Abstract
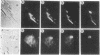
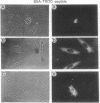
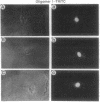
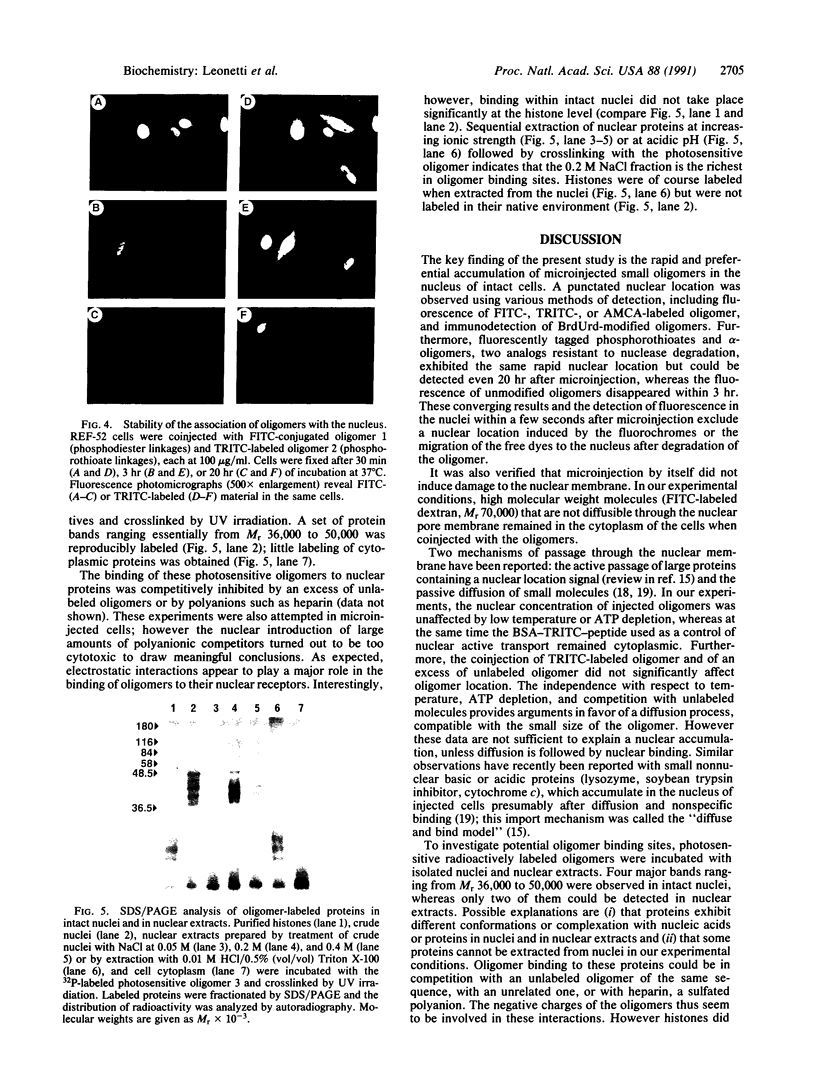
Antisense oligomers constitute an attractive class of specific tools for genetic analysis and for potential therapeutic applications. Targets with different cellular locations have been described, such as mRNA translation initiation sites, pre-mRNA splicing sites, or the genes themselves. However the mechanism(s) of action and the intracellular distribution of antisense oligomers remain poorly understood. Antisense oligomers conjugated with various fluorochromes or with BrdUrd were microinjected into the cytoplasm of somatic cells, and their cellular distribution was monitored by fluorescence microscopy in fixed and nonfixed cells. A fast translocation in the nuclei and a concentration on nuclear structures were observed whatever probe was used. Nuclear transport occurs by diffusion since it is not affected by depletion of the intracellular ATP pool, temperature, or excess unlabeled oligomer. Accumulation of the oligomers in the nuclei essentially takes place on a set of proteins preferentially extracted between 0.2 M and 0.4 M NaCl as revealed by crosslinking of photosensitive oligomers. The relationship between nuclear location of antisense oligomers and their mechanism of action remains to be ascertained and could be of major interest in the design of more efficient antisense molecules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Barsony J., Marx S. J. Immunocytology on microwave-fixed cells reveals rapid and agonist-specific changes in subcellular accumulation patterns for cAMP or cGMP. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1188–1192. doi: 10.1073/pnas.87.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutorin A. S., Gus'kova L. V., Ivanova E. M., Kobetz N. D., Zarytova V. F., Ryte A. S., Yurchenko L. V., Vlassov V. V. Synthesis of alkylating oligonucleotide derivatives containing cholesterol or phenazinium residues at their 3'-terminus and their interaction with DNA within mammalian cells. FEBS Lett. 1989 Aug 28;254(1-2):129–132. doi: 10.1016/0014-5793(89)81023-3. [DOI] [PubMed] [Google Scholar]
- Breeuwer M., Goldfarb D. S. Facilitated nuclear transport of histone H1 and other small nucleophilic proteins. Cell. 1990 Mar 23;60(6):999–1008. doi: 10.1016/0092-8674(90)90348-i. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Gariépy J., Schoolnik G., Kornberg R. D. Synthetic peptides as nuclear localization signals. Nature. 1986 Aug 14;322(6080):641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- Goodchild J. Conjugates of oligonucleotides and modified oligonucleotides: a review of their synthesis and properties. Bioconjug Chem. 1990 May-Jun;1(3):165–187. doi: 10.1021/bc00003a001. [DOI] [PubMed] [Google Scholar]
- Heikkila R., Schwab G., Wickstrom E., Loke S. L., Pluznik D. H., Watt R., Neckers L. M. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. 1987 Jul 30-Aug 5Nature. 328(6129):445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Kulka M., Smith C. C., Aurelian L., Fishelevich R., Meade K., Miller P., Ts'o P. O. Site specificity of the inhibitory effects of oligo(nucleoside methylphosphonate)s complementary to the acceptor splice junction of herpes simplex virus type 1 immediate early mRNA 4. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6868–6872. doi: 10.1073/pnas.86.18.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang I., Scholz M., Peters R. Molecular mobility and nucleocytoplasmic flux in hepatoma cells. J Cell Biol. 1986 Apr;102(4):1183–1190. doi: 10.1083/jcb.102.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre M., Bayard B., Lebleu B. Specific antiviral activity of a poly(L-lysine)-conjugated oligodeoxyribonucleotide sequence complementary to vesicular stomatitis virus N protein mRNA initiation site. Proc Natl Acad Sci U S A. 1987 Feb;84(3):648–652. doi: 10.1073/pnas.84.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManaway M. E., Neckers L. M., Loke S. L., al-Nasser A. A., Redner R. L., Shiramizu B. T., Goldschmidts W. L., Huber B. E., Bhatia K., Magrath I. T. Tumour-specific inhibition of lymphoma growth by an antisense oligodeoxynucleotide. Lancet. 1990 Apr 7;335(8693):808–811. doi: 10.1016/0140-6736(90)90934-w. [DOI] [PubMed] [Google Scholar]
- Picard D., Salser S. J., Yamamoto K. R. A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell. 1988 Sep 23;54(7):1073–1080. doi: 10.1016/0092-8674(88)90122-5. [DOI] [PubMed] [Google Scholar]
- Thenet S., Morvan F., Bertrand J. R., Gautie C., Malvy C. Alpha are more stable than beta anomer oligonucleotides in 3T3 cellular extracts. Biochimie. 1988 Dec;70(12):1729–1732. doi: 10.1016/0300-9084(88)90031-4. [DOI] [PubMed] [Google Scholar]
- Wachter L., Jablonski J. A., Ramachandran K. L. A simple and efficient procedure for the synthesis of 5'-aminoalkyl oligodeoxynucleotides. Nucleic Acids Res. 1986 Oct 24;14(20):7985–7994. doi: 10.1093/nar/14.20.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubov L. A., Deeva E. A., Zarytova V. F., Ivanova E. M., Ryte A. S., Yurchenko L. V., Vlassov V. V. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci U S A. 1989 Sep;86(17):6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]