Abstract
Objective
To assess whether weight gain above or below Institute of Medicine (IOM) recommended amounts in an ethnically diverse obstetric population with normal glucose tolerance is associated with differences in neonatal adiposity.
Study Design
In this prospective cohort study, healthy women with normal glucose tolerance based on the International Association of Diabetes and Pregnancy Study Groups guidelines were enrolled. Gestational weight at multiple time points were collected. Neonatal adiposity was measured by air displacement plethysmography at 24-72 hours of life. Analyses included Fisher's exact test, ANOVA, and a trajectory analysis using a group-based weight gain trajectory model with a censored normal distribution.
Result
Overweight and obese women were more likely to exceed IOM weight gain guidelines. Regardless, there was no significant difference in %body fat of neonates born to mothers who either met or exceeded gestational weight gain guidelines. Gestational weight gain timing influenced neonatal anthropometrics: women who gained excessively by the first prenatal visit had neonates with significantly higher birth weight (3.91 kg vs. 3.45 kg, p<0.001), and %body fat (13.7% vs. 10.9%, p=0.0001) compared to women who had steady, moderate gestational weight gain.
Conclusion
Avoidance of excessive gestational weight gain in the first trimester may prevent high amounts of neonatal adiposity.
Introduction
Excessive neonatal adiposity is a likely risk factor for development of childhood obesity and associated adverse metabolic health.1,2 Diabetes in pregnancy is regarded as the classical model of maternal over-nutrition leading to excessive neonatal fat accrual.3 An adverse intrauterine environment composed of excess maternal fuels,4 including hyperglycemia below the diagnostic threshold for diabetes, insulin resistance, high amounts of circulating lipids, and high free fatty acids may in combination lead to excessive neonatal adiposity at birth. Obesity in pregnancy and excessive gestational weight gain are both associated with this adverse intrauterine environment.5-8 However, hyperglycemia in pregnancy is thought to have the most profound effects on neonatal fat accrual,9 thus studying neonatal anthropometric outcomes requires knowledge of both maternal glucose tolerance and neonatal adiposity.
Until recently, few studies have documented neonatal adiposity as a potential marker of adverse pregnancy outcomes and/or predictor of adverse metabolic health. It is now recognized that birth weight is not an adequate surrogate of body composition,10 nor a good predictor of later adiposity.11 Direct measurements of neonatal adiposity are required. Newer, non-invasive methods to accurately measure neonatal adiposity are now available thus it is important to determine whether excessive neonatal adiposity is associated with the development of childhood obesity. Furthermore, whether excessive neonatal adiposity is associated with adverse metabolic health in later childhood, irrespective of the presence of childhood obesity, is not known. To address these research questions, prospective cohorts with accurate maternal weight gain measures and neonatal anthropometrics must be followed long-term.
The specific finding that offspring born to mothers with gestational diabetes mellitus (GDM), regardless of birth weight, were more likely to have increased neonatal adiposity,12 and become obese in later childhood,13 was described years before the current “obesity epidemic.” Whether increased childhood obesity rates are due, in part, to exposure to an adverse intrauterine environment, including maternal obesity, excessive gestational weight gain, or other factors is a topic of great research interest. Several groups have reported associations between excessive gestational weight gain and increased neonatal adiposity.8,14,15 However, few studies have excluded women with abnormal glucose tolerance or examined the timing of weight gain as it relates to neonatal adiposity.
The primary objective of this study was to determine the effect of gestational weight gain (GWG) in a diverse maternal population of varying pre-pregnancy BMI groups on neonatal adiposity. All women enrolled had normal glucose tolerance based upon 2-hr fasting 75g oral glucose tolerance tests using the thresholds recommended by the International Association of Diabetes in Pregnancy Study Groups (IADPSG) Consensus panel.16
Research Design and Methods
Subjects
Women ages 18-40 years, carrying singleton pregnancies, were recruited from obstetric practices associated with a large academic hospital. At the time of study enrollment, these practices had implemented the IADPSG recommendations utilizing a one-step approach to diagnose GDM.16 Healthy pregnant women were eligible for this study if they had normal glucose tolerance on a fasting 75-g Oral Glucose Tolerance Test (OGTT) at 24-28 weeks’ gestation, defined by the IADPSG criteria: fasting plasma glucose < 92 mg/dl, 1 hr plasma glucose <180 mg/dl, and 2 hr plasma glucose <153 mg/dl. Women were excluded from the study if they had carried more than 3 pregnancies to term, took glucocorticoids, insulin, or anti-hypertensive therapy, or delivered their newborn < 37 weeks gestation.
This study was approved by the Institutional Review Board at Northwestern University for conduct of research on human subjects. Participants gave informed, written consent for themselves and their newborns to enroll in the study.
Maternal measurements
The following gestational weights were collected on each participant: self-reported pre-pregnancy weight (confirmed by medical records in 80% of participants), measured weight at first prenatal visit, early second trimester measured weight, measured weight at the time of the OGTT and measured weight at the last prenatal visit. All weights were measured on calibrated scales by trained medical assistants. For each weight measurement, women removed shoes and wore light clothing. Measured height and self-reported pre-pregnancy weight were used to calculate pre-pregnancy BMI. Total gestational weight gain was calculated by subtracting the self-reported pre-pregnancy weight from the weight at the last prenatal visit.
Neonatal Anthropometrics
Measurements of neonates occurred between 24 and 72 hours of life and were obtained by one of two trained examiners. Neonate length was obtained with the baby positioned on a hard-surface measuring board, head in the mid-line position, legs held straight, and a moveable footboard was pressed against the balls of the feet. This length measurement was recorded to the nearest 0.1 cm, repeated, and the results averaged. The Pea Pod Infant Body Composition System (Cosmed, Rome, Italy), which uses the methodology of air displacement plethysmography, was used to determine neonatal adiposity. Air displacement testing procedures have been previously described.17 Briefly, the PEA POD was calibrated by a menu-operated system according to the manufacturer guidelines. First, a wig-cap was placed on the baby; next the baby was placed naked on the Pea Pod scale and weight was measured to the nearest 0.0001 kg. Then the neonate was placed inside the Pea Pod chamber for 2 minutes during volume measurements. Using pressure-volume equations, body composition, including fat mass and fat-free mass was calculated to provide adiposity (reported as %).
Statistical Analysis
A power calculation for sample size determined that 190 pregnant mothers would be required to have 80% power to detect differences in neonatal adiposity between those born to mothers with and without excessive gestational weight gain. The final sample size with data available for analysis was 176 maternal-neonate pairs. Descriptive data were summarized using means and standard deviations for continuous data and percentages for categorical data. Maternal pre-pregnancy weight was related to IOM weight gain categories using Fisher's exact test. Neonatal adiposity was compared across IOM guidelines using one-way analysis of variance with post-hoc comparisons using the Tukey-Kramer method. Weight gain trajectories were analyzed using the censored normal (C-Norm) group-based trajectory model which determined patient groupings based on maximal difference in weight gain trajectories over time.18 Data analyses were performed using SAS 9.4 (SAS Institute Inc. Cary, NC) and Stata 13 (StataCorp LP. College Station, TX).
Results
Maternal and neonatal characteristics of the study participants are displayed in Table 1. Of the participants, 62.5% had a healthy pre-pregnancy weight, 3% were underweight, and 35% had an overweight or obese pre-pregnancy weight; 40% of women were from a minority racial or ethnic group, and the majority of participants were married and had a college degree. Three mothers (1.8% of cohort) reported smoking cigarettes during their pregnancy. Mean glucose levels (fasting, 1-hr, 2-hr) on fasting 2-hr tolerance tests were well below the cut-off values for diagnosis of gestational diabetes mellitus (16). All neonates studied were full-term with a mean gestational age of 39 and 5/7 weeks. Neonatal body composition measurements were performed at a mean of 36 hours of life, standard deviation (sd) of 14 hours.
Table 1.
Maternal and Neonatal Characteristics
| Maternal Characteristics: N= 168 | Mean (s.d.) and/or Frequency (%) | |
|---|---|---|
| Pre-pregnancy BMI (kg/m2) | 24.8 (5.8) | |
| Underweight (<18.5) | 17.9 (0.3) | 3.0% |
| Normal Weight (18.5-24.9) | 21.7 (1.7) | 62.5% |
| Overweight (25-29.9) | 26.6 (1.4) | 15.5% |
| Obese (>30) | 34.9 (4.4) | 19% |
| Maternal Height (in) | 65.15 (2.7) | |
| Primiparous | 45.2% | |
| Race/Ethnicity | ||
| White/ Non-Hispanic | 59.5% | |
| Black | 16.7% | |
| Hispanic | 10.7% | |
| Asian/ Asian Indian | 13.1% | |
| Married | 83.9% | |
| Education (college degree) | 88.6% | |
| OGTT (fasting 75-g) mg/dl | ||
| Fasting | 76.5 (5.6) | |
| 1 hour | 115.4 (26.6) | |
| 2 hour | 100.2 (19.7) | |
| Gestational Weight Gain (lbs)* | ||
| Below IOM guidelines | 15.4 (10.2) | 26.2% |
| Within guidelines | 26.8 (5.7) | 33.3% |
| Above guidelines | 37.7 (8.9) | 40.5% |
| Cesarean Section | 23.8% | |
|
Newborn Characteristics: N= 168 | ||
| Gestational age (weeks) | 39.5 (1.1) | |
| Sex (% male) | 51.2% | |
| Length (cm) | 50.9 (2.2) | |
| Birth weight (g) | 3469 (499) | |
| Fat-free mass (g)^ | 2900 (424) | |
| Fat mass (g)^ | 367 (166) | |
| Adiposity (%)^ | 10.8 (3.70) | |
BMI: Body mass index, OGTT: oral glucose tolerance test, IOM: Institute of Medicine
IOM guidelines for gestational weight gain are based on pre-pregnancy BMI19
N= 156
Maternal measured weights were collected from medical records at four time points (mean +/− sd): weight at first prenatal visit (9.5 +/− 3.8 weeks), early second trimester weight (19.5 +/− 1.5 weeks), weight at the time of the OGTT (26.1 +/− 1.7 weeks) and weight at the last prenatal visit (39.0 +/− 1.1 weeks).
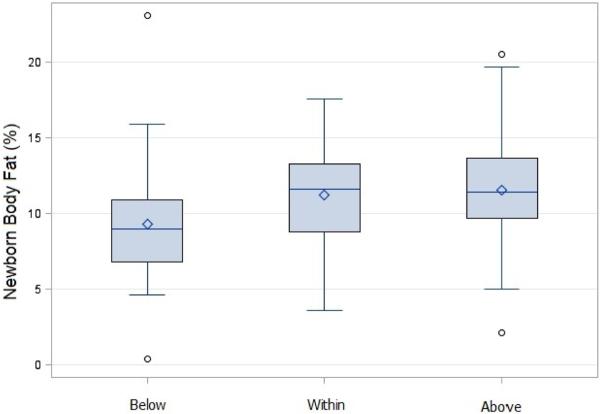
Using a cross-tabulation analysis of maternal pre-pregnancy BMI relative to IOM GWG guidelines, overweight and obese women were more likely to exceed IOM recommended amounts of weight gain compared to normal weight women (p<0.0001, Table 2). Neonatal adiposity for each IOM GWG category is displayed in Figure 1. Mean percent body fat of neonates born to women who gained within guidelines (10.6 +/− 3.4%) was not different compared to neonates of women who gained below (9.0 +/− 4.1%) or above (11.8 +/− 3.4%) guidelines. The neonates born to women who gained below guidelines had significantly lower body fat compared to neonates born to women who gained above guidelines (difference 2.8%, adjusted p=0.004).
Table 2.
Overweight and Obese Women are More Likely than Normal Weight Women to Exceed IOM Guidelines for Weight Gain in Pregnancy.
| Institute of Medicine Guidelines | Maternal Pre-Pregnancy BMI | |||
|---|---|---|---|---|
| Underweight | Normal Weight | Overweight | Obese | |
| Did not meet | 60% | 22.8% | 0 | 18.8% |
| Met | 40% | 41.0% | 26.9% | 18.8% |
| Exceeded | 0 | 36.2% | 73.1% | 62.5% |
| Totals: N= 168 | 5 | 105 | 26 | 32 |
Relative to IOM Guidelines * Maternal Pre-pregnancy BMI Cross-tabulation: Fisher's exact test p < 0.0001
Percentages represent the number of women within each BMI category (columns) who did not meet, met, or exceeded IOM guidelines for weight gain in pregnancy.
Overweight and obese women were more likely than normal weight women to exceed IOM guidelines.
Figure 1. Neonatal Adiposity Based on IOM Gestational Weight Gain Category.
Adiposity (percent body fat) of neonates (N=156) born to women according to the Institute of Medicine guidelines for gestational weight gain. Box plots represent means (solid lines) +/− standard deviations and whiskers display minimums and maximums. Mean percent body fat of neonates born to mothers who gained within guidelines (10.6 +/− 3.4%) was not different compared to neonates of mothers who gained below (9.0 +/− 4.1%) or above guidelines (11.8 +/− 3.4%). Neonates born to mothers who gained below guidelines had significantly lower body fat compared to neonates born to mothers who gained above guidelines (adjusted p=0.004).
Sex differences in neonatal adiposity were determined: the mean body fat among females was 11.7%, and among males, 10.1% (p=0.006). This difference remained significant when adjusted for gestational age, maternal age, and racial/ethnic group.
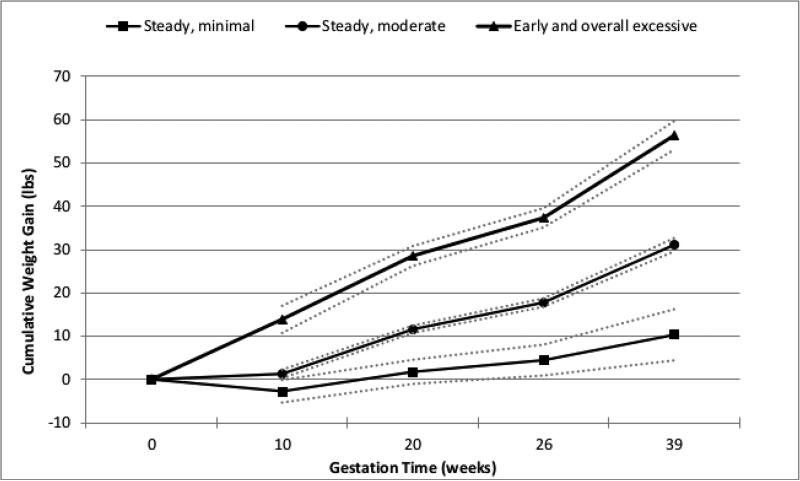
To assess the influence of GWG timing on neonatal anthropometrics, an exploratory group-based trajectory model was created (Figure 2) with the estimated marginal means of neonatal outcomes displayed in Table 3. Three distinct weight gain trajectories over the 40-week pregnancy time period were determined by this model. The majority of participants displayed GWG trajectories of steady, moderate gain (79%). A smaller group of participants (12%) were classified as having steady, minimal gain throughout pregnancy; this group gained approximately 11 lbs on average by the end of pregnancy. Finally, 9% of participants had early and overall excessive weight gain- 15 lbs on average by the first prenatal visit (at approximately 10 weeks gestation), 60 lbs on average by the end of pregnancy. The neonatal outcomes of birth weight and adiposity were different among the 3 trajectory groups. Neonates born to participants with early and overall excessive gain had significantly higher birth weight, (3.91 kg compared to 3.45 kg in participants with steady, moderate gain, p<0.001), and significantly more adiposity (13.7% compared to 10.9%, p=0.0001). In summary, neonates born to mothers with early and overall excessive gain, had 26% greater adiposity compared to neonates born to mothers with steady, moderate gain.
Figure 2. Gestational Weight Gain Trajectories.
Estimated weight gain trajectories (solid lines) and their 95% confidence intervals (dotted lines). Group-based trajectory model determined 3 distinct patterns of cumulative weight gain over the 40-week gestation period. The majority of participants were classified in the steady, moderate gain group (79%). The remainder were in the steady, minimal gain group (12%) or the early and overall excessive gain group (9%).
Table 3.
Timing of Gestational Weight Gain and Neonatal Anthropometrics
| GWG Timing | Birthweight (kg) | Adiposity (%) |
|---|---|---|
| Steady, Minimal Gain | 3.25 (0.1) | 8.3 (0.9) |
| Steady, Moderate Gain | 3.45 (0.04) | 10.9 (0.3) |
| Early and Overall Excessive Gain | 3.91 (0.1) | 13.7 (0.9) |
| p-value | <0.0001 | 0.0001 |
Estimated marginal means (standard error) adjusted for maternal age, race/ethnicity, maternal pre-pregnancy BMI, fasting glucose at the time of OGTT, gestational age, and neonatal sex.
Referent= Steady, moderate gain group
Discussion
The primary objective of this study was to assess the impact of gestational weight gain above the IOM guidelines19 on adiposity in neonates. In this study of women with documented normal glucose tolerance based upon a 2-hr 75-g fasting OGTT,16 neonatal adiposity did not differ between groups with excessive versus recommended weight gain as advised by the IOM guidelines. However, using an exploratory trajectory analysis model, and incorporating multiple measurements of maternal weight during pregnancy, we found altered neonatal anthropometric outcomes of higher birth weight and higher amounts of adiposity in a subgroup that had excessive weight gain in the first trimester. Women with excessive weight gain in the first trimester had neonates who, on average, weighed 13% more and had 26% more adiposity than neonates born to women with the recommended minimal weight gain in the first trimester.
These results of an association between excessive weight gain in early pregnancy and increased neonatal adiposity extend the findings reported by Davenport et al in a similar-sized cohort.20 In that study, neonates born to women with excessive weight gain in the first half of pregnancy were two and half times more likely to have increased percent body fat. Yet, and similar to the results in this report, neonatal body fat did not differ between women whose total GWG was within IOM guidelines compared to women who gained excessively. Neonatal body fat in the Davenport study was calculated using a validated anthropometric estimation equation incorporating measurements of neonate weight, length and suprailiac skinfold thickness by calipers.21 In the present study, the non-operator dependent method of air displacement plethysmography was used to determine neonatal body fat.
Results of this study differ from other studies reporting excessive GWG with increased neonatal adiposity.8,22 To our knowledge, six other observational studies in the literature have reported on associations between GWG and neonatal adiposity.7,8,14,15,20,22 The difference in our results compared to the majority of these other studies is likely due to the inclusion in the present study of only women with documented normal glucose tolerance. Newborn adiposity is known to be linearly related to maternal glucose across the range of fasting and post-load values below levels diagnostic of diabetes.23 The present study is the first to include stringent glucose tolerance enrollment criteria16 among participants providing assurance that none of the participants had gestational diabetes. Thus, the results of the present study may underestimate the actual number of American women who gain excessive weight in early pregnancy. Women who gain excessively in early pregnancy are more likely to have insulin resistance,24 and a portion of these women will progress to abnormal glucose tolerance by mid-pregnancy.25 It has recently been demonstrated that insulin resistance in the first half of pregnancy is highly predictive of increased neonatal adiposity.26 The excess of maternal fuels and hormones (i.e. fetal over-nutrition) associated with insulin resistance include hyperglycemia, circulating lipids, free fatty acids, and leptin, all of which, individually and in conjunction, have been correlated with fetal fat accretion.27,28
A major knowledge gap exists in our understanding of the regulation of fetal fat accretion, particularly the importance of each stage of pregnancy in determining neonatal adiposity. In this analysis, early excessive GWG was associated with increased neonatal adiposity. We, and others, suggest that excessive weight gain early in pregnancy drives maternal insulin resistance whereas fetal growth drives late pregnancy weight gain.29 A strong determinant of fetal growth that we are not able to account for is genetic contribution.30
Consistent with known differences of body composition in males and female neonates, in this study, the females had, on average, 1.6% more body fat than the males. This difference was due to higher amounts of fat mass in the females, compared to males, and higher amounts of fat free mass (i.e. muscle mass) in the males compared to females. This sexual dimorphism in fetal fat accretion has been well described.31
In this cohort, 19.6% of participants gained below the IOM guidelines for weight gain in pregnancy. The neonates born to these women had significantly lower adiposity compared to women who gained above guidelines. The significance of this lower adiposity among these neonates whose mothers gained less weight than recommended during pregnancy is not clear.
This study had some weaknesses, including the relatively small sample size and highly educated participants thus limiting the ability to generalize study findings. Additionally, participants in this study underwent a one-step GDM diagnostic test rather than the two-step approach32 utilized by the majority of obstetricians in the United States. Importantly, by studying women with normal glucose tolerance using stringent criteria, we have avoided a potential confounder of the outcome of interest: neonatal adiposity.
The results of this study suggest that excessive weight gain in early pregnancy is associated with higher adiposity in neonates. The Institute of Medicine recommends that women gain not more than 5 pounds in the first trimester.19 Women in this study who gained 15 pounds by their first prenatal visit had babies with significantly greater adiposity compared with babies born to mothers with recommended first trimester weight gain. Attempts to limit gestational weight gain, especially among overweight and obese women who are at highest risk of gaining excessively, needs to begin at or before conception. This recommendation echoes the IOM recommendations regarding achieving a healthy BMI prior to conception for optimal pregnancy health. As many pregnancies are unintended, public health campaigns addressing healthy pregnancy weight gains and the avoidance of weight gain in the first trimester are necessary.
In summary, gestational weight gain is complex and driven by a multitude of factors. The relationship between GWG and subsequent neonatal size is dependent on maternal fuels, placental efficiency, and genetic components. This list is not exhaustive and we are just beginning to understand the interplay between these factors. Modifiable factors such as avoiding early excessive GWG may improve the maternal milieu and prevent high amounts of neonatal adiposity. We speculate that this strategy could have a positive long-term impact on reducing childhood obesity rates.
Acknowledgements
We thank the participants who volunteered for this study.
Funding for this study was provided by grant number K12 HD055884 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
The authors would like to acknowledge the critical review and comments provided by Alan Peaceman, MD and Linda Van Horn, RD, PhD, both at Northwestern University Feinberg School of Medicine.
Funding Source: Funding for this study was provided by grant number K12 HD055884 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The funding source had no involvement in the study design, data analysis and interpretation, or writing of this manuscript.
Footnotes
Disclosure Statement: The authors report no conflicts of interest.
Contributor Information
Jami L. Josefson, Ann & Robert H. Lurie Children's Hospital of Chicago, Division of Endocrinology, Department of Pediatrics, Northwestern University Feinberg School of Medicine.
Hannah Simons, Planned Parenthood Federation of America.
Dinah M. Zeiss, Department of Medicine-Endocrinology, Northwestern University Feinberg School of Medicine.
Boyd E. Metzger, Department of Medicine-Endocrinology, Northwestern University Feinberg School of Medicine.
References
- 1.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J of Clin Nutr. 2009;90(5):1303–13. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vohr BR, McGarvey ST, Tucker R. Effects of maternal gestational diabetes on offspring adiposity at 4-7 years of age. Diabetes Care. 1999;22(8):1284–91. doi: 10.2337/diacare.22.8.1284. [DOI] [PubMed] [Google Scholar]
- 3.Freinkel N. Banting Lecture. Of pregnancy and progeny. Diabetes. 1980;29(12):1023–35. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 4.Metzger BE, Phelps RL, Freinkel N, Navickas IA. Effects of gestational diabetes on diurnal profiles of plasma glucose, lipids, and individual amino acids. Diabetes Care. 1980;3(3):402–9. doi: 10.2337/diacare.3.3.402. [DOI] [PubMed] [Google Scholar]
- 5.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J ObstetGynecol. 2006;195(4):1100–3. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 6.HAPO Study Cooperative Research Group Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with maternal body mass index. BJOG. 2010;117(5):575–84. doi: 10.1111/j.1471-0528.2009.02486.x. [DOI] [PubMed] [Google Scholar]
- 7.Badon SE, Dyer AR, Josefson JL. HAPO Study Cooperative Research Group. Gestational weight gain and neonatal adiposity in the Hyperglycemia and Adverse Pregnancy Outcome study-North American region. Obesity (Silver Spring) 2014;22(7):1731–8. doi: 10.1002/oby.20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P, et al. Higher infant body fat with excessive gestational weight gain in overweight women. American Journal of Obstetrics and Gynecology. 2011;205(3):211–7. doi: 10.1016/j.ajog.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitelaw A. Subcutaneous fat in newborn infants of diabetic mothers: An indication of quality of diabetic control. Lancet. 1977;1(8001):15–8. doi: 10.1016/s0140-6736(77)91654-3. [DOI] [PubMed] [Google Scholar]
- 10.Oken E, Gillman MW. Fetal origins of obesity. Obesity Research. 2003;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 11.Pettitt DJ, Knowler WC, Bennett PH, Aleck KA, Baird HR. Obesity in offspring of diabetic Pima Indian women despite normal birth weight. Diabetes Care. 1987;10(1):76–80. doi: 10.2337/diacare.10.1.76. [DOI] [PubMed] [Google Scholar]
- 12.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189(6):1698–704. doi: 10.1016/s0002-9378(03)00828-7. [DOI] [PubMed] [Google Scholar]
- 13.Silverman BL, Landsberg L, Metzger BE. Fetal hyperinsulinism in offspring of diabetic mothers. Association with the subsequent development of childhood obesity. Ann N Y Acad Sci. 1993;699:36–45. doi: 10.1111/j.1749-6632.1993.tb18835.x. [DOI] [PubMed] [Google Scholar]
- 14.Waters TP, Huston-Presley L, Catalano PM. Neonatal body composition according to the revised institute of medicine recommendations for maternal weight gain. J Clin Endocrinol Metabol. 2012;97(10):3648–54. doi: 10.1210/jc.2012-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starling AP, Brinton JT, Glueck DH, Shapiro AL, Harrod CS, Lynch AM, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J of Clin Nutr. 2015;101(2):302–9. doi: 10.3945/ajcn.114.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res. 2003;53(3):486–92. doi: 10.1203/01.PDR.0000049669.74793.E3. [DOI] [PubMed] [Google Scholar]
- 18.Jones BL, Nagin DS, Roeder K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociol Methods Res. 2001;29(3):374–93. [Google Scholar]
- 19.Institute of Medicine . Weight gain during pregnancy: Reexamining the guidelines. The National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 20.Davenport MH, Ruchat SM, Giroux I, Sopper MM, Mottola MF. Timing of excessive pregnancy-related weight gain and offspring adiposity at birth. Obstet Gynecol. 2013;122(2):255–61. doi: 10.1097/AOG.0b013e31829a3b86. [DOI] [PubMed] [Google Scholar]
- 21.Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol. 1995;173(4):1176–81. doi: 10.1016/0002-9378(95)91348-3. [DOI] [PubMed] [Google Scholar]
- 22.Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women's Survey. Am J Clin Nutr. 2010;91(6):1745–51. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J of Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 24.Walsh JM, McGowan CA, Mahony RM, Foley ME, McAuliffe FM. Obstetric and metabolic implications of excessive gestational weight gain in pregnancy. Obesity (Silver Spring) 2014;22(7):1594–600. doi: 10.1002/oby.20753. [DOI] [PubMed] [Google Scholar]
- 25.Herring SJ, Oken E, Rifas-Shiman SL, Rich-Edwards JW, Stuebe AM, Kleinman KP, et al. Weight gain in pregnancy and risk of maternal hyperglycemia. Am J Obstet Gynecol. 2009;201(1):61–67. doi: 10.1016/j.ajog.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crume TL, Shapiro AL, Brinton JT, Glueck DH, Martinez M, Kohn M, et al. Maternal Fuels and Metabolic Measures during Pregnancy and Neonatal Body Composition: The Healthy Start Study. J Clin Endocrinol Metab. 2015;100(4):1672–80. doi: 10.1210/jc.2014-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–80. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josefson JL, Zeiss DM, Rademaker AW, Metzger BE. Maternal leptin predicts adiposity of the neonate. Horm Res Paediatr. 2014;81(1):13–9. doi: 10.1159/000355387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawlor DA, Relton C, Sattar N, Nelson SM. Maternal adiposity--a determinant of perinatal and offspring outcomes? Nat Rev Endocrinol. 2012;8(11):679–88. doi: 10.1038/nrendo.2012.176. [DOI] [PubMed] [Google Scholar]
- 30.Chawla R, Badon SE, Rangarajan J, Reisetter AC, Armstrong LL, Lowe LP, et al. Genetic risk score for prediction of newborn adiposity and large-for-gestationalage birth. J Clin Endocrinol and Metab. 2014;99(11):E2377–86. doi: 10.1210/jc.2013-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gale C, Logan KM, Jeffries S, Parkinson JR, Santhakumaran S, Uthaya S, et al. Sexual dimorphism in relation to adipose tissue and intrahepatocellular lipid deposition in early infancy. Int J Obes (Lond) 2015;39(4):629–32. doi: 10.1038/ijo.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Committee on opinion no. 504: Screening and diagnosis of gestational diabetes mellitus. Obstet Gynecol. 2011;118(3):751–3. doi: 10.1097/AOG.0b013e3182310cc3. [DOI] [PubMed] [Google Scholar]