Abstract
Anthrax vaccine adsorbed (AVA, BioThrax) was recently approved by the Food and Drug Administration (FDA) for a post-exposure prophylaxis (PEP) indication in adults 18–65 years of age. The schedule is three doses administered subcutaneous (SC) at 2-week intervals (0, 2, and 4 weeks), in conjunction with a 60-day course of antimicrobials. The Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) developed an animal model to support assessment of a shortened antimicrobial PEP duration following Bacillus anthracis exposure. A nonhuman primate (NHP) study was completed to evaluate the efficacy of a two dose anthrax vaccine absorbed (AVA) schedule (0, 2 weeks) aerosol challenged with high levels of B. anthracis spores at week 4– the time point at which humans would receive the third vaccination of the approved PEP schedule. Here we use logistic regression models to combine the survival data from the NHP study along with serum anthrax lethal toxin neutralizing activity (TNA) and anti-PA IgG measured by enzyme linked immunosorbent assay (ELISA) data to perform a cross-species analysis to estimate survival probabilities in vaccinated human populations at this time interval (week 4 of the PEP schedule). The bridging analysis demonstrated that high levels of NHP protection also yield high predicted probability of human survival just 2 weeks after the second dose of vaccine with the full or half antigen dose regimen. The absolute difference in probability of human survival between the full and half antigen dose was estimated to be at most approximately 20%, indicating that more investigation of the half-antigen dose for vaccine dose sparing strategies may be warranted.
Keywords: Anthrax, Bacillus anthracis, Correlate of protection, Bootstrap, Logistic regression, Cross-species prediction
1. Introduction
Anthrax vaccine absorbed (AVA, BioThrax®) is the only FDA approved vaccine for prevention of anthrax in humans. The pre-exposure prophylaxis (PrEP) schedule for AVA is a priming series of 3 intramuscular (IM) injections (0, 1, 6 months) with boosters at 12 and 18 months, and annually thereafter for those at continued risk of infection [1]. In 2015, under the ‘animal rule’, FDA also approved a post-exposure prophylaxis (PEP) indication for AVA in adults 18–65 years of age. The schedule is three doses administered subcutaneous (SC) at 2-week intervals (0, 2, and 4 weeks), in conjunction with a 60-day course of antimicrobials [2–4]. In a 2010 FDA meeting, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) agreed on the use of PrEP animal model to establish protective antibody levels at relevant time points in support of the PEP indication [5]. These protective levels may be used to bridge to humans [6].
In 2014, the Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) announced an intention to develop an animal model to support assessment of a shortened antimicrobial PEP duration following Bacillus anthracis exposure [7]. The resulting study evaluated short term efficacy of a two dose AVA schedule (0, 2 weeks) in nonhuman primates (NHP) challenged with high levels of B. anthracis spores at week 4. In addition, to evaluate multifold expansion of the current anthrax vaccine stockpile, the PHEMCE established a human clinical trial of two-dose AVA regimens (0, 2 weeks or 0, 4 weeks) with the full antigen dose and a three-dose regimen (0, 2, 4 weeks) with full and half the standard antigen amount [8]. The objective of that study was to determine the safety and immunogenicity of reduced or divided doses of vaccine.
The primary immunogen in AVA is anthrax toxin protective antigen (PA). Serological analyses of anthrax vaccines include serum anthrax lethal toxin neutralizing activity (TNA) and anti-PA IgG measured by enzyme linked immunosorbent assay (ELISA). These measures of humoral immune response have been established as correlates of protection (COP) in animal models [6,9–12]. These COP have been used as a modeling tool with NHP survival data to predict the probability of survival in vaccinated human populations [13,14]. The cross-species modeling to estimate the probability of survival for anthrax vaccines in humans is necessary because clinical infection studies are impractical and ethically infeasible [5,13,15].
Here we report on a cross-species analysis between the accelerated two-dose NHP study data [16] and the human immunogenicity trial [8]. The primary objective of the analysis was to investigate the predicted probabilities of survival in humans at Day 28 receiving a full-dose or half-dose of AVA at only the 0 and 2 week time points of the accelerated PEP regimen (0, 2, and 4 weeks) when extrapolated from NHP data on a matching schedule. The immune response data from both studies and the survival data from the NHP study were used to generate predicted survival probabilities in humans receiving two doses (0, 2 weeks) of the full or half antigen dose accelerated AVA regimens. These data may assist in estimating the predicted probability of survival in humans of the half-dose regimen relative to the full-dose regimen. A half-dose regimen may provide significant increases in vaccination coverage during a large scale emergency when the demand for AVA may exceed availability.
2. Materials and methods
2.1. Vaccine
The human clinical trial and non-clinical study both used the same lot of AVA (FAV392A). Sterile saline was used as a diluent to generate the dilutions specified for the non-clinical study.
2.2. Non-clinical test system
The animal study is described in detail by Sivko [16]. In brief, 48 cynomolgus macaques (24 males and 24 females) were assigned to one of 5 AVA dose groups, which ranged from 1:3 to 1:243 dilutions of the human dose or to receive saline only. Animals were vaccinated intramuscularly (IM) on Days 0 and 14 and then aerosol challenged with B. anthracis Ames strain spores on Day 28. The design for the non-clinical study is presented in Table 1. Following challenge, animals were observed for survival through the morning of Day 56. Prior to challenge, blood was collected for anti-PA IgG ELISA and TNA (NF50 and ED50) on Days 0, 14, 21, and 28. The limit of detection (LOD) for the NHP assays were 1.6 μg/mL, 0.074, and 37 for anti-PA IgG, TNA NF50, and TNA ED50, respectively. The study was conducted with oversight from the Institutional Animal Care and Use Committee.
Table 1.
Pre-clinical study design and survival data.
| Group | AVA® vaccine lot | AVA® vaccine dilution (IM) | Number of animals | Vaccination days | Challenge day | Number of survivors (Percent) |
|---|---|---|---|---|---|---|
| 5 | FAV392A | 1:3 | 8 | 0 and 14 | 28 | 7 (88) |
| 1 | 1:9 | 8 | 8 (100) | |||
| 4 | 1:27 | 9 | 9 (100) | |||
| 3 | 1:81 | 9 | 6 (67) | |||
| 2 | 1:243 | 8 | 2 (25) | |||
| 6 | Saline control | 6 | 0 (0) |
IM – Intramuscular.
2.3. Immunogenicity and safety human clinical trial
The results of the human clinical trial have been detailed by Bernstein et al. [8]. The study design for the clinical trial is presented in Table 2. Subjects were scheduled to have blood samples drawn for anti-PA IgG ELISA and TNA (NF50 and ED50) testing on Days 0, 7, 14, 21, and 28. The LOD for the human assays were 0.855 μg/mL, 0.059, and 26 for anti-PA IgG, TNA NF50, and TNA ED50, respectively. Additional details and results are available at Clinicaltrials.gov NCT01641991.
Table 2.
Clinical trial study design.
| Study arm | Number of subjects in per protocol population | Vaccine | Vaccination schedulea |
|||
|---|---|---|---|---|---|---|
| Day 0 SC (mL) |
Day 14 SC (mL) |
Day 28 SC (mL) |
6 Months IM (mL) |
|||
| A | 67 | Full-Dose AVA | 0.50 | 0.50 | NA | 0.50 |
| B | 59 | Full-Dose AVA | 0.50 | NA | 0.50 | 0.50 |
| C | 59 | Full-Dose AVA | 0.50 | 0.50 | 0.50 | 0.50 |
| D | 60 | Half-Dose AVA | 0.25 | 0.25 | 0.25 | 0.50 |
| Total | 245 | |||||
SC – Subcutaneous.
IM – Intramuscular.
NA – Not applicable.
Only data through Day 28 included in analysis.
2.4. Statistical methods
From the human clinical trial, only the groups vaccinated on Days 0 and 14 (Table 2; Arms A, C, and D) were included in the analysis. Since Arms A and C had the same vaccination schedule and dose up to this point, they were combined into a single group for this analysis. In addition, the analysis only included human humoral immune response data up to and including the Day 28 time point, since only those are relevant in predicting survival subsequent to Day 28.
Immunological endpoints included in the analysis are Day 21, Day 28, and peak values for antiPA IgG ELISA, TNA NF50 and TNA ED50. Peak value was defined as the maximum observed titer across all blood collection time points for an individual animal or subject through Day 28. Assay results that were reported as less than the LOD were replaced with one-half the LOD for the primary analyses. The cross-species analysis was performed separately for each immunological endpoint. Each analysis was performed using the same methods and followed the continuous relationship approach of Kohberger et al. [17] and Fay et al. [13] for extrapolating protection from NHP to humans.
A logistic regression model for NHP survival was fitted to the base-10 log-transformed NHP humoral immune response data. The fit of the logistic regression models to the observed data was assessed by computing the max-rescaled R-square, and the predictive accuracy of each model was assessed using area under the curve (AUC). The estimated NHP logistic survival curve for each antibody assay value was directly applied to each human antibody value to estimate the human's survival probability. The predicted net human survival for each group (full-dose = Arms A and C, half-dose = Arm D) consisted of the average of predicted survival probabilities for all the individual humans in the group.
A double bootstrap procedure [18] with 10,000 iterations was implemented to estimate 95 percent confidence intervals (CI) for each estimated human group survival probability. The resampling procedure incorporates the observed variations in both the NHP and the human responses to assess the degree of variability in model conclusions that would be found if the experiments were actually repeated many times. Percentiles from the 10,000 bootstrap estimates were used for the confidence interval.
The primary cross-species analysis excluded control animals and replaced values less than the LOD with one-half the LOD. The sensitivity of the results to these methods was assessed with two alternative analyses (1) unvaccinated animals were included and (2) values less than the LOD for immunologic assays were analyzed as reported, except zeroes were replaced with one-half the minimum observed non-zero value for that species. Minimum observed non-zero values in the NHP data were 0.064 μg/mL, 0.002, and 1 for anti-PA IgG ELISA, TNA NF50 and TNA ED50, respectively. Minimum observed non-zero values in the human data were 0.289 μg/mL, 0.001, and 1 for anti-PA IgG ELISA, TNA NF50 and TNA ED50, respectively.
Additional analyses were performed that only included human immunogenicity data. Specifically, two-sample t-tests were performed on the log-transformed immunological endpoints to test for a significant difference between the full-dose and half-dose using Satterthwaite's unpooled method to account for potential unequal variances between the two groups [19]. Associated confidence intervals were also estimated. Two-sample t-tests were performed to test whether there was a significant difference in log-transformed humoral immune responses between males and females for the human data. In addition, linear regression models were fitted to each log-transformed immune response variable with simultaneous adjustment for vaccine dose, sex and weight. These models were used to test whether sex was a significant predictor of immune response after adjusting for vaccine dose and weight. Humoral immune response values reported as less than the LOD were replaced with one-half the LOD for summary statistics and these additional analyses. For the Day 28 data, a small number of values were less than the LOD. Out of 126 people vaccinated with the full-dose regimen, there were 3, 2, and 0 values less than the LOD for TNA NF50, TNA ED50 and anti-PA IgG, respectively. Out of 60 people vaccinated with the half-dose regimen, there were 10, 7, and 2 that were less than the LOD for TNA NF50, TNA ED50 and anti-PA IgG, respectively. The small number of values less than the LOD help ensure that alternative analysis methods for including assay values less than the LOD would not yield substantial differences in results.
All statistical analysis was performed using SAS software [20] with the exception of the double bootstrap analysis which was performed in R [21].
3. Results
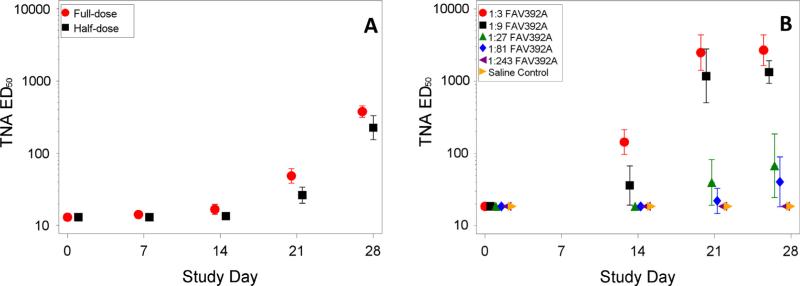
Fig. 1 presents the geometric mean TNA ED50 titer with 95 percent confidence interval (95% CI) for the full- and half-dose groups for the human data and for each vaccine dilution group from the animal study. Fig. 2 presents the individual data points and geometric means with 95% CI for the same groups separately for males and females. The TNA NF50 and anti-PA IgG data had similar patterns and are not shown.
Fig. 1.
Geometric means and 95% confidence intervals TNA ED50 data by group and day from human study (A) and NHP study (B).
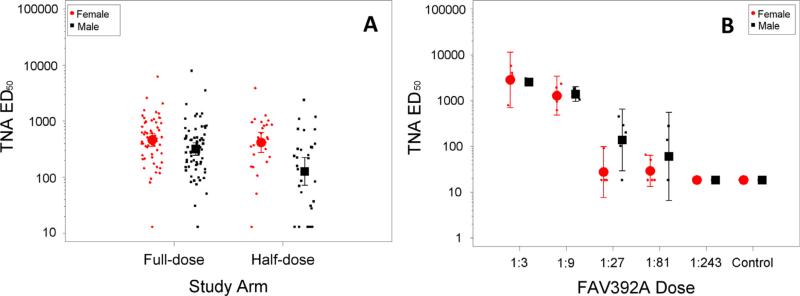
Fig. 2.
Individual data points with geometric means and 95% confidence intervals for TNA ED50 by sex and group for day 28 data from human study (A) and NHP study (B).
The human humoral immune response in the combined full-dose groups (Arms A and C) was somewhat greater than that in the half-dose group (Arm D). On Day 28 the ratio of geometric mean TNA ED50 titers for the full-dose group to the half-dose group was 1.67 (95% CI: 1.10–2.54, p = 0.017), the ratio for TNA NF50 was 1.69 (95% CI: 1.11–2.57, p = 0.015), and the ratio for anti-PA IgG was 1.85 (95% CI: 1.22–2.82, p = 0.005). Further, for the human study at Day 28, females had a significantly greater TNA response than males at full-dose (p = 0.043 for ED50 and p = 0.028 for NF50) and particularly at half-dose (p = 0.001 for ED50 and p = 0.002 for NF50). For anti-PA IgG, the difference was not significantly different between males and females for the full-dose (p = 0.152), but was significant for the half-dose (p = 0.004). The ratios of geometric mean immune responses showed that females had approximately a 1.5-fold (TNA NF50 or ED50) or 1.25-fold (anti-PA IgG) increase in immune response for the full-dose and an approximately threefold increase for the half-dose. There were no significant differences in the immunological endpoints between males and females in the animal study. The immune responses for the two human groups fall in between the second and third highest animal vaccine doses, both of which provided 100 percent protection in the animal model.
At enrollment, the males in the human clinical trial were significantly heavier than the females by 12.1 kg. We jointly assessed the role of vaccine group, sex, and weight on log-transformed humoral immune response data using a linear regression model including all pairwise interactions. Inclusion of sex in the model added significantly to the fit. Furthermore, with sex included, weight was not a significant predictor of immune response; indicating that for this dataset, sex was a more important predictor of immune response than weight.
Table 3 presents AUC and max-rescaled R-square statistics that compare the fit of the logistic regression models of NHP survival for each immune response assay. Values for the alternative analyses and additional time points are included in Table S1. Higher values of AUC indicate better prediction of the observed data. AUC estimates ranged from 0.806 to 0.830 indicating good prediction of the observed data. The values for max-rescaled R-square ranged from 0.3214 to 0.3862. Both measures were greater for the anti-PA IgG ELISA model than the TNA models. Figs. S1 and S2 [16] present the estimated logistic regression models with 95 percent confidence intervals for anti-PA IgG ELISA and TNA NF50, respectively, for Day 28. The observed data are shown as the proportion of animals that survived in binned ranges of the immune response. These plots along with high values for AUC indicate that the logistic models provide a reasonable fit to the data.
Table 3.
Max-rescaled R-square and area under the curve for day 28 logistic regression models fitted to NHP data.
| Assay | Max-rescaled Rsquare | Area under the curve (AUC) |
|---|---|---|
| Anti-PA IgG ELISA (μg/mL) | 0.3862 | 0.830 |
| TNA NF50 | 0.3214 | 0.806 |
| TNA ED50 | 0.3316 | 0.808 |
For the majority of animals and human subjects, the Day 28 assay result constituted the peak value, so the analysis results were very similar for Day 28 and peak. Additionally, the Day 28 measurement was the last immune response prior to animal challenge, and the last immune response before challenge has been shown to correlate best with protection in other NHP challenge models [12,14]. The AUC and max-rescaled R-square values show that the models fitted to Day 28 or peak provide better fit to the animal data than the Day 21 models. Therefore, only results based on Day 28 immune response data are shown.
Table 4 summarizes the estimated human probability of survival extrapolated from the animal data with bootstrap 95 percent confidence intervals for anti-PA IgG ELISA, TNA NF50 and TNA ED50 at Day 28 for the primary analysis. Table S2 presents the results for two alternative analysis methods at Day 28. Results from the primary and alternative analyses are shown graphically in Fig. 3. This analysis uses the humoral immune response and survival data from the NHP study, combined with the humoral immune response data from the human clinical trial to predict the probability of survival in humans. The primary model results exclude control animals and replace values less that the LOD with one-half the LOD.
Table 4.
Estimated probability of survival results for day 28 with control animals excluded and values less than the limit of detection (LOD) replaced with one-half the LOD.
| Assay | Estimated Percent (95% bootstrap confidence interval) |
||
|---|---|---|---|
| Full-Dose | Half-Dose | Difference | |
| Anti-PA IgG ELISA (μg/mL) | 95.8 (82.2, 100.0) | 91.1 (78.3, 100.0) | 4.7 (0.0a, 8.8) |
| TNA NF50 | 89.4 (76.2, 99.3) | 82.8 (70.0, 90.9) | 6.6 (1.3, 21.5) |
| TNA ED50 | 89.5 (76.2, 100.0) | 83.4 (70.8, 94.2) | 6.1 (1.2, 16.6) |
Estimated lower bound was 2E-5% which was rounded to 0.0%.
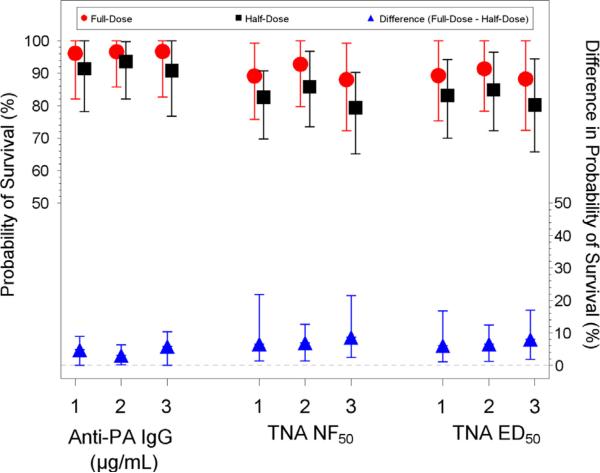
Fig. 3.
Estimated probability of survival results for full-dose and half-dose (left y-axis) and difference in probability of survival between full-dose and half-dose (right y-axis) with 95% confidence intervals for day 28. Estimated probability was determined using three different analysis methods (1) Primary: control animals excluded and values less than the limit of detection (LOD) replaced with one-half the LOD, (2) Alternate 1: control animals included and values less than the LOD replaced with one-half the LOD, and (3) Alternate 2: control animals excluded and zeroes replaced with one-half the minimum.
The analysis based on the anti-PA IgG ELISA concentration predicted a probability of survival in the full-dose group of 95.8% (95% CI: 82.2–100%) and a probability of survival in the half-dose group of 91.1% (95% CI: 78.3–100%). The estimated difference in survival probabilities (full-dose minus half-dose) was 4.7 percentage points (95% CI: 0.0–8.8%). For TNA ED50 the estimated probability of survival for full-dose was 89.5% (95% CI: 76.2–100%) and the estimated probability of survival for half-dose group was 83.4% (95% CI: 70.8–94.2%), with an estimated difference of 6.1 percentage points (95% CI: 1.2–16.6%). The TNA NF50 results were very similar to those for TNA ED50. Overall, these results indicate that the probability of survival in the full-dose group was significantly greater than that for the half-dose group. The upper bounds of the confidence intervals were 8.8%, 21.5%, and 16.6% for anti-PA IgG, TNA NF50, and TNA ED50, respectively. This finding indicates that based on the extrapolation analysis, there is statistical confidence that the difference in protection between the full-dose and half-dose regimen is less than 22 percentage points based on TNA data or less than 10 percentage points based on anti-PA IgG ELISA data. The sensitivity analyses using the two alternative modeling approaches yielded mostly similar conclusions (Fig. 3 and Table S2).
Table 5 presents the primary analysis for all three antibody measures performed separately for males and females. The predicted survival rates in the full-dose groups were approximately the same for males and females. Although not significant, the half-dose group showed a lower predicted survival for males compared to females. The lower probabilities of male survival stem from the higher immune response observed in females. This in turn resulted in the observed difference between the full-dose and half-dose being larger for males than in females. For males, the estimated difference in survival probabilities between the full- and half-dose is approximately 8% (95% CI: 0.0–15.9%) for anti-PA IgG and 11% for TNA NF50 and ED50 (95% CI TNA NF50: 2.5–38.8%; ED50: 2.4–29.0%). For females, the predicted reduction in survival for use of half-dose was only 1–2 percentage points, with confidence intervals that included zero for all three antibody measures.
Table 5.
Estimated probability of survival results for day 28 with males and females analyzed separately (control animals excluded and values less than the limit of detection (LOD) replaced with one-half the LOD).
| Assay | Sex | Estimated Percent (95% bootstrap confidence interval) |
||
|---|---|---|---|---|
| Full-Dose | Half-Dose | Difference | ||
| Anti-PA IgG ELISA (μg/mL) | Males | 95.4 (81.9, 100.0) | 87.2 (74.0, 100.0) | 8.3 (0.0a, 15.9) |
| Females | 96.2 (83.0, 100.0) | 95.3 (82.0, 100.0) | 0.8 (−0.9, 3.2) | |
| TNA NF50 | Males | 88.0 (74.5, 100.0) | 76.5 (57.7, 86.4) | 11.4 (2.5, 38.8) |
| Females | 91.2 (77.8, 100.0) | 89.6 (76.5, 100.0) | 1.6 (−3.4, 8.2) | |
| TNA ED50 | Males | 88.3 (74.8, 100.0) | 77.4 (63.0, 90.9) | 10.9 (2.4, 29.0) |
| Females | 90.9 (77.3, 100.0) | 89.8 (76.3, 100.0) | 1.1 (−3.6, 8.0) | |
Estimated lower bound was 4E-5% which was rounded to 0.0%.
4. Discussion
The human clinical trial and NHP study had complementary designs and used the same vaccine lot which allowed the data generated from these two studies to be combined for this cross-species analysis. The vaccination schedules from the two studies were the same up to Day 28. On Day 28 and after, additional vaccinations were given in the human clinical trial when the cynomolgus macaques in the pre-clinical trial were aerosol challenged with B. anthracis Ames spores on Day 28.
Bernstein et al. analyzed the anti-PA IgG and TNA data from the clinical trial and showed that the full-dose study group did have advantages over the half-dose regimen in terms of sero-conversion and early response. The differences in humoral immune responses between the two dosages were statistically significant.
Extrapolating vaccine protection from NHPs to humans indicated that immunizing with the full-dose of AVA at the 0 and 2 week time points of the PEP regimen had estimated probabilities of survival of approximately 95% and 90% based on anti-PA IgG ELISA and TNA, respectively, at Day 28. For the half-dose group, those estimated probabilities of survival were approximately 91% and 83%, respectively. The corresponding lower bounds of the 95 percent bootstrap confidence intervals for survival based on those Day 28 immune responses (anti-PA IgG ELISA, TNA NF50, and TNA ED50) all indicated greater than 70% probability of survival for the half-dose regimen.
The estimated probability of survival in humans was significantly greater in the full-dose regimen than the half-dose regimen; although the lower bound of the 95% confidence interval for the difference was quite close to zero for all three immune responses based on the primary model. Nevertheless, the estimated difference in predicted full-dose versus half-dose survival across all models and all three immune responses was between 2.8% and 8.7%. These analysis results are consistent with the significantly greater human humoral immune response for the full- versus half-dose regimen.
If we consider a scenario with a limited supply of vaccine, there are potential benefits of the half-dose regimen. Assuming there is a sufficient supply of vaccine to only vaccinate half (100,000) of an aerosol-exposed population of 200,000 with the full-dose regimen or all 200,000 with the half-dose regimen, the half-dose regimen is estimated to protect 80–95% more people than the full-dose regimen. For example, the primary analysis based on Day 28 anti-PA IgG ELISA predicts that 95,800 people would be protected using the full-dose regimen and 182,200 people would be protected using the half-dose regimen. We note that in any scenario where vaccine demand exceeds supply, more people would survive using the half-dose regimen as opposed to the full-does regimen as long as the probability of survival for the half-dose regimen is greater than half the probability of survival for the full-dose regimen. These calculations assume that the probability of survival for an unvaccinated human is zero and that all of the vaccinated individuals were exposed, which is a worst case set of assumptions for the full-dose regimen. Adjusting for the survival probability in unvaccinated humans (which may be as large as 20% [22]) would reduce the estimated difference in protection between the full and half-dose regimen, but the general conclusion is unchanged.
The analyses of the data from the individual studies and the cross-species analysis have broad implications for anthrax preparedness analysis. The reasonableness and applicability of these results depend on a number of factors. After 30 days of antibiotics, the remaining vegetative spores that may germinate would be a much lower challenge dose than the aerosol exposure of the animals. The differences between the full-dose and half-dose of AVA in this study do not reflect concomitant use of antibiotics. It is possible that such antibiotic use could increase the efficacy of both full and half-dose vaccination toward 100%, thus reducing the dose-related difference in protection or otherwise modifying the response. The human extrapolation results rest on the assumption that the protection in humans is the same as the protection in NHPs given the same humoral immune response. The analysis by Fay et al. [13] studied the interspecies uncertainty and concluded that this extrapolation may be informative.
5. Conclusions
The clinical and NHP studies combined in this analysis were designed and conducted in a way that supports the cross-species analysis presented here. The data demonstrate high levels of protection (NHP) and high predicted probability of survival (humans) 2 weeks after the second dose of vaccine. These data may support cessation of antimicrobial prophylaxis at the time the third vaccine dose is given. Shorter antimicrobial treatment courses could also increase patient adherence, reduce known drug-related adverse events and extend the capacity of the Strategic National Stockpile with no estimated decrease in emergency preparedness. The data also support the effectiveness of the AVA PEP vaccine schedule as well as further consideration of potential vaccine dose sparing strategies that entail half-dose vaccination.
Supplementary Material
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.06.041.
References
- 1.FDA Approval Letter – BioThrax. < http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm304758.htm>.
- 2.FDA BoThrax®-Package Insert. 2012 < http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/UCM074923.pdf>.
- 3.Wright JG, Plikaytis BD, Rose CE, Parker SD, Babcock J, Keitel W, et al. Effect of reduced dose schedules and intramuscular injection of anthrax vaccine adsorbed on immunological response and safety profile: a randomized trial. Vaccine. 2014 Feb 12;32(8):1019–28. doi: 10.1016/j.vaccine.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn CP, Sabourin CL, Niemuth NA, Li H, Semenova VA, Rudge TL, et al. A three-dose intramuscular injection schedule of anthrax vaccine adsorbed generates sustained humoral and cellular immune responses to protective antigen and provides long-term protection against inhalation anthrax in rhesus macaques. Clin Vaccine Immunol. 2012;19:1730–45. doi: 10.1128/CVI.00324-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.November 16-17, 2010: Vaccines and related biological products advisory committee meeting presentations. < http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm239733.htm>.
- 6.Ionin B, Hopkins RJ, Pleune B, Sivko GS, Reid FM, Clement KH, et al. Evaluation of immunogenicity and efficacy of anthrax vaccine adsorbed for post-exposure prophylaxis. Clin Vaccine Immunol. 2013;20(7):1016–26. doi: 10.1128/CVI.00099-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.2014 Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) Strategy and Implementation Plan. < http://www.phe.gov/Preparedness/mcm/phemce/Documents/2014-phemce-sip.pdf>.
- 8.Bernstein DI, Jackson L, Patel SM, El Sahly HM, Spearman P, Rouphael N, et al. Immunogenicity and safety of four different dosing regimens of anthrax vaccine adsorbed for post-exposure prophylaxis for anthrax in adults. Vaccine. 2014 Oct 29;32(47):6284–93. doi: 10.1016/j.vaccine.2014.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little SF, Ivins BE, Fellows PF, Pitt ML, Norris SL, Andrews GP. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004 Jan 2;22(3–4):422–30. doi: 10.1016/j.vaccine.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Savransky VM, Sanford D, Syar E, Austin J, Tordoff KP, Anderson M, et al. Pathology and pathophysiology of inhalational anthrax in a guinea pig model. Infect Immun. 2013;81(4):1152–63. doi: 10.1128/IAI.01289-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitt ML, Little SF, Ivins BE, Fellows P, Barth J, Hewetson J, et al. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine. 2001;19:4768–73. doi: 10.1016/s0264-410x(01)00234-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Schiffer JM, Dalton S, Sabourin CL, Niemuth NA, Plikaytis BD, et al. Comprehensive analysis and selection of anthrax vaccine adsorbed immune correlates of protection in rhesus macaques. Clin Vaccine Immunol. 2014;21(11):1512–20. doi: 10.1128/CVI.00469-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fay MP, Follmann DA, Lynn F, Schiffer JM, Stark GV, Kohberger R, et al. Anthrax vaccine–induced antibodies provide cross-species prediction of survival to aerosol challenge. Sci Transl Med. 2012;4:151ra126. doi: 10.1126/scitranslmed.3004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffer JM, Chen L, Dalton S, Niemuth NA, Sabourin CL, Quinn CP. Bridging non-human primate correlates of protection to reassess the Anthrax Vaccine Adsorbed booster schedule in humans. Vaccine. 2015 Jul 17;33(31):3709–16. doi: 10.1016/j.vaccine.2015.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gronval GK, Trent D, Borio L, Brey R, Nagao L. The FDA animal efficacy rule and biodefense. Nat Biotechnol. 2007;25(10):1084–7. doi: 10.1038/nbt1007-1084. [DOI] [PubMed] [Google Scholar]
- 16.Sivko GS, Stark GV, Tordoff KP, Taylor KL, Glaze E, VanRaden M, et al. Evaluation of early immune response-survival relationship in cynomolgus macaques after Anthrax Vaccine Absorbed vaccination and Bacillus anthracis spore challenge. Vaccine. 2016 doi: 10.1016/j.vaccine.2016.04.048. http://dx.doi.org/10.1016/j.vaccine.2016.04.048. [DOI] [PMC free article] [PubMed]
- 17.Kohberger RC, Jemiolo D, Noriega F. Prediction of pertussis vaccine efficacy using a correlates of protection model. Vaccine. 2008;26:3516–21. doi: 10.1016/j.vaccine.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Efron B, Tibshirani RJ. An introduction to the bootstrap. Chapman and Hall; New York: 1993. [Google Scholar]
- 19.Best DI, Rayner CW. Welch's approximate solution for the Behren's-Fisher problem. Technometrics. 1987;29:205–10. [Google Scholar]
- 20.SAS Copyright, SAS Institute Inc . SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. Cary, NC, USA: [Google Scholar]
- 21.R Core Team. R . A language and environment for statistical computing. R Foundation for Statistical; Computing, Vienna, Austria: 2015. URL http://www.R-project.org/ [Google Scholar]
- 22. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ucm061751.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.