of vascular smooth muscle cells induced by ox-LDL through targeting PAPP-A. Cardiovasc Res 100: 272–279, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Thangavel C, Bhoopathi E, Ertel A, Lim M, Addya S, Fortina P, Witkiewicz AK, Knudsen ES. Regulation of miR106b cluster through the RB pathway: Mechanism and functional targets. Cell Cycle 12: 98–111, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila I, Curcio A, Condorelli G, Indolfi C. MicroRNA-133 controls vascular smooth muscle cell phenotype switch in vitro and vascular remodeling in vivo. Circ Res 109: 880–893, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Van KC. [Constipation and fecal incontinence in the elderly]. Rev Med Liege 69: 337–342, 2014. [PubMed] [Google Scholar]
- 46.Vergoulis T, Vlachos IS, Alexiou P, Georgakilas G, Maragkakis M, Reczko M, Gerangelos S, Koziris N, Dalamagas T, Hatzigeorgiou AG. TarBase 6.0: Capturing the exponential growth of miRNA targets with experimental support. Nucleic Acids Res 40: D222–D229, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wermuth PJ, Addya S, Jimenez SA. Effect of protein kinase C delta (PKC-delta) inhibition on the transcriptome of normal and systemic sclerosis human dermal fibroblasts in vitro. PLoS One 6: e27110, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams AH, Liu N, van RE, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol 21: 461–469, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong CM, Wei L, Au SL, Fan DN, Zhou Y, Tsang FH, Law CT, Lee JM, He X, Shi J, Wong CC, Ng IO. MiR-200b/200c/429 subfamily negatively regulates Rho/ROCK signaling pathway to suppress hepatocellular carcinoma metastasis. Oncotarget 6: 13658–13670, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wystub K, Besser J, Bachmann A, Boettger T, Braun T. miR-1/133a clusters cooperatively specify the cardiomyogenic lineage by adjustment of myocardin levels during embryonic heart development. PLoS Genet 9: e1003793, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang S, Xie N, Cui H, Banerjee S, Abraham E, Thannickal VJ, Liu G. miR-31 is a negative regulator of fibrogenesis and pulmonary fibrosis. FASEB J 26: 3790–3799, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Zheng XL. Myocardin and smooth muscle differentiation. Arch Biochem Biophys 543: 48–56, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Zoni E, van der Pluijm G, Gray PC, Kruithof-de Julio M. Epithelial plasticity in cancer: unmasking a microRNA network for TGF-beta-, Notch-, and Wnt-mediated EMT. J Oncol 2015: 198967, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.