Abstract
Irritable bowel syndrome (IBS) is a common disorder characterized by recurrent abdominal pain, bloating, and disturbed bowel habit, symptoms that impact the quality of life of sufferers. The pathophysiological changes underlying this multifactorial condition are complex and include increased sensitivity to luminal and mucosal factors, resulting in altered colonic transit and visceral pain. Moreover, dysfunctional communication in the bidirectional signaling axis between the brain and the gut, which involves efferent and afferent branches of the peripheral nervous system, circulating endocrine hormones, and local paracrine and neurocrine factors, including immune and perhaps even microbial signaling molecules, has a role to play in this disorder. This minireview will examine recent advances in our understanding of the pathophysiology of IBS and assess how cross talk between hormones, immune, and microbe-derived factors and their neuromodulatory effects on peripheral nerves may underlie IBS symptomatology.
Keywords: interleukins, GLP-1, leptin, myenteric, submucosal
irritable bowel syndrome (IBS) is a prevalent functional bowel disorder characterized by abnormal regulation of motor and sensory function in the distal gastrointestinal (GI) tract. The pathophysiology of IBS is likely multifactorial and includes heightened sensitivity to luminal and mucosal factors such as enteroendocrine secretions, stress hormones, malabsorbed or maldigested nutrients, bile acids, or alterations in the colonic microbiome. This can result in increased barrier permeability, subsequent immune activation, and altered neural regulation of GI secretion and motility. Visceral hypersensitivity and associated abdominal pain, which is one of the most debilitating symptoms of this disorder, is also stimulated by such luminal and mucosal factors (see Ref. 10 for review).
Underlying the pathophysiology of IBS is dysregulation of the bidirectional brain-gut signaling axis, which comprises efferent and afferent neuronal pathways of the parasympathetic and sympathetic nervous systems, endocrine hormones, including the hypothalamic-pituitary-adrenal (HPA) stress axis hormones and digestive hormones, and immune and microbial signaling molecules. The importance of the central nervous system (CNS) in regulating digestive function and satiety is well established (41), as are the detrimental effects of emotional stress on GI physiology (2). Indeed, the high comorbidity between centrally mediated psychiatric disorders such as depression and anxiety with GI dysfunction (18) is further evidence of the importance of the brain-gut signaling axis. However, it is clear that communication between the brain and gut is a bidirectional system and this minireview will look at recent advances in our understanding of communication between hormones and immune factors originating in the GI tract and how they signal to the peripheral and central nervous systems.
The Contribution of Endocrine Hormones to IBS Symptoms
IBS patients present with lower pain thresholds to rectal distension and increased intensity of visceral sensation, observations that are accompanied by enhanced activity in the thalamus, insula, and anterior cingulate cortex, which are part of the CNS pain matrix (69). Moreover, this hypersensitivity has been related to mood and emotion in patients, with evidence of high comorbidity between stress-related disorders, such as depression and anxiety, and IBS. Indeed, stress has a significant role in the initiation, exacerbation, and persistence of GI symptoms during symptom flares (76), effects that may be mediated through the stress hormone corticotrophin-releasing factor (CRF), which is secreted in response to stress, by neurons of the paraventricular nucleus of the hypothalamus.
Clearly, dysregulation of central modulation, or CNS processing, contributes to IBS symptomology, particularly the exacerbating influence of psychological stressors (39). However, perceived pain sensation is initiated through activation of sensory nerves in the periphery (24), which is evident by blocking visceral pain hypersensitivity with rectally applied analgesics (69), demonstrating the two-way communication system of the brain-gut signaling axis. Colonic nociceptors are stimulated through distension of the colonic lumen but may also be activated through inflammatory mediators. Indeed, the role of immune mediators is now being recognized to play a role in IBS symptoms (24, 36, 61), and activation of the HPA axis is a well-studied response orchestrated by the brain in reaction to infection and inflammation (74).
Neuromodulatory effects of stress peptides in gut function.
Given the high comorbidity of stress-associated mood disorders in IBS patients, it's likely that sensitivity to stress is altered in these individuals. Indeed, CRF, which is secreted in response to perceived stressors and activates the HPA axis, evokes increased secretion of cortisol in IBS patients (18). CRF is key to stress-related alterations in colonic motor and secretory function (see Ref. 81 for review) and evokes its biological effects through activation of CRF1 and CRF2 receptors, which are expressed in the hypothalamus and other brain regions. Administration of CRF into the central nucleus of the amygdala, which is important in the integration of emotional and sensory information, stimulates local release of norepinephrine and sensitization of visceral nociception, an effect that is inhibited using a CRF1 receptor antagonist (79). Although the effects of CRF in the CNS are crucial to regulation of the GI tract, receptors are also expressed in the colon, where they are ideally placed to mediate the effects of stress on gastric emptying, transit, and gut motility. In support of the importance of peripheral regulation of GI function are studies demonstrating that stress-induced defecation and visceral hypersensitivity are attenuated by blocking peripheral CRF1 receptor activation (6, 30, 55). However, our studies also noted the potential role of inflammatory molecules in symptoms such as visceral pain and stress-induced defecation and the potential for cross talk between stress and immune molecules in the manifestation of IBS symptom flares (6). Indeed, Jizhong et al. (37) detected concurrent increases in expression of Toll-like receptors and CRF receptors in blood samples from IBS patients and activation of CRF receptors is also linked to increased colonic barrier permeability and inflammation (42). Moreover, stress induces degranulation of mast cells, resulting in the release of proinflammatory mediators (40, 57). The efficacy of CRF1 receptor antagonists in animal models (6, 30, 55) is encouraging; however, clinical trials using such antagonists have thus far been disappointing, primarily due to unfavorable pharmacokinetics, tissue accumulation, high protein binding, and reactive metabolite formation (31). Moreover, pexacerafont, an orally active, selective CRFR1 antagonist, had no effect on colonic transit in diarrhea-predominant IBS, indicating that CRF 1 receptors may not constitute a useful therapeutic target in humans (80).
GI peptides are key signaling molecules from the gut lumen to the brain.
Although food intolerance does not underlie IBS pathophysiology, ingestion of certain food types, particularly carbohydrate- and fat-rich meals, can result in abdominal pain and bloating. Wheat is thought to be one of the key triggers of IBS symptoms (15), and, although celiac disease is no more prevalent in IBS patients than the general population, IBS patients who carry HLA-DQ2 or HLA-DQ8 genotypes, which predispose individuals to celiac disease, display more sensitivity to gluten (84). They are also more likely to benefit from a gluten-free diet than patients without these genotypes (1, 85), a mechanism that appears to be dependent on alterations to the adaptive immune system (85). Additionally, some studies find that diets low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) show improvements in IBS symptoms (47, 59). However, others report that reducing anxiety by hypnotherapy is just as effective as this restrictive diet (68). This topic remains controversial with one recent meta-analysis supporting the efficacy of a low-FODMAP diet in the treatment of functional GI disorders (49), whereas another meta-analysis failed to prove any benefit (70). In mechanistic terms, where a low-FODMAP diet was found to be beneficial to IBS patients, immune activation was suppressed and the microbiome was altered with greater numbers of bacteria involved in the consumption of gas (51). Although release of gas by fermentation is normal, the enhanced sensitivity of IBS patients to bowel distension may underlie the debilitating visceral pain they experience following ingestion of a meal rich in FODMAPs.
The cellular and molecular mechanisms underlying the exacerbation of IBS symptoms following a meal are not yet clear. Nerve fibers are thought to terminate before reaching the epithelial layer. Thus, unless the integrity of the mucosal barrier has been lost, nerve fibers cannot directly sense luminal nutrients (25). However, enteroendocrine cells (EECs) detect ingested nutrients, thus acting as electrically excitable biosensors (13). Indeed, an important physiological response to the arrival of food in the gut lumen is the secretion of digestive and incretin hormones. Moreover, visceral afferent endings are sensitive to gut hormones released on the basolateral side of the mucosal barrier. These include hormones that regulate appetite and energy homeostasis such as glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP), and enteroendocrine secretions linked to the modulation of motility, absorptosecretory function, and sensation, such as serotonin, substance P, and peptide YY (PYY). Thus EECs could act as a lynchpin in the transmission of luminal signals to the host nervous system. Consistent with this theory, a recent paper by Bohorquez and colleagues (5) provided evidence of direct, physical mechanisms of signaling between EECs and neurons innervating the gastrointestinal tract. The authors provide evidence of PYY- and GLP-1-positive EECs producing a pseudopod-like elongation, termed a neuropod, that synapsed with efferent and afferent nerves.
Incretin hormones have neuromodulatory action in the GI tract.
The biological activities of GLP-1 include stimulation of glucose-dependent insulin secretion and insulin biosynthesis and inhibition of glucagon secretion. In the gut, GLP-1 inhibits gastric emptying and food intake. A clinical trial of a GLP-1 mimetic found that it alleviated some IBS symptoms with anti-spasmodic and pain-relieving properties (32) and may be a useful therapeutic strategy in constipation-predominant IBS (56). Although, the molecular mechanisms by which GLP-1 achieves this outcome are not completely understood, it is known that GLP-1 increases firing rates in afferent vagal nerves (52). GLP-1-expressing neurons are found in the enteric nervous system but also in brain regions such as the nucleus tractus solitarius and the ventrolateral medulla (43). GLP-1 receptors have also been detected in vagal and dorsal root ganglia and the area postrema and hypothalamus in the CNS (73), revealing that the action of GLP-1 on gut function may be centrally or peripherally orchestrated. Cross talk between this GI hormone and the HPA stress axis have been revealed through the stimulatory action of GLP-1 on CRF neurons (58), once more hinting that a complex relationship between several physiological systems has been impeding our understanding of IBS. K cells are EECs that secrete an incretin hormone, GIP, which is released in response to luminal carbohydrates to promote pancreatic insulin secretion. Although less has been reported about a potential role for GIP, mucosal biopsies from IBS patients express fewer K cells than healthy controls, which was particularly evident in diarrhea-predominant IBS patients (22). As GIP also inhibits gastric acid secretion, a reduction in the number of K cells may lead to unregulated gastric acid secretion, which could contribute to dyspepsia and gastroesophageal reflux, common symptoms in IBS (71).
Other enteroendocrine secretions in the manifestation of IBS symptoms.
Serotonin is primarily synthesized in the gut and exerts its effects through receptors expressed on intrinsic and extrinsic nerves, modulating colonic motility and secretion, and activating extrinsic nerve fibers. Circulating serotonin levels are elevated in diarrhea-predominant IBS patients but decreased in constipation-predominant patients. This has resulted in the use of selective serotonergic agonists and antagonists to treat specific subtypes of the disorder (11); however, these are not without side effects. EECs also secrete chromogranins and secretogranins, which promote the release of other GI hormones. In IBS, particularly patients with rapid colonic transit, levels of granins are altered, resulting in them being proposed as an indirect biomarker of colonic secretion or motility (9). Other GI peptides with neuromodulatory effects, PYY (21) and neuropeptide Y (88), are both reduced in IBS biopsies, although the mechanistic consequences of the altered expression has yet to be elucidated.
The GI tract is the largest endocrine organ, secreting more than two dozen different hormones, many of which are altered in the guts of IBS patients. In addition to a hyperactivated stress axis, endocrine signaling and hormonal modulation of nerve signals to the CNS are likely contributors to the manifestation of symptoms in IBS. Nonetheless, evidence is now growing that, although no overt inflammation is evident in the colons of IBS patients, immune activation is an additional consideration in the pathophysiology of the disorder, and indeed interaction between immune and endocrine factors may underlie symptom flares (7, 37).
Neuroimmune Interactions Contribute to IBS Pathophysiology
Neuromodulatory effects of cytokines.
The GI tract is unusual in that it is continuously exposed to immunogenic stimuli ingested with food. The immune system must determine whether the ingested antigens pose a threat to the host or whether they should be tolerated. Thus the lamina propria, lying below the GI mucosa, is home to many immune cells, which are primed to respond to pathogens. Compared with healthy controls, IBS patients exhibit changes in mucosal immune cell populations, with elevated numbers of mast cells and lymphocytes (29). Degranulation of mast cells causes the release of inflammatory mediators including proteases, prostaglandins, histamine, and cytokines, which can activate enteric neurons (8), although a recent report suggested that continuous exposure to these mediators may result in desensitization of enteric neurons (64). Concentrations of proinflammatory cytokines such as interleukin (IL)-6 and IL-8 are also elevated in the IBS plasma (53), although not all studies detected this change (12). Cytokines facilitate cell-to-cell signaling in an immune response but may also participate in modulating intrinsic and afferent nerve activity. Indeed, IL-6 (6, 63), IL-1β (86), and tumor necrosis factor-α (72) have direct actions on enteric neuronal activation and we observed that the neurostimulatory effects of IBS plasma on rat submucosal and myenteric neurons is dependent on IL-6, IL-8, and CRF (6, 62). Such cytokines stimulate sensitization of nociceptors, which would increase the excitability of afferent endings innervating the colon. Indeed, IL-1β can sensitize splanchnic afferent nerves to histamine and mesenteric ischemia (28) and also stimulate increased excitability of DRG neurons to mechanical or thermal stimulation (4). Thus cytokines may be important in initiating and perpetuating visceral hypersensitivity and abdominal pain in IBS.
Vagal function.
Although most dense in the proximal regions of the GI tract, vagal innervation is still significant in the distal colon (25, 83) and vagal afferent neurons are likely to act as the primary interrogators of peripheral colonic signals. Moreover, the vagus nerve has been shown to modulate the production of proinflammatory cytokines by activating the HPA axis, which is anti-inflammatory, and the cholinergic anti-inflammatory pathway (65). Vagal tone has been found to be important in cytokine secretion in Crohn's disease with an inverse relationship between vagal tone and plasma levels of TNFα. This wasn't noted in IBS, however, and no link was found between IL-6 and vagal tone in either disorder (66), indicating that this mechanism may not be important in IBS pathophysiology.
Barrier function.
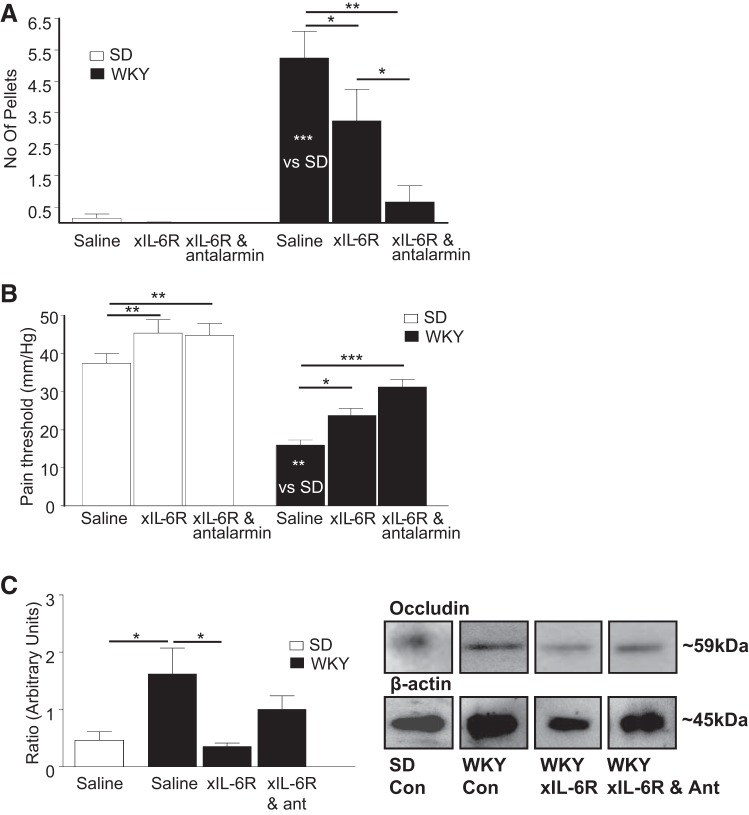
IL-6 and other proinflammatory cytokines (60) promote breakdown of the mucosal barrier, thus facilitating movement of pathogens across the epithelial barrier and resulting in activation of an immune response. In IBS patients, where plasma IL-6 levels are chronically elevated and the HPA stress axis is hyperactivated (18), a coincident compromise of the mucosal barrier is observed, which could result in sensitization of afferent nerves leading to increased sensitivity to visceral pain (89). Indeed, intervention studies in the stress-sensitive Wistar-Kyoto rat model of IBS, which exhibits visceral hypersensitivity, demonstrated that neutralizing IL-6 receptors alleviated visceral pain and altered motility with associated reductions in tight junction proteins. Moreover, cotreatment of animals with the CRF1 receptor antagonist antalarmin and anti-IL-6 receptor antibodies, further alleviated symptoms of stress-induced defecation and visceral pain sensitivity [Fig. 1, reproduced from original publication (6)].
Fig. 1.
IL-6R neutralization and antalarmin alter gastrointestinal dysfunction in WKY rats. A: histogram illustrating the number of fecal pellets excreted by Sprague-Dawley (SD, n = 7, 7, and 9) and Wistar-Kyoto (WKY, n = 10, 9, and 11) rats in the open field arena when administered saline, anti-interleukin (IL)-6 receptor antibodies (xIL-6R), or xIL-6R and antalarmin. B: histogram showing the pressure (mmHg) at which SD (n = 7, 7, and 9) and WKY (n = 10, 9, and 11) rats display pain behaviors in response to colorectal distension when treated with saline, xIL-6R, or xIL-6R and antalarmin. C: histogram illustrates the ratios of expression of occludin (n = 5) over the β-actin loading control (Con) in mucosal samples from the distal colon of SD and WKY rats treated with saline, xIL-6R, and xIL-6R with antalarmin (ant). *P < 0.05, **P < 0.01, and ***P < 0.001. Adapted from previously published work (6).
Microbial signaling to the gut and brain.
With the development of germ-free (GF) and antibiotic- or probiotic-treated rodents, we have begun to recognize the importance of the microbiome in gut and whole host homeostasis. The colon is host to over a hundred trillion microbial organisms, most of which are bacterial. These microbes have diverse but important functions that may be beneficial to their host, such as scavenging extra energy through fermentation of nondigestible foods, secreting vitamins, and ensuring the normal development of the immune system. Thus the traditional view of the host-microbiota relationship being symbiotic, where the microbes benefit more than the host, has been reassessed and the interaction is more akin to a mutualistic relationship, or, to take it to the extreme, it has been proposed that microbes may manipulate host physiology and behavior to their own benefit (77). This shift in our appreciation of the relationship between colonic bacteria and the normal development of gastrointestinal, immune, and endocrine physiology and the peripheral and central nervous systems has resulted in a recent surge of studies. Aside from its role in GI function, recent reviews have discussed the importance of gut bacteria in the development of diseases of the CNS, including depression and anxiety (19, 50), Parkinson's disease (23), Alzheimer's disease (33), and autism spectrum disorder (35). Indeed, a variety of probiotics appear to exhibit psychoactive attributes, producing neuromodulatory factors which can act on host cells (17). The vagus nerve has been implicated as a key component of the signaling axis between the colonic microbiota and the brain (48, 67, 82); however, our understanding of the molecular and cellular mechanisms, which facilitate the transmission of a luminally originating bacterial signal across the gut barrier to the peripheral nervous system, is far from clear.
Microbes and host neurological development.
The importance of gut microbiota in the development of the immune, endocrine, and nervous systems has been established using GF mice. The absence of microbiota in these mice is associated with increased anxiety-like behaviors and alterations in central neurotransmitters, effects that are proposed to be mediated through endocrine mechanisms (14). Elegant electrophysiological studies in GF mice recently demonstrated the importance of the nervous system, as excitability of intrinsic primary afferent neurons, the likely neural starting point of gut-to-brain signaling, was dampened in GF mice (54). Through coevolution with their hosts, human colonic bacteria have likely developed systems for sensing host-associated signals, including the capacity to identify and respond to human hormones. This enables bacteria to recognize that they are in the vicinity of a suitable host and offers a plausible mechanism of communication between prokaryotes and eukaryotes (26). Moreover, through a mechanism termed “microbial endocrinology” (44), gut bacteria can also potentially respond to host signals. Indeed, sensitivity to stress hormones is reported to underlie the modification of the composition and diversity of the intestinal microbiota (27).
Microbial metabolites and secretory products.
Particular strains of Lactobacillus and Bifidobacterium bacteria can secrete the inhibitory neurotransmitter γ-aminobutyric acid (3, 75) or derive it from luminal matter such as monosodium glutamate (3). Other bacterial subtypes have been shown to secrete monoamines such as norepinephrine, dopamine, and serotonin (45, 46) and the serotonin precursor tryptophan (16). Luminal bacteria stimulate synthesis of serotonin in colonic cells, which in turn modulates host GI physiology (87). In addition to these secretory products, major microbial metabolites are short-chain fatty acids, including butyrate, acetate, and propionate, which are produced during anaerobic fermentation of dietary fiber. Butyrate has been implicated in microbial-host cross talk, which may be through specific transporters or receptors or by modulating the immune system and vagus nerve activity (78). Another emerging area of research is the enzymatic modification of bile acids by gut microbes that can modify signals to the host and thereby influence immune homeostasis among other functions (38).
Microbes and the host endocrine system.
In addition to detecting and responding to ingested nutrients, EECs, which are found embedded in the gut epithelial layer throughout the GI tract, are capable of detecting bacterial by-products. A recent paper by Chimerel and colleagues (13) demonstrated that GLP-1-secreting L cells are capable of sensing indole, a bacterial metabolic product of tryptophan. Indole inhibits voltage-gated K+ channels in L cells, resulting in continued depolarization of these cells, sustained influx of calcium through voltage-gated calcium channels and increased secretion of GLP-1. Additionally, gut bacteria can metabolize prebiotics, which nourish microbes, to form PYY (34), GLP-1, leptin, and ghrelin (20).
Visceral afferent endings are sensitive to gut peptides such as GLP-1 and PYY. However, as nerve fibers are thought to terminate before reaching the epithelial layer, nerve fibers are not likely to sense luminal hormones directly (25). Nonetheless, a neuroepithelial circuit could act as a conduit for microbe-host signaling with precise, temporal transmission of gut-derived sensory signals and real-time modulatory feedback on EECs (5). Thus EECs with microbial and GI hormonal biosensing capabilities and neural connections represent a possible means of bridging the gap between microbes and the host.
Conclusions
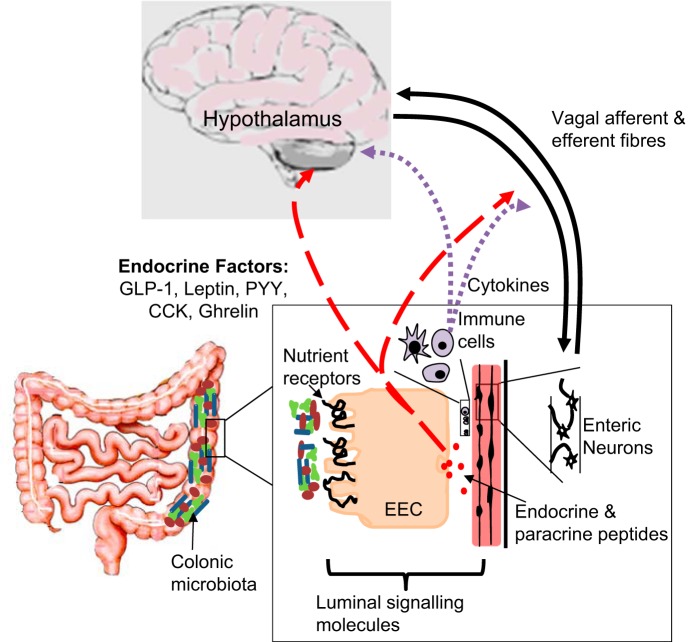
IBS is a prevalent and debilitating GI disorder, with few effective treatments that merely target symptoms rather than the cause. The heterogeneity of this disorder hints at a complex multifactorial pathophysiology and, indeed, studies in animal models of IBS and in patients have revealed alterations in endocrine, immune, and nerve physiology, with recent evidence that dysfunctional microbiome-gut-brain signaling is also a key player in the manifestation of IBS symptoms (Fig. 2). In particular, studies implicating gut microbiota in altered CNS physiology and behaviors have resulted in a paradigm shift in our appreciation of the interaction between us and our microbial colonizers. Nonetheless, the molecular and cellular mechanisms employed by the immune, endocrine, and neural systems to facilitate communication between gut microbes and the peripheral and central nervous systems are not yet understood and have revealed an emerging avenue of research.
Fig. 2.
Brain-gut-microbiome signaling. The schematic illustrates potential signaling mechanisms between the luminal microbiota, the intrinsic and extrinsic colonic nerves, and the central nervous system with the role of neurocrine, endocrine, and immune factors indicated.
GRANTS
D. O'Malley is currently supported by a Wellcome Trust Seed Award and TRAP funding from the School of Medicine, University College Cork.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.O'M. prepared figures; D.O'M. drafted manuscript; D.O'M. edited and revised manuscript; D.O'M. approved final version of manuscript.
REFERENCES
- 1.Aziz I, Trott N, Briggs R, North JR, Hadjivassiliou M, Sanders DS. Efficacy of a gluten-free diet in subjects with irritable bowel syndrome-diarrhea unaware of their HLA-DQ2/8 genotype. Clin Gastroenterol Hepatol 14: 696–703.e1, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology 114: 559–578, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 113: 411–417, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci 28: 14062–14073, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohorquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 125: 782–786, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley MM, O'Halloran KD, Rae MG, Dinan TG, O'Malley D. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J Physiol 592: 5235–5250, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckley MM, O'Mahony SM, O'Malley D. Convergence of neuro-endocrine-immune pathways in the pathophysiology of irritable bowel syndrome. World J Gastroenterol 20: 8846–8858, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, Michel K, Schemann M. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137: 1425–1434, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri M. Editorial: fecal granins in IBS: cause or indicator of intestinal or colonic irritation? Am J Gastroenterol 107: 448–450, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 367: 1626–1635, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M. Pharmacology of the new treatments for lower gastrointestinal motility disorders and irritable bowel syndrome. Clin Pharmacol Ther 91: 44–59, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Chang L, Adeyemo M, Karagiannides I, Videlock EJ, Bowe C, Shih W, Presson AP, Yuan PQ, Cortina G, Gong H, Singh S, Licudine A, Mayer M, Tache Y, Pothoulakis C, Mayer EA. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol 107: 262–272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep 9: 1202–1208, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol 28: 1221–1238, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut 65: 169–178, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 43: 164–174, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil 25: 713–719, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Dinan TG, Quigley EM, Ahmed SM, Scully P, O'Brien S, O'Mahony L, O'Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology 130: 304–311, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res 63: 1–9, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Dockray GJ. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol 592: 2927–2941, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. Densities of rectal peptide YY and somatostatin cells as biomarkers for the diagnosis of irritable bowel syndrome. Peptides 67: 12–19, 2015. [DOI] [PubMed] [Google Scholar]
- 22.El-Salhy M, Hatlebakk JG, Hausken T. Reduction in duodenal endocrine cells in irritable bowel syndrome is associated with stem cell abnormalities. World J Gastroenterol 21: 9577–9587, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felice VD, Quigley EM, Sullivan AM, O'Keeffe GW, O'Mahony SM. Microbiota-gut-brain signalling in Parkinson's disease: Implications for non-motor symptoms. Parkinsonism Relat Disord 27: 1–8, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Feng B, La JH, Schwartz ES, Gebhart GF. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 302: G1085–G1098, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol 817: 115–133, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Freestone P. Communication between bacteria and their hosts. Scientifica (Cairo) 2013: 361073, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freestone PP, Williams PH, Haigh RD, Maggs AF, Neal CP, Lyte M. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock 18: 465–470, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Fu LW, Longhurst JC. Interleukin-1beta sensitizes abdominal visceral afferents of cats to ischaemia and histamine. J Physiol 521: 249–260, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goral V, Kucukoner M, Buyukbayram H. Mast cells count and serum cytokine levels in patients with irritable bowel syndrome. Hepatogastroenterology 57: 751–754, 2010. [PubMed] [Google Scholar]
- 30.Greenwood-Van Meerveld B, Johnson AC, Cochrane S, Schulkin J, Myers DA. Corticotropin-releasing factor 1 receptor-mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol Motil 17: 415–422, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov 11: 462–478, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Hellstrom PM, Naslund E, Edholm T, Schmidt PT, Kristensen J, Theodorsson E, Holst JJ, Efendic S. GLP-1 suppresses gastrointestinal motility and inhibits the migrating motor complex in healthy subjects and patients with irritable bowel syndrome. Neurogastroenterol Motil 20: 649–659, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Hill JM, Lukiw WJ. Microbial-generated amyloids and Alzheimer's disease (AD). Front Aging Neurosci 7: 9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 46: 261–274, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsiao EY. Immune dysregulation in autism spectrum disorder. Int Rev Neurobiol 113: 269–302, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Ishihara S, Tada Y, Fukuba N, Oka A, Kusunoki R, Mishima Y, Oshima N, Moriyama I, Yuki T, Kawashima K, Kinoshita Y. Pathogenesis of irritable bowel syndrome—review regarding associated infection and immune activation. Digestion 87: 204–211, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Jizhong S, Qiaomin W, Chao W, Yanqing L. Corticotropin-releasing factor and toll-like receptor gene expression is associated with low-grade inflammation in irritable bowel syndrome patients with depression. Gastroenterol Res Pract 2016: 7394924, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joyce SA, Gahan CG. Bile acid modifications at the microbe-host interface: potential for nutraceutical and pharmaceutical interventions in host health. Annu Rev Food Sci Technol 7: 313–333, 2016. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy PJ, Clarke G, O'Neill A, Groeger JA, Quigley EM, Shanahan F, Cryan JF, Dinan TG. Cognitive performance in irritable bowel syndrome: evidence of a stress-related impairment in visuospatial memory. Psychol Med 44: 1553–1566, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konturek SJ, Brzozowski T, Konturek PC, Zwirska-Korczala K, Reiter RJ. Day/night differences in stress-induced gastric lesions in rats with an intact pineal gland or after pinealectomy. J Pineal Res 44: 408–415, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Konturek SJ, Konturek JW, Pawlik T, Brzozowski T. Brain-gut axis and its role in the control of food intake. J Physiol Pharmacol 55: 137–154, 2004. [PubMed] [Google Scholar]
- 42.Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol 60, Suppl 7: 33–46, 2009. [PMC free article] [PubMed] [Google Scholar]
- 43.Lim GE, Huang GJ, Flora N, LeRoith D, Rhodes CJ, Brubaker PL. Insulin regulates glucagon-like peptide-1 secretion from the enteroendocrine L cell. Endocrinology 150: 580–591, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyte M. Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol 12: 14–20, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog 9: e1003726, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays 33: 574–581, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Maagaard L, Ankersen DV, Vegh Z, Burisch J, Jensen L, Pedersen N, Munkholm P. Follow-up of patients with functional bowel symptoms treated with a low FODMAP diet. World J Gastroenterol 22: 4009–4019, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malick M, Gilbert K, Daniel J, Arseneault-Breard J, Tompkins TA, Godbout R, Rousseau G. Vagotomy prevents the effect of probiotics on caspase activity in a model of postmyocardial infarction depression. Neurogastroenterol Motil 27: 663–671, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Marsh A, Eslick EM, Eslick GD. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur J Nutr 55: 897–906, 2016. [DOI] [PubMed] [Google Scholar]
- 50.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 34: 15490–15496, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, Madsen K, Bercik P, Vanner S. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2016. Mar 14. pii: gutjnl-2015-311339. doi: 10.1136/gutjnl-2015-311339 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 52.McKee DP, Quigley EM. Intestinal motility in irritable bowel syndrome: is IBS a motility disorder? Part 1. Definition of IBS and colonic motility. Dig Dis Sci 38: 1761–1772, 1993. [DOI] [PubMed] [Google Scholar]
- 53.McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther 33: 1045–1052, 2011. [DOI] [PubMed] [Google Scholar]
- 54.McVey Neufeld KA, Perez-Burgos A, Mao YK, Bienenstock J, Kunze WA. The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol Motil 27: 627–636, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Million M, Zhao JF, Luckey A, Czimmer J, Maynard GD, Kehne J, Hoffman DC, Tache Y. The newly developed CRF1-receptor antagonists, NGD 98-2 and NGD 9002, suppress acute stress-induced stimulation of colonic motor function and visceral hypersensitivity in rats. PLoS One 8: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mosinska P, Salaga M, Fichna J. Novel investigational drugs for constipation-predominant irritable bowel syndrome: a review. Expert Opin Investig Drugs 25: 275–286, 2016. [DOI] [PubMed] [Google Scholar]
- 57.Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, Mantyh C, Pappas TN, Takahashi T. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol 292: G1037–G1044, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Nakade Y, Tsukamoto K, Iwa M, Pappas TN, Takahashi T. Glucagon like peptide-1 accelerates colonic transit via central CRF and peripheral vagal pathways in conscious rats. Auton Neurosci 131: 50–56, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Nanayakkara WS, Skidmore PM, O'Brien L, Wilkinson TJ, Gearry RB. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: the evidence to date. Clin Exp Gastroenterol 9: 131–142, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Natale L, Piepoli AL, De Salvia MA, De Salvatore G, Mitolo CI, Marzullo A, Portincasa P, Moschetta A, Palasciano G, Mitolo-Chieppa D. Interleukins 1 beta and 6 induce functional alteration of rat colonic motility: an in vitro study. Eur J Clin Invest 33: 704–712, 2003. [DOI] [PubMed] [Google Scholar]
- 61.O'Malley D. Immunomodulation of enteric neural function in irritable bowel syndrome. World J Gastroenterol 21: 7362–7366, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Malley D, Buckley MM, McKernan DP, Quigley EM, Cryan JF, Dinan TG. Soluble mediators in plasma from irritable bowel syndrome patients excite rat submucosal neurons. Brain Behav Immun 44: 57–67, 2015. [DOI] [PubMed] [Google Scholar]
- 63.O'Malley D, Liston M, Hyland NP, Dinan TG, Cryan JF. Colonic soluble mediators from the maternal separation model of irritable bowel syndrome activate submucosal neurons via an interleukin-6-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 300: G241–G252, 2011. [DOI] [PubMed] [Google Scholar]
- 64.Ostertag D, Buhner S, Michel K, Pehl C, Kurjak M, Gotzberger M, Schulte-Frohlinde E, Frieling T, Enck P, Phillip J, Schemann M. Reduced responses of submucous neurons from irritable bowel syndrome patients to a cocktail containing histamine, serotonin, TNF alpha, and tryptase (IBS-cocktail). Front Neurosci 9: 465, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med 9: 125–134, 2003. [PMC free article] [PubMed] [Google Scholar]
- 66.Pellissier S, Dantzer C, Mondillon L, Trocme C, Gauchez AS, Ducros V, Mathieu N, Toussaint B, Fournier A, Canini F, Bonaz B. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn's disease and irritable bowel syndrome. PLoS One 9: e105328, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perez-Burgos A, Wang B, Mao YK, Mistry B, McVey Neufeld KA, Bienenstock J, Kunze W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol 304: G211–G220, 2013. [DOI] [PubMed] [Google Scholar]
- 68.Peters SL, Yao CK, Philpott H, Yelland GW, Muir JG, Gibson PR. Randomised clinical trial: the efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 44: 447–459, 2016. [DOI] [PubMed] [Google Scholar]
- 69.Price DD, Craggs JG, Zhou Q, Verne GN, Perlstein WM, Robinson ME. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. Neuroimage 47: 995–1001, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rao SS, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther 41: 1256–1270, 2015. [DOI] [PubMed] [Google Scholar]
- 71.Rasmussen S, Jensen TH, Henriksen SL, Haastrup PF, Larsen PV, Sondergaard J, Jarbol DE. Overlap of symptoms of gastroesophageal reflux disease, dyspepsia and irritable bowel syndrome in the general population. Scand J Gastroenterol 50: 162–169, 2015. [DOI] [PubMed] [Google Scholar]
- 72.Rehn M, Hubschle T, Diener M. TNF-alpha hyperpolarizes membrane potential and potentiates the response to nicotinic receptor stimulation in cultured rat myenteric neurones. Acta Physiol Scand 181: 13–22, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, Reimann F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 63: 1224–1233, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci 15: 1088–1095, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schousboe A, Waagepetersen HS. GABA: homeostatic and pharmacological aspects. Prog Brain Res 160: 9–19, 2007. [DOI] [PubMed] [Google Scholar]
- 76.Stengel A, Tache Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol 71: 219–239, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stilling RM, Dinan TG, Cryan JF. The brain's Geppetto-microbes as puppeteers of neural function and behaviour? J Neurovirol 22: 14–21, 2016. [DOI] [PubMed] [Google Scholar]
- 78.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int 99: 110–132, 2016. [DOI] [PubMed] [Google Scholar]
- 79.Su J, Tanaka Y, Muratsubaki T, Kano M, Kanazawa M, Fukudo S. Injection of corticotropin-releasing hormone into the amygdala aggravates visceral nociception and induces noradrenaline release in rats. Neurogastroenterol Motil 27: 30–39, 2015. [DOI] [PubMed] [Google Scholar]
- 80.Sweetser S, Camilleri M, Linker Nord SJ, Burton DD, Castenada L, Croop R, Tong G, Dockens R, Zinsmeister AR. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol 296: G1299–G1306, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tache Y, Million M. Role of corticotropin-releasing factor signaling in stress-related alterations of colonic motility and hyperalgesia. J Neurogastroenterol Motil 21: 8–24, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takada M, Nishida K, Kataoka-Kato A, Gondo Y, Ishikawa H, Suda K, Kawai M, Hoshi R, Watanabe O, Igarashi T, Kuwano Y, Miyazaki K, Rokutan K. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol Motil 28: 1027–1036, 2016. [DOI] [PubMed] [Google Scholar]
- 83.Tong WD, Ridolfi TJ, Kosinski L, Ludwig K, Takahashi T. Effects of autonomic nerve stimulation on colorectal motility in rats. Neurogastroenterol Motil 22: 688–693, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O'Neill J, Carlson P, Lamsam J, Janzow D, Eckert D, Burton D, Zinsmeister AR. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 144: 903–911.e3, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wahnschaffe U, Schulzke JD, Zeitz M, Ullrich R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 5: 844–850; quiz 769, 2007. [DOI] [PubMed] [Google Scholar]
- 86.Xia Y, Hu HZ, Liu S, Ren J, Zafirov DH, Wood JD. IL-1beta and IL-6 excite neurons and suppress nicotinic and noradrenergic neurotransmission in guinea pig enteric nervous system. J Clin Invest 103: 1309–1316, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161: 264–276, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang H, Yan Y, Shi R, Lin Z, Wang M, Lin L. Correlation of gut hormones with irritable bowel syndrome. Digestion 78: 72–76, 2008. [DOI] [PubMed] [Google Scholar]
- 89.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 146: 41–46, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]