Abstract
The mechanisms involved in the weight loss seen after vertical sleeve gastrectomy (VSG) are not clear. The rat stomach has two morphologically and functionally distinct proximal and distal parts. The rat model for VSG involves complete removal of the proximal part and 80% removal of the distal part along the greater curvature. The purpose of this study was to understand the potential independent contributions of removal of these distinct gastric sections to VSG outcomes. We prepared four surgical groups of male Long-Evans rats: VSG, sham surgery (control), selective proximal section removal (PR), and selective distal section removal (DR). Gastric emptying rate (GER) was highest after VSG compared with all other groups. However, PR, in turn, had significantly greater GER compared with both DR and sham groups. The surgery-induced weight loss followed the same pattern with VSG causing the greatest weight loss and PR having greater weight loss compared with DR and sham groups. The results were robust for rats fed regular chow or a high-fat diet. Body mass analysis revealed that the weight loss was due to the loss of fat mass, and there was no change in lean mass after the surgeries. In conclusion, removal of the proximal stomach contributes to most, but not all, of the physiological impact of VSG.
Keywords: sleeve gastrectomy, weight loss, gastric emptying rate
the latest u.s. center for disease control and prevention data show that two out of every three Americans are either overweight or obese, and the prevalence of Type 2 diabetes mellitus has more than tripled during the last 30 yr (6, 15). This is confounded by the fact that effective treatment options for obesity are limited, and bariatric surgery is currently the most effective therapy for achieving long-term weight loss and improvements in its comorbidities (36, 38).
Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are currently the two most commonly performed bariatric surgeries in the United States (1, 34). Although both are effective at eliciting weight loss and an improved metabolic profile, these two surgeries are drastically different anatomically. In RYGB, the stomach is divided to create a small proximal pouch at the distal end of the esophagus that is also connected to the mid-jejunum, and the bypassed section of gut (most of stomach, duodenum, and proximal jejunum) is attached to the distal jejunum. In this rearrangement, ingested food is delivered from the small pouch of stomach directly into the middle region of the jejunum effectively bypassing most of the stomach, the entire duodenum and the proximal jejunum. In VSG, the majority of the stomach (~80%) is resected along the greater curvature to form a narrow vertical sleeve through which ingested food passes en route from the esophagus to the duodenum, and no intestinal rearrangement occurs. Despite these differences, animal (7) and even the most conservative clinical (11, 17, 30) studies demonstrate that the two surgeries are more similar than dissimilar in the degree of weight loss and improvements in comorbidities.
We have developed rat (7, 10, 39) and mouse (8, 28, 33, 42) models of VSG and have found that, similar to humans, obese rats that undergo VSG have significantly lower body mass due to predominant loss of fat, as compared with the sham-operated rats. We have also found that VSG changes several important parameters in feeding behavior and metabolism. First, after VSG, rats have reduced food intake during the initial 3 to 5 wk after the surgery (7). Second, VSG causes the animals to ingest smaller but more frequent meals, and there is no evidence of fat malabsorption compared with sham-operated animals (39). Third, VSG rats have a lower preference for high-fat relative to low-fat diets than sham-operated controls (10). Fourth, VSG causes weight loss-independent improvements in both glucose (7) and lipid (28, 40) metabolism. Lastly, we have found that following VSG there are gastric mechanical consequences, namely a more rapid gastric emptying rate (GER), that may be important for the physiological implications of the surgery (9).
Our rat model of VSG (Fig. 1) involves resection of 80% of the stomach along the greater curvature. This portion of the stomach consists of the proximal and distal anatomical sections that human and animal studies suggest have distinct mechanical roles (12, 21, 23). The proximal stomach is thin and membranous and expands to accommodate ingested nutrients without drastic increases in intragastric pressure. The distal stomach is thick and glandular and mainly involved in the physical breakdown of food into smaller-sized particles with the help of strong peristaltic movements. Given that the rat model of VSG involves resection of two physiologically and morphologically distinct parts of the stomach, in this article, we sought to determine the independent effects of resecting each of these distinct sections to mimic the physiological and behavior changes seen after VSG.
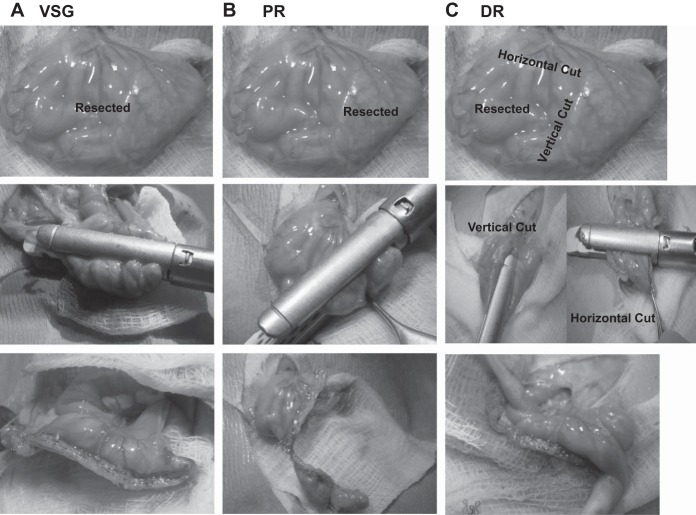
Fig. 1.
Photos of the vertical sleeve gastrectomy (VSG; A), proximal section removal (PR; B), and distal section removal (DR; C) surgeries. For VSG and PR, only one cut was made to remove tissue. For DR, two cuts were made as close to the limiting ridge as possible.
MATERIALS AND METHODS
Animals
Male Long-Evans rats (250–300 g) were purchased from Harlan Laboratories (Indianapolis, IN). They were housed in individual cages in rooms that were maintained at 25°C temperature, 50–60% humidity, and a 12:12-h light-dark cycle. Initial validations studies were performed in chow-fed rats, and a second cohort was studied in high fat-fed rats. Each diet group was randomly divided into four surgical subgroups. In a chow-fed group, the numbers of animals were distributed as sham (n = 5), proximal stomach resection (PR; n = 6), distal stomach resection (DR; n = 6), and VSG (n = 6). The high-fat diet group had the same four surgical subgroups (n = 10/group), but these animals were maintained on a high-fat diet (HFD) before and after surgery. We also studied a third cohort of rats with an objective to analyze whether gastric emptying rate changes soon (2 wk) after the VSG surgery. The rats in the third cohort were randomly assigned to either sham (n = 9) or VSG (n = 7) surgical subgroups. Similar to the HFD group, these rats were also maintained on HFD before and after surgery. There were no surgical deaths or complications in the chow-fed or third cohort of animals. However, in the HFD group, one VSG, one DR, and one sham animal died. The VSG and DR animal died within a couple of weeks of surgery, while the sham animal died 2 mo after surgery for unknown reasons. Any data collected from these animals have been excluded from analysis. All procedures for animal use were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Diet
All animals had ad libitum access to food and water throughout the experiment, except where noted. In the chow group, a low-fat chow diet (Teklad LM-485, 17% calories from fat; Harlan Laboratories, Frederick, MD) was provided both before and for 48 days after surgery. We then examined how diet manipulation influenced various end points in these rats (described below). To do this, the animals were switched to Ensure Plus liquid diet (Abbott Laboratories, Columbus, OH) for 10 days. After 1 wk wash-out period, where the animals were fed the same chow diet as before, the animals were then fed the Tso’s high-fat butter diet (D03082706, 40% calories from fat; Research Diets, New Brunswick, NJ) from day 66 to 114 after the surgery.
The HFD group of rats (n = 40) were fed Tso’s high-fat butter diet 8 wk before surgery and throughout the duration of the study. The third cohort of rats (n = 16) were also fed Tso’s high-fat butter diet for 6 wk before surgery and throughout the duration of the study.
Nuclear magnetic resonance was used to assess lean and fat mass of all rats before and periodically after surgery (Echo MRI: Echo Medical Systems, Houston, TX).
Surgery
All surgical procedures were performed under general anesthesia (isoflurane), and the VSG and sham surgeries were performed technically, as described previously (39). Pictures of the VSG, PR, and DR surgeries are depicted in Fig. 1. For PR, the proximal thin, membranous part of the stomach was completely excised with an ETS-FLEX 35-mm staple gun (Ethicon Endo-Surgery, Cincinnati, OH). In DR, one vertical and one horizontal cut was made with the stapler starting at the limiting ridge of the rat stomach. In VSG, ~80% of the thick distal glandular stomach was resected along the greater curvature using the ETS-FLEX 35-mm staple gun.
Postoperative Care
All rats were given 0.25 ml of Metacam (0.5 mg/kg of body weight once daily for 3 days), 0.25 ml of Buprenex (0.05 mg/kg twice daily for 3 days), and warm saline (10 ml twice daily for 3 days) subcutaneously. A wire grate was placed on the floor of the cages for 5 days after the surgery to prevent rats from eating their bedding. Rats in all three groups were given ad libitum Osmolite (in bottles; Abbott Laboratories, Columbus, OH) 3 days before and 3 days after surgery as an easier-to-digest alternative to solid food.
Food intake and body weight were monitored daily for 1 wk postsurgery and weekly thereafter.
Liquid Nutrient Gastric Emptying Rate Assessment
In both chow and HFD groups, at ~1 mo after the surgery (well after the initial weight loss period), the rate of liquid nutrient gastric emptying was assessed, as previously described (9). The rate of liquid nutrient gastric emptying was assessed during the initial recovery period (at 2 wk) after the surgery in the third group. Briefly, after an 8-h fast, acetaminophen (Sigma, A7085, 99.0% pure; 100 mg/kg) dissolved in 2.7 ml of 50% dextrose at 37°C was administered by oral gavage. Blood samples (~200 μl) were collected from the tail vein in heparinized tubes every 15 min for 1 h. Blood glucose was assessed with a hand-held glucose analyzer (Accuchek; Roche Diagnostics, Indianapolis, IN). Blood was centrifuged (3,000 rpm for 15 min), and plasma was stored at −80°C. Plasma acetaminophen levels were quantitatively analyzed by spectrophotometry (Sekisui Diagnostics, Charlottetown, Canada).
Solid Food Gastric Emptying Rate Assessment
In the chow group, solid gastric emptying rate was assessed at 6 mo postoperatively. The rats were given ad libitum access to Ensure Plus 3 days before the test to remove any solid residues in the stomach. After an overnight fast, the animals were given ad libitum access to chow for 10 min. The amount of chow consumed was noted, and the animals were euthanized for 2 h postprandially. The stomach was weighed before and after removal of stomach contents, and the percentge of chow left in the stomach (out of the amount consumed) was used to calculate gastric emptying rate as has been done previously (32).
To gain a better understanding of the time course of changes in solid food gastric emptying rate following these surgeries, we developed a technique to perform gastric emptying scintigraphy with parallel planar collimators on a Siemens trimodal Inveon uSPECT/CT scanner. This method is closely modeled to the Procedure Guideline for Adult Solid-Meal Gastric-Emptying Study 3.0 from the Society of Nuclear Medicine and Molecular Imaging (35). At 6 mo postoperatively, the HFD group of rats were trained to eat two pellets (~4.5 g) of a high-sucrose, high-fat diet (Research Diet ID 12331 58% and 25.5% calories from fat and carbohydrate, respectively) at 10 AM (during light cycle) for 2 days before the test. After an overnight fast, two high-fat food pellets soaked with 1 mCi (37 MBq) 99mTc sulfur colloid were offered to each rat at 10 AM for 10 min. The amount of chow consumed was noted. Then the rat was anesthetized with isoflurane, and its stomach was imaged at 0, 15, 45, 75, 105, and 135 min. The rat was allowed to wake up in between imaging time points because gastric emptying is slowed by anesthesia (37). Decay-corrected data was plotted on a time activity curve to demonstrate gastric emptying time of the rat stomach contents.
Glucose Metabolism
Mixed meal tolerance test.
At 5 mo postoperatively, HFD-fed rats were fasted for 6 h and then were orally gavaged with 2.7 ml of Ensure Plus. This volume was determined on the basis of the average 15-min consumption of Ensure Plus by rats with VSG (10). Blood was sampled from the tail vein and was analyzed for glucose at 0, 10, 30, 60, and 120 min (Accuchek, Roche Diagnostics, Indianapolis, IN). An additional volume of blood was taken at 0, 10, 30, and 60 min for assaying insulin and GLP-1 and at 0 and 20 min for assaying ghrelin. Plasma insulin was measured with a commercially available ELISA assay (Crystal Chem, Downers Grove, IL). For GLP-1 analysis, blood was collected into tubes containing an antiproteolytic cocktail (4.65 g EDTA+ 92 mg aprotinin + 40,000 U heparin in 50 ml saline), and GLP1 (7–36) was quantified using an electrochemiluminescence assay (Meso Scale Discovery, Gaithersburg, MD). For active ghrelin analysis, blood was collected into 200 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, a protease and esterase inhibitor in EDTA-coated tubes, and 1 N HCl was added to 100 μl of plasma. Active ghrelin levels were then quantified by a commercial ELISA assay (EMD Millipore, Temecula, CA).
Ingestive behavior analysis.
At 6 mo postoperatively, ingestive behavior (meal size, meal number, and daily food intake) of Ensure Plus was assessed in HFD animals using the Biodaq system (Research Diets, New Brunswick, NJ). The rats were acclimatized for 3 days to the Biodaq System and to Ensure Plus before the assessment.
Caloric density preference (high vs. low fat) was analyzed at 4 mo postoperatively in the chow-fed group, as described previously (10). In brief, both high- and low-fat diets (Research Diets D03082706 and D03082705 with calories from fat at 40% and 9%, respectively) were offered using two hoppers for 15 days. The first 3 days of assessment were used as an acclimatization period. The remaining 12 days were used to calculate relative consumption of a high- vs. low-fat diet.
Intestinal fat absorption was analyzed ~5 mo postoperatively in the chow-fed group using a labeled-fat diet, as we have done previously (22).
Statistics
GraphPad statistical software (La Jolla, CA) was used to analyze data. The results are expressed as means ± SE, and one-way and two-way independent repeated-measures ANOVAs were applied where appropriate. A Bonferroni post hoc analysis was performed to further analyze significant interactions. A P value <0.05 was considered to be statistically significant.
RESULTS
Body Mass and Composition
The timeline for all studies performed is listed in Fig. 2. In our rodent models of VSG (7, 10, 39), and consistently seen here (Fig. 3), there is an early (days 1–21) surgery-induced decrease in body weight compared with sham control animals. After this early decrease, sham and VSG animals followed a parallel growth trajectory with the VSG animals never reaching an equivalent body mass as sham animals (VSG < sham at 14–112 days on chow and 21–63 days on HFD). Importantly, this occurred whether the animals were on chow (Fig. 3A) or HFD (Fig. 3B). The fact that the VSG rats do not continue to lose weight is likely due to the fact that, in general, rats continue to increase body mass due to gains in both lean and fat mass throughout their lives (4). When animals were on chow, the body mass of PR animals was significantly lower than sham (days 77–112) and DR (days 56–112) rats. Both VSG and PR rats weighed less than both sham and DR rats. However, PR rats also maintained a higher body weight than VSG rats, suggesting an intermediate body weight phenotype. When maintained on a HFD both before and after surgery, VSG, but not PR, rats weighed significantly less than both sham and DR rats.
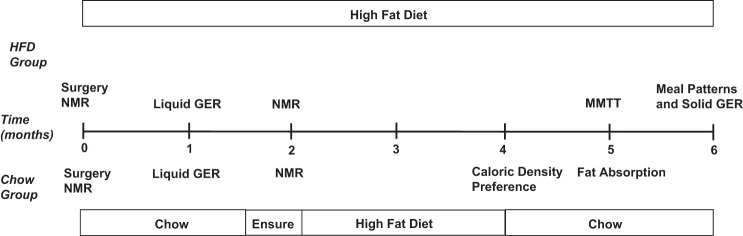
Fig. 2.
Timeline of experiments in chow and high-fat diet (HFD)-fed surgical groups.
Fig. 3.
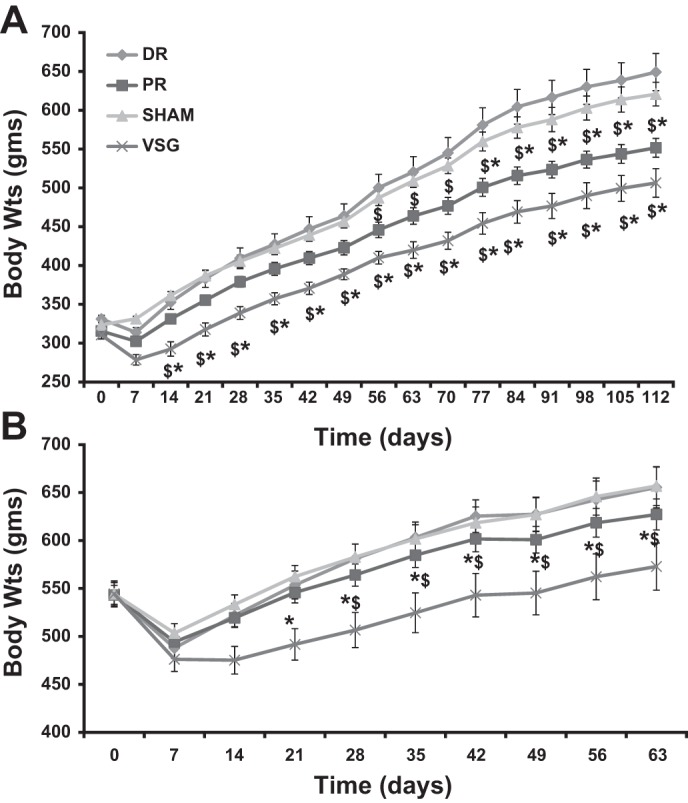
Body weight changes after surgery. Body weight trajectories after surgical procedures are depicted for the chow group (A) and for the HFD group (B). The DR group had a similar body mass compared with sham in both the chow and HFD groups. VSG body weights were significantly lower than those of sham and DR in both the chow and HFD groups. PR body weights were significantly lower than those of sham and DR only in the chow group. *Significantly different vs. sham, P < 0.05. $Significantly different vs. DR, P < 0.05.
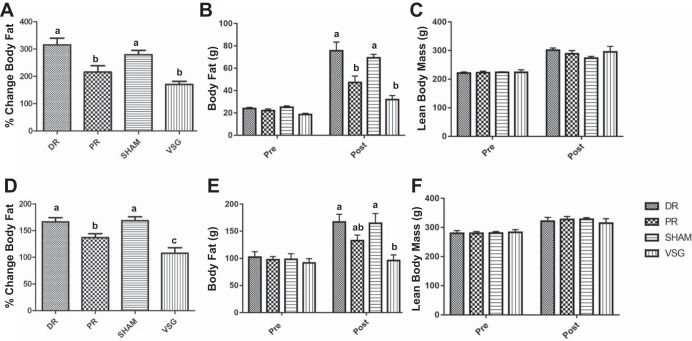
There were no surgery-induced differences detected in lean mass from presurgery to postsurgery on either chow or HFD (Fig. 4, C and F). Body fat significantly increased from presurgery to postsurgery measurements in both chow and HFD in all but the VSG group. The percentage increase in fat mass was significantly smaller from presurgery to postsurgery in both PR and VSG vs. DR and sham animals on the chow diet (Fig. 4A). In the HFD group, VSG animals had a significantly lower percentage increase in fat mass compared with all three other groups (Fig. 4D). In contrast to the lack of significance found in body mass, the gain in fat mass of the HFD PR animals was significantly lower than that of sham and DR but still significantly higher than that of VSG.
Fig. 4.
Body composition changes after surgery. NMR analysis of body composition presurgery and at day 63 postsurgery in the chow (A–C) and HFD (D–F) groups. Percent change in body fat in A and D show that the gain in body fat was significantly lower for both VSG and PR than for sham and DR in both chow and HFD groups, respectively. Body fat gain was similar in VSG and PR in the chow group, while significantly higher in PR, as compared with VSG in the HFD group. Absolute body fat presurgery and day 63 post-surgery are shown in chow (B) and HFD groups (E), respectively. Although there was a main effect of time, such that lean mass increased over time, there were no statistical differences in lean mass either preoperatively or postoperatively between surgeries in both chow (C) and HFD (F) groups. P < 0.05. Letters above bars that differ indicate a significant difference.
Energy Intake
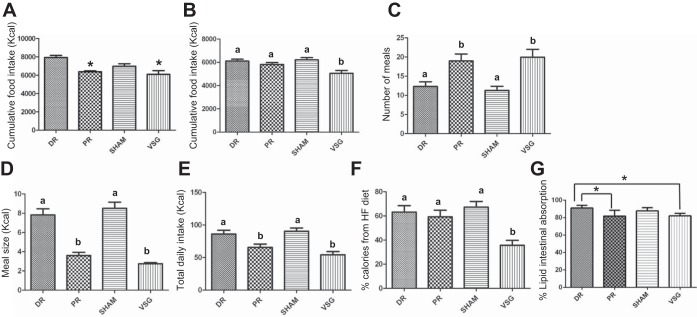
In chow-fed animals, PR and VSG both had significantly lower cumulative food intake than DR (Fig. 5A). In the HFD group, only VSG had a significantly reduced cumulative food intake as compared with the other three surgical groups (Fig. 5B).
Fig. 5.
A: energy intake and fat absorption after surgery. Cumulative food intake of PR and VSG after the surgeries were significantly lower than that of DR in the chow group. B: cumulative food intake of VSG was significantly lower than that of sham, DR, and PR in the HFD group. Both VSG and PR exhibited significantly smaller and more frequent meals than DR and sham when ad libitum access to Ensure Plus was offered to HFD group (C and D). However, the daily intake was still significantly lower than that of sham and DR (E). Only VSG had a significantly lower preference to the HFD in the chow group (F). G: behenate diet test in the chow group indicated that the lipid absorption by the gastrointestinal tract in both VSG and PR was similar to that of sham. However, DR had higher lipid absorption rate than both VSG and PR. *P < 0.05. Significant difference is indicated by letters that differ above the bars.
We analyzed feeding patterns (during the light as well as the dark cycle) in the HFD group using the Biodaq system 4 mo after recovery from surgery. During this time, the animals had ad libitum access to Ensure Plus rather than their previous HFD pellets. Rats with PR had similar feeding patterns as animals with VSG; i.e., both groups took in significantly smaller but significantly more frequent meals compared with both DR and sham groups (Fig. 5, C and D). When ingesting Ensure, overall net daily caloric intake was significantly lower in both PR and VSG compared with DR and sham (Fig. 5E).
We also assessed whether the portion of stomach removed had any influence on caloric density preference. Interestingly, only VSG animals had a significantly lower preference for calorically dense HFD as compared with the LFD (Fig. 5F).
Fat Absorption
We measured lipid absorption in the chow-fed cohort by exposing the animals to a diet with a nonmetabolized lipid (behenate) and measured the appearance of behenate in the feces and from that calculated lipid absorption (22). In the chow group, lipid absorption by the gastrointestinal tract in case of VSG and PR was similar to sham. However, DR had higher lipid absorption rate than both VSG and PR (Fig. 5G).
Glucose and Gastrointestinal Hormones
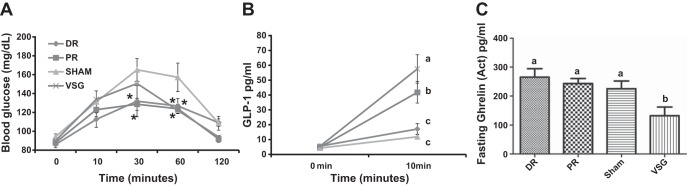
In the HFD-fed group, blood glucose excursions in response to a mixed liquid meal (Ensure Plus) were significantly lower in DR and PR vs. sham at 30 min and in all three groups vs. sham at 60 min after the gavage (Fig. 6A). GLP-1 levels 10 min after the gavage were significantly higher in VSG vs. PR and both groups were significantly greater than DR and sham (Fig. 6B). Baseline levels of ghrelin were significantly lower in VSG vs. all other groups (Fig. 6C).
Fig. 6.
Glucose and gastrointestinal hormones in response to surgery. Blood glucose values (A), change in GLP-1 levels from baseline at 10 min (B) and fasting ghrelin levels before a mixed-meal tolerance test (MMT) in the HFD rats (6C). Blood glucose values of DR, PR, and VSG were significantly lower than those of sham at 60 min. DR and PR blood glucose values were significantly lower at 30 min. Fasting ghrelin levels were significantly lower in VSG, as compared with DR, PR, and sham. GLP-1 secretion matched the gastric emptying rate (GER) for the four groups. Different letters indicate statistical significance, P < 0.05. *Significantly different vs. sham, P < 0.05.
Gastric Emptying
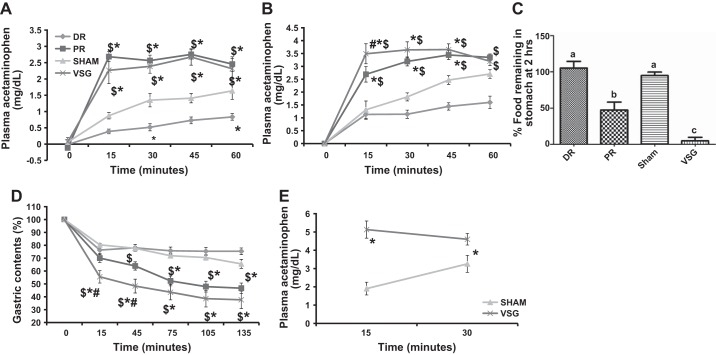
We used a previously validated (9) method of assessing liquid gastric emptying rate by mixing a gavaged nutrient solution with acetaminophen. Using this method, liquid nutrient gastric emptying rate in the chow group was found to be significantly greater in VSG and PR vs. sham and DR at all time points assessed after the gavage (Fig. 7A). Interestingly, liquid nutrient gastric emptying rate was slower in DR vs. sham groups at 30 and 60 min. We found similar results in the HFD group (Fig. 7B). Specifically, VSG animals had significantly greater plasma acetaminophen and thus a higher gastric emptying rate compared with all other groups at 15 min, and vs. sham and DR at all time points thereafter. Gastric emptying rate was significantly higher in VSG, as compared with sham as early as 2 wk after the surgery (Fig. 7E).
Fig. 7.
Gastric emptying rate after surgery. Liquid nutrient gastric emptying using the acetaminophen test is depicted for the chow group (A), for the HFD group (B) and the third cohort (E). C: solid gastric emptying, as assessed by analyzing the percent of food remaining in the stomach 2 h after feeding in the chow group. D: analysis is given of planar gamma imaging after rats consumed ad libitum 99mTc sulfur colloid-labeled HF diet to analyze solid gastric emptying in the HFD group. Solid and liquid GER were the highest in VSG, as compared with sham and DR in both HFD and chow groups. GER in PR was significantly lower than in VSG but higher than in both sham and DR except for the liquid GER assessment in the chow group, where it was as high as in VSG. GER was significantly higher in VSG as compared with sham in the third cohort soon (2 wk) after the surgery. Different letters indicate statistical significance, P < 0.05. *Significantly different vs. sham, P < 0.05. $Significantly different vs. DR, P < 0.05. #Significantly different vs. PR, P < 0.05.
We initially analyzed solid food gastric emptying in the chow group by assessing the amount of chow left in the stomach 2 h after an ad libitum meal (Fig. 7C). DR (7.43 ± 0.81 g) and sham (3.6 ± 0.88 g) animals consumed a significantly greater amount of chow during the 10-min feeding period compared with both PR (4.7 ± 0.56 g) and VSG [2.52 ± 0.34 (ANOVA; main effect of surgery; P < 0.05]. The percent of ingested food left in the stomach after 2 h was significantly lower in the VSG compared with all other groups, while the amount of food left in the stomach of PR animals was significantly lower than in the DR and sham rats (Fig. 7C). This indicates that, as we have previously observed with liquid diets, solid food gastric emptying rate was significantly highest in VSG followed by PR, which was, in turn, significantly higher than sham and DR.
We then confirmed these findings in the HFD group with planar gamma camera imaging, a more sensitive technique that allowed us to quantify gastric emptying over time (Fig. 7D). Again, sham (3.4 ± 0.58 g) and DR (2.67 ± 0.37 g) groups consumed more radiolabeled HFD compared with both PR (1.45 ± 0.15 g) and VSG (1.25 ± 0.12 g) animals. Similar to the prior experiment, the percentage of food left in the stomach was significantly lower, and thus, gastric emptying was higher, in VSG vs. PR, sham and DR at the 15 and 45 min time points (Fig. 7D). At 45 min, the GER was significantly higher in PR vs. DR. Thereafter, GERs of both VSG and PR were similar and significantly higher than in DR and sham. These data suggest that the GER was significantly greater in VSG across all time points and in the case of PR, it was intermediate between VSG vs. sham and DR groups.
DISCUSSION
Despite the well-known benefits of bariatric procedures, such as VSG, the mechanisms underlying their success are still unknown. We have previously hypothesized that increased GER drives key intestinal adaptations to VSG (9, 10). Our current data demonstrate that removal of a distinct area of the stomach, the proximal portion, drives a large portion of this increase in GER and mimics much of the weight loss and feeding behavior changes seen with VSG. Conversely, removal of the distal stomach had no effect on feeding and body weight and actually reduced GER. Together, these data suggest that removal of the proximal portion of the stomach replicates many of the physiological changes induced by VSG, but that removal of both the proximal and distal portions of the stomach are necessary for the full weight loss and metabolic effects of VSG.
Reflecting the robustness of these effects, VSG and PR had similar effects whether the animals were on chow or HFD. Additionally, the impact of VSG on body composition (39), GER (9), preference for lower-calorie density meals (10, 43), and fat absorption (40) are all similar between the current studies and our previously reported studies, in which animals were all on a high-fat butter diet. These data highlight the impact of surgery and the importance of the gastrointestinal (GI) tract, independent of diet, on physiological processes regulating nutrient assimilation.
We confirmed our previous finding that VSG increases the GER of liquid nutrients, as has been reported in both humans and rodents (3, 5, 9, 25, 26), and it is noteworthy that this effect does not occur following adjustable gastric banding (24). We also demonstrate that this change in GER happens rapidly (within 2 wk) and also occurs with solid food. Although not to the extent of VSG, PR also increased GER of both solid and liquid nutrients. Whether or not GER has a direct effect on regulating body weight remains to be determined in humans, as measures of GER in obese humans have found either rapid (44), normal (2, 16, 35, 41), or slowed emptying (14, 20). Clinically, both RYGB and VSG are associated with an approximate 30% weight loss, and both cause rapid GER. Consistent with this, a positive association between GER and weight loss has been observed after RYGB in humans (31). This increase in GER has been suggested to increase postprandial levels of GI hormones, including GLP-1, that also increase satiety (29). However, we have found that GLP-1 signaling is not necessary for weight loss, nor is rapid GER necessary for increases in GLP-1 after VSG (9, 42). Conversely, slowing of GER has been suggested to increase satiety and, consequently, to induce weight loss (19). The idea is that slowed GER would, via mechanical stretch receptors, send neural signals to the hindbrain to increase satiety. However, our data do not support this contention. DR, which slowed emptying of liquid nutrients to the extent that postprandial glucose tolerance was improved, had no impact on body mass or composition. While it could be argued that DR did not alter solid food GER and that the DR rats in this study were chronically maintained on solid food, they were placed on a liquid diet during the meal pattern assessment and even during that week they were equivalent in meal patterning and total food intake compared with sham animals. Given that the largest physiological impact of DR was on slowing GER, our data do not support the contention that slowing gastric emptying rate alone (i.e., without gastric neural signals arising from the distal stomach) can directly result in weight loss.
Alternatively, it is difficult to reconcile the general impact of GER on postprandial glucose homeostasis and long-term glucose control. The DR animals, with their slow GER, had significantly lowered postprandial glucose excursions. This might suggest that strategies to slow GER would be a viable therapeutic strategy for Type 2 diabetes mellitus. Indeed, it is thought that part of the mechanism by which long-acting GLP-1 agonists improve glucose is via slowing of GER. However, although many patients that undergo VSG experience resolution of diabetes (36), the pattern of glucose responses to an oral glucose load is an elevated peak glucose that returns more rapidly toward baseline (9, 30). This change in pattern is also seen in PR with the excursions not being quite as high.
Both VSG and PR altered meal patterns to result in smaller more frequent meals. One possibility is that this is a learned behavior to prevent visceral illness or diarrhea. We cannot rule out either of these possibilities. While there was significantly lower intestinal lipid absorption, the animals did not have visible diarrhea. One caveat to this is that we know that when VSG animals are pushed toward hyperphagia either during recovery from food restriction (39) or during lactation (18), they eat the same amount of calories as sham surgery HFD-fed animals. These data suggest that when the physiology demands, the animals can become hyperphagic, suggesting that the shift in meal patterns is due to neural or hormonal satiety signals rather than sickness.
GER has been hypothesized to account for 70% of HbA1c% (27). This presents one of the central conundrums of VSG. How does it both dramatically increase GER and still greatly improve HbA1c%. It is possible that for glucose control, slowing GER has a direct effect on lowering postprandial glucose excursions but has little effect on overall glucose homeostasis. Conversely, rapid GER requires large-scale adaptations of the GI tract and beyond, and it is possible that these adaptations result in a physiological regulatory system that pushes the animal toward an overall improvement in glucose homeostasis and defense of lowered body weight.
One systemic effect of rapid GER is its ability to stimulate nutrient-induced GI hormone release. PR animals had an intermediate increase in GER, as well as an intermediate nutrient-induced GLP-1 response that fell between that of VSG and sham animals. Exogenous GLP-1 suppresses GER (13), suggesting that the exaggerated GLP-1 response to surgery should slow GER via a negative feedback loop. However, we have previously demonstrated that the remaining sleeve remnant following VSG is no longer responsive to many of the typical inhibitory signals to GER, including the long-acting GLP-1 agonist exendin-4 (9). Conversely, when we controlled nutrient entry into the intestine with a direct duodenal infusion of glucose, there was a similar increase in GLP-1, as when that same dose was gavaged in both sham and VSG rats (9). We hypothesize that the mechanism for the increase in GLP-1 with surgery is an intestinal adaptation to the ongoing challenge of rapid nutrient entry into the gut.
Perspectives and Significance
In summary, removal of the proximal section of the stomach did not, in its entirety, replicate the effects of VSG. Rather, there was an intermediate impact of PR on both GER and fat loss on a HFD; that is, PR animals had faster GER, and lost fat compared with sham animals, but these effects were still significantly less than what occurred in VSG animals. There were also no changes in preference for the lower-calorie density diet. As DR had no impact on any of these parameters, these data suggest that the degree of weight loss and shift in dietary preference is due to a synergistic effect of removal of both the proximal and distal sections. Regardless, the key conclusion is that removal of the proximal vs. the distal section of the stomach plays a large role in mediating many of the effects of VSG.
GRANTS
The work of the laboratory is supported in part by National Institutes of Health Awards, DK-082480 (to D. Sandoval), DK-093848 (to R. Seeley) and also by Ethicon Endo-Surgery, Inc. Intramural research grant award from Center for Clinical and Translational Science and Training at University of Cincinnati funded the solid gastric emptying imaging work.
DISCLOSURES
Randy Seeley has received research support from Ethicon Endo-Surgery, Novo Nordisk, Sanofi, and Boehringer Ingelheim. Randy Seeley has served on scientific advisory boards for, Ethicon Endo-Surgery, Novartis, Nestle, Daiichi Sankyo, Circuit Therapeutics, Takeda, Boehringer Ingelheim, and Novo Nordisk. Darleen Sandoval has received research support from Ethicon Endo-Surgery, Novo Nordisk, and Boehringer Ingelheim. Darleen Sandoval is on a scientific advisory board for Ethicon Endo-Surgery. Lisa Lemen serves as a radiation safety physics consultant for Ethicon Endo-Surgery (Cincinnati).
ACKNOWLEDGMENTS
We would like to thank Dr. Jose Berger, Dr. Alfor Lewis, Kathleen Smith, and Mouhamadoul Toure for their help with surgical procedures.
Authors B. Kulkarni, R. Seeley and D. Sandoval now work at the Department of Surgery, University of Michigan, Ann Arbor, Michigan 48103.
REFERENCES
- 1.Abu-Jaish W, Rosenthal RJ. Sleeve gastrectomy: a new surgical approach for morbid obesity. Expert Rev Gastroenterol Hepatol 4: 101–119, 2010. doi: 10.1586/egh.09.68. [DOI] [PubMed] [Google Scholar]
- 2.Barkin JS, Reiner DK, Goldberg RI, Phillips RS, Janowitz WR. The effects of morbid obesity and the Garren-Edwards gastric bubble on solid phase gastric emptying. Am J Gastroenterol 83: 1364–1367, 1988. [PubMed] [Google Scholar]
- 3.Baumann T, Kuesters S, Grueneberger J, Marjanovic G, Zimmermann L, Schaefer A-O, Hopt UT, Langer M, Karcz WK. Time-resolved MRI after ingestion of liquids reveals motility changes after laparoscopic sleeve gastrectomy—preliminary results. Obes Surg 21: 95–101, 2011. doi: 10.1007/s11695-010-0317-6. [DOI] [PubMed] [Google Scholar]
- 4.Bogdanske JJ, Hubbard-Van Stelle S, Riley Rankin M, Schiffman BM. Laboratory Rat Procedural Techniques. Sarasota, FL: CRC Press, 2010. [Google Scholar]
- 5.Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, Gonzalez P, Papapietro K. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg 19: 1515–1521, 2009. doi: 10.1007/s11695-009-9954-z. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Diabetes Report Card 2014. http://www.cdc.gov/obesity/data/adult.html.
- 7.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Pérez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D’Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141: 950–958, 2011. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschöp MH, Sandoval DA, Seeley RJ. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 144: 50–52.e5, 2013. doi: 10.1053/j.gastro.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, Sorrell J, Lemmen L, LaSance K, Woods SC, Seeley RJ, D’Alessio DA, Sandoval DA. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab 306: E424–E432, 2014. doi: 10.1152/ajpendo.00469.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers AP, Wilson-Perez HE, McGrath S, Grayson BE, Ryan KK, D’Alessio DA, Woods SC, Sandoval DA, Seeley RJ. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am J Physiol Endocrinol Metab 303: E1076–E1084, 2012. doi: 10.1152/ajpendo.00211.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chouillard EK, Karaa A, Elkhoury M, Greco VJ; Intercontinental Society of Natural Orifice, Endoscopic, and Laparoscopic Surgery (i-NOELS) . Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for morbid obesity: case-control study. Surg Obes Relat Dis 7: 500–505, 2011. doi: 10.1016/j.soard.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Collins PJ, Horowitz M, Chatterton BE. Proximal, distal and total stomach emptying of a digestible solid meal in normal subjects. Br J Radiol 61: 12–18, 1988. doi: 10.1259/0007-1285-61-721-12. [DOI] [PubMed] [Google Scholar]
- 13.de Gordejuela AG, Pujol Gebelli J, García NV, Alsina EF, Medayo LS, Masdevall Noguera C. Is sleeve gastrectomy as effective as gastric bypass for remission of type 2 diabetes in morbidly obese patients? Surg Obes Relat Dis 7: 506–509, 2011. doi: 10.1016/j.soard.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Delgado-Aros S, Kim DY, Burton DD, Thomforde GM, Stephens D, Brinkmann BH, Vella A, Camilleri M. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol Gastrointest Liver Physiol 282: G424–G431, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Edelbroek M, Horowitz M, Maddox A, Bellen J. Gastric emptying and intragastric distribution of oil in the presence of a liquid or a solid meal. J Nucl Med 33: 1283–1290, 1992. [PubMed] [Google Scholar]
- 16.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315: 2284–2291, 2016. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French SJ, Murray B, Rumsey RD, Sepple CP, Read NW. Preliminary studies on the gastrointestinal responses to fatty meals in obese people. Int J Obes Relat Metab Disord 17: 295–300, 1993. [PubMed] [Google Scholar]
- 18.Grayson BE, Schneider KM, Woods SC, Seeley RJ. Improved rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. Sci Transl Med 5: 199ra112, 2013. doi: 10.1126/scitranslmed.3006505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenway F, Zheng J. Electrical stimulation as treatment for obesity and diabetes. J Diabetes Sci Technol 1: 251–259, 2007. doi: 10.1177/193229680700100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horowitz M, Collins PJ, Cook DJ, Harding PE, Shearman DJ. Abnormalities of gastric emptying in obese patients. Int J Obes 7: 415–421, 1983. [PubMed] [Google Scholar]
- 21.Hunt JN, Spurrell WR. The pattern of emptying of the human stomach. J Physiol 113: 157–168, 1951. doi: 10.1113/jphysiol.1951.sp004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology 127: 139–144, 2004. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Kelly KA. Gastric emptying of liquids and solids: roles of proximal and distal stomach. Am J Physiol Gastrointest Liver Physiol 239: G71–G76, 1980. [DOI] [PubMed] [Google Scholar]
- 24.Masuda T, Ohta M, Hirashita T, Kawano Y, Eguchi H, Yada K, Iwashita Y, Kitano S. A comparative study of gastric banding and sleeve gastrectomy in an obese diabetic rat model. Obes Surg 21: 1774–1780, 2011. doi: 10.1007/s11695-011-0512-0. [DOI] [PubMed] [Google Scholar]
- 25.Melissas J, Daskalakis M, Koukouraki S, Askoxylakis I, Metaxari M, Dimitriadis E, Stathaki M, Papadakis JA. Sleeve gastrectomy-a “food limiting” operation. Obes Surg 18: 1251–1256, 2008. doi: 10.1007/s11695-008-9634-4. [DOI] [PubMed] [Google Scholar]
- 26.Melissas J, Koukouraki S, Askoxylakis J, Stathaki M, Daskalakis M, Perisinakis K, Karkavitsas N. Sleeve gastrectomy: a restrictive procedure? Obes Surg 17: 57–62, 2007. doi: 10.1007/s11695-007-9006-5. [DOI] [PubMed] [Google Scholar]
- 27.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 26: 881–885, 2003. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 28.Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, Kohli R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 22: 390–400, 2014. doi: 10.1002/oby.20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, Standfield S, Rayner CK, Horowitz M. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring) 22: 2003–2009, 2014. doi: 10.1002/oby.20791. [DOI] [PubMed] [Google Scholar]
- 30.Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flüe M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg 250: 234–241, 2009. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 31.Pomerri F, Foletto M, Allegro G, Bernante P, Prevedello L, Muzzio PC. Laparoscopic sleeve gastrectomy–radiological assessment of fundus size and sleeve voiding. Obes Surg 21: 858–863, 2011. doi: 10.1007/s11695-010-0255-3. [DOI] [PubMed] [Google Scholar]
- 32.Robert A, Olafsson AS, Lancaster C, Zhang WR. Interleukin-1 is cytoprotective, antisecretory, stimulates PGE2 synthesis by the stomach, and retards gastric emptying. Life Sci 48: 123–134, 1991. doi: 10.1016/0024-3205(91)90405-Z. [DOI] [PubMed] [Google Scholar]
- 33.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509: 183–188, 2014. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA 294: 1909–1917, 2005. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki H, Nagulesparan M, Dubois A, Straus E, Samloff IM, Lawrence WH, Johnson GC, Sievers ML, Unger RH. Hypergastrinemia in obese noninsulin-dependent diabetes: a possible reflection of high prevalence of vagal dysfunction. J Clin Endocrinol Metab 56: 744–750, 1983. doi: 10.1210/jcem-56-4-744. [DOI] [PubMed] [Google Scholar]
- 36.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366: 1567–1576, 2012. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schurizek BA. The effects of general anaesthesia on antroduodenal motility, gastric pH and gastric emptying in man. Dan Med Bull 38: 347–365, 1991. [PubMed] [Google Scholar]
- 38.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H, Swedish Obese Subjects Study Scientific Group . Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351: 2683–2693, 2004. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 39.Stefater MA, Pérez-Tilve D, Chambers AP, Wilson-Pérez HE, Sandoval DA, Berger J, Toure M, Tschöp M, Woods SC, Seeley RJ. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 138: 2426–2436.e3, 2010. doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefater MA, Sandoval DA, Chambers AP, Wilson-Pérez HE, Hofmann SM, Jandacek R, Tso P, Woods SC, Seeley RJ. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology 141: 939–949.e4, 2011. doi: 10.1053/j.gastro.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verdich C, Lysgård Madsen J, Toubro S, Buemann B, Holst JJ, Astrup A. Effect of obesity and major weight reduction on gastric emptying. Int J Obes Relat Metab Disord 24: 899–905, 2000. doi: 10.1038/sj.ijo.0801250. [DOI] [PubMed] [Google Scholar]
- 42.Wilson-Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Pérez-Tilve D, Seeley RJ. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes 62: 2380–2385, 2013. doi: 10.2337/db12-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson-Pérez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, Seeley RJ. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes 37: 288–295, 2013. doi: 10.1038/ijo.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright RA, Krinsky S, Fleeman C, Trujillo J, Teague E. Gastric emptying and obesity. Gastroenterology 84: 747–751, 1983. [PubMed] [Google Scholar]