Abstract
Secreted Frizzled-related protein 2 (sFRP2) plays a key role in chronic fibrosis after myocardial infarction and in heart failure. The aim of this study was to elucidate the mechanisms through which sFRP2 may regulate the growth and extracellular matrix (ECM) remodeling of adult mouse cardiac fibroblasts (CFs). We found that sFRP2 activates CFs in part through canonical Wnt/β-catenin signaling, as evidenced by increased expression of Axin2 and Wnt3a, but not Wnt5a, as well as accumulation of nuclear β-catenin. In response to sFRP2, CFs exhibited robust cell proliferation associated with increased glucose consumption and lactate production, a phenomenon termed “the Warburg effect” in oncology. The coupling between CF expansion and anaerobic glycolysis is marked by upregulation of glyceraldehyde-3-phosphate dehydrogenase and tissue-nonspecific alkaline phosphatase. In conjunction with these phenotypic changes, CFs accelerated ECM remodeling through upregulation of expression of the matrix metalloproteinase (MMP) 1 and MMP13 genes, two members of the collagenase subfamily, and enzyme activities of MMP2 and MMP9, two members of the gelatinase subfamily. Consistent with the induction of multiple MMPs possessing collagenolytic activities, the steady-state level of collagen type 1 in CF-spent medium was reduced by sFRP2. Analysis of non-CF cell types revealed that the multifaceted effects of sFRP2 on growth control, glucose metabolism, and ECM regulation are largely restricted to CFs and highly sensitive to Wnt signaling perturbation. The study provides a molecular framework on which the functional versatility and signaling complexity of sFRP2 in cardiac fibrosis may be better defined.
Keywords: secreted Frizzled-related protein 2, cardiac fibroblast, extracellular matrix, matrix metalloproteinase, Wnt
cardiac fibroblasts (CFs) maintain the structure and function of the myocardium, in part through coordinated synthesis, deposition, and breakdown of extracellular matrix (ECM) components. While in a normal heart, fibroblasts are quiescent and primarily involved in routine maintenance of the ECM, they become activated by myocardial injury and expand significantly, triggering excessive ECM remodeling and fibrosis. Tissue fibrosis is also a natural sequel to chronic inflammation in response to persistent tissue damage. ECM thickening distorts organ architecture, interferes with cell-cell communication, impairs paracrine signaling, and, ultimately, leads to organ failure. It is estimated that ≥40% of mortality in the Western world is attributable to diseases associated with chronic fibrosis (55).
Chemokines and cytokines are critically involved in the inflammatory and subsequent fibrogenic cascades. While these soluble mediators participate in recruiting immune cells, fibrocytes, and various progenitor cells to the site of injury, they also directly modulate the phenotype and activity of resident CFs (10). Monocyte chemoattractant protein-1/CCL2 stimulates fibroblast collagen expression and upregulates the potent profibrotic cytokine transforming growth factor (TGF)-β1, which possesses robust activities in fibroblast recruitment, myofibroblast differentiation, and ECM deposition (38). TGFβ1 also promotes ECM deposition by inducing expression of ECM genes and suppressing expression of matrix metalloproteinase (MMP) genes (27). Monocyte chemoattractant protein-1 null mice exhibit reduced expression of many inflammatory cytokines and TGFβ1, along with diminished CF accumulation and attenuated left ventricular remodeling following myocardial infarction (9). Indeed, TGFβ1 inhibition in various animal models was found to reduce cardiac, hepatic, and renal fibrosis (14, 39, 48), and various strategies have been used to suppress the myocardial TGFβ1 secretome (54, 56). However, not all fibrosis is mediated by TGFβ1, because several independent fibrotic mechanisms have been described (21, 32). We previously documented an antifibrotic therapy based on blockade of secreted Frizzled (Fz)-related protein 2 (sFRP2) (37). Injections of sFRP2 antibody coordinately reduced myocardial fibrosis and improved cardiac function in the failing hamster heart. The cardiac therapeutic benefits mediated by sFRP2 inhibition are similar to those achieved by injections of mesenchymal stem cells (36, 43) or vascular endothelial growth factor (58, 59).
Excessive activation of Wnt signaling is associated with cardiac, pulmonary, renal, and skeletal muscle fibrosis (2, 6, 15, 53) and is involved in TGFβ1-mediated fibrosis (1). sFRP2 contains an NH2-terminal cysteine-rich domain (CRD) homologous to Fz receptor CRDs and can modulate Wnt signaling by binding to Wnt ligands and Fz receptors (34). sFRP2 has traditionally been viewed as a Wnt inhibitor, in that it competes with Fz receptors for Wnt binding. However, its Wnt-activating activity has been independently demonstrated in different organ systems (26, 37, 52), and we have observed sFRP2 blockade-mediated downregulation of myocardial Wnt signaling in the sFRP2 antibody therapy (37). These controversial findings indicate that sFRP2-mediated Wnt signaling is profoundly influenced by the cellular context. Since the regulatory pathways governing fibroblast behavior have emerged as significant points of inquiry for the development of antifibrotic strategies and much of what exists in the literature regarding fibroblast signaling mechanisms is derived from noncardiac fibroblasts, we initiated this study to determine the mechanisms through which the growth phenotype and ECM remodeling function of CFs may be regulated by sFRP2.
METHODS AND MATERIALS
Cell culture.
Cardiac and hepatic fibroblasts were prepared from young C57BL mice according to our previously established protocol (25). All animal procedures and protocols conformed to guidelines for the care and use of animals in research of the University at Buffalo. Fibroblasts and C2C12 myoblasts were maintained in advanced DMEM containing 5% fetal bovine serum, 50 μg/ml gentamicin, and 0.125 μg/ml amphotericin B (Fungizone). Fibroblasts receiving less than two trypsin passages were used for experiments. Typically 2–4 × 104 cells per well were plated on 24-well plates and allowed to reach ∼30% confluency (for proliferation assay only) or ∼90% confluency before initiation of experiments. Cells were plated on 35-mm dishes for experiments involving isolation of RNA and nuclear proteins. Our earlier experiments were based on treatment of cells with 3 nM recombinant sFRP2 protein, as documented elsewhere (35). More recently, we have adopted DNA transfection for sFRP2 treatment, because more consistent and robust results were obtained with the DNA transfection method. The compounds BIO and IWR-1 (Sigma) were prepared in DMSO for cell treatment.
Construction of sFRP2 expression vector and DNA transfection.
The CMV-based expression vector pcDNA3 was used to express sFRP2 in cultured cells. The mouse sFRP2 cDNA was amplified from C2C12 myoblast RNA using the cloning primers GTCGCTAGTCCACGATGCCG and CTAGCATTGCAGCTTGCGGA. The cDNA was first cloned into pCR2.1-TOPO and sequenced by the Roswell Park Cancer Institute DNA sequencing core facility. The sFRP2 sequence was then inserted into KpnI and NotI sites of pcDNA3, generating the expression vector pCMV-sFRP2. DNA transfection was performed using a DNA transfection kit [catalog no. N-104, University at Buffalo Biomedical Research Service Center (BRSC)]. Cells in each well were incubated in 0.3 ml of serum-free advanced DMEM containing 1 μg of pcDNA3 (as control) or pCMV-sFRP2 plasmid DNA prepared in 75 μl of calcium phosphate solution for 6 h, washed twice, and refed the growth medium. Cells and spent medium were harvested 2−3 days after transfection.
Quantitative PCR.
RNA was extracted using RNA isolation kits (Qiagen). After reverse transcription, quantitative PCR (qPCR) was performed to analyze gene expression using the SYBR Green kit on MyIQ (Bio-Rad), as previously described (30). β2-Microglobulin was used as the reference gene for data calculation based on the cycle threshold (2ΔΔCT) method. Primer sequences used for qPCR are listed in Table 1. Oligonucleotides were synthesized by Midland Oligo.
Table 1.
Oligonucleotides for quantitative PCR
| Primer Sequence |
||
|---|---|---|
| Genes | 5′ | 3′ |
| β2-Microglobulin | AGAATGGGAAGCCGAACATA | CCGTTCTTCAGCATTTGGAT |
| Col1A1 | GAGCGGAGAGTACTGGATCG | GTTCGGGCTGATGTACCAGT |
| Col1A2 | CGAGACCCTTCTCACTCCTG | GCATCCTTGGTTAGGGTCAA |
| MMP1a | GTGCTCTCCTTCCACAGAGG | GGTCCACGTCTCATCAAGGT |
| MMP2 | TGGTTTCCCTAAGCTCATCG | TTGGTTCTCCAGCTTCAGGT |
| MMP3 | TGGAGATGCTCACTTTGACG | GAGCAGCAACCAGGAATAGG |
| MMP8 | CAAGCAATCAATTCCGGTCT | AGCGCTGCATCTCTTTAAGC |
| MMP9 | CAAATTCTTCTGGCGTGTGA | GTCCACCTGGTTCACCTCAT |
| MMP13 | CAACCCTAAGCATCCCAAAA | TCCTCGGAGACTGGTAATGG |
| MMP14 | AGTCAGGGTCACCCACAAAG | TTTGGGCTTATCTGGGACAG |
| MMP15 | CAGGAAAGGCATGGAACAAT | GTACCAGCCCAGCTTCTCAG |
| Wnt3a | GGGACCCCAGTACTCCTCTC | GGGCATGATCTCCACGTAGT |
| Wnt5a | TGCCACTTGTATCAGGACCA | CTGTGCTGCAGTTCCATCTC |
| Axin2 | GGTCCAGTCCACCAAACCTA | CCATCTTCATTCCGCCTAGC |
Preparation of nuclear proteins.
All buffers were supplemented with a 1× cocktail of protease inhibitors (catalog no. P8340, Sigma). Cells isolated from 35-mm dishes were triturated in 0.3 ml of ice-cold buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EGTA, 0.1 mM EDTA, 1.5 mM MgCl2, 10 mM NaF, and 1 mM Na3VO4) with gentle agitation on ice for 30 min. Samples were centrifuged at 2,700 g for 10 min at 4°C. Pellets were resuspended in 0.1 ml of buffer A supplemented with 0.7% Nonidet P-40 and incubated on ice for 10 min. After centrifugation at 2,700 g for 10 min, the pellets were resuspended in 50 μl of buffer B (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EGTA, 0.1 mM EDTA, 1.5 mM MgCl2, 10 mM NaF, 1 mM Na3VO4, and 20% glycerol) and incubated on ice for 1 h. Samples were finally centrifuged at 23,000 g for 10 min at 4°C, and supernatants were collected as nuclear protein extracts.
Western blotting.
Cellular protein extracts and conditioned media were prepared in SDS-PAGE sample buffer, treated with 1% β-mercaptoethanol, and boiled for 10 min. Proteins were fractionated by 8% SDS-PAGE for detection of β-catenin and collagen and by 12% SDS-PAGE for detection of smooth muscle actin (SMA). Proteins were electrotransferred to an Immobilon-P membrane, which was incubated with optimally diluted primary antibody overnight at 4°C. Washed membrane was probed with a horseradish peroxidase-conjugated secondary antibody. Signals were developed using the SuperSignal chemiluminescence substrate (Pierce Biotechnology) and digitally imaged. Protein bands were quantified by densitometry. Antibodies used for Western blotting are as follows: MMP2 (catalog no. AF1488, R & D Systems), MMP9 (catalog no. AF909, R & D Systems), MMP13 (catalog no. sc-30073, Santa Cruz Biotechnology), β-catenin (catalog no. 9582, Cell Signaling Technology), collagen type 1 (catalog no. sc-28657, Santa Cruz Biotechnology), SMA (catalog no. ab5694, Abcam), and YY1 (19).
Enzyme assays.
Activities of tissue-nonspecific alkaline phosphatase (TNAP) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were determined by colorimetric TNAP and GAPDH assay kits (catalog nos. E-101 and E-102, BRSC), as documented previously (35). Typically, 2−5 μg of protein extracts were used for the enzyme assays. Assay of TNAP activity is based on conversion of p-nitrophenol phosphate to nitrophenol in an alkaline buffer. TNAP activity was measured at 414 nm and calculated using a molar extinction coefficient of 18.75 mM−1·cm−1. Assay of GAPDH activity is based on reduction of the tetrazolium salt INT in a NADH-coupled enzymatic reaction to formazan, which exhibits an absorption maximum at 492 nm. GAPDH activity was calculated using a molar extinction coefficient of 18 mM−1·cm−1. Since TNAP is an ectoenzyme, TNAP activity was measured in situ in 24-well plates in several experiments. TNAP assay solution (0.25 ml) was added to each well after cells were rinsed briefly with normal saline. Reaction was stopped by addition of 50 μl of 1 N NaOH before absorbance measurement.
Gelatin zymography.
ProMMP and mature MMP forms in harvested conditioned media were initially identified by treatment with 1 mM 4-aminophenylmercuric acetate at 37°C for 1 h followed by gelatin zymography to locate their respective gel positions, as described elsewhere (50). For gelatin zymography, four parts of each medium sample were mixed with one part of 5× sample buffer (0.25 M Tris·Cl, pH 6.8, 10% SDS, 25% glycerol, and 0.25% bromophenol blue) and fractionated by 10% SDS-PAGE. The resolving gel contained 1 mg/ml porcine skin gelatin type A (Sigma). Upon completion of electrophoresis, the gel was rinsed in a wash buffer (50 mM Tris·Cl, pH 8, 200 mM NaCl, 5 mM CaCl2, 5 μM ZnSO4 and 2.5% Triton X-100) four times for 15 min each for enzyme renaturation and then incubated with gentle agitation in a reaction buffer (50 mM Tris·Cl, pH 8, 200 mM NaCl, 5 mM CaCl2, and 5 μM ZnSO4) at 37°C for ∼20 h. MMP activity bands were revealed by staining the gel with a 3% Coomassie Blue G250 solution prepared in 50% methanol and 10% acetic acid and quantified by densitometry.
Metabolite assays.
l-Lactate, glucose, and inorganic phosphate (Pi) were assayed using kits (catalog nos. A-108, A-114, and A-110, BRSC) that are based on lactate dehydrogenase reaction, coupled hexokinase and glucose-6-phosphate dehydrogenase reaction, and ammonium molybdate-based reaction, respectively. Conditioned media were first deproteinized by precipitation with 25% polyethylene glycol-8000 on ice for 30 min. Supernatants were harvested and diluted 10- to 40-fold with distilled H2O to obtain assay linearity. Twenty microliters of each sample, along with a set of standards, were mixed with 50 or 100 μl of the respective assay solution in 96-well plates. Lactate and glucose assays were incubated at 37°C for 30 min. Phosphate assay was incubated at room temperature for 10 min. Absorbance was measured at 492 nm for lactate and glucose assays and 620 nm for phosphate assay. Sample metabolite concentrations were derived by comparison with a standard curve performed at the same time.
Statistical analysis.
Two and multiple experimental groups were compared using Student's t-test and one-way ANOVA with post hoc Tukey's HSD test, respectively. P < 0.05 is considered significant. Data are representative of at least two independent experiments and expressed as means ± SD (n = 3).
RESULTS
sFRP2-driven CF proliferation and anaerobic glycolysis.
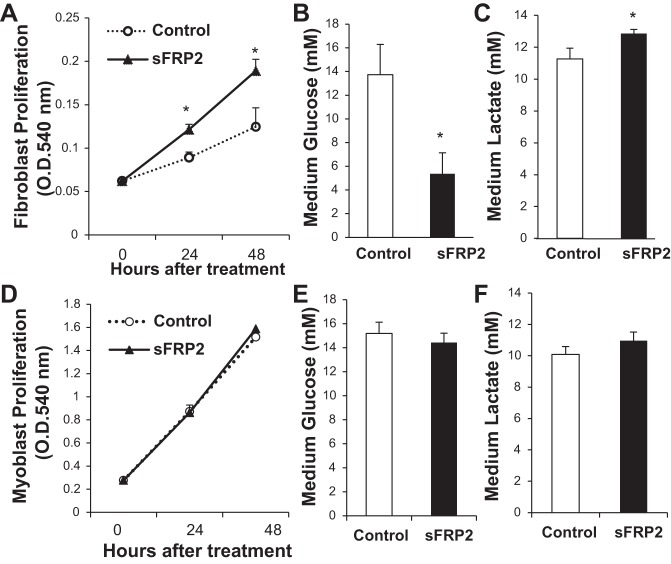
Excessive expansion of CFs is a prominent pathology in heart failure. We performed MTT assay to determine whether sFRP2 might have a mitogenic effect on adult mouse CFs. Figure 1A indicates that CFs treated with sFRP2 proliferated significantly faster than control CFs. This cell growth-promoting effect of sFRP2 was, however, not observed in C2C12 myoblasts (Fig. 1D) and several other cell types, including liver fibroblasts, 3T3 fibroblasts, HeLa cells, and HEK-293 cells (data not shown). Increased CF proliferation is associated with significantly increased glucose consumption (Fig. 1B) and lactate production (Fig. 1C), which, again, are not observed in C2C12 myoblasts (Fig. 1, E and F). These results demonstrate that sFRP2 coordinates CF proliferation and anaerobic glycolysis through “the Warburg effect,” which occurs even when oxygen is plentiful (40). Western blot analysis of SMA, a marker of myofibroblasts, did not reveal a change after sFRP2 treatment (data not shown).
Fig. 1.
Cardiac fibroblast (CF) proliferation and anaerobic glycolysis are enhanced by secreted Frizzled-related protein 2 (sFRP2). CFs (A–C) and C2C12 myoblasts (D–F) were transfected with pcDNA3 (Control) and pCMV-sFRP2 vector DNA at 0 h. A and D: cell proliferation measured at 24 and 48 h by MTT assay. OD540, optical density at 540 nm. *P < 0.05 vs. Control. B, C, E, and F: glucose and lactate concentrations in conditioned medium harvested at 48 h. *P < 0.05 vs. Control.
Induction of an acidic and phosphate-rich ECM environment.
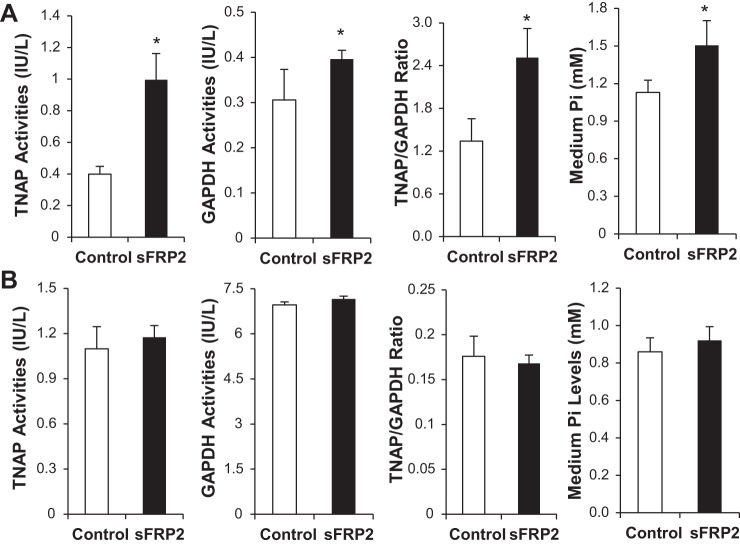
We recently demonstrated that TNAP is a downstream target of sFRP2 implicated in ECM fibrocalcification (35). Proliferating CFs treated with sFRP2 exhibited prominently increased activity of TNAP (Fig. 2A). We found that the CFs also exhibited significantly increased activity of GAPDH (Fig. 2A), a housekeeping glycolytic enzyme, which is consistent with the increased lactate output shown in Fig. 1. Cell specificity is again a feature in the enzyme regulation, because neither TNAP nor GAPDH was activated by sFRP2 in C2C12 myoblasts (Fig. 2B). Since TNAP is an ectoenzyme possessing a phosphate-splitting activity, we measured the concentration of Pi in conditioned media and found that only the sFRP2-treated CFs significantly increased the level of extracellular Pi (Fig. 2A). Thus, CFs under the influence of sFRP2 could promote an acidic and phosphate-rich ECM milieu.
Fig. 2.
Coordinated activation of tissue-nonspecific alkaline phosphatase (TNAP) and GAPDH by sFRP2 in CFs. Cell lysates and conditioned medium were harvested from CFs (A) and C2C12 myoblasts (B) 3 days after DNA transfection. Cell lysates were used for assays of TNAP and GAPDH enzyme activities. Levels of Pi in conditioned medium were also measured. *P < 0.05 vs. Control.
Increased expression of MMP1a and MMP13.
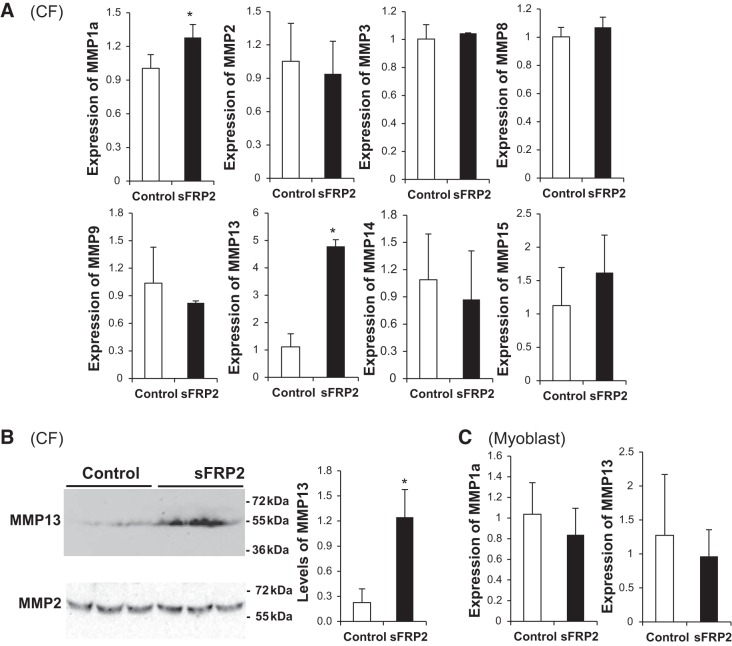
MMPs are major players in ECM remodeling and are upregulated during chronic heart failure and following myocardial infarction (44, 46). Whether sFRP2 may remodel the ECM through regulation of selective members of the MMP gene family has not been examined. We analyzed expression of the following MMP genes by qPCR: MMP1, MMP8, and MMP13 from the collagenase subfamily, MMP2 and MMP9 from the gelatinase subfamily, MMP3 from the stromelysin subfamily, and MMP14 and MMP15 from the membrane-type subfamily (33). sFRP2 significantly activated expression of MMP1a and MMP13 in CFs without an appreciable effect on the other MMP genes (Fig. 3A). In particular, expression of MMP13 was prominently increased (∼5-fold) by sFRP2, prompting us to confirm this change at the protein level by Western blotting. Figure 3B reveals a similar increase in MMP13 protein after sFRP2 treatment, which also confirms that neither MMP2 mRNA nor MMP2 protein abundance is affected by sFRP2. None of the MMP genes was affected by sFRP2 in C2C12 myoblasts (only MMP1a and MMP13 are shown in Fig. 3C). These data indicate that sFRP2 is a potent inducer of collagenase in CFs.
Fig. 3.
sFRP2 stimulates expression of matrix metalloproteinase (MMP) 1a and MMP13 in CFs. CFs (A and B) and C2C12 myoblasts (C) were transfected with pcDNA3 (Control) and pCMV-sFRP2 vector DNA. In A and C, total RNA was harvested from cells after 3 days and processed for quantitative PCR analysis of MMP gene expression. *P < 0.05 vs. Control. Only MMP1a and MMP13 are shown for C2C12 myoblasts. In B, conditioned media were analyzed by Western blotting using MMP13 and MMP2 antibodies. MMP protein abundance was quantified by densitometry, and MMP13 protein levels are presented as MMP13-to-MMP2 ratio.
Increased gelatinolytic activities of MMP2 and MMP9.
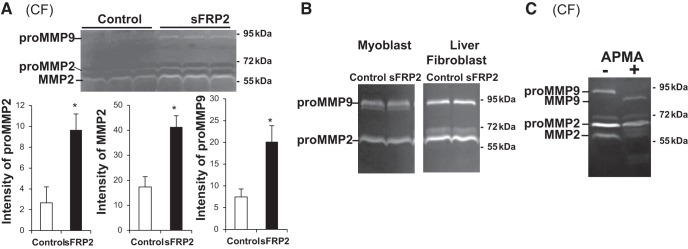
sFRP2 has been shown to possess a posttranslational regulatory function, by virtue of its enhancement of the procollagen C-proteinase activity of Tolloid-like metalloproteinases, which convert procollagen to collagen (16). We investigated whether sFRP2 might also have a posttranslational regulatory function targeting MMPs. Gelatin zymography shows that sFRP2 significantly enhanced the activities of MMP2 and MMP9 in CFs (Fig. 4A), but not in myoblasts or liver fibroblasts (Fig. 4B). Since proMMPs and mature MMPs are activated in the gelatin degradation assay, we used 4-aminophenylmercuric acetate, an organomercurial capable of converting proMMPs to mature MMPs in vitro, to identify the corresponding MMP bands on the gel (Fig. 4C). The results reveal that sFRP2 enhanced the signal intensity of proMMP2, mature MMP2, and proMMP9, but not mature MMP8, in CFs. Given that transcription of the MMP2 and MMP9 genes is not altered by sFRP2, nor is protein synthesis (Fig. 3), the results uncover a novel posttranslational regulatory function of sFRP2 targeting the enzyme activities of MMP2 and MMP9 in CFs.
Fig. 4.
sFRP2 enhances enzyme activities of MMP2 and MMP9 in CFs. Conditioned media were harvested from CFs (A and C), as well as C2C12 myoblasts and liver fibroblasts (B), 3 days after DNA transfection. Intensity of proMMP and mature MMP forms was measured by gelatin zymography (20 μl of medium loaded per lane) followed by densitometry. Quantification is presented for CF media in A. *P < 0.05 vs. Control. In C, sFRP2-treated conditioned medium was incubated with 1 mM 4-aminophenylmercuric acetate (APMA) for 1 h prior to gelatin zymography. Respective gel positions of proMMP and active MMP forms are indicated.
Upregulation of MMPs and downregulation of collagen.
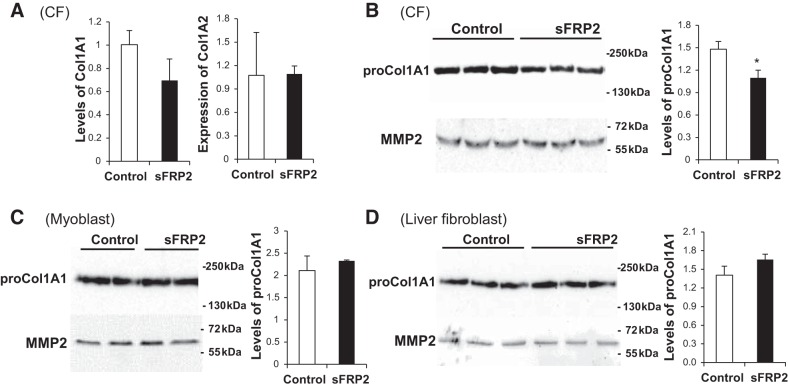
MMP1 and MMP13, also known as collagenase 1 and collagenase 3, respectively, possess robust collagenolytic activities. MMP2 and MMP9, also known as gelatinase A and gelatinase B, respectively, are also capable of breaking down collagen (33). All four collagen-degrading enzymes were upregulated by sFRP2 in CFs, albeit via different mechanisms, as shown above. We therefore predicted that the steady-state level of collagen in conditioned medium would be reduced by sFRP2. This was indeed confirmed by Western blotting (Fig. 5B). qPCR analysis reveals that expression of the collagen 1A1 and 1A2 genes was unaffected by sFRP2 (Fig. 5A), suggesting that decreased collagen in Fig. 5B is not caused by downregulation of gene expression. Cell specificity is again a feature in the ECM regulation mode, because the abundance of collagen was not affected by sFRP2 in myoblasts (Fig. 5C) or liver fibroblasts (Fig. 5D).
Fig. 5.
Downregulation of collagen type 1 by sFRP2 in CF conditioned medium. A: total RNA was harvested from CFs after 3 days and processed for quantitative PCR analysis of expression of Col1A1 and Col1A2 genes. B–D: conditioned media harvested from CFs, C2C12 myoblasts, and liver fibroblasts 3 days after DNA transfection were processed for Western blot analysis of procollagen 1 (30 μl of medium loaded per lane). Densitometric quantification of proCol1A1 intensity is presented as collagen-to-MMP2 ratio. *P < 0.05 vs. Control.
sFRP2 activates canonical Wnt signaling in CFs.
Our previous sFRP2 antibody therapy for hamster heart failure suggested a Wnt-activating function for sFRP2 in vivo (37). Having identified the multifaceted effects of sFRP2 on CFs, i.e., cell proliferation, anaerobic glycolysis, and ECM regulation, we sought to determine whether these regulatory roles of sFRP2 might intersect the Wnt signaling pathway. qPCR analysis shows that sFRP2 activates expression of the Wnt3a and Axin2 genes (Fig. 6A), indicating activation of canonical Wnt signaling, which was not observed in myoblasts (Fig. 6C). Expression of Wnt5a, a noncanonical Wnt, was unaffected by sFRP2 (Fig. 6A). To further corroborate this finding, we performed Western blot analysis of β-catenin, which reveals increased accumulation of nuclear β-catenin in sFRP2-treated CFs (Fig. 6B). These findings indicate that sFRP2 functions as an activator of canonical Wnt signaling in isolated CFs.
Fig. 6.
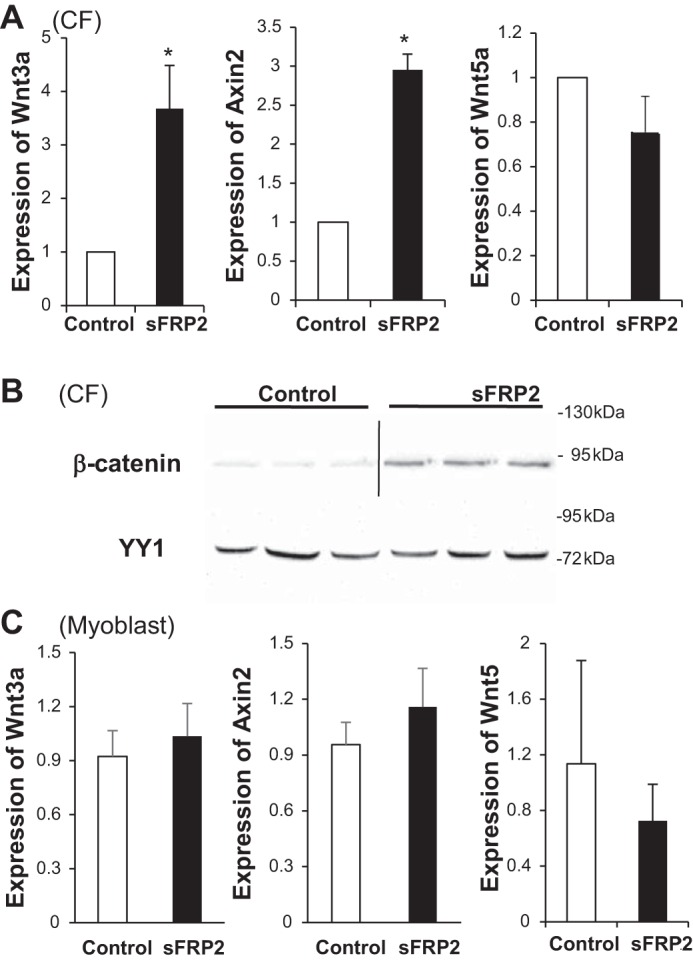
sFRP2 activates CF Wnt/β-catenin signaling. Total RNA was isolated from CFs (A) and C2C12 myoblasts (C) 3 days after DNA transfection. Expression of Wnt3a, Axin2, and Wnt5a was analyzed by quantitative PCR. *P < 0.05 vs. Control. In B, nuclear proteins were isolated from CFs and processed for Western blot analysis of β-catenin and YY1. β-Catenin blot is derived from 2 different gels, and the same samples were run again for confirmation and probed with the YYI antibody.
sFRP2-mediated CF proliferation and anaerobic glycolysis are sensitive to Wnt signaling perturbation.
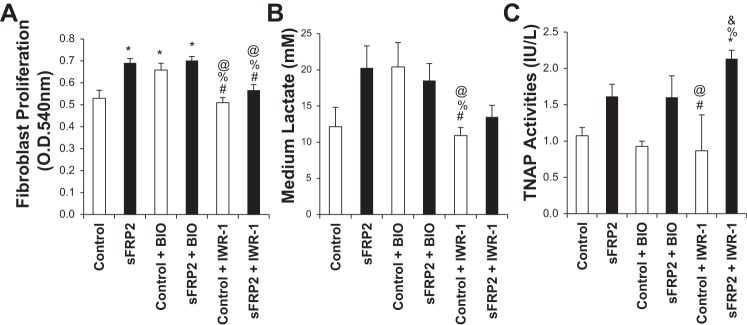
Having demonstrated sFRP2 as an activator of Wnt signaling, we further determined whether Wnt signaling might mediate the multiple CF-specific effects of sFRP2. We used two small-molecule modulators of the Wnt/β-catenin pathway (5, 41): BIO, a cell-permeable indirubin compound that acts as a highly selective ATP-competitive inhibitor of glycogen synthase kinase 3α/β, and IWR-1, which enhances proteolytic degradation of β-catenin by the proteasome through stabilization of the destruction complex member Axin 2. We found that BIO alone was sufficient to stimulate CF proliferation as observed with sFRP2 alone (Fig. 7A), and addition of BIO with sFRP2 did not further enhance CF proliferation. This Wnt-dependent effect on CF proliferation was further confirmed by the finding that IWR-1 obliterated the growth-stimulatory effect of sFRP2 without affecting the basal growth rate (Fig. 7A). A similar drug effect profile was obtained with lactate assay (Fig. 7B), indicating that cell proliferation and anaerobic glycolysis are coordinated by sFRP2-Wnt signaling. However, TNAP regulation appears more complex, because while BIO alone failed to promote TNAP activity, antagonism of Wnt/β-catenin signaling by IWR-1 treatment further enhanced the TNAP-activating effect of sFRP2 (Fig. 7C), suggesting that sFRP2 could also regulate TNAP function independent of the canonical Wnt signaling pathway.
Fig. 7.
Differential regulation of CF proliferation, glycolysis, and TNAP by sFRP2-Wnt signaling. CFs were treated with BIO (5 μM), IWR-1 (3 μM), or DMSO (Control) upon completion of the 6-h transfection protocol. MTT assay (A) and in situ TNAP assay (C) were performed 3 days after transfection. Conditioned medium was collected and analyzed by lactate assay (B). One-way ANOVA with post hoc Tukey's HSD test was used for multiple-sample comparisons. *P < 0.05 vs. Control; #P < 0.05 vs. sFRP2; %P < 0.05 vs. Control + BIO; @P < 0.05 vs. sFRP2 + BIO; & P < 0.05 vs. Control + IWR-1.
Antagonism of Wnt/β-catenin signaling enhances sFRP2-induced MMP activity.
Although BIO-induced Wnt signaling promoted CF proliferation (Fig. 7A), it did not induce TNAP (Fig. 7C), nor did it alter the activities of MMPs (Fig. 8A). On the other hand, antagonism of Wnt/β-catenin signaling by IWR-1 further increased the activities of sFRP2-induced MMPs (Fig. 8, B and C), thus mimicking the stimulatory effect of IWR-1 on TNAP (Fig. 7C). Again, increased MMP activities are associated with a corresponding decrease in the steady-state level of collagen in conditioned medium (Fig. 8D), suggesting a functional effect of MMP induction in ECM regulation. Since IWR-1 apparently synergizes with sFRP2 in the activation of TNAP and MMP, which are critical ECM remodeling enzymes, we propose an ECM regulatory mechanism (see discussion). Taken together, our findings illustrate functional versatility and signaling complexity exhibited by sFRP2 in cardiac ECM regulation.
Fig. 8.
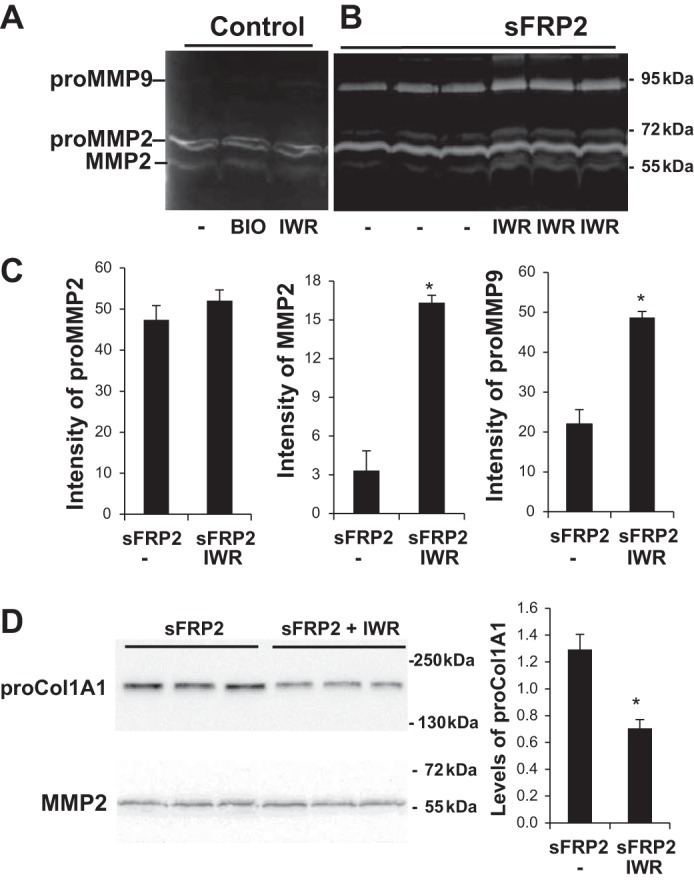
sFRP2-Wnt signaling regulation of MMPs and collagen turnover. CFs were treated with BIO (5 μM), IWR-1 (3 μM), or DMSO (Control) upon completion of the 6-h transfection protocol. Conditioned media were collected and analyzed by gelatin zymography (A and B). C: quantification of data from B. D: Western blotting of procollagen 1. MMP protein intensity was quantified for the sFRP2 treatment group and is presented as proCol1A1-to-MMP2 ratio. *P < 0.05 vs. Control.
DISCUSSION
Wnt signaling is known to promote fibrosis in different organ systems (2, 6, 15, 53). Depending on the cellular context and composition of the seven-pass transmembrane Fz receptors and diverse coreceptors, various Wnt molecules can activate β-catenin-dependent and -independent pathways that are antagonized by several types of secreted factors such as Wif, Dkk, and sFRPs (3, 22). Although sFRP2 has traditionally been viewed as a Wnt inhibitor, its Wnt-activating function has been demonstrated (26, 52). Similar to our findings, in vivo and in vitro data were obtained to show that thyroid hormone-induced expression of sFRP2 stabilizes β-catenin, leading to induction of Wnt in the intestinal epithelium (45). A cross-talk regulation between sFRP and Wnt is evident, because sFRP2 expression in the aggregating mesenchyme, for example, is induced by Wnt4 (29), and both sFRP1 and sFPR2 can also regulate the activity of Wnt4 (57). However, qPCR analysis reveals that exogenously added sFRP2 does not affect expression of sFRP2 in cultured CFs, indicating that autoregulation does not appear to be a feature of sFRP2 gene regulation (data not shown). sFRP2 could presumably promote canonical Wnt signaling through interaction between the conserved CRDs. Since the current study demonstrates sFRP2-mediated gene induction of Wnt3a, but not Wnt5a, sFRP2 could augment β-catenin activities initiated by Wnt3a, mimicking the documented effect on Wnt16B (47).
Expression of sFRP2 is elevated after the onset of the myocardial fibrotic phase (4 days after coronary artery ligation) and remains significantly elevated thereafter (24). Increased myocardial fibrosis in the failing heart also correlates with elevated expression of sFRP2. Increased expression of sFRP2 during myocardial injury may serve to promote Wnt signaling, leading to CF expansion fueled by glucose metabolism during the early fibrotic phase. We were intrigued to find that the mitogenic activity of sFRP2 is coupled to the Warburg effect, which is often associated with tumor cell expansion relying on anaerobic glycolysis even when oxygen is abundant (40). Our data indicate that the sFRP2-induced Warburg effect is at least in part mediated by increased activity of GAPDH, a key housekeeping glycolytic enzyme. Wnt signaling has also been shown to regulate hepatic metabolism (31). It is unclear whether the previously recognized growth-promoting effect of sFRP2 on human malignant glioma cells may also involve the Warburg effect (42). However, cell specificity is likely a major determinant in this regulatory mode, because sFRP2 is also known to function as a tumor suppressor (7, 23).
sFRP2 is apparently highly versatile and functionally diverse in cellular regulation. In particular, sFRP2 appears to have a knack for regulation of extracellular proteases involved in ECM regulation. Kobayashi et al. identified sFRP2 as a direct enhancer of the procollagen C-proteinase activity of bone morphogenetic protein 1 (BMP1) (24). The proposed sFRP2-BMP1 axis promotes procollagen processing and collagen deposition in the ECM. However, this ECM-regulatory function of sFRP2 was not confirmed by von Marschall and Fisher (51), and thus additional study is needed to clarify the controversy. Esteve et al. demonstrated sFRP2 binding to ADAM10 metalloprotease and inhibition of its proteolytic activity (12). Since ADAM10 cleaves multiple substrates, including Notch, N-cadherin, and amyloid precursor protein, sFRP2 may be targeted for Alzheimer's disease and cancer treatment. Adding to these mechanisms is our present finding that sFRP2 can regulate the MMP network through transcriptional regulation of MMP1 and MMP13 and through posttranslational regulation of MMP2 and MMP9. Increased collagen deposition mediated by the sFRP2-BMP1 axis and increased collagen turnover mediated by the sFRP2-MMP axis would lead to accelerated ECM remodeling, which is a pathological hallmark in heart failure (44, 46).
The diseased heart exhibits many pathological phenotypes, including adverse myocardial remodeling characterized by myocyte hypertrophy and fibrosis, affecting muscle elasticity, contractile function, and flow reserve. The architectural alteration is mediated in part by diverse MMPs possessing different regulatory modes and capable of degrading a wide array of ECM components (4, 18, 46). MMPs also have significant activity against multiple nonmatrix protein substrates, thereby directly modulating cell growth and migration behaviors. We show here that sFRP2- and Wnt/β-catenin-mediated CF proliferation and glycolysis are coupled to increased expression of MMP1 and MMP13, two critical MMPs belonging to the collagenase family involved in the breakdown of fibrillar collagen. Functional relevance of this regulatory mechanism is revealed from our demonstration that the steady-state level of collagen is concurrently reduced. This finding is consistent with the demonstration that MMP13 is a downstream target of canonical Wnt signaling in human chondrocytes (11). MMP1 and MMP13 are frequently overexpressed under inflammatory conditions and are transcriptionally regulated by similar cytokines (13). MMP1 is expressed in ischemic brain and is further upregulated upon reperfusion, suggesting a role for MMP1 in ischemia-reperfusion injury (28). Skin fibroblasts isolated from MMP13 knockout mice display altered cell morphology and impaired maturation of granulation tissue, along with decreased production of MMP2 (49).
Notably, although expression of MMP2 and MMP9, two gelatinases also capable of breaking down collagen, is not transcriptionally activated by sFRP2 in CFs, enzyme activity was significantly enhanced by sFRP2, as shown by gelatin zymography. In search of a potential mechanism underlying this apparent posttranslational regulation mode, we found that human MMP2 can be phosphorylated at serine, threonine, and tyrosine residues. MMP2 phosphorylated by protein kinase C exhibits diminished activity, whereas its activity is enhanced by alkaline phosphatase treatment (20). Since TNAP is activated by sFRP2 in CFs, we speculate that the sFRP2-induced MMP2 and MMP9 activities are mediated in part through TNAP induction, which leads to dephosphorylation of the two MMPs, thus enhancing their activities. Our experiments based on IWR-1 treatment showing that the Wnt inhibitor coordinately enhanced the activities of TNAP and MMPs in concert with sFRP2 and further reduced the extracellular level of collagen provide corroboration for this regulatory mechanism. Although this posttranslational regulatory mechanism mediated by IWR-1 in induction of MMP activity remains to be characterized, we speculate that IWR-1 may exert a stabilizing effect on sFRP2 protein analogous to its stabilizing effect on Axin 2 protein. The postulated sFRP2-TNAP-MMP regulatory axis bears therapeutic significance, because MMP2 and MMP9 have been targeted for the treatment of ischemic brain and heart injuries (4, 18).
ECM expansion is a pathophysiological response of the failing heart, irrespective of its etiological origin. Specific targeting of fibrogenic signals thus represents an ideal reparative strategy. However, clinical trials of MMP inhibitors have generated disappointing results due to the use of broad-spectrum inhibitors (8, 17). Direct targeting of TGFβ1 for antifibrotic therapy is also problematic due to the involvement of the cytokine in many other processes. On the other hand, antibody blockade of sFRP2 (37) and genetic knockout of sFRP2 (24) have been found to reduce fibrosis and improve cardiac function in rodent models. The current study demonstrates that the action of sFRP2 exhibits a high degree of cell specificity, and thus sFRP2 targeting may represent a novel clinical therapeutic target.
GRANTS
Funding for the study was provided in part by National Heart, Lung, and Blood Institute Grant R01 HL-84590 and the University at Buffalo Biomedical Research Service Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.L., M.A., K.J.C., C.E., and A.R.R. performed the experiments; H.L., M.A., K.J.C., C.E., A.R.R., and T.L. analyzed the data; H.L., M.A., K.J.C., and T.L. interpreted the results of the experiments; H.L., M.A., and T.L. prepared the figures; M.A., K.J.C., and T.L. developed the concept and designed the research; T.L. drafted the manuscript; T.L. edited and revised the manuscript; T.L. approved the final version of the manuscript.
REFERENCES
- 1.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH. Activation of canonical Wnt signalling is required for TGFβ-mediated fibrosis. Nat Commun 3: 735, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci 119: 395–402, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi M, Kaczmarek L. Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 49: 563–573, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5: 100–107, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 162: 1495–1502, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung MT, Lai HC, Sytwu HK, Yan MD, Shih YL, Chang CC, Yu MH, Liu HS, Chu DW, Lin YW. SFRP1 and SFRP2 suppress the transformation and invasion abilities of cervical cancer cells through Wnt signal pathway. Gynecol Oncol 112: 646–653, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295: 2387–2392, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96: 881–889, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Dobaczewski M, Frangogiannis NG. Chemokines and cardiac fibrosis. Front Biosci 1: 391–405, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enochson L, Stenberg J, Brittberg M, Lindahl A. GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition. Osteoarthritis Cartilage 22: 566–577, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Esteve P, Sandonis A, Cardozo M, Malapeira J, Ibanez C, Crespo I, Marcos S, Gonzalez-Garcia S, Toribio ML, Arribas J, Shimono A, Guerrero I, Bovolenta P. SFRPs act as negative modulators of ADAM10 to regulate retinal neurogenesis. Nat Neurosci 14: 562–569, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Foley CJ, Kuliopulos A. Mouse m atrix metalloprotease-1a (Mmp1a) gives new insight into MMP function. J Cell Physiol 229: 1875–1880, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukasawa H, Yamamoto T, Suzuki H, Togawa A, Ohashi N, Fujigaki Y, Uchida C, Aoki M, Hosono M, Kitagawa M, Hishida A. Treatment with anti-TGFβ antibody ameliorates chronic progressive nephritis by inhibiting Smad/TGFβ signaling. Kidney Int 65: 63–74, 2004. [DOI] [PubMed] [Google Scholar]
- 15.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins DR, Keles S, Greenspan DS. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol 26: 508–523, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov 6: 480–498, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Hughes BG, Schulz R. Targeting MMP-2 to treat ischemic heart injury. Basic Res Cardiol 109: 424, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Kalenik JL, Chen D, Bradley ME, Chen SJ, Lee TC. Yeast two-hybrid cloning of a novel zinc finger protein that interacts with the multifunctional transcription factor YY1. Nucleic Acids Res 25: 843–849, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res 85: 413–423, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGFβ independent. J Immunol 173: 4020–4029, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116: 2627–2634, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Kilinc D, Ozdemir O, Ozdemir S, Korgali E, Koksal B, Uslu A, Gultekin YE. Alterations in promoter methylation status of tumor suppressor HIC1, SFRP2, and DAPK1 genes in prostate carcinomas. DNA Cell Biol 31: 826–832, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge G, Grieskamp T, Yamada C, Liu TC, Huang G, Basson CT, Kispert A, Greenspan DS, Sato TN. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol 11: 46–55, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kositprapa C, Zhang B, Berger S, Canty JM, Lee TC. Calpain-mediated proteolytic cleavage of troponin I induced by hypoxia or metabolic inhibition in cultured neonatal cardiomyocytes. Mol Cell Biochem 214: 47–55, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Kress E, Rezza A, Nadjar J, Samarut J, Plateroti M. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor-α1 and activates β-catenin signaling in mouse intestine. J Biol Chem 284: 1234–1241, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Leask A, Abraham DJ. TGFβ signaling and the fibrotic response. FASEB J 18: 816–827, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Lenglet S, Montecucco F, Mach F, Schaller K, Gasche Y, Copin JC. Analysis of the expression of nine secreted matrix metalloproteinases and their endogenous inhibitors in the brain of mice subjected to ischaemic stroke. Thromb Haemost 112: 363–378, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Lescher B, Haenig B, Kispert A. sFRP-2 is a target of the Wnt-4 signaling pathway in the developing metanephric kidney. Dev Dyn 213: 440–451, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Lin H, Shabbir A, Molnar M, Yang J, Marion S, Canty JM, Lee T. Adenoviral expression of vascular endothelial growth factor splice variants differentially regulate bone marrow-derived mesenchymal stem cells. J Cell Physiol 216: 458–468, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Fergusson MM, Wu JJ, Rovira II, Liu J, Gavrilova O, Lu T, Bao J, Han D, Sack MN, Finkel T. Wnt signaling regulates hepatic metabolism. Sci Signal 4: ra6, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-β-dependent and -independent pathways of induction of tubulointerstitial fibrosis in β6−/− mice. Am J Pathol 163: 1261–1273, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci 11: 1696–1701, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Malinauskas T, Jones EY. Extracellular modulators of Wnt signalling. Curr Opin Struct Biol 29: 77–84, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Martin S, Lin H, Ejimadu C, Lee T. Tissue-nonspecific alkaline phosphatase (TNAP) as a target of sFRP2 in cardiac fibroblasts. Am J Physiol Cell Physiol 309: C139–C147, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mastri M, Shah Z, McLaughlin T, Greene CJ, Baum L, Suzuki G, Lee T. Activation of Toll-like receptor 3 (TLR3) amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency. Am J Physiol Cell Physiol 303: C1021–C1033, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mastri M, Shah Z, Wang W, Hsieh K, Wooldridge B, Suzuki G, Lee T. Secreted Frizzled-related protein 2 (sFRP2) as a target in anti-fibrotic intervention. Am J Physiol Cell Physiol 306: C531–C539, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng XM, Nikolic-Paterson DJ, Lan HY. TGFβ: the master regulator of fibrosis. Nat Rev Nephrol 12: 325–338, 2016. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor-β prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology 32: 247–255, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Otto AM. Warburg effect(s)—a biographical sketch of Otto Warburg and his impacts on tumor metabolism. Cancer Metab 4: 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polychronopoulos P, Magiatis P, Skaltsounis AL, Myrianthopoulos V, Mikros E, Tarricone A, Musacchio A, Roe SM, Pearl L, Leost M, Greengard P, Meijer L. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem 47: 935–946, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Roth W, Wild-Bode C, Platten M, Grimmel C, Melkonyan HS, Dichgans J, Weller M. Secreted Frizzled-related proteins inhibit motility and promote growth of human malignant glioma cells. Oncogene 19: 4210–4220, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Shabbir A, Zisa D, Lin H, Mastri M, Roloff G, Suzuki G, Lee T. Activation of host tissue trophic factors through JAK/STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am J Physiol Heart Circ Physiol 299: H1428–H1438, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a non-invasive therapeutic regimen. Am J Physiol Heart Circ Physiol 296: H1888–H1897, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skah S, Nadjar J, Sirakov M, Plateroti M. The secreted Frizzled-related protein 2 modulates cell fate and the Wnt pathway in the murine intestinal epithelium. Exp Cell Res 330: 56–65, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87: 1285–1342, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Sun Y, Zhu D, Chen F, Qian M, Wei H, Chen W, Xu J. SFRP2 augments WNT16B signaling to promote therapeutic resistance in the damaged tumor microenvironment. Oncogene 35: 4321–4334, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, Seidman JG. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgfβ. J Clin Invest 120: 3520–3529, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toriseva M, Laato M, Carpen O, Ruohonen ST, Savontaus E, Inada M, Krane SM, Kahari VM. MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability. PLos One 7: e42596, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toth M, Sohail A, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Biol 878: 121–135, 2012. [DOI] [PubMed] [Google Scholar]
- 51.von Marschall Z, Fisher LW. Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix products by three isoforms of bone morphogenetic protein-1 (BMP1). Matrix Biol 29: 295–303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Marschall Z, Fisher LW. Secreted Frizzled-related protein-2 (sFRP2) augments canonical Wnt3a-induced signaling. Biochem Biophys Res Commun 400: 299–304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs. III. Intercalated disc remodeling. J Mol Cell Cardiol 31: 333–343, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 10: 15–26, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Wynn TA. Fibrotic disease and the TH1/TH2 paradigm. Nat Rev Immunol 4: 583–594, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshino K, Rubin JS, Higinbotham KG, Uren A, Anest V, Plisov SY, Perantoni AO. Secreted Frizzled-related proteins can regulate metanephric development. Mech Dev 102: 45–55, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Zisa D, Shabbir A, Mastri M, Suzuki G, Lee T. Intramuscular VEGF repairs the failing heart: role of host-derived growth factors and mobilization of progenitor cells. Am J Physiol Regul Integr Comp Physiol 297: R1503–R1515, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zisa D, Shabbir A, Mastri M, Taylor T, Aleksic I, McDaniel M, Suzuki G, Lee T. Intramuscular VEGF activates an SDF1-dependent progenitor cell cascade and an SDF1-independent muscle paracrine cascade for cardiac repair. Am J Physiol Heart Circ Physiol 301: H2422–H2432, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]