Abstract
Several chemical and molecular factors in the intestine are reported to be altered and to have a potentially significant role in irritable bowel syndrome (IBS), particularly in IBS with diarrhea. These include bile acids; short-chain fatty acids; mucosal barrier proteins; mast cell products such as histamine, proteases, and tryptase; enteroendocrine cell products; and mucosal mRNAs, proteins, and microRNAs. This article reviews the current knowledge and unanswered questions in the pathobiology of the chemical and molecular factors in IBS. Evidence continues to point to significant roles in pathogenesis of these chemical and molecular mechanisms, which may therefore constitute potential targets for future research and therapy. However, it is still necessary to address the interaction between these factors in the gut and to appraise how they may influence hypervigilance in the central nervous system in patients with IBS.
Keywords: bile, short-chain fatty acids, permeability, proteases, tryptase, histamine, mucosal expression, mRNA, miRNA
irritable bowel syndrome (IBS) is traditionally diagnosed on the basis of symptoms: recurrent abdominal pain and alterations in bowel function experienced as diarrhea (IBS-D), constipation (IBS-C), or mixed variant with constipation alternating with diarrhea (IBS-M). A variety of central and peripheral mechanisms initiate perturbation of gastrointestinal motor and sensory functions and lead to IBS symptoms (9, 41). These observations lead to the hypothesis that identification of the peripheral factors, their dysfunctions, and altered tissue expression will lead to a greater understanding of the pathobiology, diagnosis of subgroups, and prevention or reversal of the symptoms of IBS.
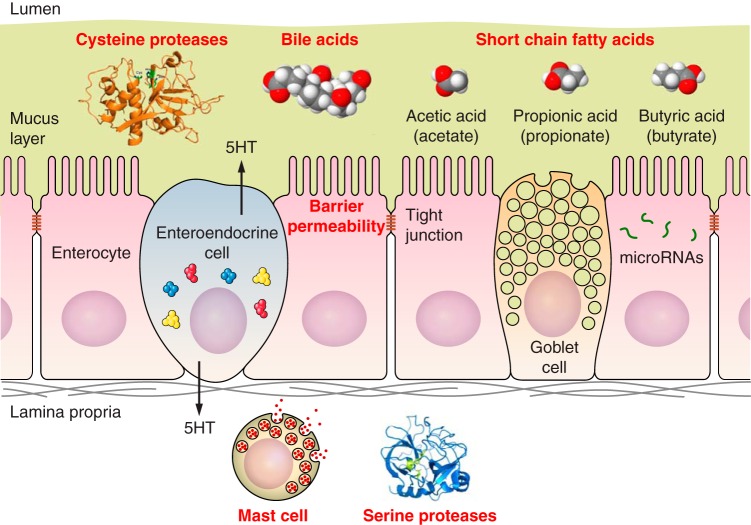
The objectives are to review the salient literature and knowledge gaps, focusing on chemical and molecular factors that could help understand the pathobiology of IBS and conceivably serve as potential therapeutic targets in IBS. The microbiota in IBS has been reviewed extensively elsewhere (4, 49) and is not included in this review of endogenous chemical and molecular mediators. Thus we will focus on bile acids (BAs), short-chain fatty acids (SCFAs), mucosal barrier proteins, proteases, mast cell products, and pathobiologically relevant mucosal molecules such as mRNA, proteins, and miRNA. These factors are linked in different ways in the complex mechanisms leading to the symptomatology in IBS (Table 1, Fig. 1).
Table 1.
Chemical and molecular factors in the multifactorial mechanisms implicated in mucosal functions in IBS
| Secretion/Absorption | Barrier | Inflammation | Sensation | |
|---|---|---|---|---|
| Lumen | Bile acids; short-chain fatty acids | Bile acids | Bacterial proteases/toxins | |
| Mucosa | Ion channel expression | Tight junction expression; microRNA expression | MicroRNA expression; neurotransmitters; mesotrypsin | |
| Lamina propria | Serine proteases | Mast cells; serine proteases |
Fig. 1.
Endogenous chemical and molecular factors in IBS.
Table 2.
Summary of published literature on the role of soluble mediators in IBS
| Supernatant of Stool or Mucosal Biopsies | Patient Group | Receptor/Mechanism of Action | Biological Effect | Citation (Ref. no.) |
|---|---|---|---|---|
| CPA from FSN | Sum of two cohorts; 26 IBS-D, 44 IBS-C, 56 HC | CPA levels are increased in IBS-C vs. HC and IBS-D | Level of CPA correlated with pain. Increased visceral hypersensitivity in PAR2-deficient and normal mice infused with FSN from high-CPA IBS-C vs. normal-CPA IBS-C and HC. | Annahazi et al. (2) |
| Decreased occludin in mice and T84 cells after repeated infusion of FSN in IBS-C vs. HC and in colonic mucosal biopsies in 8 IBS-C vs. 6 HC. | Increased intestinal permeability in PAR2 knockout mice and T84 cells infused with FSN in high-CPA IBS-C vs. normal-CPA IBS-C and HC. | |||
| Serine protease from mucosal biopsies | 10 IBS-D, 5 IBS-C, 12 HC | Activation of PAR2 receptors | Increased hyperexcitability in DRG neurons from wild-type mice vs. PAR2-deficient mice in IBS-D vs. IBS-C and HC. | Valdez Morales et al. (52) |
| CPA from mucosal biopsies | Cysteine protease receptor | Increased hyperexcitability in DRG neurons from mice in 1 IBS-D. | ||
| Serine protease (tryptase), histamine and serotonin from mucosal biopsies | 7 IBS-D, 4 IBS-C, 5 HC | Serine protease, serotonin and histamine receptors | Activation of submucosal neurons in surgical specimen of human colon, mediated by H1–H3, 5HT3 and serine protease receptors. | Buhner et al. (8) |
| Mucosal biopsies | 17 IBS-D, 19 IBS-C, 15 IBS-A, 14 HC | Incubation with IBS SUP (all subtypes) decreased ZO-1 mRNA expression in Caco-2 cells | Increased intestinal permeability in Caco-2 cells incubated with SUP of 39 IBS patient (15 IBS-C, 14 IBS-D, 14 IBS-A) vs. 14 HC. | Piche et al. (43) |
| Decreased ZO-1 expression in colonic biopsies of 21 IBS vs. 12 HC | Increased permeability in colonic biopsies of 12 IBS (3 IBS-C, 4 IBS-D, 5 IBS-A) vs. 5 HC | |||
| Serine proteases from FSN | 24 IBS-D, 18 IBS-C, 10 IBS-A, 17 UC | Activation of PAR2 receptors | Increased visceral hypersensitivity in wild-type mice vs. PAR2-deficient mice | Gesce et al. (27) |
| 23 INF, 25 HC | Increased phosphorylation of myosin light chain; internalization of ZO-1 | Increased permeability in wild-type mice vs. PAR2-deficient mice | ||
| 22 IBS-D, 17 HC | PAR2-mediated increased brain-derived neurotrophic factor expression in Caco-2 cells and mice; p38 MAPK phosphorylation in Caco-2 cells. | Increased visceral hypersensitivity in mice | Wang et al. (56) | |
| Serine proteases from mucosal biopsies | 20 IBS-D, 10 IBS-C, 10 IBS-M, 11 HC | PAR2 mediated increased 5,6-EET in DRG neurons from mice and increased stimulation of transient receptor potential vanilloid-4 in mice treated with SUP in IBS-D vs. HC. | Increased visceral hypersensitivity in mice exposed to SUP in IBS-D patients. | Cenac et al. (15) |
| Histamine | 8 IBS, 7 HC | Mouse single-serosal afferents and DRG sensitized by SUP in IBS compared with control. | Effect mediated by HRH1 interacting with TRPV1. | Wouters et al. (58) |
| 55 IBS patients (44 analyzed) | HRH1 antagonist decreased the potentiating effect of SUP on DRG neurons in IBS-D patients. | Ebastine, HRH1 antagonist, decreased rectal sensation, abdominal pain, and improved quality of life compared with placebo in a group of IBS patients. | ||
| Reactive oxygen species from mucosal biopsies | 14 PI IBS-D, 12 HC | Activation of PAR2 receptors on mast cells in rats. | Mast cell activation in rats with production of histamine. | Han et al. (29) |
| Mesotrypsin in colonic mucosal biopsies | 10 IBS, 9 HC | Response to inflammatory stimuli and induction of hyperexcitability of mouse DRG neurons in a PAR2-dependent fashion | Intestinal epithelial cells produce and release mesotrypsin in IBS and upon inflammatory stimulation, specifically at their basolateral side. | Fourcade et al. (24); Lopez-Lopez et al. (37) |
CPA, cysteine proteases; FSN, fecal supernatant; protease activated receptors, PAR2; H, histamine; HC, healthy controls; 5-HT, serotonin; ZO-1, zonula occludens-1; EET, epoxyeicosatrienoic acid; MAPK mitogen-activated protein kinase; IBS-A, alternating-pattern irritable bowel syndrome; UC, ulcerative colitis; INF, infectious acute diarrhea; SUP, supernatant; DRG, dorsal root ganglion; HRH1, histamine receptor H1; TRPV1, transient reporter potential channel V1.
Bile Acids
Patients with IBS-D may have increased total fecal bile acid excretion, and the percentage of primary fecal bile acids is positively correlated with Bristol stool score and stool frequency (20). There is a significant overall relationship between BAs in serum and stool in IBS. IBS-D is associated with increased primary and reduced secondary BAs in feces relative to healthy controls, whereas IBS-C is associated with marginally reduced primary and increased secondary BAs in feces relative to healthy controls (19). Novel observations of BA malabsorption in patients with IBS-D are reduced levels of FGF-19, variations in klotho-β and FGFR4 genes in association with looser consistency and accelerated colonic transit (10, 57), and identification of an effector G protein-coupled BA receptor (TGR5) on motor, secretory, and enteroendocrine cells in the intestine (1, 45).
Unanswered questions or gaps in knowledge.
While there have been advances in understanding the prevalence and mechanisms of BA diarrhea, further studies of the interaction between microbial flora and BAs are needed, as bacteria in the cecum deconjugate primary BAs and can thereby alter their signaling. In addition, the possible secretory and motor-stimulating effect of primary BAs and the potential role of rapid colonic transit in decreasing the conversion of primary to secondary BAs need further clarification. Finally, there is evidence of abnormal levels of total BAs (48, 57) in patients with IBS-C or chronic constipation, but these studies have involved relatively small numbers of patients and require further replication.
Short-Chain Fatty Acids
An estimated 2–20% of complex carbohydrates escapes absorption in small intestine and reaches the colon (35) in healthy volunteers. Approximately 90% of the starches entering the colon are fermented by gut bacteria into SCFAs and absorbed or used by colonocytes. A major proportion of SCFAs in the mammalian colon is comprised of butyric, acetic, and propionic acids (46). Mucosal butyrate enhances net sodium and chloride ion absorption in isolated stripped mucosa from distal rat colon to a greater extent than propionate and acetate (7). The enhanced absorption of water is attributed, at least in part, to the increased expression of sodium-hydrogen exchanger channels (40).
Abnormalities of SCFAs in stool have been reported in IBS patients. There is evidence of increased colonic fermentation of SCFAs (and gas production) with lower stool mass and slower colonic transit in patients with IBS-C (47) or IBS (39). In a third study, there were higher levels of acetate, propionate, and total organic acids and higher counts of Veillonella and Lactobacillus in IBS patients compared with controls, and those with higher organic acid levels presented with worse gastrointestinal symptoms, quality of life, and negative emotions (50).
On the other hand, some effects of the SCFAs that may be associated with diarrhea include acceleration of colonic transit by stimulation release of serotonin (5-HT) (26) and the secretory effects of SCFAs such as propionate-induced ion and fluid secretion in guinea pig distal colon in vitro, and increased expression of FFA2 receptor (the SCFA receptor) (32). In a study of 63 consecutive IBS patients who received a 4-wk FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) restricted diet, there was marked modulation of fecal fermentation: saccharolytic fermentation (e.g., acetic and n-butyric acids) decreased, while proteolytic fermentation (e.g., i-butyric and i-valeric acids) increased, though there was no relationship between fecal SCFA levels and IBS symptoms (53).
Unanswered questions or gaps in knowledge.
SCFA abnormalities have been observed in patients with IBS-D and IBS-C; however, it is not clear whether this is a cause-effect relationship. It is important to know whether altered composition or concentration of SCFAs is responsible for the transit abnormalities seen in IBS or whether the changes in SCFAs reflect the abnormality in colonic transit similar to the relationship between colonic transit and bile acid composition. The relative proportions of SCFAs may also be influenced by different microbial flora, as has been demonstrated in people of different ethnicity (30). Future studies need to further elucidate the interactions between microbiome, SCFAs, and bile acids, with standardization of diet, longitudinal or repeat studies, and studies conducted in diverse populations who ingest different diets, as well as the effects of FODMAP restriction.
Molecular Species Involved in Mucosal Barrier
The structural components of intercellular tight junctions can be classified as integral membrane proteins (occludin, claudins, and junctional adhesion molecules), junctional complex proteins [e.g., zonula occludens (ZO) proteins, ZO-1, ZO-2, ZO-3], and cell cytoskeleton structures (microtubules, intermediate filaments, and microfilaments) (14). Abnormalities in the mucosal barrier provide access of luminal factors to immune cells in the lamina propria, leading to the immune activation or inflammation in patients with IBS. In turn, inflammatory cytokines may induce changes in mucosal barrier function.
Thus several chemical and molecular mechanisms lead to initiation and maintenance of the increased mucosal permeability, including alterations in expression, localization, and regulation of tight junction proteins; dysbiosis of the microbial flora resulting in the lack of signals to maintain or increase in signals that break the mucosal barrier; active inflammation and increased proinflammatory cytokines and oxidative species; and increased density of epithelial gaps caused by increased cell shedding (14).
Conceptually, the alteration in barrier function may be related to different molecules that are expressed in the lumen, the mucosa and lamina propria, in addition to the barrier itself (Table 1) in patients with IBS. These include
Dihydroxyl BAs (16) and miRNA-29 increase intestinal permeability (60, 61).
Background stress, female sex, and immune activation can influence intestinal mucus barrier (18), gut permeability, visceral pain sensitivity, and gut dysfunction (42, 44).
Increased permeability and immune activation may be induced by chemotactic relatively small tripeptides produced by bacteria (e.g., formyl met-leu-phe) (23).
mRNA expression of barrier proteins is generally reduced in IBS-D (e.g., claudin-1 and ribonucleoprotein-1) and increased (e.g., ZO-1 and occludin) in IBS-C (11).
Proteases (such as the serine proteases and tryptase) produced by luminal bacteria or by mast cells in the lamina propria increase intestinal mucosal permeability through stimulation of type 2 protease-activated receptors (PAR-2) triggering phosphorylation of myosin light chains with subsequent changes in tight junction permeability (22).
Unanswered questions or gaps in knowledge.
In vivo methods that have been used in previous studies to assess intestinal and colonic permeability relied on the urinary measurement of absorbable and poorly metabolized sugars and sugar alcohols, such as lactulose and mannitol. Results from such studies have been difficult to interpret (13) due to limitations that include variation in the timing of urine collection and the possible contamination of urine samples by dietary and other sources of the measured molecule. As a result of these methodological challenges, the proportion of IBS patients with increased intestinal or colonic permeability is still unclear. The methods for in vivo measurement of intestinal permeability are evolving, and Grover et al. (28) have recently validated the use of the stable isotope-labeled pentose [13C]mannitol as a probe molecule. Such potentially more accurate methods are yet to be validated in IBS. Pilot studies suggest a beneficial role of probiotics and glutamine (6) in decreasing permeability in the gastrointestinal tract and thus ameliorating symptoms in IBS (5). Larger studies attempting to reverse increased intestinal permeability in patients with IBS and to study the effect of this reversal on various pathophysiological mechanisms such as immune activation and visceral hypersensitivity are needed. Better understanding of those mechanisms and validated in vivo permeability testing may then lead to specific therapies targeting a subset of patients with IBS who have increased mucosal permeability.
Enteroendocrine Cell Products
The roles of peptides and amines in IBS are extensively reviewed elsewhere (10). The best examples are serotonin and the possible role of serotonin-selective uptake transporter (36, 38), the increased plasma postprandial 5-HT in IBS-D, and reduced postprandial levels in IBS-C (3). Serotonin's effects on fluid secretion and stimulation of colonic motility and tone (54) are illustrated by the protean manifestations of carcinoid diarrhea that results predominantly from the effects of serotonin. There is some evidence that serotonin may be involved in control of small intestinal permeability in IBS (33), but confirmation is required.
Densities of rectal enteroendocrine cells in IBS have been investigated, and the density of peptide YY cells was significantly lower and somatostatin cell density higher in IBS patients than in the controls (21). However, there were generally no differences between IBS-D and IBS-C.
Enteroendocrine cells are stimulated to secrete their products by molecular entities in the intestinal or colonic lumen, such as SCFA and bile acids (1). For example, Alemi et al. (1) showed that BAs stimulated release of the peristaltic transmitters 5-HT and calcitonin gene-related peptide (CGRP); antagonists of these transmitters suppressed BA-induced peristalsis, consistent with localization of TGR5 (BA receptor) to enterochromaffin cells and intrinsic primary afferent neurons. In their elegant studies, TGR5-knockout mice did not show peristalsis or transmitter release in response to BAs (1).
Unanswered questions or gaps in knowledge.
It is unclear whether the enteroendocrine cell products influence intestinal permeability or whether they are influenced by the microbiome. A very recent metagenomic study observed chromogranin A (CgA), a protein secreted by enteroendocrine cells, was exclusively associated with 61 microbial species whose abundance collectively accounted for 53% of microbial composition. Low CgA concentrations were seen in individuals with a more diverse microbiome (59). In addition, studies using organoids show increased stimulation of enteroendocrine cell 5-HT and serotonergic receptors by the SCFA acetate (51).
Proteases
There is abundant literature documenting increased mast cells in the lamina propria (reviewed in Ref. 13), proximity of mast cells to intestinal nerves, and the effects of fecal supernatants or mucosal extracts from patients with IBS on immune activation and afferent stimulation including transient receptor potential cation channel, subfamily V, member 4 (TRPV4) channel activation in animal models (15) (Table 3). In another study (58), supernatants from biopsies of patients with IBS were shown to induce nociception in a murine model and these effects mediate via histamine receptor H1 (HRH1) sensitization of TRPV1 receptors. These interesting interactions were confirmed in humans with IBS, in whom ebastine, a nonsedating HRH1 antagonist, was associated with a decrease in pain in a placebo-controlled clinical trial. A third fecal supernatant from IBS-D patients also increased production of brain-derived neurotrophic factor (BDNF) in colonic epithelial cells and induced visceral hypersensitivity in mice (55). The increased BDNF acts on enteroglial cells in the colon of IBS patients, and the enteroglial cells that localize in proximity to colonic nerves express substance P and correlate with abnormal pain scores in IBS patients (56). Recent data also suggest that mucosal biopsies from IBS patients have increased expression of mesotrypsin (24, 37), and effects of mesotrypsin appear to be mediated by PAR-2 receptors (34, 37).
Table 3.
Mechanisms of action and possible therapeutic roles of different microRNAs involved in IBS
| Micro RNA | Target | Population studied | Results | Suggested mechanism | Potential clinical applicability | Citation (ref. #) |
|---|---|---|---|---|---|---|
| miRNA-24 | Serotonin reuptake transporter (SERT) | 10 IBS patients and 10 healthy patients; IBS mouse model | miRNA-24 upregulated in IBS patients and intestinal mucosa of mice | miRNA-24 inhibits SERT expression and aggravates IBS | miRNA-24 inhibitor | Liao et al. (36) |
| miRNA-29 | NFKB- repressing factor(NKRF) and claudin-1 genes | 183 IBS and 36 HC | Increased levels of miRNA-29 and reduced levels of NKRF and claudin-1 in patients with IBS-D | miRNA-29 targets and reduces claudins and NKRF, which increases intestinal permeability | miRNA-29 inhibitors for IBS with increased permeability | Zhou et al. (60) |
| Mir 29 knock-out mice | Decreased intestinal hyperpermeability in Mir-29 knockout mice | |||||
| Glutamine synthetase gene GLUL | 19 IBS-D and 10 HC | Increased miRNA-29a in IBS-D patients with increased intestinal permeability (42% of IBS-D patients) | miRNA-29a increases membrane permeability by decreasing GLUL gene expression and glutamine level | miRNA-29 inhibitor and glutamine for IBS with increased intestinal permeability | Zhou et al. (61) | |
| miRNA-150 and miRNA-342-3p | Multiple targets including telomerase-related proteins dyskerin, and prosurvival protein kinase AKT2 | 5 IBS-D, 5 IBS-C, 2 IBS-M, and 31 HC | Increased level of miRNA-150 and miRNA-342-3p in IBS | Inflammatory, pain, and motility pathways | Possible role as biomarkers | Fourie et al. (25) |
| miRNA-199 | Transient receptor potential vanilloid type 1 (TRPV1) signaling | 45 IBS-D and 40 HC | Decreased colonic miRNA-199 correlates with visceral pain in patients with IBS-D | miRNA-199a decreases visceral pain via inhibition of TRPV1 signaling | miRNA-199 precursors for pain in IBS | Zhou et al. (63) |
| Visceral hypersensitivity rat models | Reduced miRNA-199a in rat DRG and colon tissue associated with visceral hypersensitivity | |||||
| miRNA-510 | 5-HT3 receptor type 3 subunit gene HTR3E | 98 IBS-D, 99 IBS-C and 100 HC (UK); 119 IBS-D and 195 HC (Germany) | HTR3E variant c. *76G>A, associated with female IBS-D | Variant of HTR3E reduces binding and inhibitory effect of miRNA-510, thus increasing expression of 5-HT3E protein in IBS-D | Unclear clinical impact of miRNA-510 in IBS | Kapeller et al., (31) |
HC, healthy controls; NFKB, Nuclear factor-κB; NKRF, NFKB- repressing factor; DRG, Dorsal root ganglia.
Unanswered questions or gaps in knowledge.
The specific mediators in fecal supernatants that result in their effects need to be identified and classified based on the different IBS subtypes. Therapeutic potentials of mast cell modification and protease inhibition have not been adequately appraised with selective pharmacological agents to explore their potential mechanistic role in IBS. For example, the promising effects of ketotifen in a randomized controlled trial may be associated with its effects on mast cell stabilization or the sedating antihistaminic effects of the drug.
Similarly, the potential role of Bowman-Birk protease inhibitors and their variants, which are naturally found in legumes such as soybean, pea, lentil, and chickpea (17), would appear to be worthy of further study, if patients with increased expression of such proteases could be identified in research and, eventually, in clinical studies, thereby appraising potential health-promoting properties of these inhibitors within the gastrointestinal tract.
Altered Expression of Mucosal Molecules in IBS Pathogenesis
Ribonucleic acid (RNA) sequencing showed transcriptomic changes (secretory and barrier) in rectosigmoid mucosa in IBS-D (12), and the findings were confirmed by using RT-PCR relative to healthy controls and IBS-C patients (11).
miRNAs are short RNA molecules that do not code for protein production, but they are capable of posttranslational regulation of expression of several target mRNAs. They are released from their producing cells in protected forms and therefore are stable for long periods in biological fluids. Table 3 summarizes the role of several miRNAs that have been reported to have actions pertinent to IBS (25, 31, 36, 60, 61, 63).
Unanswered questions or gaps in knowledge.
These interesting observations require replication in larger numbers of patients. The safety and the challenging method of delivery of miRNA inhibitors and/or precursors require future dedicated trials (62).
Conclusion
Several molecular and chemical factors may contribute to the development of IBS. The interrelationships between these factors, as well as the role of the central nervous system hypervigilance and “top-down” modulation of these factors, require further exploration. However, the evidence continues to point to significant role in pathogenesis of these intraluminal and mucosal mechanisms, which constitute potential targets for further research and future specific therapy.
GRANTS
M. Camilleri is funded by grant R01-DK92179 from the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.C. prepared figures; M.C., I.O., and H.H. drafted manuscript; M.C., I.O., and H.H. edited and revised manuscript; M.C., I.O., and H.H. approved final version of manuscript.
REFERENCES
- 1.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 144: 145–154, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annahazi A, Ferrier L, Bezirard V, Leveque M, Eutamene H, Ait-Belgnaoui A, Coeffier M, Ducrotte P, Roka R, Inczefi O, Gesce K, Rosztoczy A, Molnar T, Ringel-Kulka T, Ringel Y, Piche T, Theodorou V, Wittmann T, Bueno L. Luminal cysteine-proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation-predominant IBS. Am J Gastroenterol 108: 1322–1331, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology 130: 34–43, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Barbara G, Feinle-Bisset C, Ghoshal UC, Quigley EM, Santos J, Vanner S, Vergnolle N, Zoetendal EG. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 2016 Feb 18. pii: S0016-5085(16)00219-5. doi: 10.1053/j.gastro.2016.02.028 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5.Barbara G, Zecchi L, Barbaro R, Cremon C, Bellacosa L, Marcellini M, De Giorgio R, Corinaldesi R, Stanghellini V. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clinl Gastroenterol 46 Suppl: S52–S55, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Basra S, Verne GN, Zhou Q. Randomized placebo controlled trial of glutamine for the treatment of diarrhea-predominant irritable bowel syndrome. Gastroenterology 144, Suppl 1: S160, 2013. [Google Scholar]
- 7.Binder HJ, Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology 96: 989–996, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, Michel K, Schemann M. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137: 1425–1434, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 367: 1626–1635, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol 592: 2967–2980, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camilleri M, Carlson P, Acosta A, Busciglio I. Colonic mucosal gene expression and genotype in irritable bowel syndrome patients with normal or elevated fecal bile acid excretion. Am J Physiol Gastrointest Liver Physiol 309: G10–G20, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camilleri M, Carlson P, Acosta A, Busciglio I, Nair AA, Gibbons SJ, Farrugia G, Klee EW. RNA sequencing shows transcriptomic changes in rectosigmoid mucosa in patients with irritable bowel syndrome-diarrhea: a pilot case-control study. Am J Physiol Gastrointest Liver Physiol 306: G1089–G1098, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 303: G775–G785, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 24: 503–512, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cenac N, Bautzova T, Le Faouder P, Veldhuis NA, Poole DP, Rolland C, Bertrand J, Liedtke W, Dubourdeau M, Bertrand-Michel J, Zecchi L, Stanghellini V, Bunnett NW, Barbara G, Vergnolle N. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology 149: 433–444, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med 94: 661–674, 1979. [PubMed] [Google Scholar]
- 17.Clemente A, Sonnante G, Domoney C. Bowman-Birk inhibitors from legumes and human gastrointestinal health: current status and perspectives. Curr Protein Pept Sci 12: 358–373, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Da Silva S, Robbe-Masselot C, Ait-Belgnaoui A, Mancuso A, Mercade-Loubière M, Salvador-Cartier C, Gillet M, Ferrier L, Loubière P, Dague E, Theodorou V, Mercier-Bonin M. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: prevention by a probiotic treatment. Am J Physiol Gastrointest Liver Physiol 307: G420–G429, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Dior M, Delagrèverie H, Duboc H, Jouet P, Coffin B, Brot L, Humbert L, Trugnan G, Seksik P, Sokol H, Rainteau D, Sabate JM. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol Motil 28: 1330–1340, 2016. [DOI] [PubMed] [Google Scholar]
- 20.Duboc H, Rainteau D, Rajca S, Humbert L, Farabos D, Maubert M, Grondin V, Jouet P, Bouhassira D, Seksik P, Sokol H, Coffin B, Sabaté JM. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 24: 513– 520, e246–e247, 2012. [DOI] [PubMed] [Google Scholar]
- 21.El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. Densities of rectal peptide YY and somatostatin cells as biomarkers for the diagnosis of irritable bowel syndrome. Peptides 67: 12–19, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Enjoji S, Ohama T, Sato K. Regulation of epithelial cell tight junctions by protease-activated receptor 2. J Vet Med Sci 76: 1225–1229, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferry DM, Butt TJ, Broom MF, Hunter J, Chadwick VS. Bacterial chemotactic oligopeptides and the intestinal mucosal barrier. Gastroenterology 97: 61–67, 1989. [DOI] [PubMed] [Google Scholar]
- 24.Fourcade CR, Denadai-Souza A, Motta JP, Bautzova T, Spreadbury I, Vanner SJ, Deraison C, Vergnolle N. Epithelial mesotrypsin in IBS: expression and function. Gastroenterology 148: S120, 2015. [Google Scholar]
- 25.Fourie NH, Peace RM, Abey SK, Sherwin LB, Rahim-Williams B, Smyser PA, Wiley JW, Henderson WA. Elevated circulating miR-150 and miR-342-3p in patients with irritable bowel syndrome. Exp Mol Pathol 96: 422–425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 284: R1269–R1276, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Gesce K, Roka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belqnaoui A, Rosztoczy A, Izbeki F, Fioramonti J, Wittmann T, Bueno L. Increased faecal serine protease activity in diarrheic IBS patients: a colonic lumenal factor impairing colonoic permeability and sensitivity. Gut 57: 591–599, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Grover M, Camilleri M, Hines J, Burton D, Ryks M, Wadhwa A, Sundt W, Dyer R, Singh RJ. 13C mannitol as a novel biomarker for measurement of intestinal permeability. Neurogastroenterol Motil 28: 1114–1119, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han W, Lu X, Jia X, Zhou T, Guo C. Soluble mediators released from PI-IBS patients' colon induced alteration of mast cell“ involvement of reactive oxygen species. Dig Dis Sci 57: 311–319, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Hester CM, Jala VR, Langille MG, Umar S, Greiner KA, Haribabu B. Fecal microbes, short chain fatty acids, and colorectal cancer across racial/ethnic groups. World J Gastroenterol 21: 2759–2769, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, Gassler N, Fischer C, Whorwell PJ, Atkinson W, Fell C, Büchner KJ, Schmidtmann M, van der Voort I, Wisser AS, Berg T, Rappold G, Niesler B. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet 17: 2967–2977, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Karaki S, Kuwahara A. Propionate-induced epithelial K+ and Cl−/HCO3− secretion and free fatty acid receptor 2 (FFA2, GPR43) expression in the guinea pig distal colon. Pflügers Arch 461: 141–152, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Keszthelyi D, Troost FJ, Jonkers DM, van Eijk HM, Lindsey PJ, Dekker J, Buurman WA, Masclee AA. Serotonergic reinforcement of intestinal barrier function is impaired in irritable bowel syndrome. Aliment Pharmacol Ther 40: 392–402, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Knecht W, Cottrell GS, Amadesi S, Mohlin J, Skåregärde A, Gedda K, Peterson A, Chapman K, Hollenberg MD, Vergnolle N, Bunnett NW. Trypsin IV or mesotrypsin and p23 cleave protease-activated receptors 1 and 2 to induce inflammation and hyperalgesia. J Biol Chem 282: 26089–26100, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Levitt MD. Malabsorption of starch: a normal phenomenon. Gastroenterology 85: 769–770, 1983. [PubMed] [Google Scholar]
- 36.Liao XJ, Mao WM, Wang Q, Yang GG, Wu WJ, Shao SX. MicroRNA-24 inhibits serotonin reuptake transporter expression and aggravates irritable bowel syndrome. Biochem Biophys Res Commun 469: 288–293, 2016. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Lopez CD, Jaramillo-Polanco JO, Rolland-Fourcade C, Vergnolle N, Vanner S. Mesotrypsin evokes PAR2 dependent excitability of nociceptive dorsal root ganglia (DRG) neurons. Gastroenterology 150: S596, 2016. [Google Scholar]
- 38.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10: 473–486, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mortensen PB, Andersen JR, Arffmann S, Krag E. Short-chain fatty acids and the irritable bowel syndrome: the effect of wheat bran. Scand J Gastroenterol 22: 185–192, 1987. [DOI] [PubMed] [Google Scholar]
- 40.Musch MW, Bookstein C, Xie Y, Sellin JH, Chang EB. SCFA increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. Am J Physiol Gastrointest Liver Physiol 280: G687–G693, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Öhman L, Törnblom L, Simren M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol 12: 36–49, 2015. [DOI] [PubMed] [Google Scholar]
- 42.O'Malley D, Julio-Pieper M, O'Mahony SM, Dinan TG, Cryan JF. Differential visceral pain sensitivity and colonic morphology in four common laboratory rat strains. Exp Physiol 99: 359–367, 2014. [DOI] [PubMed] [Google Scholar]
- 43.Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, Neunlist M. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 58: 196–201, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Pigrau M, Rodiño-Janeiro BK, Casado-Bedmar M, Lobo B, Vicario M, Santos J, Alonso-Cotoner C. The joint power of sex and stress to modulate brain-gut-microbiota axis and intestinal barrier homeostasis: implications for irritable bowel syndrome. Neurogastroenterol Motil 28: 463–486, 2016. [DOI] [PubMed] [Google Scholar]
- 45.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil 22: 814–825, e227–e228, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rechkemmer G, Rönnau K, von Engelhardt W. Fermentation of polysaccharides and absorption of short chain fatty acids in the mammalian hindgut. Comp Biochem Physiol A Comp Physiol 90: 563–568, 1988. [DOI] [PubMed] [Google Scholar]
- 47.Ringel-Kulka T, Choi CH, Temas D, Kim A, Maier DM, Scott K, Galanko JA, Ringel Y. Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am J Gastroenterol 110: 1339–1346, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin A, Camilleri M, Vijayvargiya P, Busciglio I, Burton D, Ryks M, Rhoten D, Lueke A, Saenger A, Girtman A, Zinsmeister AR. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 11: 1270–1275, e1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG; Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 62: 159–176, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil 22: 512–519, e115, 2010. [DOI] [PubMed] [Google Scholar]
- 51.Tsuruta T, Saito S, Osaki Y, Hamada A, Aoki-Yoshida A, Sonoyama K. Organoids as an ex vivo model for studying the serotonin system in the murine small intestine and colon epithelium. Biochem Biophys Res Commun 474: 161–167, 2016. [DOI] [PubMed] [Google Scholar]
- 52.Valdez-Morales EE, Overington J, Guerrero-Alba R, Ochoa-Cortes F, Ibeakanma CO, Spreadbury I, Bunnett NW, Beyak M, Vanner SJ. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel sundrome patients: a role for PAR2. Am J Gastroenterol 108: 1634–1643, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Valeur J, Røseth AG, Knudsen T, Malmstrøm GH, Fiennes JT, Midtvedt T, Berstad A. Fecal fermentation in irritable bowel syndrome: influence of dietary restriction of fermentable oligosaccharides, disaccharides, monosaccharides and polyols. Digestion 94: 50–56, 2016. [DOI] [PubMed] [Google Scholar]
- 54.von der Ohe MR, Camilleri M, Kvols LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med 329: 1073–1078, 1993. [DOI] [PubMed] [Google Scholar]
- 55.Wang P, Chen FX, Du C, Li CQ, Yu YB, Zuo XL, Li YQ. Increased production of BDNF in colonic epithelial cells induced by fecal supernatants from diarrheic IBS patients. Sci Rep 5: 10121, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang P, Du C, Chen FX, Li CQ, Yu YB, Han T, Akhtar S, Zuo XL, Tan XD, Li YQ. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep 6: 20320, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong BS, Camilleri M, Carlson P, McKinzie S, Busciglio I, Bondar O, Dyer RB, Lamsam J, Zinsmeister AR. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol 10: 1009–1015, e3, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA, Valdez-Morales EE, Nasser Y, Van Veldhoven PP, Vanbrabant W, Van der Merwe S, Mols R, Ghesquière B, Cirillo C, Kortekaas I, Carmeliet P, Peetermans WE, Vermeire S, Rutgeerts P, Augustijns P, Hellings PW, Belmans A, Vanner S, Bulmer DC, Talavera K, Vanden Berghe P, Liston A, Boeckxstaens GE. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 150: 875–887, 2016. [DOI] [PubMed] [Google Scholar]
- 59.Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, Wang J, Imhann F, Brandsma E, Jankipersadsing SA, Joossens M, Cenit MC, Deelen P, Swertz MA; LifeLines cohort study , Weersma RK, Feskens EJ, Netea MG, Gevers D, Jonkers D, Franke L, Aulchenko YS, Huttenhower C, Raes J, Hofker MH, Xavier RJ, Wijmenga C, Fu J. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352: 565–569, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Q, Costinean S, Croce CM, Brasier AR, Merwat S, Larson SA, Basra S, Verne GN. MicroRNA 29 targets nuclear factor-κB-repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology 148: 158–169, e8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 59: 775–784, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Q, Verne GN. miRNA-based therapies for the irritable bowel syndrome. Expert Opin Biol Ther 11: 991–995, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Q, Yang L, Larson S, Basra S, Merwat S, Tan A, Croce C, Verne GN. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 65: 797–805, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]