Abstract
Neck masses are frequently encountered in pediatric medicine, and can present a diagnostic dilemma for the clinicians involved. There are several means by which neck masses in children can be subdivided, for example by age at presentation, anatomical location including compartments and fascia of the neck, their classical appearance when imaged, or by etiology. When imaging children the clinicians must be mindful of radiation exposure and as such ultrasound (US) is often attempted first. Cross sectional imaging can be helpful for problem solving with CT being particularly useful for assessing the patient in more acute scenarios, for example when there is airway compromise. Nuclear medicine scintigraphy has a role in specific circumstances and can aid in staging in the presence of malignancy. If required, additional acquisition by means of magnetic resonance imaging (MRI) and computed tomography (CT) can be considered. This pictorial review describe the diagnostic imaging of (I) congenital and Developmental Pathologies, including thyroglossal duct cyst, branchial cleft cyst, cystic hygroma, dermoid cyst, thymic cyst and ectopic thymus; (II) neoplastic lesions, including hemangiomas and vascular malformations, pilomatrixoma, neurofibroma, neuroblastoma, rhabdomyosarcoma, papillary thyroid cancer, lymphoma & leukemia; (III) neck masses of Infective causes, including lymphadenitis, retropharyngeal and peritonsilar abscess, salivary gland inflammation; and (IV) other miscellaneous lesions, including ranula, sternocleidomastoid fibromatosis coli, and goiter. Neck masses are common in the pediatric population with a broad and varied differential; malignant etiologies are less frequently encountered when compared with adults but an awareness of its potential is important when reviewing imaging.
Keywords: Pediatric, neck, mass, benign, malignant, tumor, ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI)
Introduction
Neck masses are frequently encountered in pediatric medicine, and can present a diagnostic dilemma for the clinicians involved (1,2). There are several means by which neck masses in children can be subdivided, for example by age at presentation, anatomical location including compartments and fascia of the neck, their classical appearance when imaged, or by etiology. In this pictorial review the latter approach is adopted and subdivides the topic broadly to include congenital and developmental pathologies, neoplastic, infective and miscellaneous causes. When imaging children the clinicians must be mindful of radiation exposure and as such ultrasound (US) is often attempted first. If required, additional acquisition by means of magnetic resonance imaging (MRI) and computed tomography (CT) can be considered. These modalities can help aid in improving diagnostic confidence as well provide additional information such as involvement of deeper structures, especially since this is a limit of US penetration depth while high frequency probes are usually used for evaluating superficial pathologies (2).
Congenital and developmental pathologies
Thyroglossal duct cyst
Thyroglossal duct cysts are the most frequently encountered neck mass in the pediatric population with half presenting within the first decade of life. They typically present as a mobile midline structure which increases in size over time and moves in a cranial direction in relation to the tongue being extruded. As a consequence of their embryological origin thyroglossal duct cysts are closely related to the hyoid bone with the majority found to lie just inferior to this palpable anatomical landmark. These can become symptomatic if infected and a sinus tract can subsequently develop, should a communication with the skin arise (Figures 1,2) (2-5).
Figure 1.
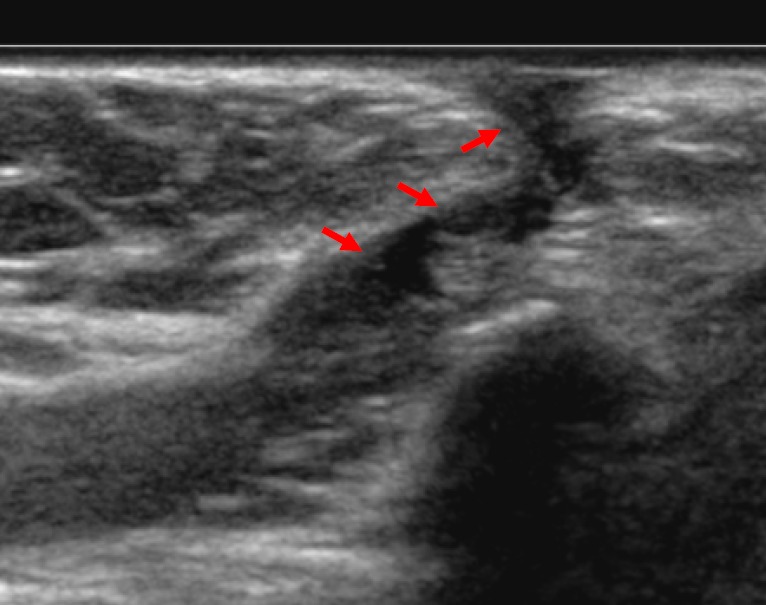
On this longitudinal ultrasound view of a 10-year old child’s neck a hypoechoic tract from the surface of the skin is demonstrated (arrows); this extended posteriorly towards the base of the tongue and appeared to move with protrusion of the tongue. This appearance is consistent with a thyroglossal duct sinus.
Figure 2.
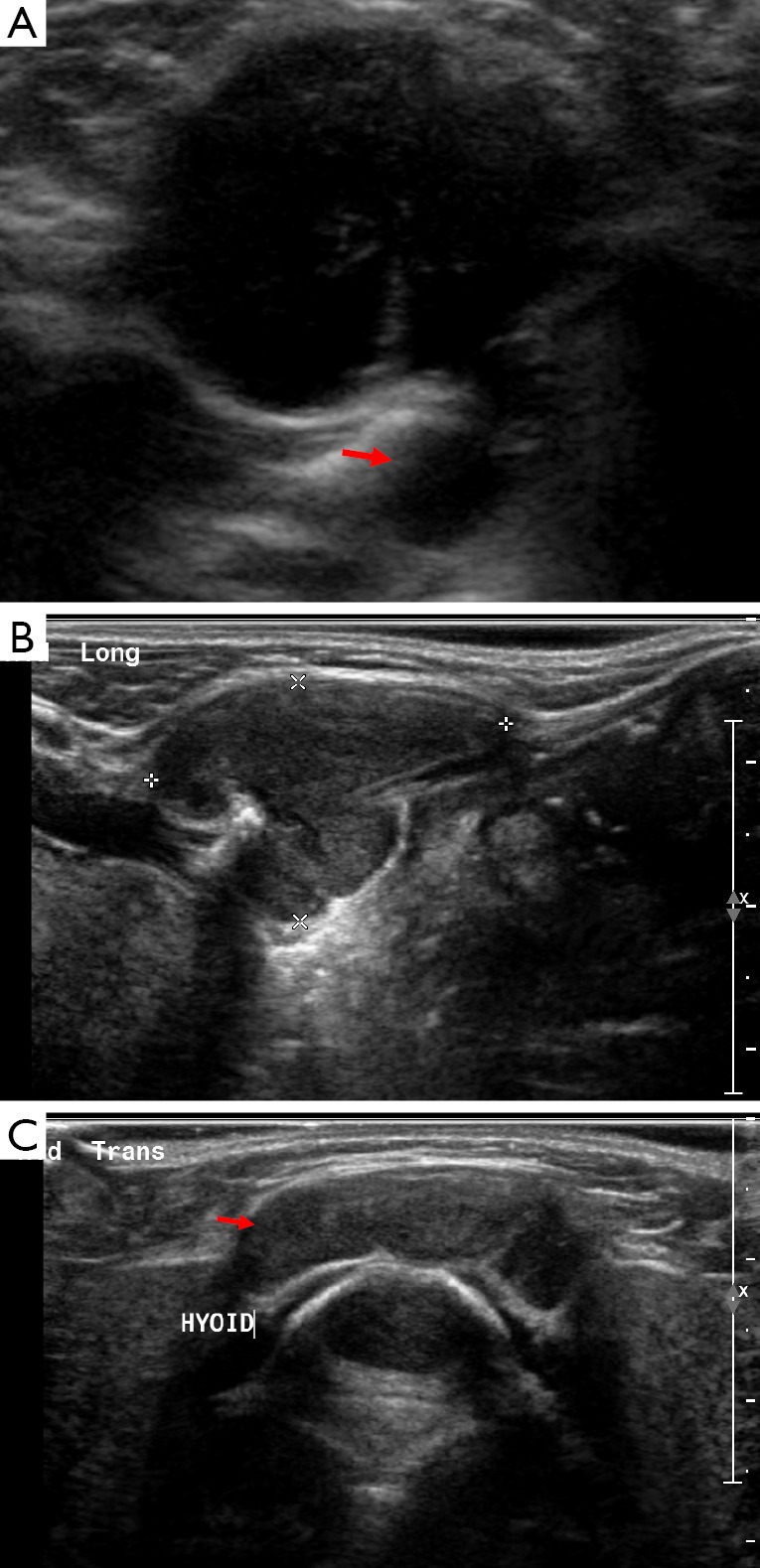
Image A is an ultrasound showing a well-defined hypoechoic structure in the neck at the level of the hyoid, there is posterior acoustic enhancement and a tract connecting it with deeper structures (arrow). On Doppler imaging there was no increased internal vascularity. The imaging characteristics and midline location are consistent with the diagnosis of a thyroglossal duct cyst. Images B and C are of the same child who represented when the thyroglossal duct cyst became infected, note how the internal echogenicity has altered.
A simple thyroglossal duct cyst will appear as an avascular, anechoic structure with posterior acoustic enhancement on US. When typical in their appearance US examination alone should suffice. If performed, on CT the cyst should be simple in its appearance and measure less than twenty Hounsfield Units. On MRI water characteristics are seen, i.e. dark on T1 and bright on T2 weighted images. In the presence of infection their appearance can vary with wall thickening and increased internal echogenicity on US, additional wall enhancement can also be demonstrated on CT. However, the cyst fluid can also contain protein causing the normal uniform hypoechoic appearance to be lost in the absence of infection. It is important that the presence and location of thyroidal tissue is determined when thyroglossal duct cyst excision is being planned. This is because the only functioning thyroid tissue may be located within the cyst itself and excision would render the patient hypothyroid (6). There is an association of papillary thyroid cancer affecting approximately 1 in every 100 cases of thyroglossal duct cysts; this should be suspected when the cyst appears more complex with areas of soft tissue and calcification (2-5,7).
Branchial cleft cyst
The face, neck and pharynx are formed from the branchial arches. Abnormalities in the embryological development of one of the four branchial arches can result in a cyst, sinus or fistula forming, the location of which alters with the branchial arch involved. The second branchial apparatus is the most frequently affected, typically resulting in cyst formation often located in the submandibular region. They can be found to lie lateral to the midline within the anterior triangle of the neck, often closely related to the path of the anterior border of the sternocleidomastoid muscle and are typically fluctuant on palpation. Branchial cleft cysts are the second most frequently encountered congenital abnormalities of the neck within the pediatric population (2,3,7-9).
When uncomplicated they are typically visualised as well delineated cystic structures appearing centrally hypoechoic on US with posterior acoustic enhancement. Their US appearances can vary however, appearing sometimes septated, inhomogeneous and even solid (Figure 3). They are uniformly hypoattenuating on CT and can demonstrate the ‘notch’ or ‘beak sign’ between the internal and external carotid arteries where there is a non-linear narrowing of the cyst between these two structures medially. MRI demonstrates the cystic properties of high signal on T2 weighted imaging but T1 appearances can alter with variation in proteinaceous contents. MRI can aid in determining the presence and path of sinus formation and to determine if there is associated inflammatory changes within the adjacent soft tissues. Repeated infections can occur which can consequently alter the internal echogenicity on US and can cause peripheral enhancement on contrast CT. In the event of multiple infections occurring surgical excision may be considered (3,7,10,11).
Figure 3.
Image A is from an ultrasound of a 4-year old child and demonstrates a well-circumscribed cystic structure which contains some faint internal echoes and demonstrates a pseudo-solid appearance but does show posterior acoustic enhancement (arrow) typical of a cyst. The corresponding MRI images are of the same child, they are labelled B, C and D and correspond to axial T1, T2 and coronal STIR sequences respectively. These illustrate a well-defined cystic lesion to the left of the midline (arrows), at the level of the vocal cords which demonstrates low signal on T1 and high signal on both T2 and STIR. The imaging characteristics are that of a branchial cleft cyst.
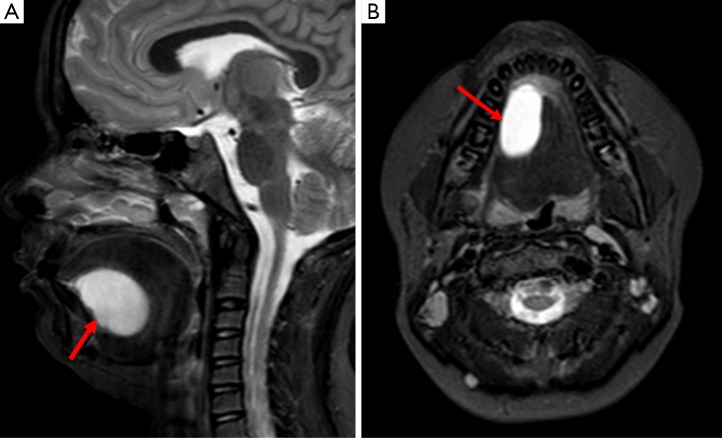
Cystic hygroma
Cystic hygromas are the commonest subtype of lymphangiomas (congenital lymphatic malformation), they are most often found to lie within the posterior triangle, however, they can occasionally be found anteriorly (3,5,12). They are associated with chromosomal defects, such as Turner’s syndrome, Trisomy 13, 18 and 21 as well as Noonan syndrome. Additional investigations by means of amniocentesis are therefore indicated when detected on antenatal US (3,7,12).
On US they can appear as poorly defined complex cystic structures with internal septations. They can have an anechoic appearance, however, this can change if there has been hemorrhage within. Additionally, they can have increased vascularity seen within the internal stroma when doppler is applied. Cystic hygromas can cross tissue planes and cause significant mass effect when large (3,5,12).
Their appearance on CT and MRI is variable but they are typically ill-defined; in the absence of hemorrhage or infection they demonstrate the usual cystic properties expected for the modality, as previously described (see Figure 4) (5). A differential diagnosis to consider for this typically posterior neck mass is an occipital meningocele; they can be distinguished form a cystic hygroma as they will be related to an underlying skull abnormality and there will be an absence of internal septations on US (13).
Figure 4.
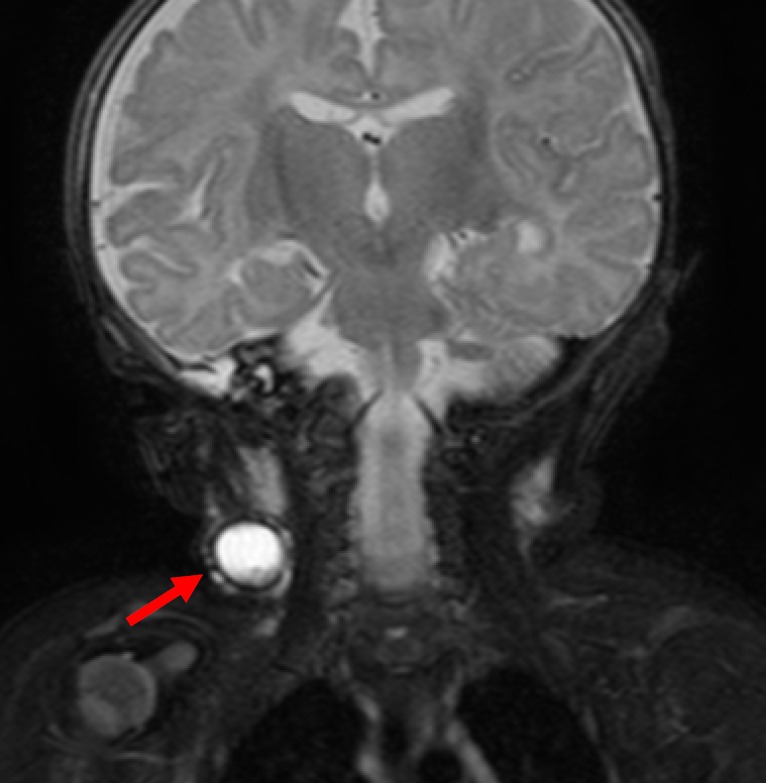
This MRI of an infant demonstrates a unilocular cystic structure in the lateral aspect of the right lower neck on a coronal fluid-sensitive STIR sequence, in keeping with the clinical suspicion of a cystic hygroma (arrow).
Dermoid cyst
Dermoid cysts are broadly subdivided by their histology: epidermoid, dermoid and teratoma, however, the broad term of ‘dermoid cyst’ is often quoted within the literature. They are an unusual cause of neck swelling but awareness of the lesions is necessary as they can be confused with thyroglossal duct cysts since they too are conventionally located adjacent to the hyoid bone in the midline. They can be located within the floor of the mouth and can appear as a visible, palpable swelling when located superficially. Furthermore, they may not present until later in childhood or early adulthood as they increase in volume slowly. Their imaging appearances can vary with their differing content, however, if there is fat present within the cyst then this is favorable for a teratoma (1,4,14,15). Figure 5 shows an example of a dermoid cyst.
Figure 5.
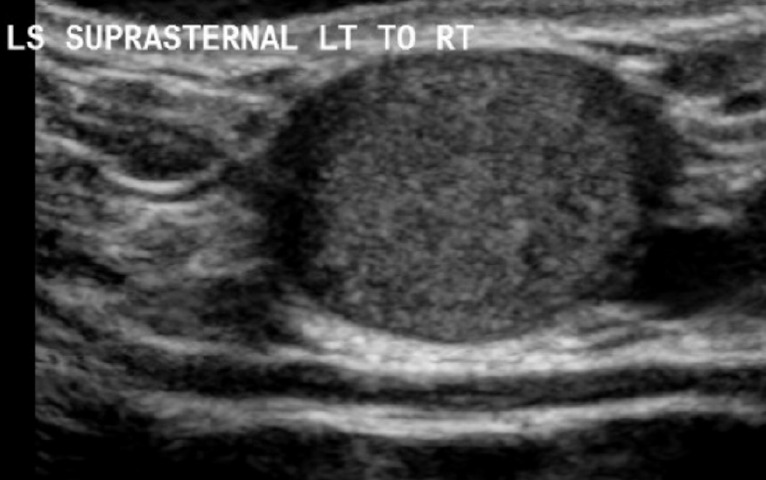
This is a longitudinal view of a suprasternal dermoid cyst on ultrasound which demonstrates it as a superficial, well demarcated ovoid structure with increased internal echogenicity and posterior acoustic enhancement.
Thymic cyst and ectopic thymus
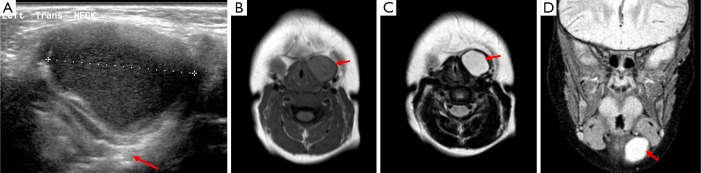
The thymus originates from the third pair of branchial arches; abnormalities can occur when the normal descent of the tissue to the anterior mediastinum is affected during development. Ectopic thymuses can be found within the anterior triangle and deeper fascial layers and demonstrate an echotexture similar to that of liver parenchyma on US (Figure 6). Thymic cysts are rarely encountered and can result from degenerated thymic tissue causing cyst formation or from remaining thyropharyngeal duct tissue. They are typically a single cyst with an associated soft tissue component with up to half remaining connected to thoracic thymic tissue. Their contents can vary resulting in differing appearances on US (2,3,15).
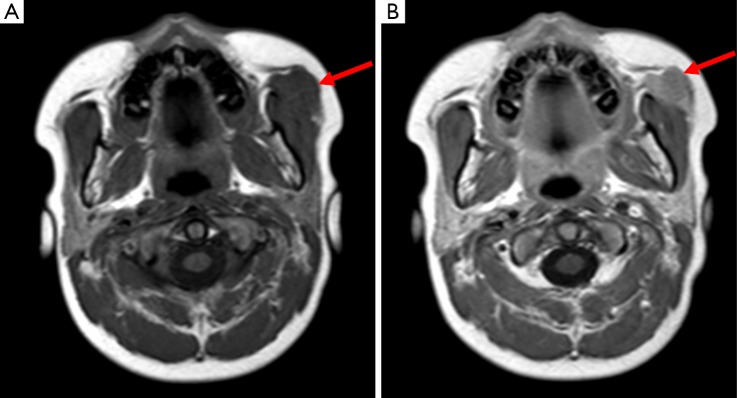
Figure 6.
Image A demonstrates the normal appearance of thymic tissue within the mediastinum and image B shows a structure within the neck displaying the same echogenicity, this indicates ectopic thymic tissue within the neck. Image C highlights the same signal intensity of thymus within the mediastinum and neck on a coronal STIR sequence (arrows).
Neoplastic lesions
Benign neoplastic lesions
Hemangiomas and vascular malformations
Congenital vascular malformations are commonly encountered in the head and neck and can be broadly classified into two distinct subtypes: infantile hemangiomas and vascular malformations. The former demonstrates an increased endothelial turnover rate and expand in an incommensurate manner, unlike the latter which are formed of endothelial tissues that increase in size in proportion to surrounding structures. US is an excellent, non-invasive means of assessing the degree of involvement of surrounding soft tissues as well as the area involved (Figure 7). The caliber and degree of flow demonstrated within a vascular malformation can help in determining the vessels involved, slow internal flow is seen in those with capillary, venous or lymphatic involvement. Arterial supply is suggested by higher flow rates with doppler imaging. This distinction can subsequently aid in determining therapeutic approach. US can also detect calcium formation within vascular malformations by detecting an echo-bright area with posterior acoustic shadowing. On CT vascular malformations have similar attenuation properties to muscle but their appearance in the presence of intravenous contrast can alter greatly (2,3,16).
Figure 7.
Image A is an ultrasound of an 18-month old child demonstrating a superficial soft tissue mass with mixed internal echogenicity with no deep structure involvement. When doppler is applied (B) this area shows avid vascularity. The imaging and clinical characteristics were that of an infantile hemangioma.
Pilomatrixoma
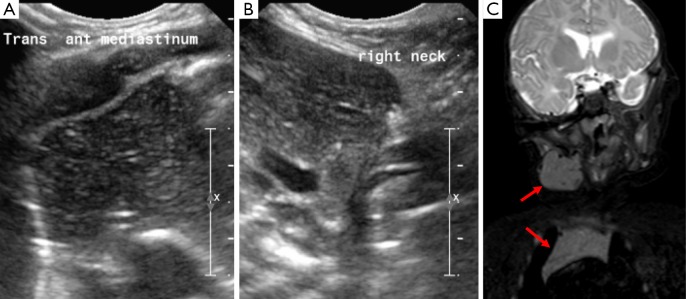
Pilomatrixomas are uncommon, benign congenital neoplastic growths originating from hair follicles. They can be encountered in the neck, in the region of the cheek or preauricular space and can cause discomfort. They typically appear as a well delineated ovoid mass with increased inhomogeneous internal echogenicity and peripheral hypo-echogenicity on US (Figure 8) (16,17).
Figure 8.
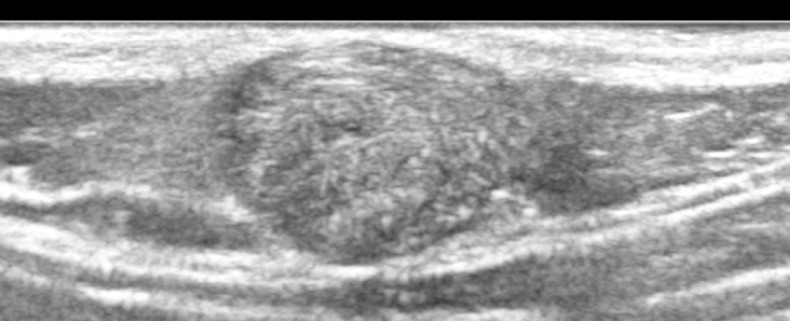
This is an ultrasound image of a superficial mass in an 8-year old child on the back of his neck which demonstrates mixed internal echogenicity with a peripheral rim of low echogenicity; on doppler imaging this did not demonstrate increased internal vascularity; there are some internal hyperechoic foci that suggest possible calcification; overall the appearances are consistent with a pilomatrixoma.
Neurofibroma
Neurofibromas are benign neurogenic tumors which arise from nerve sheaths; they can be sporadic but are frequently seen in patients with the hereditary disease neurofibromatosis type one. They grow along the path of nerves typically appearing as a slow growing uniform, elongated mass of soft tissue density with no associated lymph nodes. They can be seen to arise from the carotid sheath in the neck and can cause bony remodeling as they increase in size. An example is illustrated in Figure 9 (10,18).
Figure 9.
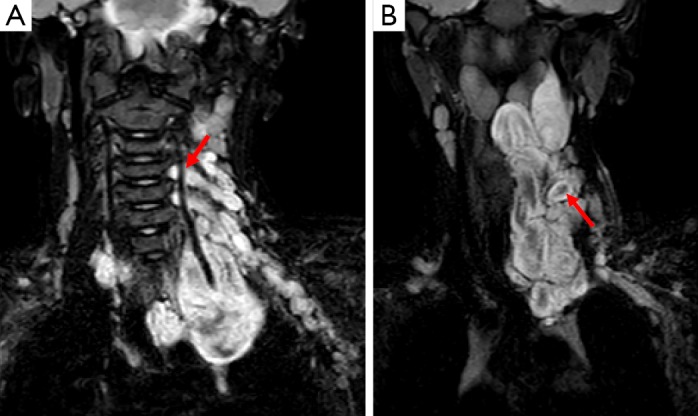
Images A and B are of an mDixon fat suppressed MRI sequence of a child with a neurofibroma. This is demonstrated as a high signal mass originating from the neural foramina of the cervical spine (see arrow on image A). Image B demonstrates the mass extending superiorly and inferiorly into the mediastinum and highlights a characteristic finding in neurofibromas whereby there is peripheral high signal with internal low signal (arrow), producing a target-like appearance on T2 weighted imaging.
Malignant neoplastic lesions
Neuroblastoma
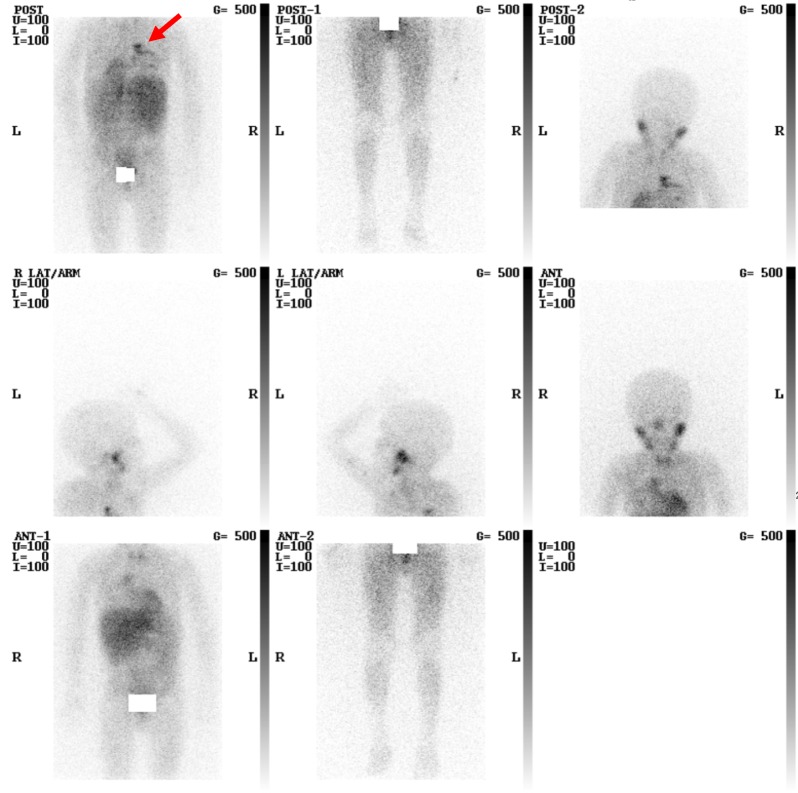
Primitive neuroectodermal tumors (PNETs) are rare tumors of neuroectodermal origin with many subtypes, including neuroblastomas. Neuroblastomas are common tumors in children and when they are encountered within the neck they usually represent metastatic disease. However, in up to 5% of children with neuroblastoma a neck mass can represent the primary site of disease and this conveys a more favorable outcome when compared with other primary sites. When occurring in the neck the presenting feature may not be simply that of a swelling, for example it can cause a Horner’s syndrome, dysphagia or stridor and furthermore neuroblastomas can be secretory resulting in additional systemic symptoms. Imaging typically highlights the presence of a heterogeneous soft tissue mass which may coalesce with surrounding lymph nodes. MRI usually demonstrates low signal on T1 and high signal on T2 weighted imaging with areas of internal restricted diffusion. CT and occasionally US can demonstrate the presence of calcification. Cross sectional imaging can also aid in assessing involvement and mass effect on adjacent structures. Highly specific imaging is available in the form of I-123 Meta-IodoBenzyl-Guanidine (MIBG) scintigraphy which demonstrates significantly increased uptake in relation to the sites of disease (Figure 10); MIBG has also been successfully used as a means of therapy in selected patients when MIBG has been radiolabeled with beta emitting I-131. Care must be taken when interpreting I-123 MIBG studies as normal physiological uptake is also seen in several sites and adequate prior thyroid blockade is also essential. A rare form of PNETs originates from peripheral nerves; they are also malignant and can present as a neck mass. Figure 11 demonstrates such a case (3,19-23).
Figure 10.
This is an Iodine-123 MIBG study of a young child with metastatic neuroblastoma who has had treatment. There is physiological uptake diffusely within the soft tissues, as well as the salivary glands, heart, liver and bowel. There is an additional focal site of tracer uptake within the mediastinum (arrow). Of note, the tracer retention within the bladder has been masked out.
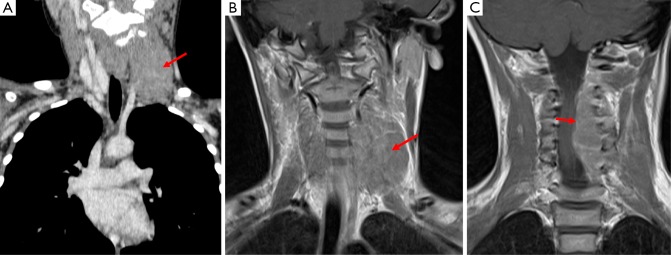
Figure 11.
This patient has a primitive neuroectodermal tumor which presented as a neck mass, this is demonstrated in image A (Coronal CT Neck) as a mass which is isodense to surrounding muscle (arrow) and on T1 weighted MRI (image B) this structure demonstrates an intermediate signal. Image C is also from a T1 weighted MRI acquisition and shows the tumor to be encroaching on the spinal canal and causing cord compression.
Rhabdomyosarcoma
Rhabdomyosarcoma is the commonest form of soft tissue sarcoma encountered within the pediatric population and is found within the head and neck in up to 40% of affected children; however, the soft tissues of the neck are the least likely location for the primary lesion within this subset. It is an aggressive malignancy and can invade adjacent structures such as the salivary glands and metastasize early in its course resulting in lymphadenopathy. Indeed, cervical lymph nodes may become involved in the presence of a primary ocular rhabdomyosarcoma. The primary neck lesion is typically solid on US but can demonstrate internal inhomogeneity and increased vascularity with doppler. MRI shows high signal on T2 weighted imaging with variable T1 signal and response to contrast (see Figure 12). MRI with contrast can also aid in the detection of intracranial and meningeal spread whilst CT and Tc99m-Methylene Diphosphonate (MDP) scintigraphy can be used in the detection and evaluation of bone metastases. Positron Emission Tomography (PET) CT has also shown promise in staging these patients (3,7,19,24-26).
Figure 12.
Image A is a T1 weighted acquisition demonstrating a soft tissue mass anterior to the left masseter muscle (arrows); image B is following gadolinium which shows uniform enhancement of this abnormality. This was found to be a rhabdomyosarcoma.
Papillary thyroid cancer
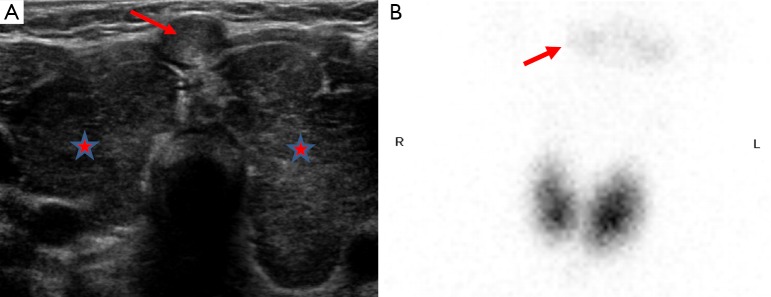
Papillary thyroid cancer is the commonest subtype of pediatric thyroid malignancy and carries a good prognosis. US demonstrates a well delineated, hypoechoic lesion with micro calcifications within the primary lesion as well as in the involved lymph chains. Nodes may also be large in caliber and demonstrate central necrosis, resulting in hypoattenuation on CT (Figure 13). As mentioned previously there is an association with thyroglossal duct cysts (2,3,19,27).
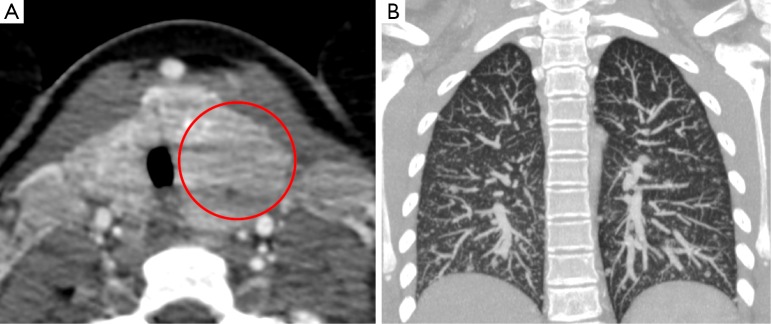
Figure 13.
This patient was found to have papillary thyroid cancer; image A shows an enlarged left lobe of thyroid with internal areas of hypoattenuation on CT (circle). This patient also had military lung metastases at presentation (B).
Lymphoma & leukemia
Lymphoma commonly presents as a neck mass with non-Hodgkin’s and Hodgkin’s subtypes typically affecting younger and older children respectively. Lymphomas can be further subdivided and graded according to the cell line involved and histological findings with some behaving more aggressively than others. For example, Burkitt lymphoma is a particularly aggressive form which typically presents as a neck/jaw swelling; its incidence varies with geographical location and has been linked with Epstein-Barr and Plasmodium falciparum malaria infection. Distinguishing subtypes on imaging, however, is not possible and tissue diagnosis is essential. Features common to lymphomas on US include a rounded appearance with reduced internal echogenicity, which is well delineated with altered vascularity and an absent hilum. Indeed, these features are not specific to lymphoma and so the differential can remain broad on initial assessment alone. Calcification within involved lymph nodes is usually only a feature following treatment. Cross sectional imaging and Tc-99m MDP scintigraphy is important to allow accurate staging and US can be helpful when assessing superficial nodes over time. On CT lymphomatous nodes appear isodense to muscle. One of the uses of CT is to assess involvement of the Waldeyer ring, however, unlike adults with lymphoma, the Waldeyer ring is usually spared in the pediatric population. The main role of MRI is in assessing central nervous system involvement when suspected. Gallium-67 scintigraphy has also proven to be a useful imaging modality during initial diagnosis and for follow-up; however, it does have its limitations particularly when assessing thymic involvement in children. The thyroid gland can also be affected by lymphoma, usually in the context of long standing inflammation as this typically results in the entire gland appearing expanded and echo-poor, but this can be variable (Figure 14). The salivary glands, most commonly the parotid gland, can also be infiltrated by lymphoma resulting in a disproportionately large and hypoechoic gland; however, this is still an uncommon cause of salivary gland swelling (2,3,27-30).
Figure 14.
Image A is a short axis view of the thyroid on ultrasound, it demonstrates a large complex mass within the left lobe of the thyroid (arrow) with a mixed pattern of internal echogenicity and some posterior acoustic enhancement. There is evidence of slight lateral deviation of the trachea which is confirmed on image B, which is the CT from the same patient. Image B also demonstrates areas of central hypoattenuation secondary to necrosis (arrow) and there is loss of surrounding fat planes. Although not demonstrated on this image there was also compression of the trachea and adjacent carotid artery and jugular vein. This was later diagnosed as non-Hodgkin’s lymphoma of the thyroid gland.
Leukemia is a common childhood cancer which can present as a lymph node swelling in the neck. The acute forms are the most frequently encountered and acute lymphoblastic leukemia is the commonest form in childhood with approximately half presenting with lymphadenopathy. Acute lymphoblastic leukemia is seen typically in those aged two to three years; acute myeloid leukemia is most often encountered within the first two years of life. Imaging is not diagnostic but it is a differential diagnosis to be aware of when scanning patients with additional non-specific symptoms (31,32).
Neck masses of Infective cause
Lymphadenitis
Lymphadenitis is lymph node inflammation usually relating to infection; it typically causes swelling within the anterior triangle in the presence of bacterial and viral etiologies. It can be focal or widespread when there is involvement of more than one nodal station. Lymphadenitis can also be secondary to tuberculous infection and more commonly involves level five lymph nodes in this setting. When tuberculous lymphadenitis occurs there is loss of the normal hilar appearance with internal septations and adjacent soft tissue swelling. Such US imaging characteristics are also present when an abscess develops within an affected lymph node and these may require intervention. On CT the findings are typical of any abscess with an area of central hypoattenuation with surrounding contrast enhancement. However, on CT fatty deposition within the lymph nodes can often be discerned when Tuberculosis is the causative agent, this may be of help when narrowing a differential diagnosis based on the radiological findings since lymphadenitis can mimic many other disease processes (1,2,10,15,17,19,25,31).
Retropharyngeal and peritonsilar abscess
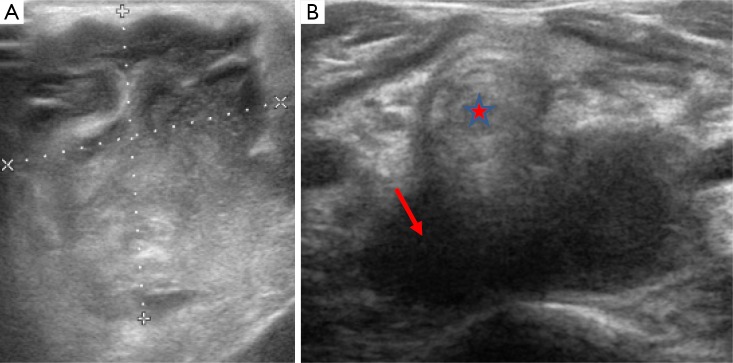
The peritonsillar and retropharyngeal spaces are the most common sites for abscess formation respectively, typically as a result of bacterial infection. Peritonsilar abscesses are unusual in children aged less than twelve years whereas most cases of retropharyngeal abscess are seen in younger children, typically those under six years of age. The effects from retropharyngeal abscess formation can be severe, and can result in airways obstruction and atlantoaxial subluxation. Airway compromise becomes less common in children over two years of age due to regression of the retropharyngeal nodes. US shows hypoechoic material with adjacent reactive lymph nodes (Figure 15). CT and lateral plain films can demonstrate locules of air within thickened soft tissues of the prevertebral space. Contrast enhancement at the periphery of the abscess may also occur on both CT and MRI. It is important to note that in young children the prevertebral tissues can appear prominent as a consequence of soft tissue laxity and head positioning, thus cross sectional imaging with contrast may be required in order to fully evaluate the presence and extent of an abscess and when formed its effects on the surrounding tissues and structures, since surgical intervention may be necessary. On rare occasions Lemierre syndrome (septic thrombophlebitis) can complicate abscess formation within the neck (1,2,5,17,19,25,33).
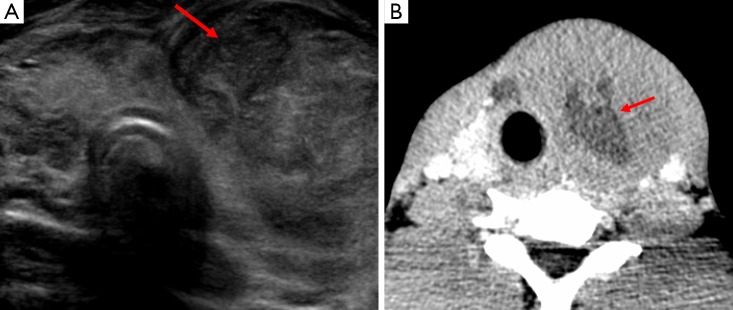
Figure 15.
Image A is a trans-axial ultrasound view which demonstrates a complex, heterogeneous mass within the neck of a child which was a superficial abscess. Image B demonstrates a hypoechoic lesion sited behind the trachea (star) due to a retropharyngeal abscess (arrow).
Salivary gland inflammation
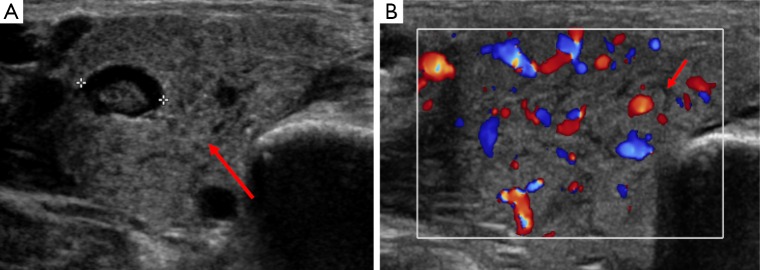
Normal salivary glands appear uniform and echo bright on US and can be clearly delineated from adjacent structures with subsequent fatty replacement of salivary tissue developing with advancement of age. Salivary gland inflammation can be the result of numerous disease processes with the parotid gland most frequently involved in children. It is most commonly the result of bacterial or viral infection, such as Mumps; however, chronic conditions such as human immunodeficiency virus, tuberculosis, sarcoidosis and other autoimmune conditions can also give rise to inflammatory changes. US may demonstrate enlargement of the affected salivary glands (Figure 16). Malignant neoplasms of the salivary glands are rare in this age group, but as previously mentioned lymphomatous involvement may occur in disseminated disease. In chronic inflammatory conditions, for example in those who have previously undergone radiotherapy, MRI sialography can be performed as an alternative to more invasive methods in the presence of obstructive symptoms. US or unenhanced CT or can also be performed when inflammatory changes are suspected to be secondary to stone disease (2,3,19).
Figure 16.
This is an ultrasound of an adolescent with unilateral parotid swelling. Image A shows an enlarged parotid gland in transverse section (arrow) with an intraparotid lymph node (identified between the calipers). Image B shows the parotid gland to be hypervascular on Doppler imaging and highlights prominent, hypoechoic ducts throughout the gland parenchyma (arrow). The cause of this patient’s parotitis was paramyxovirus (mumps) infection.
Miscellaneous lesions
Ranula
Ranulas are non-vascular, cystic midline structures typically formed as a result of sublingual duct occlusion causing subsequent mucous retention. They occur in the floor of the mouth and may protrude producing a ‘plunging’ appearance when involving the submandibular space. If they are confined to within the sublingual space, they are said to be simple. Ranulas demonstrate typical cystic appearances on all imaging modalities, i.e., thin walled and hypoechoic on US, water density on CT, hypointense on T1 and hyperintense on T2 weighted imaging. Cross sectional imaging can also demarcate the presence of a ‘tail sign’ in the presence of a communication between the ranula and sublingual gland. Wall thickening and altered internal characteristics may be observed in the presence of infection; see Figure 17 (1,2,10,19).
Figure 17.
Images A and B are sagittal and axial STIR sequences respectively of the floor of the mouth in a 10-year old child; they demonstrate a well-defined lesion of high (fluid) signal in the sublingual space (arrows). It was of low signal on T1 and demonstrated free diffusion on diffusion weighted imaging; overall this is in keeping with a ranula. If there was peripheral enhancement after gadolinium had been administered, this would raise the suspicion of infection.
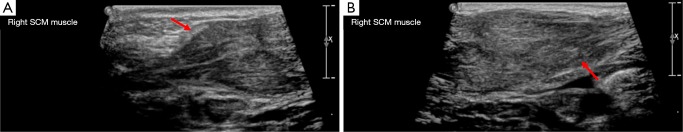
Sternocleidomastoid fibromatosis coli
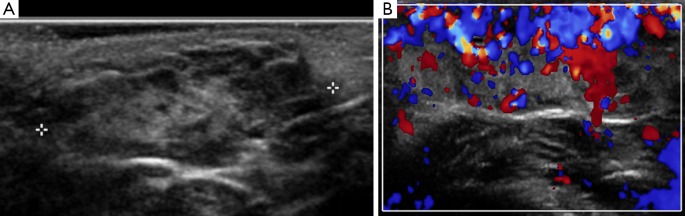
Sternocleidomastoid fibromatosis coli causes a benign, firm, unilateral neck mass which results in torticollis in infants. It can be from the result of trauma sustained to the sternocleidomastoid during delivery or from venous occlusion causing subsequent fibrosis. On US the sternocleidomastoid on the affected side appears enlarged but can demonstrate either increased or reduced echogenicity (see Figure 18). On CT it measures the same attenuation as muscle, however, calcification may be identified if there has been prior hemorrhage within the muscle fibers. It typically resolves without the need for intervention (1,2,5,19,27,33).
Figure 18.
Images A and B demonstrate the two ends of a spindle shaped mass within the sternocleidomastoid of a newborn infant on ultrasound (arrows). It did not demonstrate any increased internal flow on doppler assessment. The contralateral sternocleidomastoid muscle appeared normal. This was in-keeping with the clinical suspicion of sternocleidomastoid coli.
Goiter
Thyroid disorders are common and the development of a goiter can be the result of several pathological processes within the thyroid gland, including Grave’s disease, dyshormonogenesis, thyroiditis and nodular change. In a hypothyroid infant it is important to identify the presence and functional status of thyroidal tissue since a persisting hypothyroid state can negatively impact on physical and mental development. US and Tc99m-pertechnetate imaging can aid in thyroid gland evaluation as can the use of the perchlorate discharge test to evaluate the presence of dyshormonogenesis (3,34,35). An example has been shown in Figure 19.
Figure 19.
This is a child with hyperthyroidism and a goiter; image A shows both thyroid lobes to be enlarged with altered internal echogenicity (stars). Doppler shows increased vascularity in both lobes. There is an incidental area of nodular tissue demonstrating the same echogenicity within the isthmus (arrow). Image B is a Technetium-99m pertechnetate scan which shows diffusely increased tracer uptake in both thyroid lobes with suppression of background tissues. The salivary glands are just visible (arrow). This child was subsequently diagnosed with Grave’s disease.
Conclusions
Neck masses are a common presenting complaint within the pediatric population with a broad and varied differential; malignant etiologies are less frequently encountered when compared with adults but an awareness of its potential is important when reviewing imaging. US is the most frequently used imaging modality due to its accessibility and absence of ionizing radiation. Cross sectional imaging can be helpful for problem solving with CT being particularly useful for assessing the patient in more acute scenarios, for example when there is airway compromise. Nuclear medicine scintigraphy has a role in specific circumstances and can aid in staging in the presence of malignancy.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Badawy MK. Pediatric Neck Masses. Clinical Pediatric Emergency Medicine 2010;11:73-80. 10.1016/j.cpem.2010.05.004 [DOI] [Google Scholar]
- 2.Meuwly JY, Lepori D, Theumann N, Schnyder P, Etechami G, Hohlfeld J, Gudinchet F. Multimodality imaging evaluation of the pediatric neck: techniques and spectrum of findings. Radiographics 2005;25:931-48. 10.1148/rg.254045142 [DOI] [PubMed] [Google Scholar]
- 3.de Bruyn R. Pediatric Ultrasound: How, Why and When. 2nd Edition. Edinburgh: Elsevier, 2010. [Google Scholar]
- 4.Koeller KK, Alamo L, Adair CF, Smirniotopoulos JG. Congenital cystic masses of the neck: radiologic-pathologic correlation. Radiographics 1999;19:121-46; quiz 152-3. 10.1148/radiographics.19.1.g99ja06121 [DOI] [PubMed] [Google Scholar]
- 5.Malik A, Odita J, Rodriguez J, Hardjasudarma M. Pediatric neck masses: a pictorial review for practicing radiologists. Curr Probl Diagn Radiol 2002;31:146-57. 10.1067/cdr.2002.125778 [DOI] [PubMed] [Google Scholar]
- 6.Zander DA, Smoker WR. Imaging of ectopic thyroid tissue and thyroglossal duct cysts. Radiographics 2014;34:37-50. 10.1148/rg.341135055 [DOI] [PubMed] [Google Scholar]
- 7.Weissleder R, Wittenberg J, Harisinghani MM, Chen JW. Primer of Diagnostic Imaging, 5th Edition. United States of America: Elsevier, 2011. [Google Scholar]
- 8.Adams A, Mankad K, Offiah C, Childs L. Branchial cleft anomalies: a pictorial review of embryological development and spectrum of imaging findings. Insights Imaging 2016;7:69-76. 10.1007/s13244-015-0454-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahuja AT, King AD, Metreweli C. Second branchial cleft cysts: variability of sonographic appearances in adult cases. AJNR Am J Neuroradiol 2000;21:315-9. [PMC free article] [PubMed] [Google Scholar]
- 10.Mittal MK, Malik A, Sureka B, Thukral BB. Cystic masses of neck: A pictorial review. Indian J Radiol Imaging 2012;22:334-43. 10.4103/0971-3026.111488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaillard F, et al. Second branchial cleft cyst. Available online: http://radiopaedia.org/articles/second-branchial-cleft-cyst
- 12.Di Serafino M, Mercogliano C, Severino R, Lisanti F, Esposito F, Rocca R, Abate R, Cavallo T. Cystic Hygroma of the Neck: Ultrasound Findings. Open Journal of Radiology 2016;6:121-4. 10.4236/ojrad.2016.62018 [DOI] [Google Scholar]
- 13.Di Muzio B, Shetty A. Cystic hygroma vs. occipital meningocoele. Available online: http://radiopaedia.org/articles/cystic-hygroma-vs-occipital-meningocoele-2
- 14.Thabet H, Gaafar A, Nour Y. Thyroglossal duct cyst: Variable presentations. Egyptian Journal of Ear, Nose, Throat and Allied Sciences 2011;12:13-20. [Google Scholar]
- 15.Koch BL. Cystic malformations of the neck in children. Pediatr Radiol 2005;35:463-77. 10.1007/s00247-004-1388-0 [DOI] [PubMed] [Google Scholar]
- 16.Flis CM, Connor SE. Imaging of head and neck venous malformations. Eur Radiol 2005;15:2185-93. 10.1007/s00330-005-2828-4 [DOI] [PubMed] [Google Scholar]
- 17.Meier JD, Grimmer JF. Evaluation and management of neck masses in children. Am Fam Physician 2014;89:353-8. [PubMed] [Google Scholar]
- 18.Fortman BJ, Kuszyk BS, Urban BA, Fishman EK. Neurofibromatosis type 1: a diagnostic mimicker at CT. Radiographics 2001;21:601-12. 10.1148/radiographics.21.3.g01ma05601 [DOI] [PubMed] [Google Scholar]
- 19.Kadom N, Lee EY. Neck masses in children: current imaging guidelines and imaging findings. Semin Roentgenol 2012;47:7-20. 10.1053/j.ro.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 20.David R, Lamki N, Fan S, Singleton EB, Eftekhari F, Shirkhoda A, Kumar R, Madewell JE. The many faces of neuroblastoma. Radiographics 1989;9:859-82. 10.1148/radiographics.9.5.2678295 [DOI] [PubMed] [Google Scholar]
- 21.Chu CM, Rasalkar DD, Hu YJ, Cheng FW, Li CK, Chu WC. Clinical presentations and imaging findings of neuroblastoma beyond abdominal mass and a review of imaging algorithm. Br J Radiol 2011;84:81-91. 10.1259/bjr/31861984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papaioannou G, McHugh K. Neuroblastoma in childhood: review and radiological findings. Cancer Imaging 2005;5:116-27. 10.1102/1470-7330.2005.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikitakis NG, Salama AR, O'Malley BW, Jr, Ord RA, Papadimitriou JC. Malignant peripheral primitive neuroectodermal tumor-peripheral neuroepithelioma of the head and neck: a clinicopathologic study of five cases and review of the literature. Head Neck 2003;25:488-98. 10.1002/hed.10260 [DOI] [PubMed] [Google Scholar]
- 24.Radzikowska J, Kukwa W, Kukwa A, Czarnecka A, Krzeski A. Rhabdomyosarcoma of the head and neck in children. Contemp Oncol (Pozn) 2015;19:98-107. 10.5114/wo.2015.49158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnelly LF. Pediatric Imaging: The Fundamentals, 1st ed. Philadelphia: Elsevier, 2009. [Google Scholar]
- 26.Federico SM, Spunt SL, Krasin MJ, Billup CA, Wu J, Shulkin B, Mandell G, McCarville MB. Comparison of PET-CT and conventional imaging in staging pediatric rhabdomyosarcoma. Pediatr Blood Cancer 2013;60:1128-34. 10.1002/pbc.24430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imhof H, Czerny C, Hörmann M, Krestan C. Tumors and tumor-like lesions of the neck: from childhood to adult. Eur Radiol 2004;14 Suppl 4:L155-65. 10.1007/s00330-003-2035-0 [DOI] [PubMed] [Google Scholar]
- 28.Rowe M, Fitzsimmons L, Bell AI. Epstein-Barr virus and Burkitt lymphoma. Chin J Cancer 2014;33:609-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toma P, Granata C, Rossi A, Garaventa A. Multimodality imaging of Hodgkin disease and non-Hodgkin lymphomas in children. Radiographics 2007;27:1335-54. 10.1148/rg.275065157 [DOI] [PubMed] [Google Scholar]
- 30.Ahuja AT, Ying M, Ho SY, Antonio G, Lee YP, King AD, Wong KT. Ultrasound of malignant cervical lymph nodes. Cancer Imaging 2008;8:48-56. 10.1102/1470-7330.2008.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang S, Kansy B. Cervical lymph node diseases in children. GMS Curr Top Otorhinolaryngol Head Neck Surg 2014;13:Doc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Averill LW, Acikgoz G, Miller RE, Kandula VV, Epelman M. Update on pediatric leukemia and lymphoma imaging. Semin Ultrasound CT MR 2013;34:578-99. 10.1053/j.sult.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 33.Davies SG. editor. Chapman & Nakielny's Aids to Radiological Differential Diagnosis, 6th Edition. Edinburgh: Elsevier, 2014. [Google Scholar]
- 34.Muirhead S. Diagnostic approach to goitre in children. Paediatr Child Health 2001;6:195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leslie WD. Thyroid scintigraphy and perchlorate discharge test in the diagnosis of congenital hypothyroidism. Eur J Nucl Med 1996;23:230. 10.1007/BF01731854 [DOI] [PubMed] [Google Scholar]