We characterized the stimuli that best activate the pharyngeal swallow response to esophageal stimulation (EPSR) that occurs primarily in human infants and animals and identified the type and location of receptors that mediate this response. The EPSR is activated in a probabilistic manner and is best activated by acidic solutions in more proximal areas of the esophagus. Our findings suggest that this swallow response is very important in human infants to prevent supraesophageal reflux.
Keywords: pharyngeal swallow, esophagus, superior laryngeal nerve
Abstract
Stimulation of the esophagus activates the pharyngeal swallow response (EPSR) in human infants and animals. The aims of this study were to characterize the stimulus and response of the EPSR and to determine the function and mechanisms generating the EPSR. Studies were conducted in 46 decerebrate cats in which pharyngeal, laryngeal, and esophageal motility was monitored using EMG, strain gauges, or manometry. The esophagus was stimulated by balloon distension or luminal fluid infusion. We found that esophageal distension increased the chance of occurrence of the EPSR, but the delay was variable. The chance of occurrence of the EPSR was related to the position, magnitude, and length of the stimulus in the esophagus. The most effective stimulus was long, strong, and situated in the cervical esophagus. Acidification of the esophagus activated pharyngeal swallows and sensitized the receptors that activate the EPSR. The EPSR was blocked by local anesthesia applied to the esophageal lumen, and electrical stimulation of the recurrent laryngeal nerve caudal to the cricoid cartilage (RLNc) activated the pharyngeal swallow response. We conclude that the EPSR is activated in a probabilistic manner. The receptors mediating the EPSR are probably mucosal slowly adapting tension receptors. The sensory neural pathway includes the RLNc and superior laryngeal nerve. We hypothesize that, because the EPSR is observed in human infants and animals, but not human adults, activation of EPSR is related to the elevated position of the larynx. In this situation, the EPSR occurs rather than secondary peristalsis to prevent supraesophageal reflux when the esophageal bolus is in the proximal esophagus.
NEW & NOTEWORTHY
We characterized the stimuli that best activate the pharyngeal swallow response to esophageal stimulation (EPSR) that occurs primarily in human infants and animals and identified the type and location of receptors that mediate this response. The EPSR is activated in a probabilistic manner and is best activated by acidic solutions in more proximal areas of the esophagus. Our findings suggest that this swallow response is very important in human infants to prevent supraesophageal reflux.
swallowing has been defined as comprising oral, pharyngeal, and esophageal phases (9, 25, 32). The pharyngeal phase can be activated voluntarily as part of deglutition (9, 25, 32) or reflexly as part of the pharyngeal swallow reflex (11, 22, 42). The pharyngeal swallow reflex is primarily activated by chemical or mechanical stimulation of the larynx or pharynx (11, 22, 42); however, a few studies (10, 17, 26, 31) have found that it is possible to activate the pharyngeal swallow response by the appropriate stimulation of the esophagus.
It has been reported (17) that distension of the esophagus in dogs with a balloon using “rapid inflation to excessive size” could activate “primary peristalsis.” However, no recordings of such a response were actually published, and no data or statistics were provided (17). We have found (26) that balloon distension of the esophagus in cats can activate the pharyngeal swallow response, but, unlike other responses to esophageal stimulation, e.g., esophago-upper esophageal sphincter (UES) contractive reflex (EUCR), its activation was unpredictable. Therefore, we did not characterize the nature of the stimulus that best activates this response. Numerous other studies in various animal species have been conducted (5, 8, 16, 18, 32, 37) that have not reported activation of the pharyngeal swallow response activated by esophageal distension. Whether this lack of reporting is because the response did not occur or because the investigators were not looking for this response is unknown. Regardless, no animal study has defined or characterized the stimulus that best activates the pharyngeal swallow response to esophageal stimulation.
We could find only two human adult studies that provided some data regarding this type of response (10, 31). One study of the effects of balloon stimulation of the esophagus on the UES found that, in one subject at one time, balloon distension of the cervical esophagus activated peristalsis that “apparently originated in or above the UES” (10). This might be the pharyngeal swallow response, but there are too little data to be certain. In the other study (31), the investigators injected fluid into the distal esophagus and found that, in one out of eight subjects, out of 10 injections of fluid at pH = 7, “three peristaltic contractions followed a pharynx contraction (not significant).” Although this might be a case of a pharyngeal swallow response activated by stimulation of the esophagus, this conclusion is uncertain because a fluid rather than balloon was used. It is possible in this case that the pharyngeal swallow response was activated by the reflux of the fluid to the pharynx or larynx.
On the other hand, there is strong evidence that stimulation of the esophagus in human infants can activate the pharyngeal swallow response. Prior studies have found (19–21) that midesophageal injection of air or fluid (0.1–2.0 ml) in 33-wk-old neonates activated the pharyngeal swallow response in about 50% of the injections in 18 infants, and this effect was confirmed statistically not to be a random event. Therefore, the strongest data to date that stimulation of the esophagus can activate the pharyngeal swallow response exist in human infants, and this might explain the paucity of data in the literature on this response given that most human esophageal physiological studies have been conducted in adults. It is also known that swallowing in human adults and infants differs significantly (7, 40, 43), which might explain the strong difference between human adults and infants in the ability of stimulation of the esophagus to activate the pharyngeal swallow response.
Although swallowing in human adults and infants is significantly different, the swallowing pattern of animals and human infants is very similar. For example, both animals and human infants do not swallow food in one bolus; they fill the valeculae with food in a series of laps or sucks and then swallow when the valeculae are at capacity (39). Therefore, it is possible that animals and human infants may be very similar regarding the ability of stimulation of the esophagus to activate the pharyngeal swallow response.
Evidence exists that the sensory pathway for activating the esophagus-activated pharyngeal swallow response (EPSR) is by way of the recurrent laryngeal nerve (RLN) caudal to the cricoid cartilage (RLNc) (17, 26) and superior laryngeal nerve (SLN) (26) but not the cervical vagus nerve (26). However, the type of receptor mediating the EPSR is unknown.
Given the paucity of data regarding the ability of esophageal stimulation to activate the pharyngeal swallow response and the lack of characterization of this response, the aims of this study were as follows: 1) to characterize the appropriate stimulus for activation of the EPSR and the nature of the EPSR response, 2) to determine the type of receptor mediating the EPSR, and 3) to understand the function of the EPSR.
METHODS AND METHODS
Animal Preparation
The experiments were approved by the IACUC of the Medical College of Wisconsin. These studies were conducted in 46 decerebrate cats. We chose to use the decerebrate model because it allowed us to investigate reflexes, i.e., reflex pharyngeal swallow, that are known to be fully controlled by the brain stem (23, 25) without using anesthetics, which significantly inhibit all neural activity. In addition, prior studies (24) have found that the sensitivity of esophageal reflexes in the decerebrate cat is comparable to that in awake humans. Although the cortex controls volitional swallowing (13) and stimulation of the cortex can alter swallow motor responses (2, 3), there is no evidence that suprabulbar regions of the brain are involved in the control of the pharyngeal swallow reflex.
The cats were fasted overnight and anesthetized using isoflurane (3%). The ventral neck region was exposed, the trachea was intubated, and the carotid arteries were ligated. The skull was exposed, and a hole over a parietal lobe was made using a trephine. The hole was enlarged using rongeurs, the central sinus ligated and cut, and the brain severed midcollicularly using a metal spatula. The forebrain was then suctioned out of the skull, and the blood vessels of the circle of Willis were coagulated by suction through cotton balls soaked in warm saline. The boney sinuses were filled with bone wax, the exposed brain was covered with paraffin oil-soaked cotton balls, and the skin over the skull was sewn closed. The anesthesia was removed, and the animals were then placed supine on a heating pad (Harvard Homeothermic monitor) with the body temperature maintained between 38° and 40°C.
Surgical Preparation
The cervical region was opened along the ventral midline to implant electrodes or strain gauges in appropriate muscles and place an endotracheal tube. To monitor the pharyngeal and esophageal phases of swallowing, we recorded from muscles representative of the different phases. For the pharyngeal phase, we recorded from muscles representative of the different sets of muscles activated, i.e., pharyngeal and hyoid. Thus we implanted in n (number) animals electromyographic (EMG) electrodes in the cricopharyngeus (CP, n = 46), geniohyoideus (GH, n = 24), thyrohyoideus (TH, n = 42), thyropharyngeus (TP, n = 19), and cricothyroideus (CT, n = 16) muscles. For the esophageal phase, we implanted EMG electrodes in the striated muscle esophagus at 1 cm (E1-EMG, n = 27), 4 cm (E4-EMG, n = 18), and 7 cm (E7-EMG, n = 7) from the CP. Strain gauges were implanted on the CP (n = 6). In all animals, a gastric fistula was created to allow insertion of the manometric catheter and stimulating balloon into the esophagus through the lower esophageal sphincter (LES) without disturbing the pharynx.
In some animals, the esophagus was ligated firmly, but not tightly, using 2-0 nylon suture at the UES, LES, and the most distal extent of the cervical esophagus available, thereby separately cannulating the cervical and thoracic esophageal segments. This preparation allowed perfusion of each segment with a different fluid without affecting other areas of the digestive tract (n = 4). In some (n = 4) animals, a cut rubber band was applied around the dorsal side of the cervical esophagus just below the UES, and its ends exited the suture. When occlusion of the proximal cervical esophagus was required to prevent supraesophageal reflux (SER), i.e., during esophageal infusion of lidocaine, the rubber band was gently pulled, ventrally trapping the esophagus against the trachea, and held in place with an external clamp. In some animals, the RLNc were isolated below the cricoid cartilage, severed 1 to 2 cm below the cricoid cartilage, and stimulating electrodes were placed on the rostral segment (n = 6). In all animals, the femoral artery and vein were cannulated to monitor blood pressure and maintain hydration with infusion of saline.
Recording Techniques
Electromyography.
Bipolar Teflon-coated stainless steel wires (AS 632; Cooner Wire) bared for 2–3 mm were placed in each muscle, and the wires were fed into differential amplifiers (A-M Systems 1800). The threshold for identification of an EMG response of all muscles but the CP was a doubling of the voltage and for the CP was a change in response of 25%. In all cases, the response must have been temporally correlated with the stimulus. The electrical activity was filtered (bandpass of 0.1–3.0 KHz) and amplified 1,000–10,000 times before feeding into the computer.
Strain gauge recording.
The strain gauge force transducers (EA-06-031DE-120 option SE; Micro-Measurements) were glued to thin heat-tempered copper-beryllium shims shaped to the contour of the cat CP. Teflon-coated silver-plated copper wires were soldered to the strain gauges. The solder points of the gauges were electrically insulated with acrylic and waterproofed using polysulfide. The gauges were then embedded in Silastic for biocompatibility and to allow sewing to the CP. Each strain gauge was connected electrically to a quarter Wheatstone bridge circuit before amplification by a DC amplifier with the high-frequency filter set at 15 Hz (Grass Model 7P122). The signals from the strain gauges were fed into a computer.
Esophageal and blood pressure manometry.
The intraluminal pressures of the esophagus were recorded using a solid-state manometric catheter (Gaeltec Medical Instruments). The individual recording sites were 3 cm apart with the most proximal site 1 cm from the CP, i.e., E1-SG (n = 17), E4-SG (n = 9), E7-SG (n = 12), E10-SG (n = 9), or E13-SG (n = 3). The threshold pressure for identification of a muscle response was 5 mmHg and a response temporally associated with the stimulus. Inspiration caused a negative pressure artifact, and expiration caused minimal changes in recorded esophageal pressure. Blood pressure was recorded using a Statham pressure transducer. The output signals from these devices were attached to low-level DC preamplifiers (Grass P122) set at 3-Hz high-frequency cutoff filtration. The manometric signals were stored on computer.
Esophageal Stimulation Techniques
The esophagus was stimulated by distension or luminal fluid infusion. The esophagus was distended using a balloon so that the stimulus location and magnitude could be readily controlled and because the rough surface of an inflated balloon allows for stimulation of both mucosal and muscular tension receptors. Two types of distensions were used: slow ramp distension (n = 14) or moderate speed square wave distension (n = 18) at 2, 5, 8, 11, 14, or 17 cm from the CP. After characterizing the responses at these locations, we simplified the studies by limiting the stimuli to the cervical (1–6 cm from the CP), proximal thoracic (7–12 cm from the CP), and distal thoracic (13–17 cm from the CP) esophagus (n = 11). The balloon used in these experiments was 3 cm long, but we also compared the effects of square wave distension using 1-, 3-, or 5-cm-long balloons (n = 7). The slow ramp rate of infusion was 0.5 ml/s until a response was observed given twice per esophageal location, and the square wave infusion was by hand for 5 s to attain 1.0–2.5 cm of distension diameter. The volumes of air required to distend the balloons to specific diameters were calibrated before each experiment. The possible responses to balloon distension were secondary peristalsis (SP), EUCR, or the EPSR (22, 24).
In some animals, the esophagus was stimulated by fluid infusion at 1 ml/min in two ways: 1) infusion into the proximal thoracic esophagus 8 cm from the CP (n = 6), or 2) perfusion through cannulated cervical and thoracic segments of the esophagus (n = 4). We tested the effects of PBS (pH = 7.2), 0.9% NaCl (pH = 5.6), or 0.1 N HCl (pH = 1.2).
Nerve Stimulation
Bipolar electrodes were placed on the RLNc, and current was applied at 5–20 Hz, 1–5 V, 10–30 Hz, 0.2-s duration for 5–10 s using a Grass S88 stimulator.
Local Anesthesia of Esophageal Mucosa
Two sets of mechanoreceptors have been identified in the esophagus (24, 30, 34): muscular and mucosal. The goal of these studies was to determine whether the receptors that initiate the EPSR are located in the mucosa or muscularis because this identification would help in identifying the function of the EPSR. Prior studies (24) found that perfusion of the esophagus with lidocaine had the same result as removal of the mucosa/submucosa, which was to block the reflexes mediated by the receptors of the mucosa but to preserve the reflexes of the muscularis. Therefore, in the present study, lidocaine (2%) was infused through a PE 90 catheter inserted through the gastric fistula and LES just below the pharyngo-esophageal junction at 1 ml/min for 5 min using an infusion pump. A vacuum tube was inserted through the gastric fistula into the proximal stomach just below the LES to suck out the infused lidocaine to prevent it from affecting gastric function.
Experimental Protocols
More than one experiment was tested in each animal; therefore, the total number of animals used is less than the total number of experiments.
Characterization and quantification of the esophageal stimuli that activate the EPSR.
the relationship between stimulus and response.
First, the effects of the inflation of a 3-cm-long balloon at different distensions and locations along the total length of the esophagus on the activation of the EPSR were tested (n = 32). We compared the effects of distension in a slow ramp (n = 14) and square wave pattern (n = 18) of the applied stimulus.
Second, the effects of inflation of a 1-, 3-, or 5-cm-long balloon in the cervical, proximal thoracic, or distal thoracic esophagus were tested (n = 6). Only the square wave pattern of balloon inflation was used.
effects of the luminal acidification.
First, the effects of perfusion of NaCl (0.9% NaCl; n = 4) or HCl (0.1 N HCl, n = 6) into the proximal thoracic esophagus on the occurrence of pharyngeal swallowing were compared with spontaneous pharyngeal swallowing with no fluid perfusion or by comparing the effects of NaCl infusion followed by HCl infusion (n = 4).
Second, we tested the effects of selective perfusion of cervical and thoracic esophagus with PBS or HCl on the occurrence of pharyngeal swallow (n = 4).
Third, we tested the effects of esophageal acidification for 10–15 min on the EPSR (n = 4).
Determination of the sensory mechanisms that mediate the EPSR.
neural pathway.
We tested the effects of centripetal electrical stimulation of the RLNc (n = 6) on the ability to activate the pharyngeal swallow response.
type of esophageal receptor.
We tested the effects of lidocaine (2%) infusion (n = 6) into the esophagus on EPSR and EUCR.
Data Reduction and Analysis
These studies were designed to investigate the mechanism of initiation of the EPSR, but pharyngeal swallows can occur spontaneously even in decerebrate cats. To determine whether a response was spontaneous or activated by the stimulus, we waited for 30–60 min before beginning the experiments to determine whether spontaneous swallows occurred and if so their rate of occurrence. If swallows occurred spontaneously, at least six swallows were recorded, and the means ± SD of the rate were determined for each cat. We then only considered swallows that occurred sooner than the mean − 2 SD after a spontaneous swallow to be activated by the stimulus. The stimulus was applied at least 15 s before the next swallow was expected to occur, and no EPSR occurring 10 s after the beginning of the stimulus was considered activated by the stimulus.
The relationship between stimulus and response can be assessed by examining the magnitude of the stimulus related to the magnitude of the response. The magnitude of the response can be quantified based on the size or timing of the response. Therefore, considering that each swallow was of very similar size, for magnitude we quantified the number of swallows activated by the response, and for timing we quantified the percent occurrence of the response as well as the delay from stimulus to response.
Differences between two groups were tested using Student's paired t-test when each animal was used as its own control; otherwise Student's nonpaired t-test was used. In some cases of paired studies, the values were not normally distributed, and we used Wilson's signed rank test. When testing differences among more than two groups, we used ANOVA. In all cases, the data are expressed as means ± SD, and a P value of 0.05 or less was considered statistically significant.
RESULTS
Characterization and Quantification of the EPSR
Relationship between stimulus and response.
magnitude and location of the stimulus.
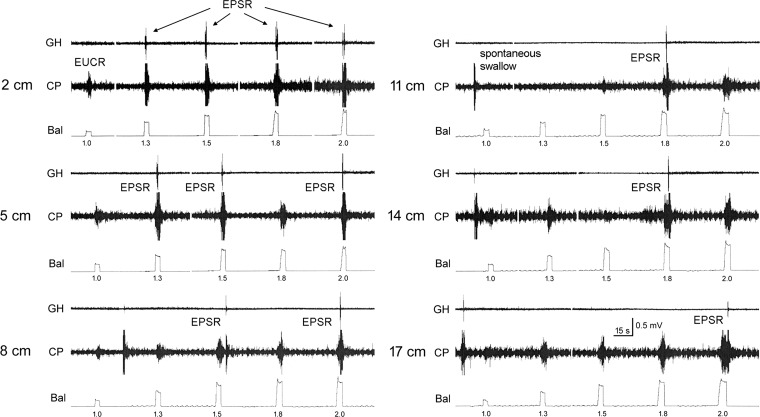
The effects of the inflation of a 3-cm-long balloon in a square wave pattern at six sites along the total length of the esophagus on the activation of the EPSR were tested (n = 18). Twelve out of the 18 cats tested had spontaneous swallows with delay between swallows of 120 ± 57 s. We found that esophageal distension at all levels of the esophagus and at all diameters was capable of activating the EPSR, but the response magnitude was not related to the magnitude of the stimulus (Fig. 1). That is, every response was just one EPSR of very similar magnitude, and the delay to activation of the EPSR was very variable (0.2–10.0 s) and not related to the magnitude of the stimulus (Fig. 2, Table 1). The delay between stimulus and the EPSR was also not related to the region of the esophagus that received the stimulus (Table 2). Although the delay increased from 3.9 ± 2.9 s to 7.9 ± 4.8 s as the stimulus moved distally in the esophagus from 2 to 17 cm from the UES, this change was not statistically significant. The relationship of the stimulus to the EPSR was probabilistic; that is, at any distension and at any level of the esophagus a stimulus could activate an EPSR, but its occurrence was a matter of chance (Table 3). The chance of occurrence ranged from 6% for a 1.0-cm distension at 17 cm from the UES to 46% for a 1.5-cm distension at 8 cm from the UES. There were relationships between the location and magnitude of the stimulus in the esophagus and the chance of activating an EPSR (Table 3). In general, stronger distensions at more proximal locations were most likely to activate an EPSR (Table 3). In addition, we found that slow ramp distensions of the esophagus were also able to activate the EPSR (Fig. 3), but we found no significant differences in probability of occurrence or threshold pressure at different locations in the esophagus (Table 4). The percent occurrence ranged from 20 ± 10% at the cervical esophagus to 32 ± 10% at the proximal thoracic esophagus, and the threshold ranged from 11 ± 6 mmHg at the cervical esophagus to 22 ± 7 mmHg at the distal thoracic esophagus.
Fig. 1.
The effect of balloon distension of the esophagus. These are recordings of geniohyoideus (GH) and cricopharyngeus (CP) electromyography (EMG) during esophago-upper esophageal sphincter (UES) contractile reflex (EUCR) or esophagus-stimulated pharyngeal swallow response (EPSR) in response to distensions of the esophagus at various esophageal locations. The EUCRs are associated with increased CP EMG, and the EPSRs are associated with increased GH and CP EMG activated in a sequential fashion. Note that only 1 EPSR was activated per stimulus, and the delays between stimulus and EPSR were variable. EPSRs were more likely to occur at higher distensions and at more rostral esophageal locations of the stimulus. Occasionally, spontaneous swallows occurred between stimuli. Bal, balloon. Balloon distensions were 1.0–2.0 cm in diameter of a 3-cm-long balloon in the esophagus at 2–17 cm from the CP.
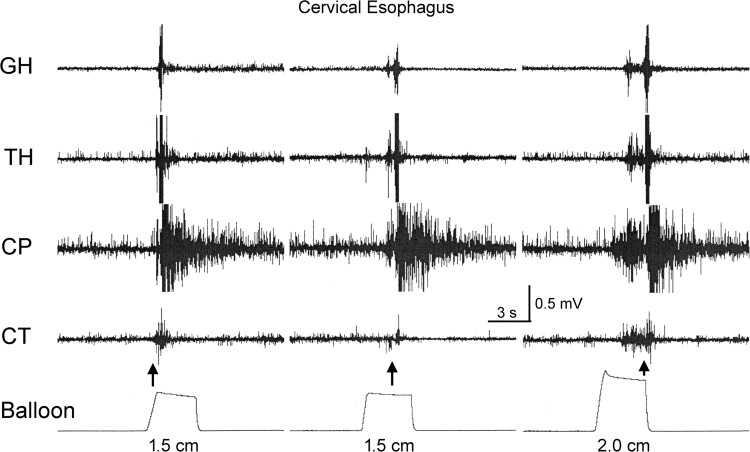
Fig. 2.
The variability of the delay between stimulus and EPSR. These are recordings of GH, thyrohyoideus (TH), CP, and cricothyroideus (CT) EMG in response to various distensions of the cervical esophagus 5 cm from the CP. Note that the delay between stimulus and response for the EPSR varied considerably. The arrows indicate the time of initiation of the EPSR. The dimensions below the distension recordings are the diameters of the balloon distensions.
Table 1.
Relationship between stimulus magnitude (esophageal distension diameter) and response magnitude (number of pharyngeal swallows activated) and time delay to the response
| Esophageal Distention, cm |
|||||
|---|---|---|---|---|---|
| 1.0 | 1.3 | 1.5 | 1.8 | 2.0 | |
| Delay, s | 3.5 ± 2.7 | 3.5 ± 2.1 | 4.4 ± 3.2 | 4.1 ± 2.7 | 3.7 ± 3.1 |
| Number of swallows | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| N | 9 | 8 | 16 | 12 | 16 |
Values of delay and number of swallows are means ± SD. There were no significant differences among delays to activation of the esophagus-stimulated pharyngeal swallow response (EPSR) caused by esophageal distension in a square wave pattern (P > 0.05) using ANOVA.
n, number of animals.
Table 2.
Relationship between stimulus sites and response magnitude (number of pharyngeal swallows activated) and time delay
| Location, cm from CP |
||||||
|---|---|---|---|---|---|---|
| 2 | 5 | 8 | 11 | 14 | 17 | |
| Delay, s | 3.9 ± 2.9 | 4.2 ± 3.4 | 3.4 ± 2.5 | 4.9 ± 2.5 | 5.3 ± 3.3 | 7.9 ± 4.8 |
| Number of swallows | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| N | 13 | 12 | 14 | 10 | 8 | 3 |
Values of delay and number of swallows are means ± SD. There were no significant differences among delays to activation of the EPSR caused by esophageal distension in a square wave pattern (P > 0.05) using ANOVA.
CP, cricopharyngeus; n, number of animals.
Table 3.
Probability of initiating the EPSR at various stimulus magnitudes (esophageal distension diameter) and locations of the stimulus in the esophagus
| Distance from CP, cm | 1.0 | 1.3 | 1.5 | 1.8 | 2.0 | R2 | P |
|---|---|---|---|---|---|---|---|
| 2 | 20 | 35 | 41 | 39 | 45 | 0.792 | 0.043 |
| 5 | 17 | 22 | 30 | 57 | 40 | 0.687 | 0.083 |
| 8 | 12 | 32 | 46 | 40 | 32 | 0.369 | 0.277 |
| 11 | 13 | 17 | 14 | 33 | 33 | 0.790 | 0.044 |
| 14 | 17 | 12 | 26 | 22 | 16 | 0.045 | 0.731 |
| 17 | 6 | 14 | 20 | 7 | 10 | 0.001 | 0.978 |
| R2 | 0.554 | 0.702 | 0.424 | 0.725 | 0.928 | ||
| P | 0.090 | 0.037 | 0.160 | 0.030 | 0.002 |
Values are % occurrence of EPSR caused by esophageal distension in a square wave pattern.
n = 16; R2; Pearson R2 for a relationship with distance from CP or magnitude of distension; P, probability of R2.
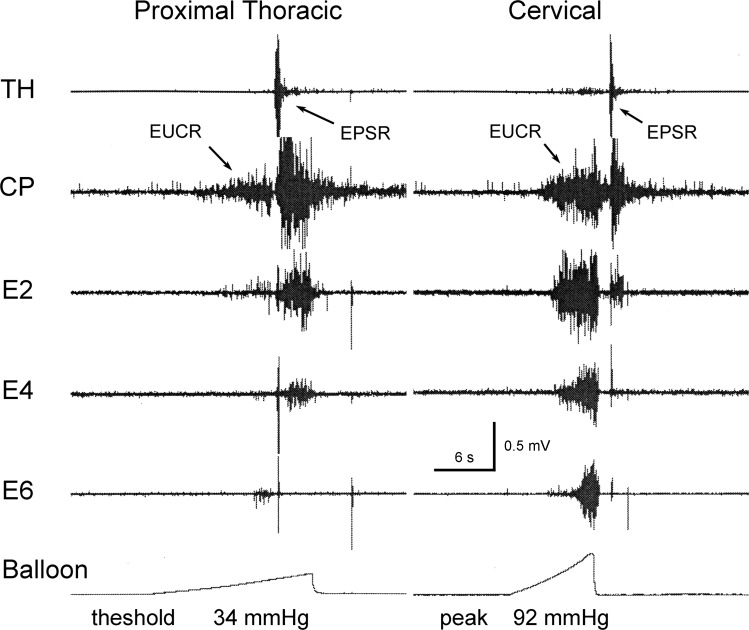
Fig. 3.
Effect of slow ramp distension of the esophagus on esophageal responses. These are TH, CP, esophagus 2 cm from the CP (E2), E4, and E6 EMG recordings during EUCR or EPSR in response to a slow ramp distension of the esophagus at the cervical (5 cm from the CP) and proximal thoracic (11 cm from CP) regions of the esophagus. Note that the EPSR occurred either during the slow distension (threshold at 34 mmHg) or at the end of the slow distension. These distensions activated the EUCR before the EPSR.
Table 4.
Effect of slow ramp distension of the esophagus on the initiation of the EPSR
| Cervical, 4 cm | Prox Thor, 9 cm | Distal Thor, 14 cm | |
|---|---|---|---|
| % occurrence | 20 ± 10 | 32 ± 10 | 26 ± 10 |
| Threshold, mmHg | 11 ± 6 | 20 ± 7 | 22 ± 7 |
Values are means ± SD (n = 14). There were no significant differences among % occurrence of or threshold stimulus to activate the EPSR caused by esophageal distension in a square wave pattern (P > 0.05) using ANOVA.
Prox, proximal; thor, thoracic esophagus.
length of esophagus stimulated.
We also found a relationship between the length of esophagus distended and the chance of activating an EPSR (Table 5). This relationship was not apparent at specific locations in the esophagus due to the high variability of the occurrence and the low number of locations tested but was statistically significant when tested using average percent occurrence at all levels of the esophagus. That is, the Pearson correlation coefficients (R2) for a relationship between the length of the balloon and the percent occurrence of the EPSR at the cervical, proximal thoracic, or distal thoracic esophagus were not statistically significant, but the R2 (0.999) for a relationship between the average percent occurrence at all levels of the esophagus and the length of the distending balloon was statistically significant (P = 0.014).
Table 5.
Probability of initiating the EPSR by distension of the esophagus at different sites using different length balloons
| Cervical, 4 cm | Prox Thor, 9 cm | Distal Thor, 14 cm | Total | |
|---|---|---|---|---|
| 1 cm | 17 | 15 | 23 | 18 |
| 3 cm | 58 | 26 | 10 | 31 |
| 5 cm | 60 | 49 | 25 | 45 |
| R2 | 0.785 | 0.960 | 0.015 | 0.999 |
| P | 0.307 | 0.128 | 0.922 | 0.014 |
Values are % occurrence of EPSR. N = 6; R2; Pearson correlation coefficient for a relationship with length of balloon or distance of center of balloon from CP; P, probability of R2. Total is the average of all responses in all locations in the esophagus.
Effect of the pH of the esophageal infusate on the spontaneous activation of pharyngeal swallowing.
effects of infusion of saline or acid.
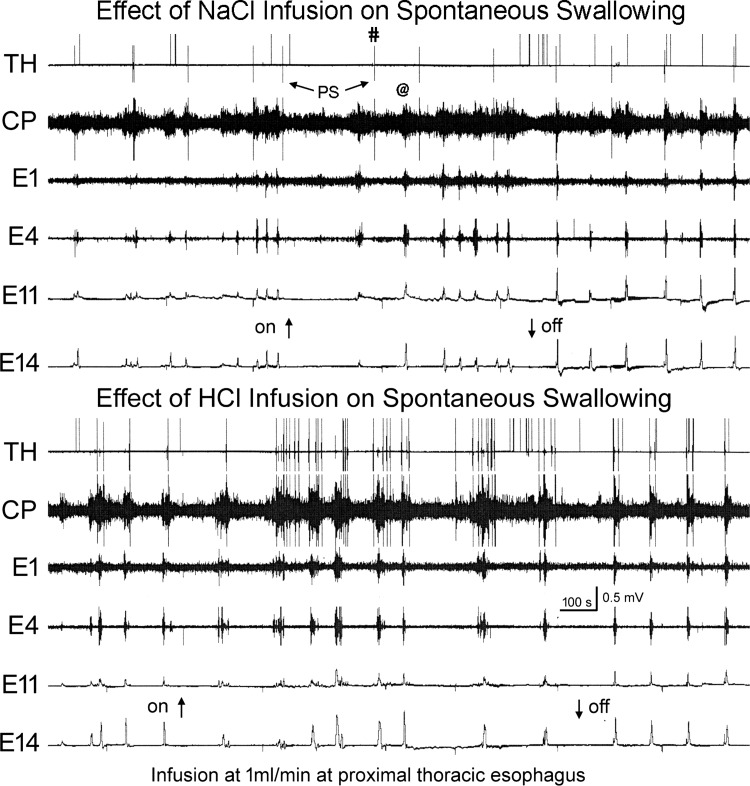
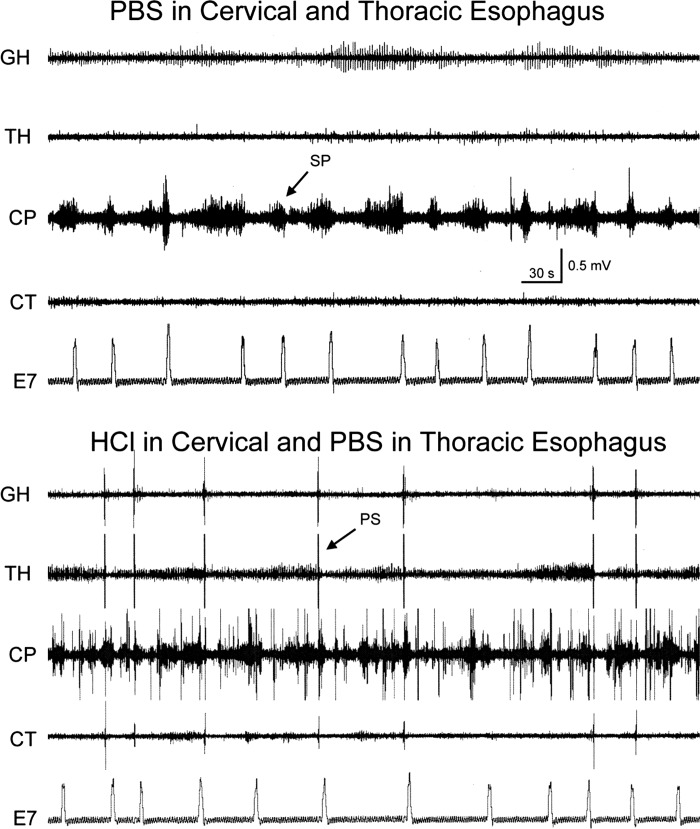
We compared the effects of infusion of saline (0.9% NaCl) or acid (0.1 N HCl) into the proximal thoracic esophagus on spontaneous swallowing rate. We found that saline infusion (Fig. 4, top) slightly, but significantly, reduced the spontaneous rate of swallowing compared with no fluid infusion (0.62 ± 0.34/min to 0.26 ± 0.15/min, P = 0.018, n = 4). On the other hand, acid infusion (Fig. 4, bottom) significantly increased the spontaneous rate of swallowing compared with no fluid infusion (0.74 ± 0.18/min to 2.47 ± 0.37/min; P = 0.0011, n = 6). When we compared the rate of swallowing during saline (n = 4) to during acid (n = 6), we found that the rate of spontaneous swallowing was significantly larger when acid was infused (P = 0.011).
Fig. 4.
Comparison of the effects of NaCl and HCl on rate of spontaneous pharyngeal swallows (PS). These are TH, CP, E1, and E4 EMG recordings and E11 and E14 manometric recordings in response to fluid infusion, NaCl or HCl, into the proximal thoracic esophagus (8 cm from the CP). Note that HCl, but not NaCl, infusion significantly increased the rate of spontaneous pharyngeal swallows. The pharyngeal swallows are indicated by the increased TH and CP EMG in a sequential fashion. Not all pharyngeal swallows were accompanied by esophageal peristalsis (#), and not all esophageal peristalses were accompanied by pharyngeal swallows (@).
effects of selective perfusion.
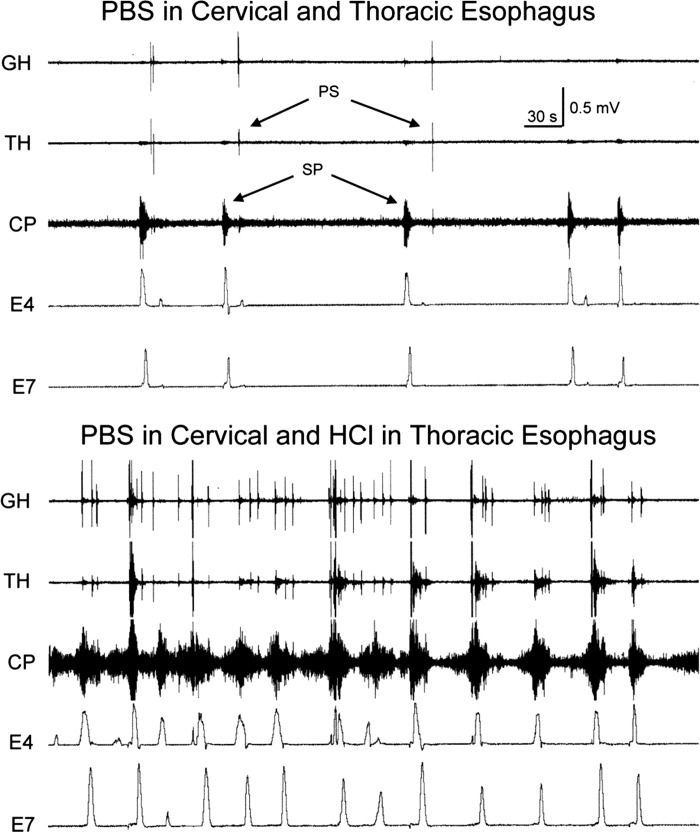
We also tested the effects of selective perfusion of cervical and thoracic esophagus with fluid of pH = 7.2 (PBS) or 1.2 (0.1 N HCl) on the occurrence of the spontaneous pharyngeal swallows and SP in the same animals at the same time (n = 4). This study was done with ligation and infusion of PBS to fully control all aspects of the fluid infusion. We found that perfusion of cervical or thoracic esophagus with fluid of pH = 7.2 for 30 min resulted in low levels of occurrences of both pharyngeal swallows (cervical: 29 ± 44; thoracic: 17 ± 7) and SPs (cervical: 25 ± 25; thoracic: 8 ± 5), but when either the cervical (Fig. 5) or thoracic (Fig. 6) esophagus was perfused with fluid of pH = 1.2, while the other segment was perfused with a fluid of pH = 7.2, the rate of pharyngeal swallows was significantly increased (cervical: 53 ± 48; P = 0.001, n = 4; thoracic: 49 ± 19, P = 0.05). Although infusion of the thoracic esophagus with fluid of pH 1.2, in the example shown (Fig. 6), caused an increase in the rate of SPs, this was not a generalized effect, as the mean rate of SPs (16 ± 12; P = 0.15, n = 4) did not change with a change in pH from 7.2 to 1.2.
Fig. 5.
Effect of changing pH of cervical esophageal perfusate on spontaneous occurrence of pharyngeal swallows and secondary peristalses (SP). These are GH, TH, CP, and CT EMG recordings and the E7 manometric recording in response to a change in pH of the fluid perfused through the cervical esophagus. Note that selective change in pH of the thoracic esophagus from 7.2 to 1.2 greatly increased the spontaneous rate of pharyngeal swallows.
Fig. 6.
Effect of changing pH of thoracic esophageal perfusate on spontaneous occurrence of pharyngeal swallows and secondary peristalsis. These are GH, TH, and CP EMG recordings and E4 and E7 manometric recordings in response to a change in pH of the fluid perfused through the thoracic esophagus. Note that selective change in pH of the thoracic esophagus from 7.2 to 1.2 greatly increased the spontaneous rate of pharyngeal swallows and secondary peristalses.
Effect of esophageal acid exposure on the sensitivity of activation of the EPSR.
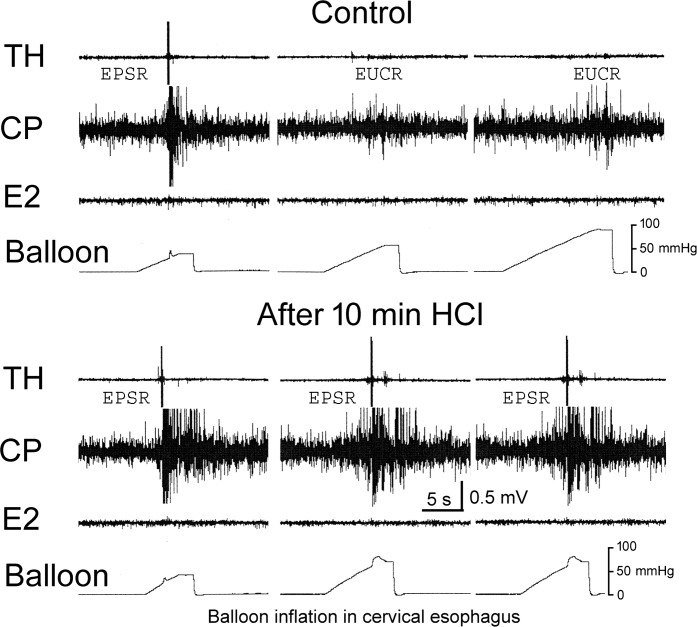
We tested the effects of perfusion of the esophagus with fluid of pH = 1.2 for 12.5 ± 1.4 min (n = 4) on the EPSR. We found that acid perfusion significantly increased the chance of activating the EPSR from the cervical (32 ± 16% to 81 ± 24%, P = 0.021) and proximal thoracic (23 ± 23% to 85 ± 14%, P = 0.035), but not the distal thoracic (7 ± 8% to 19 ± 17%, P = 0.37), esophagus (Fig. 7).
Fig. 7.
The effect of esophageal acid exposure on the EPSR. These are TH, CP, and E2 EMG recordings during EUCR and EPSR responses to distension of the cervical esophagus before and after exposure of the esophagus to HCl for 10 min. Acid exposure increased the chance of activating the EPSR. The pressures within the distending balloons are indicated by the mmHg bar.
Determination of the Sensory Mechanisms That Mediate Activation of the EPSR
Neural pathway.
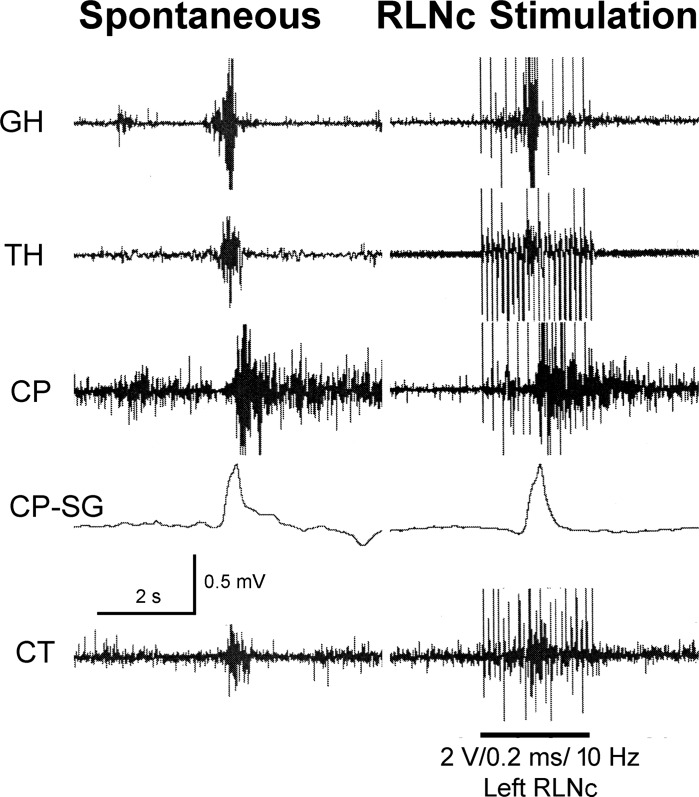
We tested the effects of electrical stimulation of the RLNc (n = 6) on the pharynx and esophagus. We found that RLNc stimulation activated pharyngeal swallows (Fig. 8) in 83% (5/6) of the animals and 40% (15/35) of stimulations.
Fig. 8.
The effect of recurrent laryngeal nerve caudal to the cricoid cartilage (RLNc) stimulation on activation of the pharyngeal swallow response. These are GH, TH, CP, and CT EMG recordings and CP strain gauge (CP-SG) recording in response to centripetal electrical stimulation of the RLNc. Note that RLNc stimulation activated a pharyngeal swallow during the stimulation period. The vertical electrical bars on the EMG signals are the electrical artifacts of the stimulus.
Type of esophageal receptor.
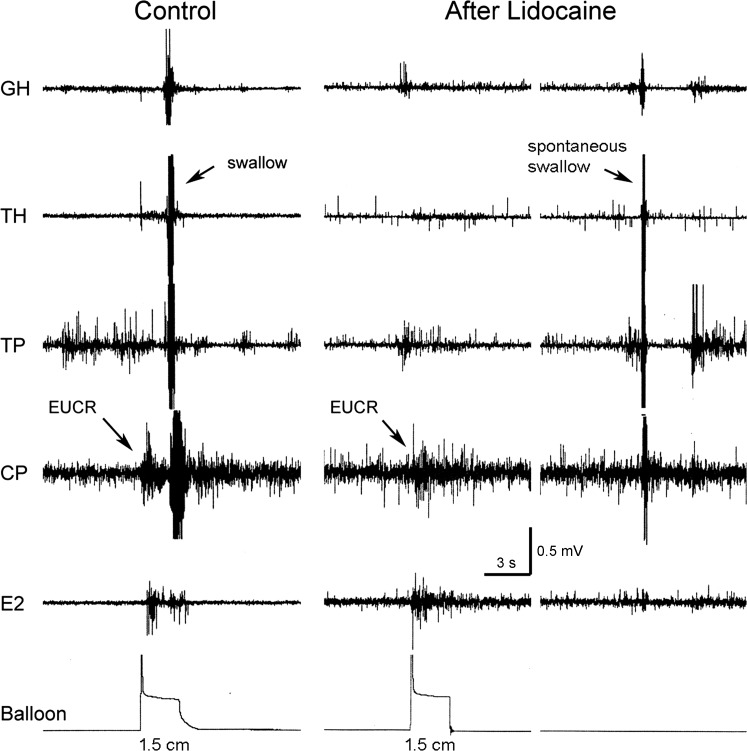
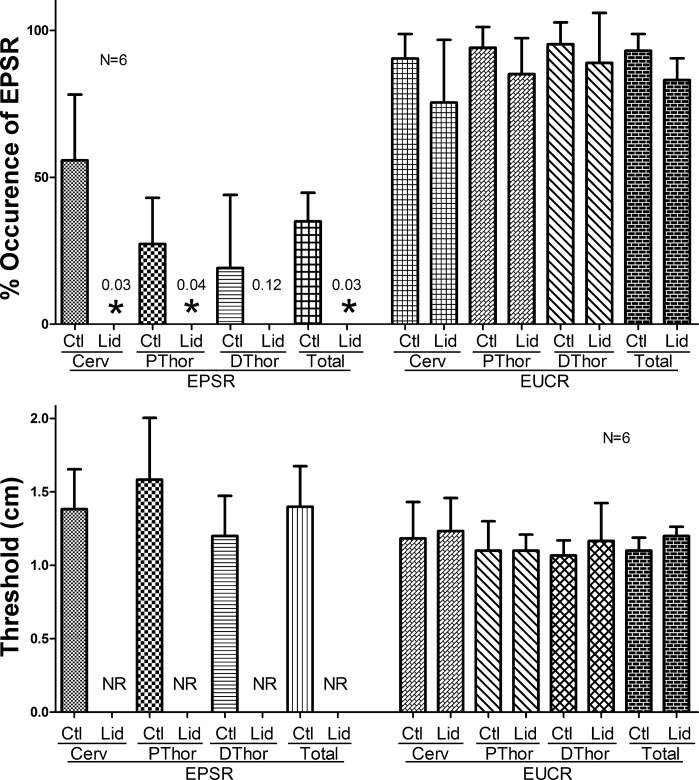
We tested the effects of cervical esophageal infusion (n = 6) of lidocaine (2%) at 1 ml/min for 5 min on activation of the EPSR as well as the EUCR. We found that, although lidocaine blocked activation of the EPSR, it had no significant effect on the percent occurrence or threshold stimulus needed to activate EUCR (Figs. 9 and 10).
Fig. 9.
Effects of lidocaine perfusion of the esophagus on EPSR and EUCR. These are GH, TH, TP, CP, and E2 EMG recordings in response to distension of a balloon in the proximal thoracic esophagus to 1.5 cm in diameter before and after lidocaine administration. Lidocaine blocked the EPSR but not the EUCR or spontaneous pharyngeal swallowing.
Fig. 10.
Graph of the effects of lidocaine perfusion of the esophagus on percent occurrence and threshold stimulus for activation of the EPSR and EUCR. Lidocaine administration significantly blocked the percent occurrence and threshold stimulus for cervical (Cerv), proximal thoracic (PThor), or average of all areas of the esophagus (Total) activation of the EPSR but not EUCR. Values are means ± SD, and the test used for EPSR was the Wilcoxon signed rank test and for EUCR was the Student's paired t-test. DThor, distal thoracic esophagus.
DISCUSSION
We found that esophageal stimulation by luminal distension activates a pharyngeal swallow response, i.e., the EPSR. Although the magnitude of the EPSR was not related to either the magnitude of esophageal distension or the length of esophagus distended, the probability of occurrence was related to both factors. The probabilistic nature of activation of the EPSR is different from activation of other esophageal reflexes (24, 26, 27), e.g., EUCR and the esophago-UES relaxation reflex (EURR), which are activated at specific thresholds using specific types of stimuli. On the other hand, the SP is also activated in a probabilistic manner. Studies (6, 10, 12, 38, 41) have found that distension the esophagus never activates SP 100% of the time but at some lower percentage similar to the EPSR. That is, distension of the thoracic esophagus activated SP 18–60% of the time in humans (6, 10, 12, 41). Therefore, although no prior study (6, 10, 12, 38, 41) labeled the SP response as being probabilistic in nature, that is what all studies found. Given this similarity between SP and EPSR, there may be a close relationship between the mechanisms governing activation of these responses, as discussed below.
The function of the EPSR may be to remove 1) esophageal contents that do not activate SP or 2) contents from the proximal cervical esophagus that require peristaltic activity arising from a more rostral site than would occur with SP. The first function is unlikely because it has been found that SP can be activated from all areas of the esophagus, including the proximal cervical esophagus, in animals (16, 17). On the other hand, the second function is supported by the anatomical and physiological differences between human adults and infants. The EPSR occurs in animals (17, 26) and human infants (22) but not in human adults (10). The oral-pharyngeal transport phase of swallowing in human infants and animals is a two-step process of a series of sucks or laps followed by a pharyngeal swallow, rather than forming a bolus in the mouth and swallowing in one continuous movement from oral cavity to esophagus (36). In both human infants and animals, but not in adult humans, the larynx is high in the neck (29), and thus the pharyngeal vestibule is smaller and holds less material. In addition, the epiglottis and velum are in close contact, which helps prevent aspiration (29, 36) while the food is stored in the vallecula before the pharyngeal phase is activated. Therefore, if food gets stuck in the proximal esophagus in human infants or cats, the choice to remove this material is either to activate the EPSR or SP. The SP always begins orad of the stimulus to ensure movement of the bolus caudally (5, 8, 9, 16), but, when the bolus is in the proximal cervical esophagus, there is little or no esophagus orad of the bolus. Activating SP under these conditions could push the bolus in both directions, causing SER and its consequences. In addition, esophageal peristalsis includes longitudinal contraction of the esophagus, which could pull the epiglottis away from the velum, exposing the airway to aspiration. Even in adult humans, it has been found that it is difficult to activate SP from the cervical esophagus (10). A safer method to propel contents out of the cervical esophagus in human infants and cats might be to activate peristalsis above the esophagus by activating the EPSR.
There are other reasons that the function of the EPSR might be protective, especially in human infants and animals. We found that the proximal esophagus is more sensitive than distal esophagus in activating the EPSR, and stimulation of this response does not require distension of the esophagus. We also found that the sensitivity for activation of the EPSR is increased when the luminal area of the esophagus exposed to the stimulus is increased. This suggests that the most appropriate physiological stimulus for the EPSR may be ingested fluid, as it would affect large areas of the esophageal mucosa. Therefore, the function of the EPSR is similar to SP of the striated muscle esophagus; however, these reflex functions seem to be inversely related to each other, as those organisms that have one response tend to have less of the other. We hypothesize that the neural mechanisms controlling striated muscle SP and EPSR are similar, and the change in response of the esophagus to stimulation from EPSR to SP is a physiological adaptation that occurs during infancy. As the laryngeal anatomy develops by descending, so also the brain stem mechanisms controlling peristalsis develop by changing the response to stimulation of the esophagus from EPSR to striated muscle SP. Further studies are needed to test this hypothesis.
The probabilistic nature of the EPSR and SP fits the proposed protective function of the EPSR. When material is in the esophagus, two reflex responses are available, i.e., the SP and the EPSR. The response selected depends on multiple factors, e.g., location in the esophagus, length of esophagus stimulated, magnitude of stimulation, pH of the material, and height of the larynx. The closer the material is to the UES, the more esophageal mucosa affected, the lower the pH, the more material is in the esophagus, and the higher the larynx, the more likely EPSR is activated. The probabilistic nature of EPSR and SP fits very well with their ability to prevent SER. That is, there is no one event or situation that results in SER; there are a range of possibilities, and an efficient way to control for such a range of possibilities is in a probabilistic manner.
Although distension of the esophagus in adult humans does not activate the EPSR, studies (4) have found that pharyngeal swallowing is not only found to occur in association with exposure of the esophagus to acid; it is the major factor in the clearance of gastro-esophageal reflux (GER). Therefore, even in adult humans, the EPSR may serve a protective function against the reflux of gastric acid.
Although clearance of GER from the thoracic esophagus is important, removal of acidic substances from the cervical esophagus has more urgency, especially in human infants. Any material that is getting closer to pharynx or airway introitus can be of risk in activating laryngeal chemoreflex or glottal closure (21, 44). Acidic media are more noxious than nonacidic media. In high-risk infants, esophago-pharyngeal provocation can cause cardio-respiratory rhythm disturbances (apnea, bradycardia, and desaturations) along with changes in vital functions that in turn cause delays in adaptation and restoration of normalcy. Exaggeration of such reflexes can result in apparent life-threatening events (ALTEs) or Brie resolved unexplained events. Recently, we have explained the mechanistic nature of ALTEs as those associated with esophageal dysmotility reflexes (15). In general, infants presenting with ALTEs recover after peristaltic mechanisms are activated and the bolus is cleared. On the other hand, the exact mechanism of sudden infant death syndrome (SIDS) is not known (because the infants die); therefore, controversy exists about inadequacy of aspiration-preventing mechanism under vulnerable states or high-risk infants. We believe that esophageal peristalsis helps in the clearance of the noxious stimulus, and EPSR may prevent SER. Studies suggest that acid reflux of gastric contents causes apnea in animals or human infants (45), and GER is associated with SIDS (1, 35, 45). In addition, it has been found that sleep is associated with decreased swallowing and longer esophageal acid clearance time (33). Therefore, the EPSR may be very important to prevent SER in human infants, and the absence or lack of EPSR in human infants may predispose them to ALTEs or potentially lead to SIDS.
The primary receptors of the esophagus that are activated by luminal acid are the mucosal mechanoreceptors (14, 34). This is consistent with our finding that activation of the EPSR is not related to the magnitude of esophageal distension, as the mucosal mechanoreceptors are not sensitive to wall distension but to mucosal stroking or chemical stimulation (34). In addition, we found that luminal lidocaine administration blocks the EPSR but does not block the EUCR. The EUCR has been shown to be mediated by tension receptors of the muscularis (24), not the mucosa, and, when applied for short periods of time, lidocaine has previously been shown to block receptors of the mucosa and not the muscularis (24). Therefore, it is highly likely that the EPSR is activated by stimulation of mucosal mechanoreceptors.
There are two types of esophageal mucosal tension receptors, rapidly and slowly adapting (30, 34). Considering that the EPSR does not require a rapid stimulus as do functions activated by the rapidly adapting receptors, i.e., EURR (24, 27) and the belch response (28), we hypothesize that it is the slowly adapting mucosal tension receptors that mediate the EPSR. The slowly adapting mucosal tension receptors have been shown to be activated by acid (30, 34). Interestingly, studies (46) have found that afferent nerves in the proximal esophageal mucosa are more superficial than those in the distal esophagus, which may explain the greater sensitivity of the cervical esophagus to activation of the EPSR, and we found that acid sensitizes the esophagus to activation of the EPSR. Therefore, we hypothesize that these superficial mucosal afferent nerves may be the sensory nerves for the acid-sensitive tension receptors that mediate the EPSR.
Prior studies have found that the EPSR is mediated by the SLN (26) and RLNc (17, 26), and we confirmed these findings when we activated pharyngeal swallows by the centripetal electrical stimulation of the RLNc. This finding that the SLN afferents mediate the EPSR is very consistent with the function of the SLN. It has been well documented that afferents in the SLN mediate activation of the pharyngeal swallow from pharyngeal and laryngeal receptors (9, 25, 32, 42). In addition, anatomical studies have found that proximal esophageal slowly adapting mucosal tension receptors project their afferents through the RLNc and SLN (28). We hypothesize that it is these receptors that mediate the EPSR.
In conclusion, mechanical or chemical stimulation of the slowly adapting receptors of the mucosa of the striated muscle esophagus activates the EPSR in a probabilistic manner. The afferents of this response are mediated by the RLNc and SLN. The function of the EPSR is the same as striated muscle SP, and we hypothesize that the EPSR is activated instead in nonhuman species and human infants with an elevated larynx to prevent SER that might otherwise occur if striated muscle SPs had occurred.
GRANTS
This work was supported in part by NIH grants RO1 DK-25731 and PO1 DK-068051.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M.L. and R.S. conception and design of research; I.M.L. and B.K.M. performed experiments; I.M.L. analyzed data; I.M.L., S.R.J., and R.S. interpreted results of experiments; I.M.L. prepared figures; I.M.L. drafted manuscript; I.M.L. edited and revised manuscript; I.M.L., B.K.M., S.R.J., and R.S. approved final version of manuscript.
REFERENCES
- 1.Al-Adnani M, Cohen MC, Scheinberg I. Gastroesophageal reflux disease and sudden infant death syndrome: Mechanisms behind an under-recognized association. Pediatr Dev Pathol 14: 53–56, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Amarasena J, Ootaki S, Yamamura K, Yamada Y. Effect of cortical masticatory area stimulation on swallowing on anesthetized rabbits. Brain Res 965: 222–238, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Aziz Q, Rothwell JC, Barlow J, Thompson DG. Modulation of esophageal responses to magnetic stimulation of the human brain by swallowing and by vagal stimulation. Gastroenterology 109: 1437–1445, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Bremner RM, Hoeft SF, Costanti M, Crookes PF, Bremner CG, Demeester TR. Pharyngeal swallowing: The major factor in clearance of esophageal reflux episodes. Ann Surg 218: 364–370, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen J. Patterns and origin of some esophageal responses to stretch and electrical stimulation. Gastroenterology 59: 909–916, 1970. [PubMed] [Google Scholar]
- 6.Creamer B, Schlegel J. Motor responses of the esophagus to distension. J Appl Physiol 10: 498–504, 1957. [DOI] [PubMed] [Google Scholar]
- 7.Delaney AL, Arvedson JC. Development of swallowing and feeding: Prenatal through first year of life. Dev Disabil Res Rev 14: 105–117, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Diamant NE. Physiology of esophageal motor function. Gastroenterol Clin North Am 18: 179–194, 1989. [PubMed] [Google Scholar]
- 9.Dodds WJ. Physiology of swallowing. Dysphagia 3: 171–178, 1989. [DOI] [PubMed] [Google Scholar]
- 10.Enzmann DR, Harell GS, Zboralske FF. Upper esophageal responses to intraluminal distension in man. Gastroenterology 72: 1292–1298, 1989. [PubMed] [Google Scholar]
- 11.Ertekin C. Voluntary versus spontaneous swallowing in man. Dysphagia 26: 183–192, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Fleshler B, Hendrix TR, Kramer P, Ingelfinger FJ. The characteristics and similarity of primary and secondary peristalsis in the esophagus. J Clin Invest 38: 110–116, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamdy S. Role of cerebral cortex in the control of swallowing. GI Motility Online. 2006. http://www.nature.com/gimo/contents/pt1/full/gimo8.html.
- 14.Harding R, Titchen DA. Chemosensitive vagal endings in the esophagus of the cat. J Physiol 246, 52P–53P, 1975. [PubMed] [Google Scholar]
- 15.Hesenstab KA, Jadcherla SR. Respiratory events in infants presenting with apparent life threatening events: Is there an explanation from esophageal motility? J Pediatr 165: 250–255, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellemans J, Vantrappen G. Physiology. In: Diseases of the Esophagus. New York: Springer Verlag, 1974, pp. 40–102. [Google Scholar]
- 17.Hwang K. Mechanism of transportation of the content of the esophagus. J Appl Physiol 6: 781–7964, 1954. [DOI] [PubMed] [Google Scholar]
- 18.Ingelfinger FJ. Esophageal motility. Physiol Rev 38: 533–584, 1958. [DOI] [PubMed] [Google Scholar]
- 19.Jadcherla SR, Duing HQ, Hoffman RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr 143: 31–38, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Jadcherla SR, Hoffman RG, Shaker R. Effect of maturation of the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J Pediatr 149: 77–82, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophago-glottal closure reflex in human infants: A novel reflex elicited with concurrent manometry and ultrasonography. Am J Gastroenterol 102: 2286–2293, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadcherla SR, Gupta A, Stoner E, Fernandez S, Shaker R. Pharyngeal swallowing: Defining pharyngeal and upper esophageal sphincter relationships in human neonates. J Pediatr 151: 597–603, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jean A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiol Rev 81: 929–969, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distension. Am J Physiol Gastrointest Liver Physiol 281: G1246–G1263, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Lang IM. Brainstem control of the phases of swallowing. Dysphagia 24: 333–348, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Lang IM, Medda BK, Jadcherla S, Shaker R. The role of the superior laryngeal nerve in esophageal reflexes. Am J Physiol Gastrointest Liver Physiol 302: G1445–G1457, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang IM, Medda BK, Shaker R. Mechanism of UES relaxation initiated by gastric air distension. Am J Physiol Gastrointest Liver Physiol 307: G452–G458, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang IM. The physiology of eructation. Dysphagia 31: 121–133, 2016. [DOI] [PubMed] [Google Scholar]
- 29.Laitman JT, Reidenberg JS. Specializations of the human upper respiratory and upper digestive systems as seen through comparative and developmental anatomy. Dysphagia 8: 318–325, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Lennerz JKM, Dentsch C, Bernardini N, Hummel T, Neuhuber WL, Reeh PW. Electrophysiological characterization of vagal afferents relevant to mucosal nociception in the rat upper oesophagus. J Physiol 582: 229–242, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madsen T, Wallin L, Boesby S, Larsen VH. Oesophageal peristalsis in normal subjects. Scand J Gastroenterol 18: 513–518, 1983. [DOI] [PubMed] [Google Scholar]
- 32.Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev 14: 77–86, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Orr WC, Heading R, Johnson LF, Krygers M. Review article: Sleep and its relationship to gastro-esophageal reflux. Aliment Pharmacol Ther 20: 39–46, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol 512: 907–916, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page M, Jeffery H. The role of gastro-oesophageal reflux in the oetiology of SIDS. Early Hum Dev 59: 127–149, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Palmer JB, Rudin NJ, Lara G, Crompton AW. Coordination of mastication and swallowing. Dysphagia 7: 187–200, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Paterson WG. Esophageal peristalsis. GI Motility Online. 2006. http://www.nature.com/gimo/contents/pt1/full/gimo13.html
- 38.Paterson WG, Rattan S, Goyal RK. Esophageal responses to transient and sustained esophageal distension. Am J Physiol Gastrointest Liver Physiol 255: G587–G595, 1988. [DOI] [PubMed] [Google Scholar]
- 39.Pouderoux P, Logemann JA, Kahrilas PJ. Pharyngeal swallowing elicited by fluid infusion: Role of volition and vallecular containment. Am J Physiol Gastrointest Liver Physiol 270: G347–G354, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Rommel N, van Wijk M, Boets B, Hebbard G, Haslam R, Davidson G, Omari T. Development of pharyngo-esophageal physiology during swallowing in the preterm infant. Neurogastroenterol Motil 23: e401–408, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Schoeman MN, Holloway RH. Stimulation and characteristics of secondary oesophageal peristalsis in normal subjects. Gut 35: 152–158, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: A review. Dysphagia 25: 323–333, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevenson RD, Allaire JH. The development of normal feeding and swallowing. Pediatr Clin North Am 38: 1439–1453, 1991. [DOI] [PubMed] [Google Scholar]
- 44.Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration form fetal to adult life. Am J Med 111: 69S–77S, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Wetmore RF. Effects of acid on the larynx of the maturing rabbit and their possible significance to the sudden infant death syndrome. Laryngoscope 103: 1242–1252, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Woodland P, Aktar R, Mthunzi E, Lee C, Peiris M, Preston SL, Blackshaw LA, Sifrim D. Distinct afferent innervation patterns within the human proximal and distal esophageal mucosa. Am J Physiol Gastrointest Liver Physiol 308: G525–G531, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]