Abstract
Objective
To compare neurodevelopmental outcomes in postnatal growth-restricted infants born < 29 weeks with and without postnatal head-sparing.
Study Design
We analyzed developmental outcomes at 2 years of age among postnatally growth-restricted infants with and without head-sparing. The primary outcome was Bayley III cognitive composite score; secondary outcomes included Bayley III motor composite score, moderate/severe cerebral palsy, GMFCS level ≥2, and presence or absence of neurodevelopmental impairment (NDI).
Results
Of 1098 infants evaluated at 18–22 months, 658 were postnatally growth-restricted, of whom 301 had head-sparing. In the multivariate model including independent risk factors for poor growth and poor developmental outcome, infants with head-sparing had higher adjusted motor composite scores (mean difference 4.65, p<0.01), but no differences in other neurodevelopmental outcomes.
Conclusion
Postnatal head-sparing is associated with improved neurodevelopmental outcome in extremely preterm infants, specifically Bayley III motor scores, but whether beneficial effects of PHS persist later in life is unknown.
Introduction
The influence of postnatal somatic growth on neurodevelopmental outcomes in preterm infants has been well-described.1, 2, 3 Although much emphasis has been placed on achieving adequate weight gain in these infants, the importance of postnatal head growth in determining early childhood neurodevelopment has also been well-established.2, 4, 5 Efforts by neonatal care providers have decreased the incidence and severity of postnatal growth restriction; however, a decline in growth percentiles from birth to discharge still occurs commonly in those born prematurely. Standard deviation scores (z-scores) at birth for weight, length, and head circumference are similar and cluster around zero, suggesting in utero growth consistent with the population mean for any given gestational age.6 By hospital discharge, however, a decrease in z-score is routinely observed for weight, length, and head circumference.5, 6, 7 This decrease is more pronounced for weight and length than for head circumference z-score.5, 6, 7 This pattern, whereby postnatal head growth appears to be “spared” relative to weight, is neither well-understood nor well-studied. We refer to this phenomenon as postnatal head sparing (PHS). We postulated that this relative head sparing may be analogous in impact to in utero head sparing with respect to later neurodevelopmental outcome. Small for gestational age neonates born term and preterm with relative head sparing have neurodevelopmental outcomes that are significantly better than those babies whose head circumference percentiles are the same or lower than their birthweight percentiles.8, 9, 10 The objective of this study was to compare neurodevelopmental outcomes in postnatal growth-restricted extremely preterm infants with and without PHS.
Methods
This is an observational, retrospective study of prospectively collected data from the National Institute of Child Health and Human Development Neonatal Research Network’s Generic Database (GDB) and Follow-Up studies. Infants 22 0/7 to 28 6/7 weeks gestation or with a birthweight of 401–1000 grams born 1/1/2009 to 12/31/2010 at participating NICHD Neonatal Research Network centers with postnatal growth restriction (PGR, defined below) and who underwent neurodevelopmental assessment at 18–22 months corrected age (CA) were included. Additional exclusion criteria included infants with missing growth data, congenital defects, hydrocephalus with shunt placement, birth head circumference (HC) > 2 standard deviations above the mean for gestational age, and missing neurodevelopmental assessment at 18–22 months CA. Collection of comprehensive information on infants continued until “status,” defined as hospital discharge or age 120 days, whichever came first.
Traditionally, growth percentiles at discharge have been used to characterize postnatal growth restriction in preterm infants. However, this approach has the potential to over-represent infants born small-for-gestational age (SGA, <10%), who may very well experience a desired pattern of growth (ex: along the 5th percentile) but are discharged <10%. In order to better describe patterns of postnatal growth regardless of centiles at birth and discharge, standard deviation scores (z-scores) were used to define PGR and PHS. PGR and PHS were defined using the following formulas:
The Fenton (2013) growth chart served as the reference for growth percentiles and corresponding z-scores, which were determined using the bulk calculator provided at http://www.ucalgary.ca/fenton/2013chart, used with permission.11 Data were collected for in-hospital morbidities, including rates of sepsis (culture-positive early or late septicemia/bacteremia treated for ≥ 5 days with antibiotics), necrotizing enterocolitis (NEC; modified Bell’s stage IIa or greater), grade 3–4 intraventricular hemorrhage (IVH) and/or periventricular leukomalacia (PVL), bronchopulmonary dysplasia (BPD; defined as receiving supplemental O2 at 36 weeks PMA or discharge if discharged before 36 weeks), and postnatal corticosteroid use for BPD/chronic lung disease. Standardized neurodevelopmental examinations were performed at 18–22 months CA by certified examiners at each Neonatal Research Network center. Examiners undergo an annual re-certification process (described elsewhere) to ensure agreement with gold-standard examiners.12
The primary outcome for this study was Bayley III13 composite cognitive score. Additional measures of neurodevelopment comprised secondary outcomes, including Bayley III composite motor score, incidences of moderate-to-severe cerebral palsy, gross motor functional classification scale (GMFCS)14 level ≥ 2 and neurodevelopmental impairment (NDI), defined as having one or more of the following: Bayley III composite cognitive score < 70, Bayley III composite motor score < 70, GMFCS level ≥ 2, bilateral blindness, or hearing impairment. A second definition for NDI was also explored using a cognitive score cutoff of < 85, rather than < 70, based upon recent reports that higher cut points for the Bayley III composite cognitive scores may better identify infants with moderate-to-severe neurodevelopmental impairment.15, 16
Bivariate analyses were conducted to compare demographic characteristics, in-hospital morbidities, and outcomes between children with vs. without postnatal head sparing (PHS), using chi-square tests for categorical variables and t-tests for continuous variables. To determine whether PHS independently influenced neurodevelopmental outcomes, two sets of mixed effects regression models were conducted to compare outcomes for infants based on PHS after controlling for other factors and including center as a random effect. The first included PHS and demographic/birth characteristics only and the second added in-hospital morbidities and therapies to the model. The variables, chosen a priori, were selected if likely to influence growth and/or neurodevelopment. All analyses were performed using SAS version 9.4 with a p-value < 0.05 indicating statistical significance. PROC MIXED was used for analyses of continuous outcomes (e.g., Bayley cognitive and motor composite scores) and PROC GLIMMIX for categorical outcomes (e.g., moderate/severe CP, GMFCS level ≥2, and NDI).
Results
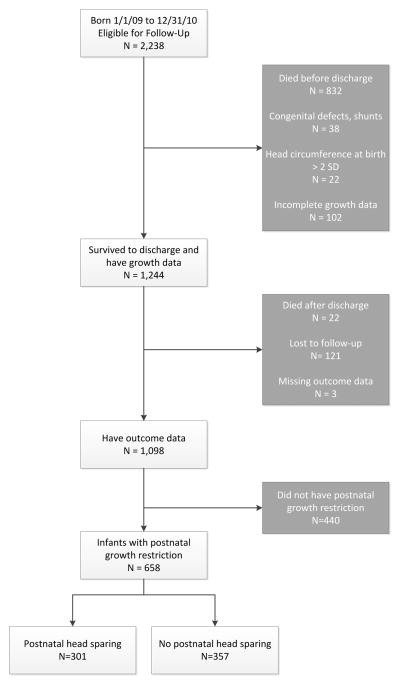
Of 2238 infants born 1/1/2009 through 12/31/2010 and eligible for follow-up, 832 died (37%) and an additional 308 were excluded. The most common reasons for exclusion were missing growth or outcome data (Figure 1). After exclusions, 658/1098 infants (59.9%) experienced PGR, survived to follow-up, and were subsequently included in the analyses. Of 658 infants with PGR, 301 (45.7%) met criteria for PHS.
Figure 1.
Flowchart demonstrating study population.
Demographic and birth characteristics for infants with and without PHS are compared in Table 1. No significant differences were observed between groups with respect to postmenstrual age (PMA) at status, SGA at birth (defined as weight z-score at birth < -1), gender, race, or ethnicity. The observed difference in mean GA at birth was statistically, but not clinically significant. At birth, mean weight and length z-scores were similar between groups with and without PHS, while mean HC z-score was greater in infants without PHS (0.09 ± 0.82 vs −0.36 ± 1.13, p<0.001).
Table 1.
Patient Demographic and Birth Characteristics
| Variable | PHS (n=301) | No PHS (n=357) | p-value |
|---|---|---|---|
|
| |||
| GA at birth, wks | 25.16 (1.10) | 24.97 (1.09) | 0.034 |
|
| |||
| PMA at statusa, wks | 39.40 (2.34) | 39.12 (2.93) | 0.175 |
|
| |||
| SGA, n (%) | 37 (12) | 31 (9) | 0.130 |
|
| |||
| Male sex, n (%) | 149 (50) | 168 (47) | 0.532 |
|
| |||
| Race, n (%) | |||
| Black | 141 (47) | 134 (38) | 0.055 |
| White | 138 (46) | 192 (54) | |
| Other | 19 (6) | 27 (8) | |
|
| |||
| Hispanic Ethnicity, n (%) | 45 (16) | 57 (17) | 0.686 |
|
| |||
| Maternal education less than high school, n (%) | 45 (21) | 56 (21) | 0.984 |
|
| |||
| Birth z-scores | |||
| Weight | 0.11 (0.92) | 0.10 (0.81) | 0.818 |
| Length | 0.05 (1.12) | 0.00 (1.04) | 0.594 |
| HC | −0.36 (1.13) | 0.09 (0.82) | <0.001 |
Data displayed as mean (SD) unless otherwise noted.
Discharge or 120 days of age
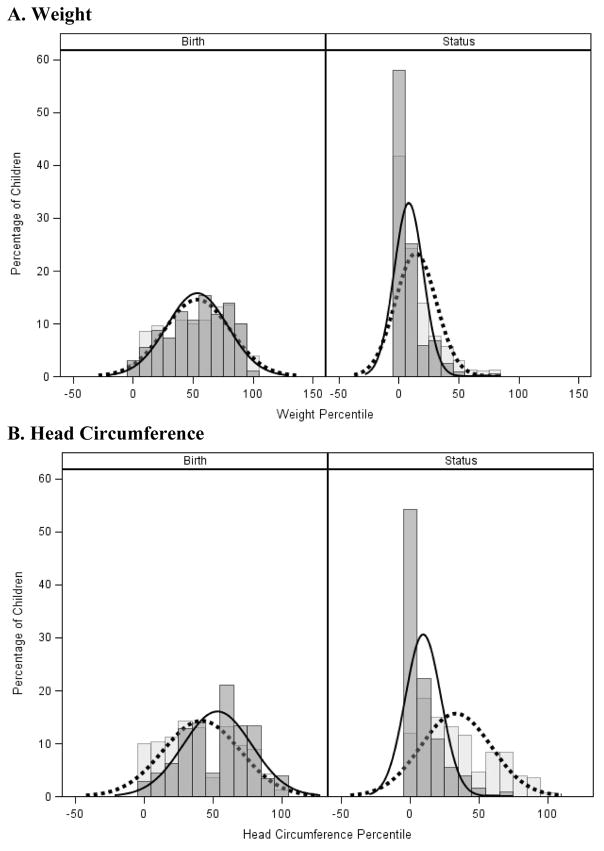
The distributions of weight and head circumference percentiles at birth and status for PHS and no PHS groups are displayed as density curves with histograms in Figure 2. As expected for infants with PGR, the distribution curves for weight for both groups are shifted leftward from birth to status, which indicates a negative z-score change between birth and status. However, it was evident that a separation had occurred in the HC percentile distribution curves at the time of discharge (or 120 days), so that the curve for infants with PHS was relatively preserved while that for infants without PHS had shifted leftward.
Figure 2. Distribution Curves at Birth and Discharge.
Graphical depiction of weight and HC percentiles at birth and status in growth-restricted infants with (light gray, dashed line) and without (dark gray, solid line) PHS. The bars are constructed as histograms and the lines represent smoothed density curves. Note the expected leftward shift in weight percentiles between birth and status (A), and the preservation of head growth percentile in infants with PHS (B).
Table 2 displays bivariate analyses comparing in-hospital morbidities between groups. Infants with PHS had a lower incidence of NEC and sepsis, and similar rates of grade 3–4 IVH/PVL and BPD. Postnatal steroid treatment was similar between infants with and without PHS (19% vs 16%, p=0.365).
Table 2.
Comparison of Morbidities Between Infants With and Without PHS
| Outcome | PHS | No PHS | p-value |
|---|---|---|---|
| Sepsis, n (%) | 79 (26) | 141 (40) | < 0.001 |
| NEC, n (%) | 24 (8) | 62 (17) | < 0.001 |
| IVH grade 3–4/PVL, n (%) | 51 (17) | 72 (20) | 0.291 |
| BPD, n (%) | 144 (48) | 194 (55) | 0.091 |
In unadjusted analyses, mean composite cognitive Bayley III scores at 18–22 months CA were not different between infants with and without PHS (90 vs 89) as shown in Table 3. However, infants with PHS had significantly higher motor scores and were less likely to have GMFCS ≥ 2 or NDI (using composite cognitive score < 70) when compared with infants without PHS (Table 3). Results of both multivariable regression models are displayed in Table 4. When controlling for demographic and birth characteristics (Model 1), compared to infants without PHS those with PHS had higher Bayley III cognitive and motor scores and were significantly less likely to experience neurodevelopmental impairment when a composite cognitive score of < 70 was used in the definition of NDI. In the second model, which controlled for in-hospital morbidities, the difference in motor score persisted, while the adjusted mean difference for cognitive score and odds of NDI were no longer significant. PHS did not impact the odds of GMFCS level ≥ 2 in either model.
Table 3.
Unadjusted Neurodevelopmental Outcomes
| Outcome | PHS | No PHS | p-value |
|---|---|---|---|
|
| |||
| Bayley III cognitive composite score | 90.78 (15.83) | 89.14 (16.88) | 0.205 |
|
| |||
| Bayley III motor composite score | 90.75 (16.36) | 86.77 (17.62) | 0.004 |
|
| |||
| Moderate/severe CP, n (%) | 20 (7) | 35 (10) | 0.148 |
|
| |||
| GMFCS level 2+, n (%) | 23 (8) | 44 (12) | 0.048 |
|
| |||
| NDI, n (%) | |||
| With cognitive score < 70 | 46 (16) | 75 (22) | 0.046 |
| With cognitive score < 85 | 87 (29) | 118 (34) | 0.193 |
Data displayed as mean (SD) unless otherwise noted
Table 4.
Influence of PHS on Neurodevelopmental Outcomes in Regression Models
| Outcome | Model 1: Demographics | Model 2: Demographics + Morbidities | ||
|---|---|---|---|---|
| Mean Diff/OR (CI) | p-value | Mean Diff/OR (CI) | p-value | |
| Bayley III cognitive score | 2.38 (0.54, 5.61) | 0.018 | 2.47 (−0.01, 5.04) | 0.06 |
| Bayley III motor score | 5.1 (2.39, 7.81) | <0.001 | 4.65 (1.93, 7.38) | <0.001 |
| GMFCS level 2+ | 0.62 (0.35, 1.10) | 0.104 | 0.61 (0.33, 1.13) | 0.117 |
| NDI (cognitive score < 70) | 0.60 (0.38, 0.94) | 0.026 | 0.5 (0.39, 1.02) | 0.058 |
| NDI (cognitive score < 85) | 0.73 (0.50, 1.08) | 0.114 | 0.77 (0.51, 1.15) | 0.199 |
Model 1 variables: GA at birth, sex, race/ethnicity, maternal education, PMA at status, SGA, HC z-score at birth
Model 2 variables: Model 1 + sepsis, NEC, IVH grade 3–4/PVL, postnatal steroids, BPD
Both models also controlled for clustering by center
Discussion
The association between postnatal growth failure and poorer neurodevelopmental outcomes in preterm infants has been well-established in several large cohort studies.1, 2, 3 Despite improvements in postnatal growth, due in part to more aggressive inpatient nutritional strategies, restricted growth between birth and discharge in those born prematurely remains a significant burden. Approximately 40% of nearly 26,000 VLBW infants in the California Perinatal Quality Collaborative database born 2005–2012 experienced a fall in weight z-score from birth to discharge of > 1,17 while 60% of infants in our study experienced PGR using the same definition. In the face of restricted somatic growth, however, our findings from this large multicenter study suggest that a relative preservation of head growth between birth and discharge is associated with neurodevelopmental benefit, particularly in motor scores, in this at-risk population.
To our knowledge, this is the first study formally describing postnatal head-sparing and the first to assess outcomes in extremely preterm infants experiencing head-sparing growth during hospitalization. While the exact mechanisms behind PHS remain unclear, it is reasonable to consider several plausible factors that may contribute to preserved head growth. The influence of nutrient delivery on postnatal head growth should be considered, given that the developing brain of preterm infants relies on an adequate supply of macro- and micronutrients during a critical period of growth and development.18 Greater protein and caloric intake during the first 4 postnatal weeks has been shown to increase head circumference at both 28 days of life and 36 weeks postmenstrual age among infants < 29 weeks gestation.19 Similarly, protein and fat intake during the first 10 postnatal weeks correlate positively with head growth in those born less than 27 weeks20, and very low birth weight infants experienced improved head growth following strategies implemented to increase caloric and protein intake.21 In extremely preterm infants, particularly those critically-ill, providing adequate nutrition to support growth is often limited by fluid restriction, inability to use the gut for feeding, or feeding tolerance, resulting in postnatal growth restriction similar to that experienced by infants in this study. However, detailed nutritional data are not available for our cohort, limiting the ability to assess the potential influence of protein and caloric intake on head growth in these infants.
In our population, infants without PHS were more likely to have NEC or sepsis. Evidence suggests that inflammation may also play a role in PHS. The accrual of lean body mass, considered a surrogate for organ growth, is altered by systemic inflammation. A sepsis model in rats results in decreased protein synthesis in skeletal muscle; the effect is mediated by TNF-α.22, 23 Increased TNF-α levels in breast milk from mothers of healthy one month old infants born at term are associated with lower infant lean body mass at one month of age.24 In preterm infants, improved anthropometric and bone growth occurs in the setting of lower levels of inflammatory mediators.25, 26 In addition to influencing growth, inflammation is known to be associated with poorer neurodevelopmental outcomes in extremely preterm infants. For example, elevated levels of inflammation-related proteins in the first two weeks of life in infants born < 28 weeks increase the odds of microcephaly and lower Mental Developmental Index scores on the BSID-II at 2 years of age.27 Likewise, preterm infants with proven neonatal sepsis or necrotizing enterocolitis demonstrate structural changes in the brain and smaller head circumferences at 2 years of age.28 Moreover, adverse neurodevelopmental outcomes and poor head growth have been associated with neonatal infection in extremely low birthweight infants.29 In this observational cohort, we cannot determine whether inflammation contributed to the slower head growth seen in these infants or to poorer ND outcomes.
Although our results implicate the pattern of postnatal head growth as an independent predictor of ND outcomes, the study is not without limitations, some of which have already been mentioned. First, in the absence of a standard definition, we chose to define PHS using change in HC z-score between birth and discharge in a population of infants with restricted somatic growth. A recent study of outcomes in preterm infants growth-restricted at birth used z-scores to define restricted growth and classify infants as either symmetric or asymmetric, but did so with measurements obtained at a single time point (birth).8 Using a single measurement, such as z-score at birth or at discharge, prevents the ability to draw conclusions about the pattern of in-hospital growth. In addition, doing so would lead to exclusion and inclusion bias, whereby some infants with poor growth would be excluded (HC remains greater than a specified z-score or percentile) and others with ‘normal’ postnatal growth might be included (HC remains less than specified z-score or percentile). By using a change in z-score from birth to discharge to define both PGR and PHS, a pattern of head growth relative to somatic growth emerges, irrespective of any classification that could be applied at either birth or discharge.
Secondly, we defined PGR and PHS based on measurements taken at only two time points, which, while more informative than a single measurement, may not adequately describe the true pattern of postnatal growth. Our database (GDB) does not capture more frequent anthropometric measurements, limiting the ability to explore postnatal growth patterns in these infants. We would predict that, given the apparent association between head growth and both sepsis and NEC, nutrition as well as increases in weight and head circumference over the period of illness and recovery would be suboptimal. Other contributory factors might also become apparent if weekly measurements were available and analyzed.
Postnatal growth patterns, such as somatic growth relative to length and head growth relative to weight gain, and their relationship to long-term development require additional investigation. Defining more- or less-desirable patterns of growth in preterm infants requires more detailed growth data. Whether beneficial growth patterns are modifiable or achievable through targeted interventions remains in question.
Conclusion
Postnatal growth-restricted extremely preterm infants with postnatal head-sparing have improved ND outcome, specifically Bayley III motor scores, when compared to those without head sparing. Further investigation is needed to determine the impact of postnatal growth patterns on long-term health outcomes, whether patterns of growth are modifiable by clinicians, and if improved growth results in improvements in neurodevelopmental outcome in these fragile neonates when they are school-age and beyond.
Acknowledgments
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network’s Generic Database and Follow-up Study through cooperative agreements. While NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of the NICHD.
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Drs. Abhik Das (DCC Principal Investigator) and Carla M. Bann (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chair: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine; Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Abbot R. Laptook, MD; Angelita M. Hensman, MS RNC-NIC; William Oh, MD; Martin Keszler, MD; Betty R. Vohr, MD; Robert Burke, MD; Melinda Caskey, MD; Katharine Johnson, MD; Elisabeth C. McGowan MD; Barbara Alksninis, PNP; Mary Lenore Keszler, MD; Theresa M. Leach, MEd CAES; Bonnie E. Stephens, MD; Victoria E. Watson, MS CAS; Suzy Ventura; Kristin M. Basso, MS BSN; Elisa Vieira, RN BSN; Andrea M. Knolls; Kristin M. Basso, MaT RN; Elisa Vieira, RN BSN.
Case Western Reserve University, Rainbow Babies & Children's Hospital (U10 HD21364, M01 RR80) – Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Anna Marie Hibbs, MD; Deanne E. Wilson-Costello, MD; Nancy S. Newman, BA RN; Bonnie S. Siner, RN; Monika Bhola, MD; Gulgun Yalcinkaya, MD; Harriet G. Friedman, MA.
Cincinnati Children's Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084) – Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Jody Hessling, RN; Estelle E. Fischer, MHSA MBA; Lenora D. Jackson, CRC; Kristin Kirker, CRC; Holly L. Mincey, RN BSN; Greg Muthig, BS; Teresa L. Gratton, PA; Jean J. Steichen, MD; Kimberly Yolton, PhD.
Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital (U10 HD40492, M01 RR30, UL1 TR83) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; William F. Malcolm, MD; Patricia L. Ashley, MD; Kimberley A. Fisher, PhD FNP-BC IBCLC; Sandra Grimes, RN BSN; Joanne Finkle, RN JD; Kathryn E. Gustafson, PhD; Matthew M. Laughon, MD MPH; Carl L. Bose, MD; Janice Bernhardt, MS RN; Gennie Bose, RN; Janice K. Wereszczak, CPNP-AC/PC.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, UL1 TR454) – David P. Carlton, MD; Ellen C. Hale, RN BS CCRC; Yvonne Loggins, RN BSN; Diane Bottcher, RN MN; Ira Adams-Chapman, MD; Maureen Mulligan LaRossa, RN; Sheena L. Carter, PhD.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Rosemary D. Higgins, MD; Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Eskenazi Health (U10 HD27856, M01 RR750, UL1 TR6) – Brenda B. Poindexter, MD MS; Gregory M. Sokol, MD; Anna M. Dusick, MD (deceased); Heidi Harmon, MD MS; Dianne E. Herron, RN; Lucy Smiley, CCRC; Carolyn Lytle, MD MPH; Lucy C. Miller, RN BSN CCRC; Heike M. Minnich, PsyD HSPP; Abbey C. Hines, PsyD; Leslie Dawn Wilson, BSN CCRC.
RTI International (U10 HD36790) – Abhik Das, PhD; Dennis Wallace, PhD; Jamie E. Newman, PhD MPH; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS CCRP; Marie G. Gantz, PhD; Carolyn M. Petrie Huitema, MS CCRP; Kristin M. Zaterka-Baxter, RN BSN CCRP.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children's Hospital (U10 HD27880, M01 RR70, UL1 TR93) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; Susan R. Hintz, MD MS Epi; Marian M. Adams, MD; M. Bethany Ball, BS CCRC; Andrew W. Palmquist, RN BSN; Melinda S. Proud, RCP; Barbara Bentley, PhD; Elizabeth Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP; Lynne C. Huffman, MD Jean G. Kohn, MD MPH; Casey E. Krueger, PhD; Brian Tang, MD; Hali E.Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN; Anne Furey, MPH; Elisabeth C. McGowan, MD; Cecelia E Sibley PT MHA; Ana K. Brussa, MS OTR/L.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Fred J. Biasini, PhD; Kristen C. Johnston, MSN CRNP; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN BSN; Sally Whitley, MA OTR-L FAOTA; Amanda D. Soong, MD; Carin Kiser, MD; Leigh Ann Smith, CRNP; Sara Kryzwanski, MS; Richard V. Rector, PhD; Sarah Ryan, PhD; Kristy Domnanovich, PhD; Leslie Rodrigues, PhD.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) – Neil N. Finer, MD; Yvonne E. Vaucher, MD MPH; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Chris Henderson, RCP CRTT; Wade Rich, BSHS RRT; Radmila West PhD.
University of Iowa and Mercy Medical Center (U10 HD53109, M01 RR59) –Dan L. Ellsbury, MD; John A. Widness, MD; Tarah T. Colaizy, MD MPH; Jane E. Brumbaugh, MD; Michael J. Acarregui, MD; Diane L. Eastman, RN CPNP MA; Karen J. Johnson, RN BSN; Donia B. Campbell, RNC-NIC; Jacky R. Walker, RN.
University of New Mexico Health Sciences Center (U10 HD53089, UL1 TR41) – Kristi L. Watterberg, MD; Robin K. Ohls, MD; Janell F. Fuller, MD; Conra Backstrom Lacy, RN; Rebecca A. Montman, BSN RNC; Jean R. Lowe, PhD; Carol Hartenberger, BSN MPH.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women's and Children's Hospital of Buffalo (U10 HD68263, U10 HD40521, M01 RR44, UL1 TR42 – Carl T. D’Angio, MD.
University of Texas Health Science Center at Houston Medical School, Children's Memorial Hermann Hospital (U10 HD21373)– Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Allison Dempsey, PhD; Patricia W. Evans, MD; Charles E. Green, PhD; Margarita Jiminez, MD MPH; Janice John, CPNP; Patrick M. Jones, MD MA, Georgia E. McDavid, RN; M. Layne Poundstone, RN BSN; Saba Siddiki, MD; Daniel Sperry, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT (ASCP).
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children's Medical Center Dallas (U10 HD40689, M01 RR633) – Pablo J. Sánchez, MD; Roy J. Heyne, MD; Luc P. Brion, MD; LiJun Chen, RN, PhD; Alicia Guzman; Melissa H. Leps, RN; Nancy A. Miller, RN; Diana M. Vasil, RNC NIC; Lizette E. Torres, RN; Sally S. Adams, MS RN CPNP; Linda A. Madden, RN CPNP; Elizabeth Heyne, PsyD PA-C; Catherine Twell Boatman, MS CIMI.
University of Utah University Hospital, Intermountain Medical Center, LDS Hospital, and Primary Children's Medical Center (U10 HD53124, M01 RR64, UL1 TR105) – Roger G. Faix, MD; Bradley A. Yoder, MD; Anna Bodnar, MD; Karen A. Osborne, RN BSN CCRC; Shawna Baker, RN; Karie Bird, RN BSN; Jill Burnett, RNC BSN; Jennifer J. Jensen, RN BSN; Manndi C. Loertscher, BS CCRP; Carrie A. Rau, RN BSN CCRC; Cynthia Spencer, RNC BSN; Mike Steffen, PhD; Kimberlee Weaver-Lewis, RN MS; Sarah Winter, MD; Karen Zanetti, RN.
Wayne State University, University of Michigan, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; Athina Pappas, MD; John Barks, MD; Martha Carlson, MD; Angela Argento; PhD; Rebecca Bara, RN BSN; Laura A. Goldston, MA; Mary Johnson, RN BSN; Mary Christensen, RT; Stephanie Wiggins, MS.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1-RR024139, UL1 TR142) – Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Christine G. Butler, MD; Patricia Cervone, RN; Sheila Greisman, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Joanne Williams, RN BSN; Elaine Romano, MSN.
Footnotes
Disclosures: The authors have no disclosures or conflicts of interest to resolve
Conflict of interest
The authors declare no conflict of interest
References
- 1.Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011;128(4):e899–906. doi: 10.1542/peds.2011-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 3.Latal-Hajnal B, von Siebenthal K, Kovari H, Bucher HU, Largo RH. Postnatal growth in VLBW infants: significant association with neurodevelopmental outcome. J Pediatr. 2003;143(2):163–170. doi: 10.1067/S0022-3476(03)00243-9. [DOI] [PubMed] [Google Scholar]
- 4.Kan E, Roberts G, Anderson PJ, Doyle LW Victorian Infant Collaborative Study G. The association of growth impairment with neurodevelopmental outcome at eight years of age in very preterm children. Early Hum Dev. 2008;84(6):409–416. doi: 10.1016/j.earlhumdev.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Neubauer V, Griesmaier E, Pehbock-Walser N, Pupp-Peglow U, Kiechl-Kohlendorfer U. Poor postnatal head growth in very preterm infants is associated with impaired neurodevelopment outcome. Acta Paediatr. 2013;102(9):883–888. doi: 10.1111/apa.12319. [DOI] [PubMed] [Google Scholar]
- 6.Ramel SE, Demerath EW, Gray HL, Younge N, Boys C, Georgieff MK. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology. 2012;102(1):19–24. doi: 10.1159/000336127. [DOI] [PubMed] [Google Scholar]
- 7.Modi M, Saluja S, Kler N, Batra A, Kaur A, Garg P, et al. Growth and neurodevelopmental outcome of VLBW infants at 1 year corrected age. Indian Pediatr. 2013;50(6):573–577. doi: 10.1007/s13312-013-0170-5. [DOI] [PubMed] [Google Scholar]
- 8.Bocca-Tjeertes I, Bos A, Kerstjens J, de Winter A, Reijneveld S. Symmetrical and asymmetrical growth restriction in preterm-born children. Pediatrics. 2014;133(3):e650–656. doi: 10.1542/peds.2013-1739. [DOI] [PubMed] [Google Scholar]
- 9.Guellec I, Marret S, Baud O, Cambonie G, Lapillonne A, Roze JC, et al. Intrauterine Growth Restriction, Head Size at Birth, and Outcome in Very Preterm Infants. J Pediatr. 2015 doi: 10.1016/j.jpeds.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Leitner Y, Fattal-Valevski A, Geva R, Eshel R, Toledano-Alhadef H, Rotstein M, et al. Neurodevelopmental outcome of children with intrauterine growth retardation: a longitudinal, 10-year prospective study. J Child Neurol. 2007;22(5):580–587. doi: 10.1177/0883073807302605. [DOI] [PubMed] [Google Scholar]
- 11.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman JE, Bann CM, Vohr BR, Dusick AM, Higgins RD, et al. Follow-Up Study Group of Eunice Kennedy Shriver National Institute of Child H. Improving the Neonatal Research Network annual certification for neurologic examination of the 18–22 month child. J Pediatr. 2012;161(6):1041–1046. doi: 10.1016/j.jpeds.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayley N. Bayley scales of infant and toddler development. San Antonio (TX): Harcourt Assessment; 2006. [Google Scholar]
- 14.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res. 2014;75(5):670–674. doi: 10.1038/pr.2014.10. [DOI] [PubMed] [Google Scholar]
- 16.Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. 2012;161(2):222–228. e223. doi: 10.1016/j.jpeds.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin IJ, Tancredi DJ, Bertino E, Lee HC, Profit J. Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch Dis Child Fetal Neonatal Ed. 2015 doi: 10.1136/archdischild-2014-308095. [DOI] [PubMed] [Google Scholar]
- 18.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85(2):614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 19.Morgan C, McGowan P, Herwitker S, Hart AE, Turner MA. Postnatal head growth in preterm infants: a randomized controlled parenteral nutrition study. Pediatrics. 2014;133(1):e120–128. doi: 10.1542/peds.2013-2207. [DOI] [PubMed] [Google Scholar]
- 20.Stoltz Sjöström E1ÖI, Ahlsson F, Engström E, Fellman V, Hellström A, Källén K, Norman M, Olhager E, Serenius F, Domellöf M. Nutrient intakes independently affect growth in extremely preterm infants: results from a population-based study. Acta Paediatr. 2013;102(11):1067–1074. doi: 10.1111/apa.12359. [DOI] [PubMed] [Google Scholar]
- 21.Roggero P, Gianni ML, Orsi A, Amato O, Piemontese P, Liotto N, et al. Implementation of nutritional strategies decreases postnatal growth restriction in preterm infants. PLoS One. 2012;7(12):e51166. doi: 10.1371/journal.pone.0051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang CH, Frost RA. Glucocorticoids and TNFalpha interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med. 2006;12(11–12):291–299. doi: 10.2119/2006-00071.Lang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang CH, Frost RA. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor alpha. Metabolism. 2007;56(1):49–57. doi: 10.1016/j.metabol.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Fields DA, Demerath EW. Relationship of insulin, glucose, leptin, IL-6 and TNF-alpha in human breast milk with infant growth and body composition. Pediatr Obes. 2012;7(4):304–312. doi: 10.1111/j.2047-6310.2012.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad I, Zaldivar F, Iwanaga K, Koeppel R, Grochow D, Nemet D, et al. Inflammatory and growth mediators in growing preterm infants. J Pediatr Endocrinol Metab. 2007;20(3):387–396. doi: 10.1515/jpem.2007.20.3.387. [DOI] [PubMed] [Google Scholar]
- 26.Eliakim A, Nemet D, Ahmad I, Zaldivar F, Koppel R, Grochow D, et al. Growth factors, inflammatory cytokines and postnatal bone strength in preterm infants. J Pediatr Endocrinol Metab. 2009;22(8):733–740. doi: 10.1515/jpem.2009.22.8.733. [DOI] [PubMed] [Google Scholar]
- 27.Kuban KC, O'Shea TM, Allred EN, Fichorova RN, Heeren T, Paneth N, et al. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr Neurol. 2015;52(1):42–48. doi: 10.1016/j.pediatrneurol.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee I, Neil JJ, Huettner PC, Smyser CD, Rogers CE, Shimony JS, et al. The impact of prenatal and neonatal infection on neurodevelopmental outcomes in very preterm infants. J Perinatol. 2014;34(10):741–747. doi: 10.1038/jp.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]