Abstract
Previous studies reported that administration of somatostatin (SST) to human patients mitigated their diarrheal symptoms. Octreotide (an analog of SST) treatment in animals resulted in upregulation of sodium/hydrogen exchanger 8 (NHE8). NHE8 is important for water/sodium absorption in the intestine, and loss of NHE8 function results in mucosal injury. Thus we hypothesized that NHE8 expression is inhibited during colitis and that SST treatment during pathological conditions can restore NHE8 expression. Our data showed for the first time that NHE8 is expressed in the human colonic tissue and that NHE8 expression is decreased in ulcerative colitis (UC) patients. We also found that octreotide could stimulate colonic NHE8 expression in colitic mice. Furthermore, the somatostatin receptor 2 (SSTR2) agonist seglitide and the somatostatin receptor 5 (SSTR5) agonist L-817,818 could restore NHE8 expression via its role in suppressing ERK1/2 phosphorylation. Our study uncovered a novel mechanism of SST stimulation of NHE8 expression in colitis.
Keywords: NHE8, somatostatin, inflammatory bowel disease, SSTR2, SSTR5
inflammatory bowel disease (IBD) is a chronic condition characterized by colitis in ulcerative colitis (UC) and in Crohn's disease. Genetic and environmental factors are implicated in the pathogenesis of IBD (8, 12). Diarrhea and abdominal pain are the common symptoms in IBD patients, and these symptoms are attributed to mucosal injury and impaired intestinal homeostasis. Abnormal water/sodium absorption has been established as having a vital role in the pathogenesis of inflammatory diarrhea (27). In the intestinal tract, Na+/H+ exchanger isoform 3 (NHE3) and NHE8 have been identified as important players in water/electrolyte transport. NHE3 knockout mice developed diarrhea, acid-base imbalance, and alteration in Na+ homeostasis (26). Loss of NHE8 resulted in altered mucosal protection and colonic inflammation (19, 32). In TNBS- and DSS-induced models of colitis, NHE8 expression was decreased in the intestine (32, 34). Thus improvement of NHE8 expression may have a role in restoration of mucosal function and also improving diarrhea in inflammatory colitis.
Somatostatin (SST), a neuropeptide produced by D cells, has multifunctions in protecting mucosal epithelial cells, regulating immune function, and inhibiting intestinal motility (9, 31). Dharmsathaphorn et al. (10, 11) found in the 1980s that SST could stimulate sodium and chloride absorption and inhibit diarrhea, and this discovery was confirmed later by others (2, 24). In clinical practice, SST analogs such as octreotide, are also used to treat intractable diarrhea, intestinal fistula, short-bowel syndrome, and carcinoid syndrome (6a, 13). We have previously reported that SST treatment could stimulate NHE8 expression in the intestine in normal mice and that SST could regulate tight junctions in mice with colitis (18, 33). However, whether the SST-mediated antidiarrheal effect is the result of stimulating NHE8 and/or NHE3 expression in colitis remains to be defined.
The current study demonstrated that NHE8 is expressed in the human intestine and that NHE8 expression is decreased in ulcerative colitis (UC) patients. NHE8 expression is also decreased in mice with DSS colitis, as well as in TNF-α-treated Caco-2 cells. SST treatment could ameliorate diarrhea by stimulating NHE8 but not NHE3 expression. Furthermore, SST stimulates NHE8 expression during inflammation through the SSTR2/5-ERK1/2 signaling pathway.
MATERIALS AND METHODS
Human subjects.
Human colorectal tissues from age- and gender-matched control individuals and UC patients were obtained during colonoscopy at West China Hospital of Sichuan University. Control individuals were patients with colorectal cancer or polyps, and the colonic tissue biopsies were obtained at least ten centimeters away from the lesion. UC patients were all diagnosed with active disease by endoscopy and pathology tests. Ulcerative colitis is a distal disease and large number of patients has left sided disease. Therefore, majority of the biopsies in this study were collected from rectum and the left colon (Table 1). Tissue biopsies for protein extraction were immediately stored in a liquid nitrogen container at the time they were collected. Tissue biopsies for the immunofluorescence (IF) study were frozen and embedded in OCT compound. This study was approved by the Ethics Committee of West China Hospital of Sichuan University (Chengdu, China).
Table 1.
Clinical characteristics of the included patients
| CT (n = 18) | UC (n = 26) | |
|---|---|---|
| Gender | ||
| Female | 10 | 11 |
| Male | 8 | 15 |
| Age, yr | ||
| <30 | 4 | 6 |
| 30–60 | 12 | 18 |
| ≥60 | 2 | 2 |
| Sample location | ||
| Rectum | 14 | 20 |
| Colon | 4 | 5 |
| Cecum | 0 | 1 |
CT, control; UC, ulcerative colitis.
Animals.
Female C57BL/6 mice (6–8 wk old) were purchased from Experimental Animal Center of West China Hospital (Sichuan University). Mice were fed with 3% DSS water for 7 days to induce colitis. From the 8th day, the treatment group received octreotide administration at doses of 50 μg/kg body wt three times a day for 3 days, while the control group received PBS injection. On the 11th day, all mice were euthanized and colonic tissues were collected. During the experiment, body weight, stool consistency and occult blood were assessed daily. Diarrheal scores were recorded following the previously described method (7). Scores higher than 2.0 were considered to have diarrheal symptoms. Disease activity index (DAI) was determined from combined scores of body weight loss, stool consistency, and fecal hemoccult following the method described previously (25). All experiments were approved by the Institutional Animal Care and Use Committee of Sichuan University.
Cell culture.
Caco-2 cells were purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in a MEM-NEAA medium containing 50 U/ml penicillin, 50 μg/ml streptomycin, and 20% fetal bovine serum in a 5% CO2 incubator. For the TNF-α study, cells were pretreated with 100 ng/ml TNF-α for 18 h, followed by exposure to 1 μM SST or SSTR agonist (1 μM seglitide or 500 nM L-818,817) according to the previously described method (18, 23, 33). For the signaling pathway study, TNF-α-pretreated cells were exposed to 25 μM SB202190 or 25 μM PD98059 for 1 h, respectively (33).
RNA purification and RT-PCR analysis.
To identify the expression of SSTRs in Caco-2 cells, specific primers of SSTRs (Table 2) were used to amplify by RT-PCR. Briefly, total RNA was purified from Caco-2 cells using Trizol reagent (Takara, Japan). Then, 100 ng total RNA were reverse transcribed to cDNA by Moloney murine leukemia virus reverse transcriptase (Thermo). RT-PCR was conducted under the following conditions: initial denaturation at 94°C for 10 min, followed by 35 cycles of 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C. PCR products were then detected by electrophoresis in 1.5% agarose gel.
Table 2.
Primers for SSTR PCR
| Sense | Anti-sense | Product, bp | Genbank Accession No. | |
|---|---|---|---|---|
| SSTR1 | GCGCCATCCTGATCTCTTTCA | ATGACGAGCAGCGATAGCAC | 397 | NM001049 |
| SSTR2 | TGGCTATCCATTCCATTTGACC | GCCAGGTATCGGTCGATGC | 389 | NM001050 |
| SSTR3 (33) | TGGGCACCCTCGTGCCAGCGG | GGGCGGCCGCTCCTGCCCGC | 447 | NM001051 |
| SSTR4 (33) | CTGAACCTCTTCGTGACCAGCCTT | CTGGTTGCAGGGCTTCCTGCT | 278 | NM001052 |
| SSTR5 (30) | CTGGTGTTTGCGGGATGTT | GAAGCTCTGGCGGAAGTTGT | 182 | NM001053 |
SSTR, somatostatin receptor.
Protein isolation and Western blot detection.
Total protein from colonic tissues and Caco-2 cells was prepared in a Cell Lyses Buffer for Western and IP kit (Beyotime, Beijing, China) following manufacturer's instructions. Tissue and cell lysates were used for Western blot detection. Briefly, proteins were separated by 10% SDS-PAGE gel and transferred to a polyvinylidene fluoride membrane. Then the membranes were incubated with NHE8 antibody (35), NHE3 antibody (Santa Cruz Biotechnology), ERK1/2 antibody (R&D System, America), phosphorylated ERK1/2 (pERK1/2) antibody (R&D System, America), and GAPDH antibody (Good Here, Hangzhou, China). The membranes were then incubated with appropriate secondary antibody for 2 h. The target bands were detected using a BM Chemiluminescence Western Blotting Kit (Roche, Germany). Targeting protein expression was normalized by GAPDH. The primary antibody dilution used in this study is: 1:2,000 for NHE8 antibody, 1:2,000 for NHE3 antibody, 1:1,000 for ERK1/2 or pERK1/2 antibody, and 1:2,000 for GAPDH antibody.
Histological assessment of colitis and NHE8 IF.
Colonic tissues were embedded in paraffin after being fixed in 10% formalin and were stained with hematoxylin and eosin (H&E) for tissue histological examination. To detect NHE8 expression, an immunofluorescence assay was conducted. Briefly, frozen colorectal tissues were embedded in OCT compound and sliced into 5-μm sections. The sections were then fixed with acetone for 10 min followed by incubation with 5% goat serum. Sections were subsequently incubated with NHE8 antibody (1:100) overnight at 4°C. The specimens were washed three times with PBST before incubation with Alexa Fluor-594 conjugated goat anti-rabbit IgG secondary antibodies (Santa Cruz Biotechnology) for 1 h at room temperature. After incubation with the nuclear-staining agent DAPI (Sigma), sections were mounted on glass slides. Images were examined and analyzed with a Zeiss Imager Z2 inverted fluorescence microscope (Carl Zeiss, Jena, Germany).
SST detection.
Colonic tissues were collected from mice and used for SST radioimmunoassay. SST radioimmunoassay was done by Beijing DORUN International Technology (Beijing, China).
Statistical analysis.
Data were analyzed with SPSS 16.0 software using ANOVA post hoc test for comparison of three and more groups. Student's t-test was used for comparison of two groups. P < 0.05 was considered significant.
RESULTS
NHE8 is expressed in human colorectal tissue and its expression is reduced in UC patients.
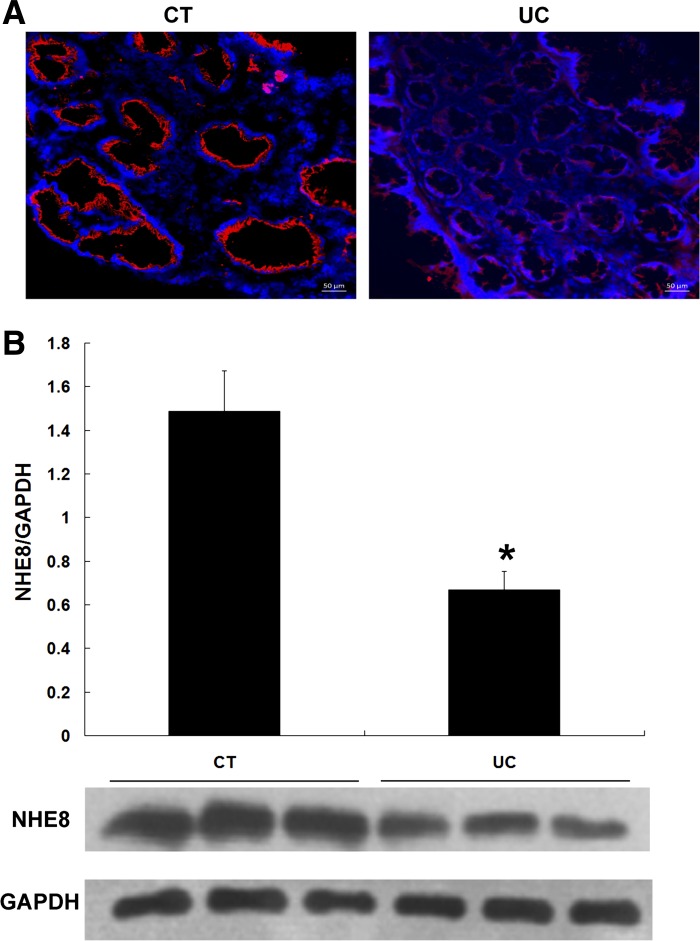
Tissue biopsies were obtained from left colon and rectum from 18 controls and 26 newly diagnosed UC patients. Immunofluorescence stain was used to locate NHE8 protein in the tissue. Total proteins were extracted and used for subsequent NHE8 detection by Western blot. As shown in Fig. 1A, fluorescence microscopy detected apically expressed NHE8 protein in human colorectal epithelia. The signal intensity of NHE8 in non-UC tissues was stronger than that in UC tissues. Since our preliminary observation showed a similar expression level of NHE8 protein in the right colon, the left colon, and the rectum, we compared NHE8 protein abundance in all biopsies obtained from left colon and rectum from control and UC patients. As indicated in Fig. 1B, Western blot confirmed the dramatic decrease in NHE8 protein abundance in UC patients compared with controls (0.67 ± 0.08 in UC vs. 1.49 ± 0.18 in control, n = 44, P < 0.05).
Fig. 1.
Sodium/hydrogen exchanger 8 (NHE8) expression in human colorectal mucosa in controls (CT) and UC patients (UC). A: NHE8 immunofluorescence (IF) in the colorectal region of normal human subjects (left) and UC patients (right). Red fluorescence represents NHE8 protein; DAPI was used to stain the nuclei (blue). The scale bar in IF images is 50 μm. B: NHE8 protein expression comparison between controls and UC patients. NHE8 protein expression is calculated by the ratio of optical density of NHE8 over GAPDH. Data are mean ± SE from 44 samples (18 for control group, 26 for UC group). *P < 0.05 for UC patients vs. controls.
Octreotide treatment ameliorates clinical symptoms and histological scores in DSS colitis mice.
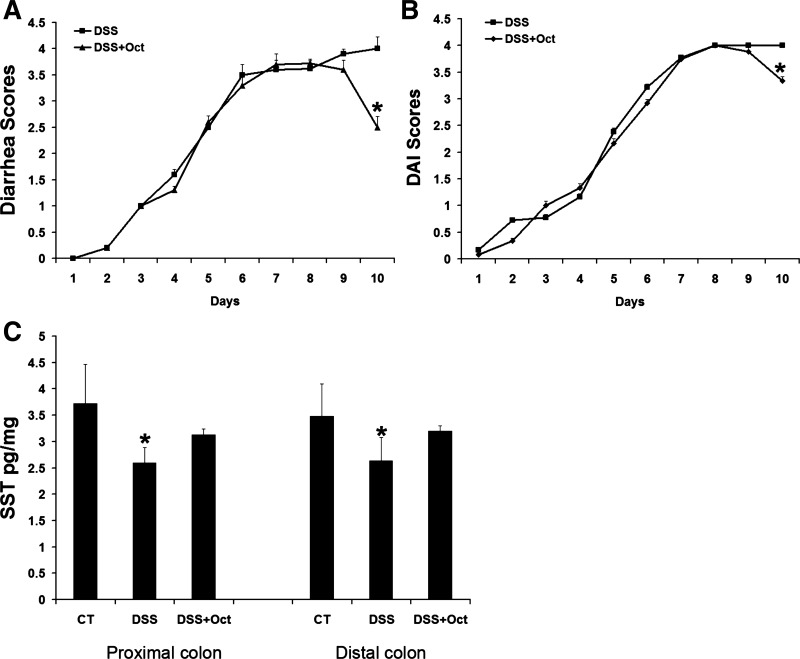
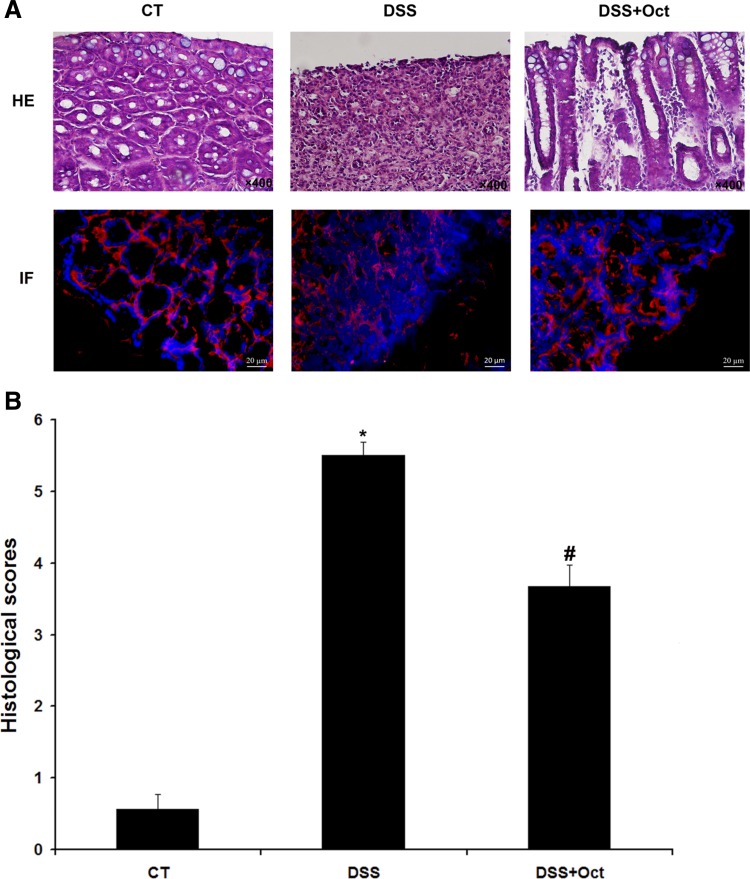
DSS induced diarrhea occurred at the fifth day with a diarrhea score of 2.50 ± 0.03. The symptom was exacerbated through the rest of the experiments. However, octreotide administration (from days 8 to 10) significantly reduced diarrhea scores (from 4.00 ± 0.23 in nontreated DSS mice to 2.50 ± 0.21 in octreotide-treated DSS mice, n = 18, P < 0.05; Fig. 2A). DAI scores were also decreased in octreotide-treated DSS mice on the 10th day (4.0 ± 0.0 in nontreated DSS mice vs. 3.33 ± 0.09 in octreotide-treated DSS mice, n = 23, P < 0.05; Fig. 2B). Furthermore, a reduced SST expression was detected in colitis tissues by SST radioimmunoassay. In the proximal colon, SST expression was significantly reduced from 3.72 ± 0.75 pg/mg tissue in control mice to 2.59 ± 0.3 pg/mg tissue in DSS mice (n = 12 mice, P < 0.05). In the distal colon, SST expression was decreased from 3.48 ± 0.62 pg/mg tissue in control mice to 2.63 ± 0.44 pg/mg tissue in DSS mice (n = 12 mice, P < 0.05). Octreotide treatment also restored SST expression to levels similar to control mice (Fig. 2C). H&E staining indicated that DSS disrupted the colonic epithelial layer, and octreotide treatment partially restored the integrity of the epithelial layer. IF detection on NHE8 protein showed that a weaker NHE8 staining was seen in DSS mice, and octreotide treatment increased NHE8 protein intensity (Fig. 3A). Using a colon injury assessment method (20), we found that the histological score in DSS mice was dramatically increased compared with control mice (5.5 ± 0.19 in DSS mice vs. 0.57 ± 0.2 in control mice, n = 18, P < 0.05). Octreotide treatment partially restored the mucosal injury score (3.67 ± 0.3 in treated DSS mice vs. 5.5 ± 0.19 in DSS mice, n = 18, P < 0.05; Fig. 3B).
Fig. 2.
Diarrhea scores, disease activity index (DAI) scores, somatostatin (SST), assay, and tissue histological observation in control mice (CT), DSS colitis mice (DSS), and octreotide-treated mice (DSS + Oct). Mice were fed with DSS water before octreotide treatment. Diarrhea scores and DAI scores were recorded and SST was measured. Data are mean ± SE from 27 mice (9 for control mice, 9 for DSS colitis mice, and 9 for octreotide-treated colitis mice). A: diarrhea scores. B: DAI scores. C: SST level in the proximal and distal colon. *P < 0.05 for DSS colitis mice (DSS) vs. octreotide treatment mice (DSS + Oct) and control mice (CT).
Fig. 3.
Histological observation and NHE8 IF detection. A: hematoxylin and eosin (H&E) stain (top) and NHE8 IF (bottom) in colon tissues from control mice (CT), DSS colitis mice (DSS), and octreotide-treated colitis mice (DSS + Oct). The magnification for H&E images is ×400. The scale bar in IF images is 20 μm. B: histological scores of mice colonic epithelia. Data are mean ± SE from 27 mice (9 for control mice, 9 for DSS colitis mice, and 9 for octreotide-treated colitis mice). *P < 0.05 for DSS colitis mice (DSS) vs. octreotide-treated colitis mice (DSS + Oct) and control mice (CT); #P < 0.05 for octreotide-treated mice vs. DSS colitis mice (DSS) and controls (CT).
Octreotide stimulates NHE8 but not NHE3 expression in DSS colitis mice.
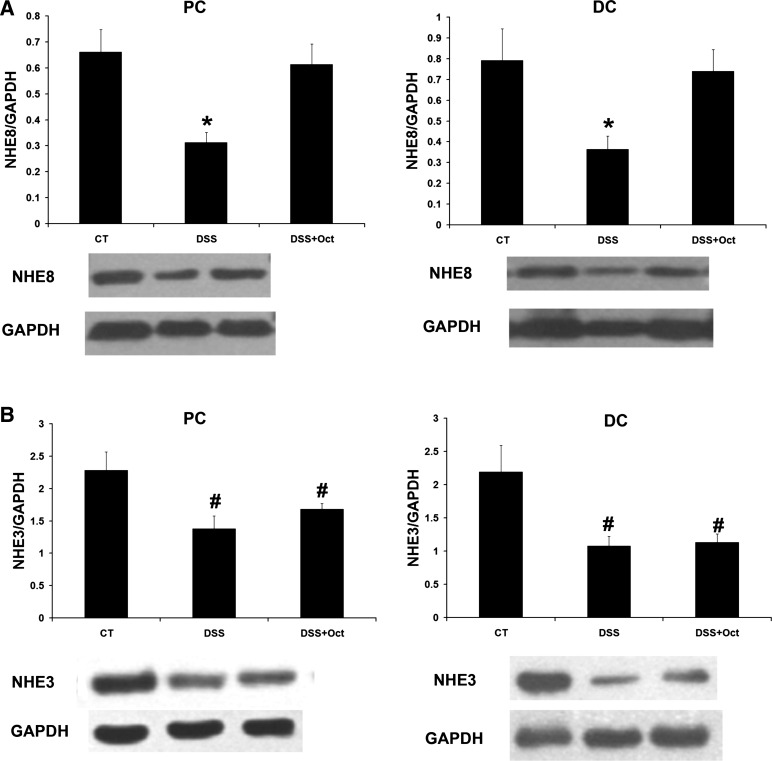
DSS treatment significantly reduced NHE8 protein abundance in mice. In the proximal colon, NHE8 protein abundance was significantly decreased in DSS mice compared with control mice (0.66 ± 0.08 in control mice vs. 0.31 ± 0.04 in DSS mice, n = 18, P < 0.05). In the distal colon, NHE8 protein abundance was also significantly reduced from 0.79 ± 0.15 in control mice to 0.36 ± 0.06 in DSS colitis mice (n = 18, P < 0.05). After octreotide treatment, NHE8 expression in the proximal colon was increased from 0.31 ± 0.04 in untreated DSS mice to 0.61 ± 0.08 in treated DSS mice (n = 18, P < 0.05); NHE8 expression in the distal colon was increased from 0.36 ± 0.06 in untreated DSS mice to 0.74 ± 0.1 in treated DSS mice (n = 18, P < 0.05; Fig. 4A). At the meantime, NHE3 expression in DSS mice was also significantly decreased from 2.28 ± 0.29 in control mice to 1.38 ± 0.2 in DSS mice in the proximal colon (n = 18, P < 0.05) and from 2.19 ± 0.4 in control mice to 1.08 ± 0.14 in DSS mice in the distal colon (n = 18, P < 0.05). However, the expression of NHE3 was not changed by octreotide treatment (Fig. 4B).
Fig. 4.
Expression of NHE8 and NHE3 in mouse colonic tissues. Colonic tissues were collected from control mice (CT), DSS mice (DSS), and octreotide-treated colitis mice (DSS + Oct). Total tissue lysates were prepared and used for Western blot detection of NHE8 and NHE3 protein. Data are mean ± SE from 27 mice (9 for control, 9 for DSS colitis mice, and 9 for octreotide-treated colitis mice). A: NHE8 expression comparison in the proximal and distal colon in mice. B: NHE3 expression comparison in the proximal and distal colon in mice. *P < 0.05 for DSS mice (DSS) vs. octreotide-treated colitis mice (DSS + Oct) and control mice (CT). #P < 0.05 for octreotide-treated mice (DSS + Oct) and DSS colitis mice (DSS) vs. control mice (CT).
SST restores NHE8 protein levels in TNF-α-treated Caco-2 cells.
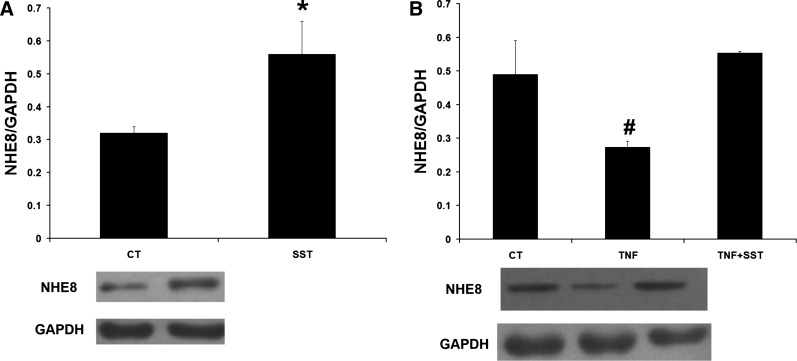
To confirm the stimulatory effect of SST on NHE8 in Caco-2 cells, we treated Caco-2 cells with 1 μM SST for 18 h. As shown in Fig. 5A, SST stimulated NHE8 expression in Caco-2 cells (0.32 ± 0.02 in control cells vs. 0.56 ± 0.1 in SST-treated cells, n = 3, P < 0.05), which was similar to our previous study (33). When Caco-2 cells were treated with 100 ng/ml TNF-α, NHE8 expression was significantly decreased from 0.49 ± 0.1 in control cells to 0.28 ± 0.01 in TNF-α-treated cells (n = 3, P < 0.05). In TNF-α-pretreated cells, SST treatment (1 μM for 1 h) increased NHE8 expression from 0.28 ± 0.01 in cells without SST treatment to 0.56 ± 0.01 in cells with SST treatment (n = 3, P < 0.05; Fig. 5B).
Fig. 5.
Expression of NHE8 in SST, TNF-α, and SST/TNF-α-treated Caco-2 cells. Caco-2 cells were-treated with 1 μM SST with or without TNF-α preincubation. Data are Mean ± SE from 3 separate experiments. A: NHE8 expression in Caco-2 cells-treated with SST without TNF-α preincubation. B: NHE8 expression in Caco-2 cells-treated with TNF-α or SST + TNF-α. *P < 0.05 for SST-treated cells (SST) vs. control cells (CT). #P < 0.05 for TNF-α-treated cells (TNF) vs. control cells (CT) and SST + TNF-α-treated cells (TNF + SST).
SST regulates NHE8 expression through SSTR2 and SSTR5.
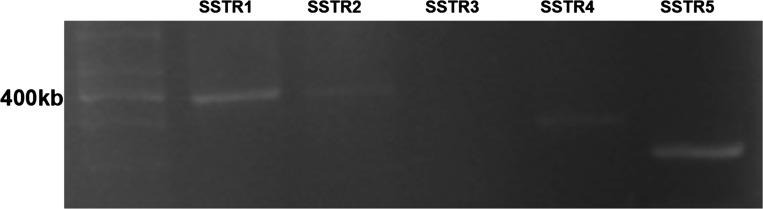
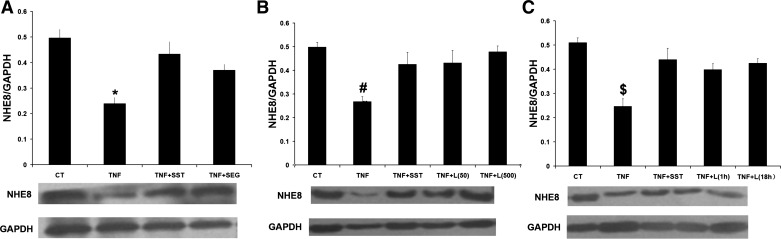
To detect the subtype of SSTR expression in Caco-2 cells, conventional RT-PCR was used with SSTR-specific primers (Table 2). As shown in Fig. 6, SSTR1, SSTR2, SSTR4, SSTR5, but not SSTR3 were amplified from Caco-2 cells. Previous studies have shown that SST is involved in NHE8 regulation and anti-inflammation in Caco-2 cells through SSTR2 and SSTR5 (17, 33). To explore whether SSTR2 or SSTR5 is involved in SST-modulated NHE8 expression in TNF-α-treated Caco-2 cells, selective SSTR2 agonist (seglitide) and SSTR5 agonist (L-817,818) were used. As shown in Fig. 7A, seglitide could stimulate NHE8 expression in TNF-α-treated cells (0.37 ± 0.02 in seglitide/TNF-α-treated cells vs. 0.24 ± 0.02 in TNF-α-treated cells, n = 3, P < 0.05). Similarly, L-817,818 at concentrations of 50 nM and 500 nM could also increase NHE8 expression from 0.26 ± 0.02 in TNF-α-treated cells to 0.43 ± 0.05 in 50 nM L-817,818-treated cells and 0.47 ± 0.02 in 500 nM L-817,818-treated cells (n = 3, P < 0.05; Fig. 7B). To determine the effect of exposure time of L-817,818 on NHE8 expression in TNF-α-treated cells, 500 nM L-817,818 were used to treat cells and NHE8 expression was determined at different time points. As shown in Fig. 7C, L-817,818 exposure for 1 h or 18 hrs stimulated NHE8 expression in TNF-α-treated cells with comparable levels.
Fig. 6.
Expression of SST receptor (SSTR) in Caco-2 cells. RNA was extracted from Caco-2 cells and was reverse-transcripted to cDNA. RT- PCR was used to amplify SSTRs cDNA with specific primers.
Fig. 7.
Effect of SSTR2 and SSTR5 agonist on NHE8 expression in Caco-2 cells. Total cell lysates were prepared from Caco-2 cells and used for Western blot detection of NHE8 protein. A: NHE8 protein expression in Caco-2 cells treated with TNF-α, SST, and seglitide. Data are mean ± SE from 3–5 separate experiments. *P < 0.05 for TNF-α-treated cells (TNF) vs. control (CT), SST-treated cells (TNF + SST), and seglitide + TNF-α-treated cells (TNF + seglitide). B: NHE8 protein expression in Caco-2 cells treated with TNF-α, 50 nM, or 500 nM L-817,818. Data are mean ± SE from 3–5 separate experiments. TNF + L(50): 50 nM L-817,818 treatment in Caco-2 cells for 18 h; TNF + L(500): 500 nM L-817,818 treatment in Caco-2 cells for 1 h. #P < 0.05 for TNF-α-treated cells (TNF) vs. control (CT), SST-treated cells (TNF + SST), and L-817,818-treated cells (TNF + L-817,818). C: NHE8 protein expression in Caco-2 cells treated with TNF-α and L-817,818 for 1 or 18 h. Data are mean ± SE from 3–4 separate experiments. TNF + L(1h): 500 nM L-817,818 treatment in Caco-2 cells for 1 h; TNF + L(18h): 500 nM L-817,818 treatment in Caco-2 cells for 18 h. $P < 0.05 for TNF-α-treated cells (TNF) vs. control (CT), SST-treated cells (TNF + SST), and L-817,818-treated cells (TNF + L).
SST regulates NHE8 expression through suppressing ERK1/2 phosphorylation.
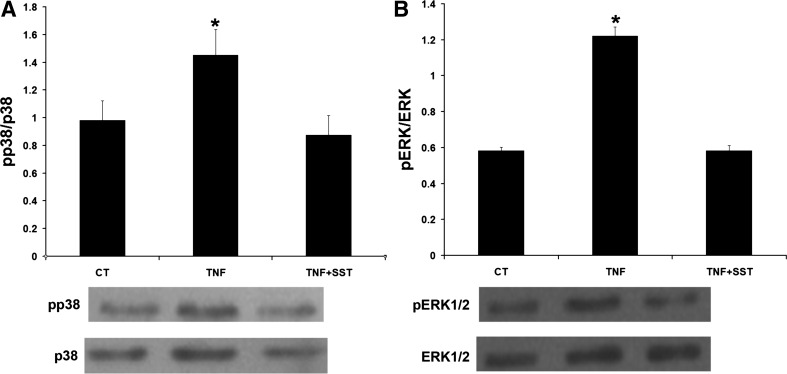
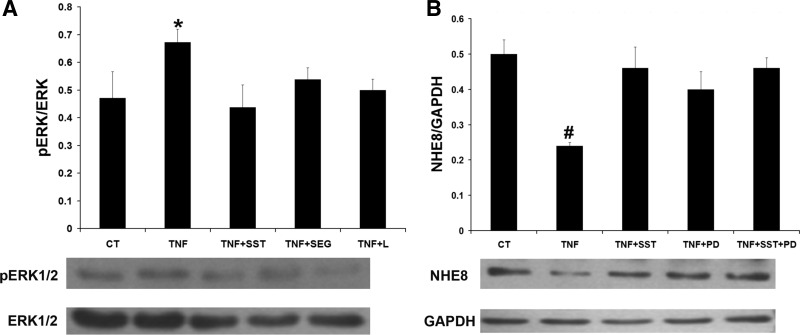
Our previous study has shown SST simulating NHE8 via activation of p38 phosphorylation (pp38) (33). SST has also been shown to be involved in regulating phosphorylation of ERK1/2 (pERK1/2) in Caco-2 cells (17). To explore the signaling pathway involved in SST treatment, the level of pp38 and pERK1/2 in TNF-α and SST-treated cells were compared by Western blot. As shown in Fig. 8A, TNF-α treatment increased p38 phosphorylation in Caco-2 cells (1.45 ± 0.18 in TNF-α-treated cells vs. 0.98 ± 0.14 in control cells, n = 3, P < 0.05). SST treatment brought p38 phosphorylation down to basal level. Similarly, TNF-α treatment increased ERK1/2 phosphorylation in Caco-2 cells, and SST treatment restored ERK1/2 phosphorylation to basal level (1.22 ± 0.05 in TNF-α-treated cells vs. 0.58 ± 0.03 in SST-treated cells, n = 3, P < 0.05; Fig. 8B). To assess whether SST stimulates NHE8 expression via suppressing phosphorylation of p38 and/or ERK1/2, SB202190 (p38-specific inhibitor), and PD98059 (ERK1/2-specific inhibitor) were used. As shown in Fig. 9, SB202190 treatment failed to restore NHE8 expression in TNF-α-treated cells (0.32 ± 0.10 in SB202190-treated cells vs. 0.23 ± 0.10 in TNF-α-treated cells, n = 3; Fig. 9A). However, when TNF-α-treated cells were exposed to PD98059, TNF-α mediated NHE8 expression inhibition was restored (0.42 ± 0.05 in PD98059-treated cells vs. 0.23 ± 0.10 in TNF-α-treated cells, n = 3, P < 0.05; Fig. 9B). Furthermore, cells treated with seglitide or L-817,818 showed reduced ERK1/2 phosphorylation in TNF-α-treated cells (Fig. 10A). To further compare the effect of SST and PD98059 on NHE8 expression, TNF-α-treated Caco-2 cells were exposed to SST and/or PD98059. As shown in Fig. 10B, all treatment conditions (SST, PD98059, SST/PD98059) restored NHE8 expression in TNF-α-treated cells (0.23 ± 0.02 in TNF-α-treated cells vs. 0.46 ± 0.06 in SST-treated cells, 0.4 ± 0.05 in PD98059-treated cells, 0.46 ± 0.03 in SST-PD98059-treated cells, n = 3, P < 0.05).
Fig. 8.
Effect of TNF-α and SST on p38 and ERK1/2 phosphorylation. Caco-2 cells were treated with 1 μM SST for 1 h. Total protein was prepared from cells and used for Western blot analysis. Data are mean ± SE from 3–5 separate experiments. A: detection of p38 phosphorylation in TNF-α and SST-treated Caco-2 cells. B: detection of ERK1/2 phosphorylation in TNF-α and SST-treated Caco-2 cells. *P < 0.05 for TNF-α-treated cells (TNF) vs. control cells (CT) and SST-treated cells (TNF + SST).
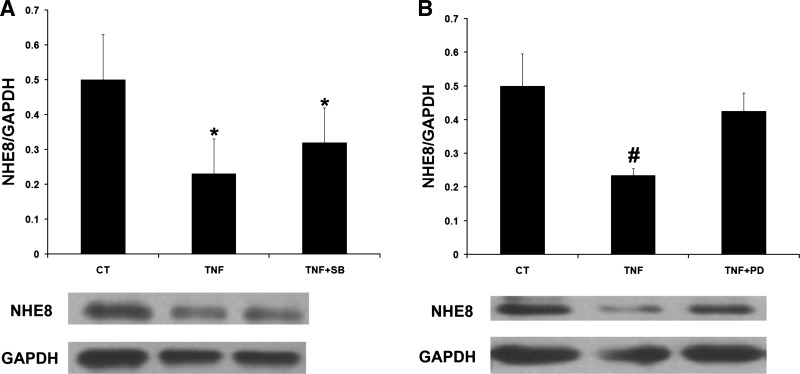
Fig. 9.
Effect of SB202190 and PD98059 on NHE8 expression in TNF-α-treated Caco-2 cells. Caco-2 cells were pretreated with TNF-α for 18 h and then exposed to 25 μM SB202190 and 25 μM PD98059 for 1 h, respectively. Total protein was prepared from cells and used for Western blot analysis. A: NHE8 protein expression in TNF-α and SB202190-treated Caco-2 cells. Data are mean ± SE from 3–4 separate experiments. *P < 0.05 for TNF-α-treated cells (TNF) and SB202190-treated cells (TNF + SB) vs. control cells (CT). B: NHE8 protein expression in TNF-α and PD98059-treated Caco-2 cells. Data are mean ± SE from 3–4 separate experiments. #P < 0.05 for TNF-α-treated cells (TNF) vs. controls (CT) and PD98059-treated cells (TNF + PD).
Fig. 10.
Signaling pathway of SST regulation on NHE8 expression in TNF-α-treated Caco-2 cells. Cells were preincubated with TNF-α for 18 h and then coincubated with 1 μM SST, 1 μM seglitide, and 500 nM L-817,818 for 1 h. Total protein was prepared from cells and used for Western blot analysis. A: pERK1/2 detection in TNF-α, SST, seglitide, and L-817,818-treated Caco-2 cells. Data are mean ± SE from 3–5 separate experiments. *P < 0.05 for TNF-α-treated cells (TNF) vs. control cells (CT), SST-treated cells (TNF + SST), seglitide-treated cells (TNF + SEG), and L-817,818-treated cells (TNF + L). B: NHE8 protein expression in TNF-α, SST, and PD98059-treated Caco-2 cells. Data are mean ± SE from 3–6 separate experiments. #P < 0.05 for TNF-α-treated cells (TNF) vs. controls cells (CT), SST-treated cells (TNF + SST), PD98059-treated cells (TNF + PD), and SST/PD98059-treated cells (TNF + SST + PD).
DISCUSSION
The intestinal epithelium plays a key role in mucosal protection and ion movement (4, 6). NHEs expressed at the apical membrane are vital electroneutral sodium transporters. NHE3 and NHE8, which are expressed at the apical membrane of the intestinal epithelial cells, are involved in intestinal ion transport and in diarrheal illnesses (16, 26, 33). Moreover, studies have demonstrated that NHE8 plays an important role in protecting the mucosa of the gastrointestinal tract (19, 36, 37). Thus stimulating NHE expression may have a role in intestinal mucosal protection. In a previous study, we have reported that SST could modulate NHE8 but not NHE3 expression in mice under normal physiological conditions (33). However, it remains unclear if such stimulation also occurs in inflammatory states, such as IBD, and whether this effect would reduce the diarrhea seen in these disorders. The intestinal inflammation has been shown to inhibit NHE3 expression in colitis patients and in animal models of colitis (29). Although studies have also shown that NHE8 expression could be inhibited in chemically induced colitis in animals and by TNF-α in cultured intestinal epithelial cells (34), the effect of inflammation on NHE8 expression in human colitis has not been explored. In the present study, we noted that NHE8 expression is expressed in the colorectal tissue in humans and its expression is significantly reduced in active UC patients.
Somatostatin is a polypeptide that is widely distributed in the nervous system, gastrointestinal tract and endocrine cells. SST could modulate various physiological processes such as proabsorption, antisecretion, and antiproliferation (33). Thus, a decrease in SST expression would impair intestinal function. Previous studies reported that SST levels were reduced in IBD patients and in mice with colitis (15, 18). We observed similar findings in DSS colitis mice. The decreased SST levels in DSS colitis mice could be partially restored after octreotide treatment. The diarrheal scores, DAI scores, and colonic mucosa histology were also improved in octreotide-treated DSS colitis mice. Thus SST treatment could improve diarrheal symptom and restore intestinal mucosa.
Somatostatin has been shown to stimulate NHE8 expression under normal physiological conditions in mice, however, the role of SST as an antidiarrheal and anti-inflammatory agent and whether it involves NHE8 are not clear. Consistent with previous studies (29, 34), the expression of both NHE8 and NHE3 was decreased in DSS colitis mice. Octreotide treatment restored NHE8 expression in the colon to normal levels in DSS colitis mice. However, octreotide did not increase NHE3 expression in the colon in DSS colitis mice. Thus, these observations suggested that the role of SST in ameliorating diarrhea is most likely involved in regulating NHE8 expression in the colon.
To study the mechanism(s) of somatostatin modulating NHE8 expression in inflammatory conditions, we tested the effect of TNF-α on NHE8 expression in Caco-2 cells (34). In the current study, we observed a stimulation of NHE8 expression by SST and a reduction of NHE8 expression by TNF-α in Caco-2 cells. These changes were in agreement with previous studies (33, 34). Moreover, SST treatment restored NHE8 expression levels in TNF-α-treated Caco-2 cells. This observation suggested that SST could upregulate NHE8 expression under normal physiological and pathophysiological conditions.
Somatostatin triggers its downstream biological responses via interaction with its receptors (SSTRs). Multiple SSTRs are expressed heterogeneously in various cells, and different receptor activation has different roles (22). To determine the subtype of SSTRs involving in SST-mediated NHE8 expression regulation during inflammation, we detected the SSTRs expression in Caco-2 cells by RT-PCR. Our result indicated the presence of SSTR1, 2, 4, and 5 in Caco-2 cells. Interestingly, we reported previously that SSTR1, 2, 3, and 5 were expressed in Caco-2 cells (33). The difference on SSTR isoform expression between these Caco-2 cells may be secondary to the heterogeneity of Caco-2 cell lines. Since previous studies showed that SST stimulated NHE8 expression through SSTR2 activation in Caco-2 cells (33) and SST regulated tight junction expression in LPS-treated Caco-2 cells by activating SSTR5 (17), we wanted to know if SSTR2 and/or SSTR5 participate in SST-mediated NHE8 expression regulation during inflammation. Using the SSTR2-specific agonist seglitide and SSTR5-specific agonist L-817,818, we found that both seglitide and L-817,818 could restore NHE8 expression in TNF-α-treated Caco-2 cells in a similar pattern in cells-treated with SST. These observations suggested that SST could restore NHE8 expression via activating SSTR2/5 in the intestinal epithelial cells during inflammatory settings.
The MAPK signaling pathway is closely modulated by SST (21). p38 phosphorylation is involved in SST-mediated NHE8 expression in the intestinal epithelial cells (33). ERK1/2 phosphorylation is associated with inflammation and cytokine expression (1, 14). SST has been shown to inhibit ERK1/2 phosphorylation in endothelial cells (3, 5). To elucidate the signaling transduction pathways involved in SST-mediated NHE8 activation during inflammation, we focused our study on p38 and ERK1/2 signaling pathways. Our results showed that the phosphorylation level of p38 and ERK1/2 was upregulated in TNF-α-treated cells, and this increase was significantly inhibited by SST treatment. By using the p38 MAPK-specific inhibitor SB202190 and the ERK1/2 MAPK-specific inhibitor PD98059, we found that SB202190 could not restore NHE8 expression in TNF-α-treated cells, while PD98059 treatment could restore NHE8 expression in TNF-α-treated cells. Therefore, the ERK1/2 pathway is most likely involved in TNF-α-mediated NHE8 expression inhibition during inflammation. To determine if SSTR2 and SSTR5 modulate NHE8 expression through the ERK1/2 pathway during inflammation, we detected the phosphorylation level of ERK1/2 in the presence of seglitide and L-817,818 in TNF-α-treated Caco-2 cells. As expected, both seglitide and L-817,818 supressed ERK1/2 phosphorylation in TNF-α-treated Caco-2 cells. In the meantime, the expression of NHE8 was restored by seglitide and L-817,818. These observations suggested that SST-mediated restoration of NHE8 expression during inflammation was mainly involved in SSTR2 and SSTR5 activation. Furthermore, we confirmed that SST, PD98059, and SST/PD98059 could restore NHE8 expression at a comparable level. Taken together, these results suggest that ERK1/2 phosphorylation induced by inflammation could be inhibited by SST via SSTR2/5 in the intestinal epithelial cells. Since SST reduces intracellular cAMP concentration (38), we used H-89, a potent inhibitor of cAMP-dependent protein kinase (mainly PKA), to treat Caco-2 cells (20 μM 1 h). Interestingly, NHE8 expression was not affected by H-89 treatment in Caco-2 cells (data not shown). Thus the cAMP-mediated PKA pathway is unlikely involved in NHE8 regulation in the intestinal epithelial cells.
In summary, we have shown for the first time that NHE8 is expressed in the human intestinal tissues and its expression is decreased in UC patients. We also identified that somatostatin could restore NHE8 expression in colitis, which results in improving diarrheal symptoms and mucosal structure. Furthermore, SST exerts its effect by restoring NHE8 expression during intestinal inflammation via the suppression of ERK1/2 phosphorylation through activation of SSTR2/5.
GRANTS
This investigation was funded by National Natural Science Foundation of China Grant 81070294 and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-073638.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.L., L.C., C.G., J.L., L.T., and D.S. performed experiments; X.L., L.C., H.X., C.G., J.L., L.T., D.S., and C.W. analyzed data; X.L., L.C., and C.W. interpreted results of experiments; X.L. and L.C. prepared figures; X.L. and L.C. drafted manuscript; H.X. and C.W. conception and design of research; H.X., F.K.G., and C.W. edited and revised manuscript; H.X., F.K.G., and C.W. approved final version of manuscript.
REFERENCES
- 1.Al-Sadi R, Guo S, Ye D, Ma TY. TNF-alpha modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol 183: 1871–1884, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthone GJ, Bastidas JA, Orandle MS, Yeo CJ. Direct proabsorptive effect of octreotide on ionic transport in the small intestine. Surgery 108: 1136–1141; discussion 1141–1142, 1990. [PubMed] [Google Scholar]
- 3.Badway AC, West FM, Tente SM, Blake AD. Somatostatin regulates intracellular signaling in human carotid endothelial cells. Biochem Biophys Res Commun 319: 1222–1227, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Barrett KE. New ways of thinking about (and teaching about) intestinal epithelial function. Adv Physiol Educ 32: 25–34, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Basivireddy J, Somvanshi RK, Romero IA, Weksler BB, Couraud PO, Oger J, Kumar U. Somatostatin preserved blood brain barrier against cytokine induced alterations: possible role in multiple sclerosis. Biochem Pharmacol 86: 497–507, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52: 439–451, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Bloom SR. Somatostatin and the gut. Gastroenterology 75: 145–147, 1978. [PubMed] [Google Scholar]
- 7.Boshuizen JA, Reimerink JH, Korteland-van Male AM, van Ham VJ, Koopmans MP, Buller HA, Dekker J, Einerhand AW. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J Virol 77: 13005–11306, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowers Y, Cahalon L, Lahav M, Schor H, Tal R, Bar-Meir S, Levite M. Somatostatin through its specific receptor inhibits spontaneous and TNF-alpha- and bacteria-induced IL-8 and IL-1 beta secretion from intestinal epithelial cells. J Immunol 165: 2955–2961, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Csaba Z, Dournaud P. Cellular biology of somatostatin receptors. Neuropeptides 35: 1–23, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Dharmsathaphorn K, Binder HJ, Dobbins JW. Somatostatin stimulates sodium and chloride absorption in the rabbit ileum. Gastroenterology 78: 1559–1565, 1980. [PubMed] [Google Scholar]
- 11.Dharmsathaphorn K, Sherwin RS, Cataland S, Jaffe B, Dobbins J. Somatostatin inhibits diarrhea in the carcinoid syndrome. Ann Intern Med 92: 68–69, 1980. [DOI] [PubMed] [Google Scholar]
- 12.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931–943, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerich JE, Patton GS. Somatstatin: physiology and clincial applications. Med Clin North Am 62: 375–392, 1978. [DOI] [PubMed] [Google Scholar]
- 14.Harper SJ, Wilkie N. MAPKs: new targets for neurodegeneration. Expert Opin Ther Targets 7: 187–200, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Koch TR, Carney JA, Morris VA, Go VL. Somatostatin in the idiopathic inflammatory bowel diseases. Dis Colon Rectum 31: 198–203, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Laubitz D, Larmonier CB, Bai A, Midura-Kiela MT, Lipko MA, Thurston RD, Kiela PR, Ghishan FK. Colonic gene expression profile in NHE3-deficient mice: evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol 295: G63–G77, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei S, Cheng T, Guo Y, Li C, Zhang W, Zhi F. Somatostatin ameliorates lipopolysaccharide-induced tight junction damage via the ERK-MAPK pathway in Caco2 cells. Eur J Cell Biol 93: 299–307, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Wang Q, Xu H, Tao L, Lu J, Cai L, Wang C. Somatostatin regulates tight junction proteins expression in colitis mice. Int J Clin Exp Pathol 7: 2153–2162, 2014. [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, Xu H, Zhang B, Johansson ME, Li J, Hansson GC, Ghishan FK. NHE8 plays an important role in mucosal protection via its effect on bacterial adhesion. Am J Physiol Cell Physiol 305: C121–C128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 20: 157–198, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Patel YC, Srikant CB. Somatostatin receptors. Trends Endocrinol Metab 8: 398–405, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Petrich A, Mann A, Kliewer A, Nagel F, Strigli A, Martens JC, Poll F, Schulz S. Phosphorylation of threonine 333 regulates trafficking of the human sst5 somatostatin receptor. Mol Endocrinol 27: 671–682, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues CA, Lennard-Jones JE, Thompson DG, Farthing MJ. The effects of octreotide, soy polysaccharide, codeine and loperamide on nutrient, fluid and electrolyte absorption in the short-bowel syndrome. Aliment Pharmacol Ther 3: 159–169, 1989. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki M, Mathis JM, Jennings MH, Jordan P, Wang Y, Ando T, Joh T, Alexander JS. Reversal of experimental colitis disease activity in mice following administration of an adenoviral IL-10 vector. J Inflamm 2: 13, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Seidler U, Lenzen H, Cinar A, Tessema T, Bleich A, Riederer B. Molecular mechanisms of disturbed electrolyte transport in intestinal inflammation. Ann NY Acad Sci 1072: 262–275, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan S, Alex P, Dassopoulos T, Zachos NC, Iacobuzio-Donahue C, Donowitz M, Brant SR, Cuffari C, Harris ML, Datta LW, Conklin L, Chen Y, Li X. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis 15: 261–274, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taboada GF, Luque RM, Bastos W, Guimaraes RF, Marcondes JB, Chimelli LM, Fontes R, Mata PJ, Filho PN, Carvalho DP, Kineman RD, Gadelha MR. Quantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur J Endocrinol 156: 65–74, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Tang C, Lan C, Wang C, Liu R. Amelioration of the development of multiple organ dysfunction syndrome by somatostatin via suppression of intestinal mucosal mast cells. Shock 23: 470–475, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Wang A, Li J, Zhao Y, Johansson ME, Xu H, Ghishan FK. Loss of NHE8 expression impairs intestinal mucosal integrity. Am J Physiol Gastrointest Liver Physiol 309: G855–G864, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, Xu H, Chen H, Li J, Zhang B, Tang C, Ghishan FK. Somatostatin stimulates intestinal NHE8 expression via p38 MAPK pathway. Am J Physiol Cell Physiol 300: C375–C382, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Chen H, Dong J, Li J, Chen R, Uno JK, Ghishan FK. Tumor necrosis factor-α downregulates intestinal NHE8 expression by reducing basal promoter activity. Am J Physiol Cell Physiol 296: C489–C497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36–G41, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Li J, Chen H, Wang C, Ghishan FK. NHE8 plays important roles in gastric mucosal protection. Am J Physiol Gastrointest Liver Physiol 304: G257–G261, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, Zhang B, Li J, Wang C, Chen H, Ghishan FK. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver Physiol 303: G335–G343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang N, Liu SM, Zheng LF, Ji T, Li Y, Mi XL, Xue H, Ren W, Xu JD, Zhang XH, Li LS, Zhang Y, Zhu JX. Activation of submucosal 5-HT(3) receptors elicits a somatostatin-dependent inhibition of ion secretion in rat colon. Br J Pharmacol 159: 1623–1625, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]