Abstract
Posterior fossa syndrome (PFS), or cerebellar mutism syndrome (CMS), is a collection of neurological symptoms that occur following surgical resection of a posterior fossa tumour, and is characterised by either a reduction or an absence of speech. Some authors suggest that CM is only one symptom of the CMS complex that also includes ataxia, hypotonia and irritability as well as cranial nerve deficits, neurobehavioral changes and urinary retention or incontinence. It is seen almost exclusively in children. In 1985 Rekate et al. published the first work describing CM as a clinical entity, occurring as a consequence of bilateral cerebellar injury. Other associated symptoms include visual impairment, altered mood, impaired swallowing and significant gross and fine motor deficits. The effects of this can have a devastating impact on both the patient and their carers, posing a significant clinical challenge to neurorehabilitation services. The reported incidence was between 8% and 31% of children undergoing surgery for posterior fossa tumour. The underlying pathologies include vasospasm, oedema, and axonal/neuronal injury. Neuroimaging has contributed to a better understanding of the anatomical location of postoperative injury. There have been a number of suggestions for treatment interventions for PFS. However, apart from some individual reports, there have been no clinical trials indicating possible benefit. Occupational therapy, speech and language therapy, as well as neurocognitive support, contribute to the recovery of these patients.
Keywords: Posterior fossa syndrome (PFS), posterior fossa tumour, cerebellar mutism syndrome (CMS), neuroimaging
Introduction
Posterior fossa syndrome (PFS) is a collection of neurological symptoms that occurs following surgical resection of a posterior fossa tumour. It is seen almost exclusively in children. A number of early publications described the effects of posterior fossa surgery on speech. Hirsch et al. were the first to report the phenomenon of PFS as a group of neuropsychological complications, including speech disturbance, in children who underwent surgical treatment for medulloblastoma between 1964 and 1972 (1). However, it was Rekate et al. who published the first work describing cerebellar mutism (CM) as a clinical entity, occurring as a consequence of bilateral cerebellar injury (2). They described six children undergoing posterior fossa surgery who sustained a similar postoperative course. Many reports of similar cases followed this publication. The collection of neurological symptoms is now well established as a syndrome; its pathogenesis, however, is still poorly understood.
Clinical presentation and evolution
PFS is characterised by either a reduction or an absence of speech. There are a number of terms which are used in the literature interchangeably when discussing PFS. These include CM and cerebellar mutism syndrome (CMS). However, some authors argue that these terms do not describe exactly the same entity, but only define individual components of the syndrome. Gudrunardottir proposed that CM is a muteness following lesioning of the cerebellum, as opposed to its occurrence with lesions of the cerebrum (3). Thomale et al. also agree that CM is only one symptom of the CMS complex that also includes ataxia, hypotonia and irritability as well as cranial nerve deficits, neurobehavioral changes and urinary retention or incontinence (3,4). Other authors argue that these distinctions are not clinically relevant, as no studies have specifically evaluated whether different anatomical changes underlie the various components of the syndrome.
The reported incidence of PFS following surgery of the posterior fossa in children is variable. A review reported an incidence of 8 to 31% (5). A higher incidence has been reported in some clinical settings (6-10). The onset of symptoms is generally within one week of surgery, manifesting in the first one to two postoperative days. Gross mutism is normally transient, lasting from one day to six months (5,11,12). However, cognitive, neurobehavioral and associated symptoms often persist for longer periods. Other associated symptoms include visual impairment, altered mood, impaired swallowing and significant gross and fine motor deficits. The effects of this can have a devastating impact on both the patient and their carers, posing a significant clinical challenge to neurorehabilitation services. It is because these effects are so significant that it is crucial to understand the risk factors and try to establish methods to reduce its incidence.
Pathogenesis
The pathogenesis of PFS is poorly understood. Several hypotheses have been proposed, including cerebellar perfusion deficits due to vasospasm, oedema or axonal injury due to direct surgical injury, neuronal dysfunction and disaschisis. Neuroimaging has made a significant contribution to the understanding of PFS and has helped to identify the anatomical location of postoperative injury.
Vasospasm
Some authors have attributed postoperative vasospasm of the arteries supplying the cerebellum and brainstem as the primary cause for transient ischaemic injury. The time interval between surgery and the development of symptomatic vasospasm averages eight days (13). Unlike tumours elsewhere, tumours of the posterior fossa tend to cause more diffuse vasospasm with involvement of both the anterior and posterior cerebral circulation, causing more extensive neurological deficits (14-16). However, there are no dedicated studies or imaging evidence to substantiate the hypothesis of vasospasm as a causal factor in PFS. Indeed, if the average time to development of vasospasm is eight days and the average time to develop PFS is one day this hypothesis alone cannot account for PFS.
Oedema
Oedema, occurring within the surgical site as a direct consequence of surgery, peaks at two hours but persists for three days, subsiding by the seventh day (17). On the basis of immediate post-operative MRI findings oedema involving the superior and middle cerebellar peduncles has been associated with PFS (18,19). Postsurgical oedema of the pontine tegmentum has also been associated with PFS (20). In addition, changes in imaging have been seen in the dentate-thalamo-cortical (DTC) pathway, particularly in its proximal part (pECP). The evidence from neuroimaging is discussed in more detail later in this review.
Axonal/neuronal injury
Axonal injury related directly to surgery has also been implicated in the development of PFS. Radiological evidence of axonal injury may be obtained from studies using diffusion tensor imaging (DTI) which evaluate the white matter tracts.
Neuronal dysfunction may also be important. Siffert et al. attribute the delay in the onset of symptoms in PFS to alterations in neurotransmitter levels and synaptic or trans-synaptic degeneration (21). Crossed cerebello-cerebral diaschisis resulting from injury to the proximal DTC pathway is a form of trans-synaptic degeneration. The abnormal DTI and perfusions noted in the frontal and temporal lobes serve as indirect evidence of this phenomenon (22-25).
Risk factors
As PFS does not invariably occur after posterior fossa surgery in children, specific risk factors must play a role in predisposing an individual to it. Identification of risk factors may suggest changes in surgical management in some children, hoping to reduce the incidence or severity of PFS.
In an extensive review, Reed-Berendt et al. (26) concluded that tumours located in the vermis (7,9,27,28), medulloblastomas (7,28,29) and tumours invading the brainstem (9,19,27,29) were more likely to develop PFS. Factors identified as consistently not being associated with CM included tumour size (7), gender (7,9), postoperative infection and aseptic meningitis (9,19). For some other factors, different studies reported conflicting results. These include the age at diagnosis, vermian incision, the degree of resection/presence of residual tumour and oedema in the middle and superior cerebellar peduncles.
Law et al. also conducted a review on the clinical and neuroanatomical predictors of CM (30). They found that children with a larger tumour size, diagnosis of medulloblastoma and children who were left handed were all more likely to develop PFS.
Telovelar or transvermian approach to posterior fossa lesions
In an attempt to reduce the incidence of CM some variations in surgical technique have been employed. Whilst there are conflicting results as to whether vermian incision is a risk factor to developing PFS, this is potentially avoidable in the surgical approach to posterior fossa tumours. Matsushima et al. first described the microsurgical anatomy of the telovelar approach, underlining its potential to gain access to the fourth ventricle through the cerebellomedullary fissure (CMF) without splitting the cerebellar vermis (31,32). The detailed microsurgical anatomy of this approach was described by Mussi and Rhoton (33).
In the telovelar approach, the CMF is exposed by dissection in a caudal to cranial dissection beginning between the uvula and the tonsil; access to the fourth ventricle is then obtained by incising the tela choroidea and the inferior medullary velum. This approach enables the exploration of the entire fourth ventricle from the obex to the aqueduct. This allows early visualisation of the floor of the fourth ventricle. There are no randomised controlled trials demonstrating that the telovelar approach leads to a lower incidence of PFS, but some case series have reported no occurrence of PFS with this approach (34).
Critiques of this approach suggest that it provides a narrowed corridor, making complete resection of the tumour potentially more difficult. However, most suggest that with a wide dissection of the CMF and gentle retraction of the tonsil an adequate exposure can be obtained. Large tumours in the fourth ventricle expand the telovelar space and facilitate the approach. In addition, improved access to the lateral recess of the ventricle makes the telovelar approach particularly effective in lesions attached to the cerebellar peduncles. Tanriover et al. compared the transvermian and telovelar approaches on ten formalin fixed specimens (35). They found that the transvermian approach provided slightly better visualization of the medial part of the superior half of the roof of the fourth ventricle. The telovelar approach, which does not involve incision of any part of the cerebellum, provides additional exposure to the lateral recesses and foramina of Luschka. Deshmukh et al. compared the telovelar approach with and without C1 posterior arch removal to the transvermian approach (36). They found that the additional removal of the C1 arch offered a larger working area than the transvermian approach, obviating the sole advocated advantage of the transvermian approach to the rostral half of the fourth ventricle. However, this approach was only necessary if the tonsils were pushed below the level of the foramen magnum by the tumour.
Many surgeons now consider the telovelar approach the standard approach for most lesions within the fourth ventricle.
Imaging
The development of new magnetic resonance imaging (MRI) techniques has offered invaluable insight into the anatomy and pathophysiology of PFS. A postoperative MRI scan is generally obtained within 72 hours of surgical resection. The assessment of postsurgical injury on conventional MRI sequences is challenging due to a number of factors, including pre-existing mass effect of the tumour on adjacent structures and peritumoral oedema. Diffusion weighted imaging (DWI) has been suggested to be sensitive in identifying postsurgical changes. This however is dependent on the timing of the scan and postoperative changes such as haemorrhage, intracranial air and artefacts.
One of the first insights from diffusion tractography was the implication of disruption of the DTC pathway. This is the efferent pathway from the dentate nucleus, extending through the thalamus to the frontal cortex, and represents one of the most important outputs of the cerebellum. In view of its proximity to the tumour and surgical field most authors believe that damage to the proximal segment of the DTC pathway is responsible for PFS (Figure 1) (24,30,37). Several imaging studies have implicated the dentate nucleus, the superior cerebellar peduncle and the mesencephalic tegmentum, which constitute the proximal efferent pathway (pECP), in the development of PFS (Figure 2) (18-20,23, 38-40). As discussed above, other studies have also demonstrated that surgical injury to the cerebellar vermis is also related to the aetiology of PFS (40,41).
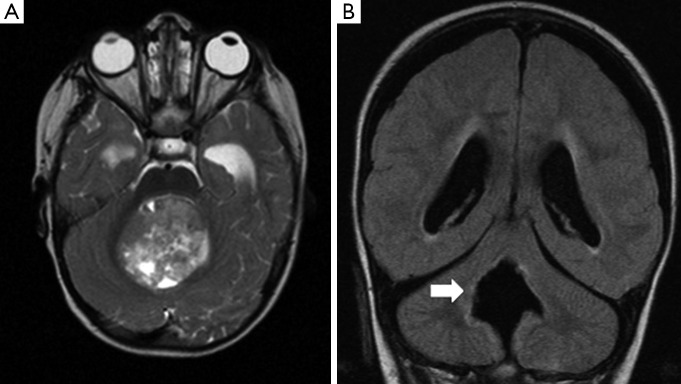
Figure 1.
Denate nucleus injury and PFS. (A) T2-weighted pre-operative MRI scan shows a large midline posterior fossa tumour, consistent with a medulloblastoma (arrow). Post-operative FLAIR imaging in (B), taken six months after resection of the tumour, demonstrates hyperintensity in the region of the right dentate nucleus (arrow). This is consistent with injury in this region. Clinically, the child has PFS. PFS, posterior fossa syndrome; MRI, magnetic resonance imaging; FLAIR, fluid attenuation inversion recovery.
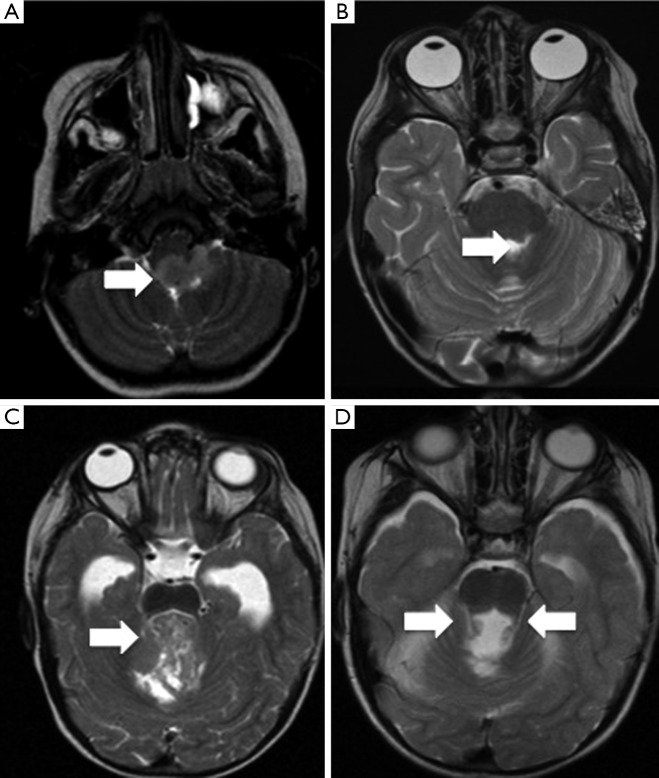
Figure 2.
Unilateral and bilateral superior cerebellar peduncle injury in PFS. (A,B) Pre- and post-operative T2-weighted MRI scans show a midline fourth ventricular tumour extending under the left superior cerebellar peduncle; after resection this structure is attenuated (arrows); (C,D) in these images, also T2-weighted MRI scans, the tumour compresses both superior cerebellar peduncles (C). In the post-operative image (D), both superior cerebellar peduncles are attenuated (arrows). Both children had PFS. These imaging data illustrate the association between loss of integrity of the superior cerebellar peduncle and PFS. PFS, posterior fossa syndrome; MRI, magnetic resonance imaging.
A recent study overcame the limitations found by variations in timing in post-operative MRI scanning (up to 72 hours) by evaluating the final intra-operative MRI scan, where the diffusion abnormalities can be directly attributed to surgical injury (42). They concluded that diffusion abnormalities on intra-operative MRI were an early predictor of PFS. The authors identified a significant association between abnormalities involving pECP structure and development of PFS. Bilateral involvement of pECP was a highly specific risk factor for predicting development of PFS. Diffusion abnormalities of the inferior vermis were also significantly associated with PFS but did not represent a risk factor in isolation. Miller at al. also found a positive association between bilateral damage to DTC and PFS, in which patients with bilateral damage were 12 times more likely to develop PFS (23). All this strengthens the body of evidence that bilateral damage to the DTC is seen in patients with PFS.
The pECP is also part of another functional circuit, the dentate-rubro-olivary triangle, also known as the Guillain-Mollaret triangle (GMT). A lesion to either the ascending (superior cerebellar peduncle) or the descending (pontine tegmentum) limbs of the GMT leads to MRI-detectable signal changes in the inferior olivary nucleus (ION), contralateral to the superior cerebellar peduncle, but ipsilateral to the pontine tegmentum tract. A retrospective, comparative MRI evaluation of the ION in postoperative paediatric patients with and without PFS revealed that hypertrophic olivary degeneration (HOD) was a delayed but fairly reliable surrogate imaging marker of damage to the contralateral pECP (Figure 3) (43). Patay et al. recently published a study showing significant association of PFS with HOD, which was characterised by degeneration of the ION following damage to structures including the superior cerebellar peduncle (SCP) and dentate nucleus (DN) (44). These results reinforce the association between pECP and PFS.
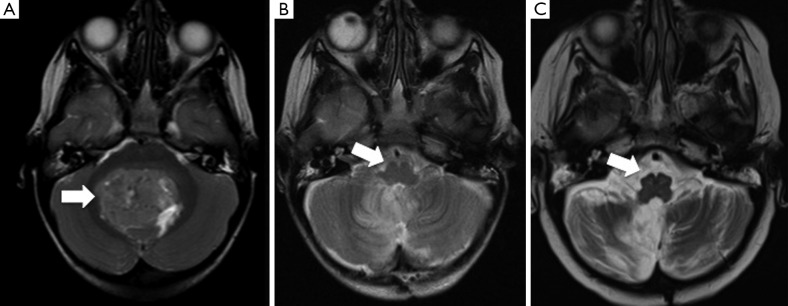
Figure 3.
Hypertrophic olivary degeneration in PFS. (A) Pre-operative T2-weighted MRI scan demonstrates a large fourth ventricular medulloblastoma (arrow). Post-operatively, this child developed PFS. Although not evident in the early post-operative MRI scan in (B) (arrow), the scan in (C), performed one year after surgery, shows hypertrophic degeneration of the inferior olivary nucleus (arrow). PFS, posterior fossa syndrome; MRI, magnetic resonance imaging.
In addition, clinically diagnosed PFS was commonly associated with bilateral HOD by MRI and vice versa. However, one study reports that in left-handed children, unilateral (right sided) pECP injury may be enough to produce CM (30). They found that the majority of left-handed children in their study sample presented with PFS following surgery.
Damage to other anatomical structures has also been implicated in association with PFS. Morris et al. reported a significant association between patients with T2 signal abnormalities in the pons, midbrain and the SCP (38). Assessment of T2 abnormalities within the pons, midbrain, DN and SCP demonstrated that 90% of patients with PFS showed abnormalities in three or more of these structures. A recent study (39) evaluating the SCP in patients with PFS using DTI and tractography reported bilateral injury to the SCP in patients with PFS.
DTI and 18FDG-PET studies have also offered insight into possible changes associated with PFS. As PFS presents postoperatively, there is an implication that the insult must occur during the surgical procedure. However, as discussed earlier, PFS is seen to begin after a period of normal neurological function following surgery. The timing of this may vary from a few hours to a day or more. Some have proposed that although the injury to particular anatomical structures may be due to direct surgical injury, the effect this has in causing PFS must be an indirect process. The concept of crossed cerebro-cerebellar diaschisis, representing an indirect functional disturbance, has been described. This phenomenon was first recognised by Brown Sequard as early as 1885 and subsequently reported by von Monakow in 1914 (45). It is defined as a sudden suspension of inhibition of function in one area of the brain after damage to another distant region that normally provides it input. This lack of input leads to a decrease in activity and loss of excitability in an otherwise normal region. Diaschisis is a dynamic process with the potential for improvement and, in some cases, total functional resolution. There are no imaging changes in the areas of the brain that have been indirectly affected, at least in the short to medium term or in the mature brain. Diffusion tensor imaging studies show that parenchyma affected by diaschisis, in particularly subcortical white matter, may undergo long-lasting, possibly permanent structural changes (44,46).
The phenomenon of crossed cerebro-cerebellar diaschisis has been known for several decades in the nuclear medicine community (47). 18FDG-PET studies have shown glucose hypotmetabolism, and SPECT studies indicate hypoperfusion in the cerebellar hemisphere contralateral to large supratentorial destructive lesion such as stroke. This phenomenon is believed to reflect reduced neuronal and synaptic activity, assuming that neurovascular coupling is still preserved (48). Resting state functional MRI studies are expected to provide additional insight into the phenomenon of diaschisis in the future.
Reversible depression of cerebral blood perfusion and hypometabolism in supratentorial structures, such as the frontal lobes, bilateral thalami and the left temporal lobe of PFS patients have been confirmed repeatedly by nuclear medicine techniques but the mechanism of hypoperfusion in remote supratentorial structures remained unexplained for a while (6,22,49). One of the earlier considerations for PFS was reversed cerebello-cerebral diaschisis. Dynamic susceptibility contrast MRI (DSC-MRI) was found to be sensitive to blood perfusion changes (24). A retrospective study analysing DSC-MRI perfusion data in patients with clinically diagnosed PFS after posterior fossa surgery for midline intraventricular medulloblastoma showed that cerebral blood volume (CBV) in supratentorial brain cortex in these patients was less than in those without PFS (23). This could be attributed to bilateral, reversed crossed cerebello-cerebral diaschisis. In PFS patients this drop in CBV was most prominent in the frontal regions, causing speculation that cerebral mutism may be related to a predominantly frontal lobe dysfunction and therefore represent a form of speech apraxia. These observations suggest a major role of the cerebellum in maintaining baseline cerebral cortical activity and modulation.
Soelva et al. (25) investigated the relationship between fronto-cerebellar association fibres (FCF), involved in neurocognitive regulatory circuitry, and PFS. Using diffusion weighted MR imaging at 3T and tractography of FCF using fibre tracking algorithm software, they concluded that volumes of FCF were significantly diminished in paediatric patients with symptoms of CM when compared to patients without symptoms. They also identified differences in fibre tract signals in the superior cerebellar peduncles and midline cerebellar structures in children with symptoms of CMS. This study highlighted the role of the neural circuitry between the frontal lobes and the cerebellum, and its relevance to neurocognitive impairment after posterior fossa tumour treatment in children.
Treatment
There have been a number of suggestions for treatment interventions for PFS. However, apart from some individual reports, there have been no clinical trials indicating possible benefit.
There are groups who consider dopaminergic cell groups in monoaminergic pathways to be important and therefore recommend the use of Bromocriptine, a dopamine agonist known to reverse the symptoms of akinetic mutism (25,50). However, it has not been shown to have this effect on patients with CM (20). Thus far there has not been a single or combination of medical therapies that have proven to be of any benefit in PFS. There is no convincing evidence that medical therapy is an avenue through which resolution of this condition may be accelerated.
One of the most significant aspects of management of PFS includes counselling of patients and their parents about the basis of CM. Occupational therapy, speech and language therapy, as well as neurocognitive support, help to the improved recovery of these patients.
Conclusions
PFS, with a reported incidence between 8% and 31% of children undergoing surgery for posterior fossa tumour, is a devastating condition with long-term effects. Whilst it is now thirty-one years since the first published work describing CM by Rekate, there has been no significant reduction in its incidence. The lack of a consistent definition has hampered detailed description and research. Although the pathogenesis of PFS is unclear, advances in imaging techniques has led to a better understanding of the anatomical structures, and their inter-relationships, affected in PFS.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hirsch JF, Renier D, Czernichow P, Benveniste L, Pierre-Kahn A. Medulloblastoma in childhood. Survival and functional results. Acta Neurochir (Wien) 1979;48:1-15. 10.1007/BF01406016 [DOI] [PubMed] [Google Scholar]
- 2.Rekate HL, Grubb RL, Aram DM, Hahn JF, Ratcheson RA. Muteness of cerebellar origin. Arch Neurol 1985;42:697-8. 10.1001/archneur.1985.04060070091023 [DOI] [PubMed] [Google Scholar]
- 3.Gudrunardottir T, Sehested A, Juhler M, Grill J, Schmiegelow K. Cerebellar mutism: definitions, classification and grading of symptoms. Childs Nerv Syst 2011;27:1361-3. 10.1007/s00381-011-1509-7 [DOI] [PubMed] [Google Scholar]
- 4.Thomale UW, Driever PH. Inconsistent terminology for cerebellar mutism. Childs Nerv Syst 2013;29:717-8. 10.1007/s00381-013-2074-z [DOI] [PubMed] [Google Scholar]
- 5.De Smet HJ, Baillieux H, Catsman-Berrevoets C, De Deyn PP, Mariën P, Paquier PF. Postoperative motor speech production in children with the syndrome of 'cerebellar' mutism and subsequent dysarthria: a critical review of the literature. Eur J Paediatr Neurol 2007;11:193-207. 10.1016/j.ejpn.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 6.Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the Posterior Fossa Syndrome in children after cerebellar tumour surgery. Cortex 2010;46:933-46. 10.1016/j.cortex.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 7.Catsman-Berrevoets CE, Van Dongen HR, Mulder PG, Paz y Geuze D, Paquier PF, Lequin MH. Tumour type and size are high risk factors for the syndrome of "cerebellar" mutism and subsequent dysarthria. J Neurol Neurosurg Psychiatry 1999;67:755-7. 10.1136/jnnp.67.6.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudrunardottir T, Sehested A, Juhler M, Schmiegelow K. Cerebellar mutism: incidence, risk factors and prognosis. Childs Nerv Syst 2011;27:513-4; author reply 515. 10.1007/s00381-010-1383-8 [DOI] [PubMed] [Google Scholar]
- 9.Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, Dias MS, Allen JC, Children's Oncology Group . Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg 2006;105:444-51. [DOI] [PubMed] [Google Scholar]
- 10.Dineen RC, Gutierez DR, Wilne S, Walker D, Grundy R, Auer D. Persisting cerebellar efferent pathway degradation following postoperative cerebellar mutism. 36th ESNR Annual Meeting. Neuroradiology, Edinburgh, 2012:S77. [Google Scholar]
- 11.Erşahin Y, Mutluer S, Cağli S, Duman Y. Cerebellar mutism: report of seven cases and review of the literature. Neurosurgery 1996;38:60-5;discussion 66. 10.1097/00006123-199601000-00015 [DOI] [PubMed] [Google Scholar]
- 12.Gudrunardottir T, Sehested A, Juhler M, Schmiegelow K. Cerebellar mutism: review of the literature. Childs Nerv Syst 2011;27:355-63. 10.1007/s00381-010-1328-2 [DOI] [PubMed] [Google Scholar]
- 13.Alotaibi NM, Lanzino G. Cerebral vasospasm following tumor resection. J Neurointerv Surg 2013;5:413-8. 10.1136/neurintsurg-2012-010477 [DOI] [PubMed] [Google Scholar]
- 14.Jacob JT, Hunt CH, Wijdicks EF, Rabinstein AA, Cloft H, Link MJ. Diffuse cerebral vasospasm after resection of a posterior fossa ependymoma. Neurocrit Care 2011;14:86-90. 10.1007/s12028-010-9464-9 [DOI] [PubMed] [Google Scholar]
- 15.Lee TT, Ragheb J, Bruce JC, Altman N, Morrison G. Diffuse cerebral vasospasm with ischemia after resection of a cerebellopontine angle primitive neuroectodermal tumor in a child. Pediatr Neurosurg 1998;29:300-3. 10.1159/000028741 [DOI] [PubMed] [Google Scholar]
- 16.LeRoux PD, Haglund MM, Mayberg MR, Winn HR. Symptomatic cerebral vasospasm following tumor resection: report of two cases. Surg Neurol 1991;36:25-31. 10.1016/0090-3019(91)90128-V [DOI] [PubMed] [Google Scholar]
- 17.Sherchan P, Kim CH, Zhang JH. Surgical brain injury and edema prevention. Acta Neurochir Suppl 2013;118:129-33. [DOI] [PubMed] [Google Scholar]
- 18.Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery 1995;37:885-93. 10.1227/00006123-199511000-00006 [DOI] [PubMed] [Google Scholar]
- 19.Wells EM, Khademian ZP, Walsh KS, Vezina G, Sposto R, Keating RF, Packer RJ. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. J Neurosurg Pediatr 2010;5:329-34. 10.3171/2009.11.PEDS09131 [DOI] [PubMed] [Google Scholar]
- 20.van Dongen HR, Catsman-Berrevoets CE, van Mourik M. The syndrome of 'cerebellar' mutism and subsequent dysarthria. Neurology 1994;44:2040-6. 10.1212/WNL.44.11.2040 [DOI] [PubMed] [Google Scholar]
- 21.Siffert J, Poussaint TY, Goumnerova LC, Scott RM, LaValley B, Tarbell NJ, Pomeroy SL. Neurological dysfunction associated with postoperative cerebellar mutism. J Neurooncol 2000;48:75-81. 10.1023/A:1006483531811 [DOI] [PubMed] [Google Scholar]
- 22.Germanò A, Baldari S, Caruso G, Caffo M, Montemagno G, Cardia E, Tomasello F. Reversible cerebral perfusion alterations in children with transient mutism after posterior fossa surgery. Childs Nerv Syst 1998;14:114-9. 10.1007/s003810050191 [DOI] [PubMed] [Google Scholar]
- 23.Miller NG, Reddick WE, Kocak M, Glass JO, Löbel U, Morris B, Gajjar A, Patay Z. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. AJNR Am J Neuroradiol 2010;31:288-94. 10.3174/ajnr.A1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagiuchi T, Ishii K, Aoki Y, Kan S, Utsuki S, Tanaka R, Fujii K, Hayakawa K. Bilateral crossed cerebello-cerebral diaschisis and mutism after surgery for cerebellar medulloblastoma. Ann Nucl Med 2001;15:157-60. 10.1007/BF02988609 [DOI] [PubMed] [Google Scholar]
- 25.Soelva V, Hernáiz Driever P, Abbushi A, Rueckriegel S, Bruhn H, Eisner W, Thomale UW. Fronto-cerebellar fiber tractography in pediatric patients following posterior fossa tumor surgery. Childs Nerv Syst 2013;29:597-607. 10.1007/s00381-012-1973-8 [DOI] [PubMed] [Google Scholar]
- 26.Reed-Berendt R, Phillips B, Picton S, Chumas P, Warren D, Livingston JH, Hughes E, Morrall MC. Cause and outcome of cerebellar mutism: evidence from a systematic review. Childs Nerv Syst 2014;30:375-85. 10.1007/s00381-014-2356-0 [DOI] [PubMed] [Google Scholar]
- 27.Korah MP, Esiashvili N, Mazewski CM, Hudgins RJ, Tighiouart M, Janss AJ, Schwaibold FP, Crocker IR, Curran WJ, Jr, Marcus RB, Jr. Incidence, risks, and sequelae of posterior fossa syndrome in pediatric medulloblastoma. Int J Radiat Oncol Biol Phys 2010;77:106-12. 10.1016/j.ijrobp.2009.04.058 [DOI] [PubMed] [Google Scholar]
- 28.Küpeli S, Yalçın B, Bilginer B, Akalan N, Haksal P, Büyükpamukçu M. Posterior fossa syndrome after posterior fossa surgery in children with brain tumors. Pediatr Blood Cancer 2011;56:206-10. 10.1002/pbc.22730 [DOI] [PubMed] [Google Scholar]
- 29.Doxey D, Bruce D, Sklar F, Swift D, Shapiro K. Posterior fossa syndrome: identifiable risk factors and irreversible complications. Pediatr Neurosurg 1999;31:131-6. 10.1159/000028848 [DOI] [PubMed] [Google Scholar]
- 30.Law N, Greenberg M, Bouffet E, Taylor MD, Laughlin S, Strother D, Fryer C, McConnell D, Hukin J, Kaise C, Wang F, Mabbott DJ. Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro Oncol 2012;14:1294-303. 10.1093/neuonc/nos160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushima T, Fukui M, Inoue T, Natori Y, Baba T, Fujii K. Microsurgical and magnetic resonance imaging anatomy of the cerebello-medullary fissure and its application during fourth ventricle surgery. Neurosurgery 1992;30:325-30. 10.1227/00006123-199203000-00003 [DOI] [PubMed] [Google Scholar]
- 32.Matsushima T, Abe H, Kawashima M, Inoue T. Exposure of the wide interior of the fourth ventricle without splitting the vermis: importance of cutting procedures for the tela choroidea. Neurosurg Rev 2012;35:563-71; discussion 571-2. 10.1007/s10143-012-0384-3 [DOI] [PubMed] [Google Scholar]
- 33.Mussi AC, Rhoton AL, Jr. Telovelar approach to the fourth ventricle: microsurgical anatomy. J Neurosurg 2000;92:812-23. 10.3171/jns.2000.92.5.0812 [DOI] [PubMed] [Google Scholar]
- 34.Tomasello F, Conti A, Angileri FF, Cardali S. Telo-velar approach to fourth-ventricle tumours: how I do it. Acta Neurochir (Wien) 2015;157:607-10. 10.1007/s00701-015-2358-z [DOI] [PubMed] [Google Scholar]
- 35.Tanriover N, Ulm AJ, Rhoton AL, Jr, Yasuda A. Comparison of the transvermian and telovelar approaches to the fourth ventricle. J Neurosurg 2004;101:484-98. 10.3171/jns.2004.101.3.0484 [DOI] [PubMed] [Google Scholar]
- 36.Deshmukh VR, Figueiredo EG, Deshmukh P, Crawford NR, Preul MC, Spetzler RF. Quantification and comparison of telovelar and transvermian approaches to the fourth ventricle. Neurosurgery 2006;58:ONS-202-6; discussion ONS-206-7. [DOI] [PubMed]
- 37.Aguiar PH, Plese JP, Ciquini O, Marino R. Transient mutism following a posterior fossa approach to cerebellar tumors in children: a critical review of the literature. Childs Nerv Syst 1995;11:306-10. 10.1007/BF00301766 [DOI] [PubMed] [Google Scholar]
- 38.Morris EB, Phillips NS, Laningham FH, Patay Z, Gajjar A, Wallace D, Boop F, Sanford R, Ness KK, Ogg RJ. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain 2009;132:3087-95. 10.1093/brain/awp241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ojemann JG, Partridge SC, Poliakov AV, Niazi TN, Shaw DW, Ishak GE, Lee A, Browd SR, Geyer JR, Ellenbogen RG. Diffusion tensor imaging of the superior cerebellar peduncle identifies patients with posterior fossa syndrome. Childs Nerv Syst 2013;29:2071-7. 10.1007/s00381-013-2205-6 [DOI] [PubMed] [Google Scholar]
- 40.Puget S, Boddaert N, Viguier D, Kieffer V, Bulteau C, Garnett M, Callu D, Sainte-Rose C, Kalifa C, Dellatolas G, Grill J. Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer 2009;115:1338-47. 10.1002/cncr.24150 [DOI] [PubMed] [Google Scholar]
- 41.Grill J, Viguier D, Kieffer V, Bulteau C, Sainte-Rose C, Hartmann O, Kalifa C, Dellatolas G. Critical risk factors for intellectual impairment in children with posterior fossa tumors: the role of cerebellar damage. J Neurosurg 2004;101:152-8. [DOI] [PubMed] [Google Scholar]
- 42.Avula S, Kumar R, Pizer B, Pettorini B, Abernethy L, Garlick D, Mallucci C. Diffusion abnormalities on intraoperative magnetic resonance imaging as an early predictor for the risk of posterior fossa syndrome. Neuro Oncol 2015;17:614-22. 10.1093/neuonc/nou299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patay Z, Enterkin J, Harreld JH, Yuan Y, Löbel U, Rumboldt Z, Khan R, Boop F. MR imaging evaluation of inferior olivary nuclei: comparison of postoperative subjects with and without posterior fossa syndrome. AJNR Am J Neuroradiol 2014;35:797-802. 10.3174/ajnr.A3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patay Z, Parra C, Hawk H, George A, Li Y, Scoggins M, Broniscer A, Ogg RJ. Quantitative longitudinal evaluation of diaschisis-related cerebellar perfusion and diffusion parameters in patients with supratentorial hemispheric high-grade gliomas after surgery. Cerebellum 2014;13:580-7. 10.1007/s12311-014-0575-2 [DOI] [PubMed] [Google Scholar]
- 45.von Monakow C. Die Lokalization in gross him und de abbau der function durch korticale herde. J.F.Bergmann, Wiesbaden, 1914. (in German).
- 46.Kim J, Lee SK, Lee JD, Kim YW, Kim DI. Decreased fractional anisotropy of middle cerebellar peduncle in crossed cerebellar diaschisis: diffusion-tensor imaging-positron-emission tomography correlation study. AJNR Am J Neuroradiol 2005;26:2224-8. [PMC free article] [PubMed] [Google Scholar]
- 47.Baron JC, Bousser MG, Comar D, Castaigne P. "Crossed cerebellar diaschisis" in human supratentorial brain infarction. Trans Am Neurol Assoc 1981;105:459-61. [PubMed] [Google Scholar]
- 48.Baron JC, Rougemont D, Soussaline F, Bustany P, Crouzel C, Bousser MG, Comar D. Local interrelationships of cerebral oxygen consumption and glucose utilization in normal subjects and in ischemic stroke patients: a positron tomography study. J Cereb Blood Flow Metab 1984;4:140-9. 10.1038/jcbfm.1984.22 [DOI] [PubMed] [Google Scholar]
- 49.Erşahin Y. SPECT in cerebellar mutism. Childs Nerv Syst 1998;14:611-3. 10.1007/s003810050283 [DOI] [PubMed] [Google Scholar]
- 50.Caner H, Altinörs N, Benli S, Calişaneller T, Albayrak A. Akinetic mutism after fourth ventricle choroid plexus papilloma: treatment with a dopamine agonist. Surg Neurol 1999;51:181-4. 10.1016/S0090-3019(98)00120-7 [DOI] [PubMed] [Google Scholar]