Abstract
Alpha hemolysin (Hla) is a pore-forming toxin produced by most Staphylococcus aureus isolates. Hla is reported to play a key role in the pathogenesis of staphylococcal infections, such as skin and soft tissue infection, pneumonia, and lethal peritonitis. This study makes use of a novel recombinant subunit vaccine candidate (AT62) that was rationally designed based on the Hla heptameric crystal structure. AT62 comprises a critical structural domain at the N terminus of Hla, and it has no inherent toxic properties. We evaluated the efficacy of AT62 in protection against surgical wound infection and skin and soft tissue infection. Mice were vaccinated on days 0, 14, and 28 with 20 μg AT62 or bovine serum albumin (BSA) mixed with Sigma adjuvant system®. Mice immunized with AT62 produced a robust antibody response against native Hla. In the surgical wound infection model, mice immunized with AT62 and challenged with a USA300 S. aureus strain showed a significantly reduced bacterial burden in the infected tissue compared to animals given BSA. Similarly, mice passively immunized with rabbit IgG to AT62 showed reduced wound infection and tissue damage. Subcutaneous abscess formation was not prevented by immunization with AT62. However, in a skin necrosis infection model, immunization with the AT62 vaccine resulted in smaller lesions and reduced mouse weight loss compared to controls. Although AT62 immunization reduced tissue necrosis, it did not reduce the bacterial burdens in the lesions compared to controls. Our data indicate that AT62 may be a valuable component of a multivalent vaccine against S. aureus.
Keywords: Staphylococcus aureus, alpha hemolysin, vaccine, skin and soft tissue infection, mouse infection models
Introduction
Staphylococcus aureus is a Gram-positive bacterial pathogen that is an important threat to humans, most commonly causing skin and soft tissue infections, but also responsible for bacteremia and sepsis, with metastatic complications. An efficacious vaccine to prevent S. aureus infections in humans is needed, but the design of an effective vaccine has proven to be challenging. A successful vaccine will likely target multiple S. aureus virulence factors, including surface polysaccharides, cell wall associated proteins, and detoxified toxins. Alpha hemolysin (Hla) is a pore-forming secreted toxin encoded by the chromosomal gene hla, and it is expressed by most S. aureus isolates [1]. Hla plays a key role in the pathogenesis of staphylococcal infections, such as skin and soft tissue infections [2], pneumonia [3], and lethal peritonitis [4]. Hla is a prime vaccine target for prevention of tissue damage and complications of staphylococcal infections. An attenuated Hla mutant (most commonly HlaH35L) has been investigated either as a single component vaccine or included in a multicomponent vaccine formulation by a large number of groups, including both academic and corporate researchers [2-9].
Whereas HlaH35L is an effective vaccine, a single point mutation is not generally considered sufficiently safe to be developed as a vaccine for human use. Several studies have identified mutations that have restored toxicity to the H35 mutants of Hla [10, 11]. This study was designed to evaluate in relevant preclinical infection models the efficacy of a novel recombinant subunit vaccine candidate for Hla, called AT62, that was rationally designed based on the heptameric crystal structure of Hla [8]. AT62 comprises the first 62 amino acids of Hla, a structural domain at the amino terminus of Hla that is critically involved in toxin oligomerization but has no inherent toxic properties. In previous studies, AT62 was shown to protect mice against staphylococcal sepsis and pneumonia [8]. In this study we evaluate the protective efficacy of AT62 in murine models of skin and soft tissue infection (SSTI), including surgical wound infection, subcutaneous abscess formation, and necrotic skin lesions.
Methods
Hemolytic assay
The relative toxicity of Hla and AT62 was evaluated in a standard rabbit erythrocyte hemolytic assay. Briefly, anticoagulated rabbit blood (Colorado Serum Company) was centrifuged, and the erythrocyte pellet was washed twice before suspension in sufficient PBS to achieve an 8% (vol/vol) concentration. Serial two-fold dilutions of AT62 or native Hla (IBT Bioservices) were mixed in 96-well plates with an equal volume of erythrocytes to achieve final concentrations of 4% erythrocytes and initial concentrations of 200 μg/ml for AT62 and 25 μg/ml for native Hla. After incubation at 37°C for 30 min, the plates were centrifuged, and 100 μl aliquots of the supernatants were transferred into wells of a microtiter plate (Nunc). The absorbance at 416 nm was measured in a Versamax™ plate reader (Molecular Devices). The percent hemolysis of each sample was compared to 100% lysis achieved with 1% Triton X-100. The toxin concentration yielding 50% hemolysis was determined using four parameter logistic curves.
Immunization
Animal experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the protocols were approved by the Institutional Animal Care and Use Committee of Harvard Medical School. AT62 was produced and purified as described previously [8]. Female BALB/c mice (4 weeks of age; Charles River Laboratories) were vaccinated in the back of the neck by the subcutaneous (SC) route using a 1 ml syringe with a 27-guage 13 mm needle. The animals were injected on days 0, 14, and 28 with 20 μg AT62 or BSA (Sigma) mixed with Sigma Adjuvant System [SAS] at a final concentration of 20%. SAS is a stable oil in water emulsion containing 0.5 mg monophosphoryl lipid A (MPL) and 0.5 mg synthetic trehalose dicorynomylate in 44 μL of squalene oil, 0.2% tween 80 and water. Each mouse received 20 μg protein and 10 μg MPL. Blood was collected from the mice by tail vein puncture before each vaccination and again before challenge. Sera were diluted 1:100 and tested by enzyme-linked immunosorbent assay (ELISA) on 96-well plates coated with 1 μg/ml native Hla (List Biologicals).
Polyclonal antibodies were raised in rabbits to purified AT62, and total IgG was recovered by Protein A affinity chromatography. Naïve rabbit IgG was purchased from Equitech-Bio, Inc., Kerrville, TX. For passive immunization of mice, female BALB/c mice (7-8 weeks old) were given 1 mg of IgG in 0.2 ml delivered intravenously (IV) in the tail vein 24 hours before bacterial challenge in the surgical wound infection model.
Bacterial cultures
S. aureus USA300 strain LAC [12] was cultivated in tryptic soy broth (Difco) to an OD at 600 nm of 0.8. After washing the bacterial cells with phosphate buffered saline (PBS), the inoculum was diluted in PBS to the appropriate bacterial cell concentration, and CFU/ml determinations were made by plating to verify the inoculum.
Mouse model of surgical wound infection
Two weeks after the third immunization, the mice were subjected to surgical wound infection as previously described [13]. Briefly, the mice were anesthetized with ketamine and xylazine, and the right thigh of each mouse was shaved and disinfected with betadine and 70% ethanol. An incision was made in the skin layer, exposing the thigh muscle. A 1-cm incision was made in the muscle to the depth of the bone and then closed with one 4-0 silk suture. A 3 μl suspension of S. aureus LAC was introduced into the muscle incision under the suture. The skin was then closed with surgical clips. Three days post-surgery, the mice were euthanized, and the infected muscle tissue was excised, weighed, homogenized, and cultured quantitatively. The results were expressed as the log CFU of S. aureus/g of tissue, and the results were compared with the Mann Whitney test. For certain experiments, the thigh muscle tissue was excised, fixed in formalin or Bouin's fixative, embedded in paraffin, and stained with hematoxylin and eosin for microscopic examination. Tissue damage and inflammation were scored for severity by an investigator (JCL) blinded to the identity of the samples.
Mouse model of subcutaneous abscess formation
SC abscess formation was evaluated as described previously [14, 15]. Briefly, groups of mice were anesthetized with ketamine and xylazine, and their backs were shaved and disinfected with betadine and 70% ethanol. An inoculum of 4 × 105 S. aureus LAC was mixed with an equal volume of sterile dextran beads (Cytodex-1; Sigma), and 0.2 mL of the mixture was injected SC into the shaved flanks of immunized mice. After 48 h, the mice were euthanized and the abscesses were aseptically excised and homogenized in 1 ml TSB. Serial dilutions of the homogenates were cultured quantitatively. The results were expressed as the log CFU of S. aureus per abscess and compared with the Mann Whitney test.
Mouse model of necrotic skin lesions
The skin infection model was performed as described by Voyich et al. [12]. Briefly, mice were anesthetized, shaved, and disinfected as described above. The flanks were inoculated with 50 μl containing 1 × 107 to 4 × 107 CFU of S. aureus LAC. The lesions, measured daily, were relatively flat, and the area of each lesion was calculated as the area of an ellipse (A = πab, where a = the major radius and b = the minor radius). Tissue necrosis was scored for severity as 0, no lesion; 1, small bump or lesion; 2, inflamed bump or lesion; 3, open lesion with crusty scab; 4, necrotic, crusty lesion. The animals were weighed before inoculation and at 48 to 72 h intervals for 14 days. The data were analyzed by two-way ANOVA with multiple comparisons and corrected for repeated measures.
To determine the bacterial burden at the site of infection, groups of mice were euthanized on day four. The abscessed skin lesions were removed, weighed, homogenized, and dilutions of the homogenates were plated quantitatively. The results were expressed as the log CFU of S. aureus per lesion and compared with the Mann Whitney test.
RESULTS
In vitro toxicity of AT62
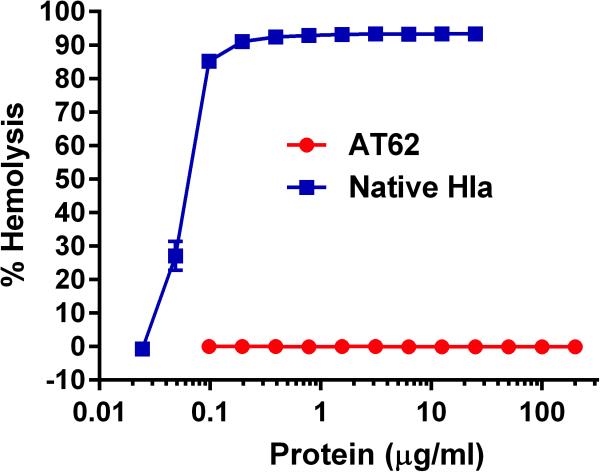
To evaluate the relative toxicity of AT62 compared with native Hla, we performed in vitro hemolysis assays on both proteins. As indicated in Fig. 1, AT62 did not lyse rabbit erythrocytes at concentrations up to 200 μg/ml AT62. In contrast, native Hla lysed rabbit erythrocytes at concentrations <0.1 μg/ml.
FIG 1.
Hemolytic assay of rabbit erythrocyte hemolysis following incubation with native Hla or AT62. Native Hla showed 50% hemolysis at 0.088 μg/ml, whereas AT62-His was nontoxic at concentrations as high as 200 μg/ml.
Protective efficacy of AT62 in a surgical wound infection model
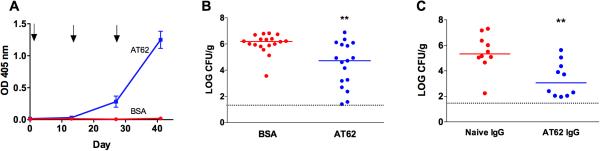
BALB/c mice were immunized with 20 μg AT62 or BSA, and their serum antibody levels were measured by ELISA. As shown in Fig. 2A, AT62-immunized mice produced a robust antibody response against native Hla. Two weeks after the third immunization, surgical incisions were made in the thigh muscles of anesthetized mice, and 3 μl containing 176 to 300 CFU of S. aureus LAC was introduced into the incision under the suture. Three days later, the mice were euthanized, and the wounded muscle tissue was excised, weighed, homogenized, and cultured quantitatively. As shown in Fig. 2B, the bacteria proliferated in the tissues of mice given BSA + SAS, resulting in a median bacterial burden of ~106 CFU/g tissue. In contrast, mice immunized with AT62 showed a 1.5 log reduction in the median bacterial CFU recovered from the surgical site (P = 0.0018).
FIG 2.
Immunogenicity and protective efficacy of AT62 in a murine surgical wound infection model. (A) Mice were immunized on days 0, 14, and 28 with 20 μg AT62 or BSA + MPL. Serum samples were diluted 1:100, and antibody levels to native Hla were measured by ELISA. (B) Mice actively immunized with AT62 or BSA had surgical site wounds inoculated with 3 μl containing 176 to 300 CFU S. aureus LAC. Three days after bacterial challenge, quantitative cultures were performed on homogenized tissues to determine the bacterial burden. The data are pooled from two independent experiments. (C) Mice were passively immunized by the IP route with 1 mg of rabbit AT62 IgG or naïve IgG and challenged 24 h later with 43 to 92 CFU S. aureus LAC. Quantitative cultures were performed after 3 d on homogenized tissues to determine the bacterial burden. Horizontal lines represent group medians, and dashed lines represent lower limit of detection by culture. *, P <0.05; **, P <0.01, determined by the Mann Whitney test.
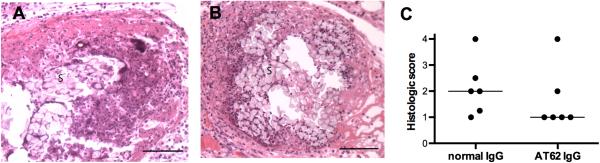
To determine whether the AT62-mediated protection in the surgical wound infection was antibody-mediated, we performed several passive immunization experiments. Mice passively immunized with naïve rabbit IgG or IgG purified from AT62-immunized rabbits were inoculated with 3 μl containing 43 to 92 CFU S. aureus LAC. The infected mice were euthanized three days later. As shown in Fig. 2C, quantitative cultures of the infected tissues revealed that mice given IgG to AT62 had ~2 logs fewer S. aureus recovered from the wounded tissue compared to mice give naïve IgG (P = 0.0042). Tissue sections processed for histology on day 3 showed numerous neutrophils and mononuclear cells infiltrating the wound site around the suture and in the surrounding muscle tissue (Fig. 3A, B). The histologic score did not differ significantly between mice given normal IgG vs. AT62 IgG, although there was a trend toward reduced inflammation and tissue damage in mice given AT62 antibodies (Fig. 3C).
FIG 3.
Histologic analysis of wound tissues from mice passively immunized 24 h prior to surgery with (A) normal IgG or (B) IgG from rabbits immunized with AT62. Fixed tissue sections were stained with hematoxylin and eosin and scored (C) for severity of inflammation and tissue damage. Photomicrographs were taken at 400x magnification; bar = 1 mm, S, silk suture material.
Protective efficacy of AT62 in a murine model of subcutaneous abscess formation
When mice are injected SC with S. aureus mixed with cytodex beads, the animals form well defined and vascularized abscesses with a thick fibrin wall. Previous histologic analysis and confocal microscopic analysis of these abscesses indicated the presence of neutrophils, bacteria, cytodex beads, and CD3+ T cells [13]. Skin necrosis does not occur in this infection model. Immunized mice inoculated with 1.1 × 105 CFU S. aureus LAC were euthanized after 48 h, and the abscesses were aseptically excised, weighed, homogenized, and cultured quantitatively. As shown in Fig. 4A, S. aureus replicated in the tissues of the mice, resulting in ~107 CFU S. aureus/abscess. Animals vaccinated with AT62 did not show a reduced bacterial burden compared to control animals (Fig. 4A), but they did show a trend toward smaller abscesses compared to mice given BSA (Fig. 4B).
FIG 4.
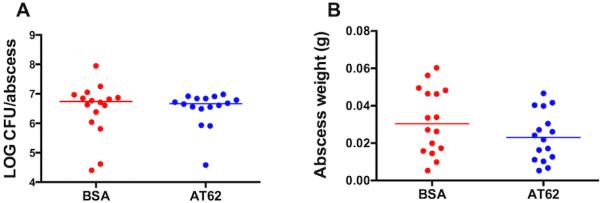
Lack of protective efficacy of AT62 in a subcutaneous abscess model. Mice were immunized as shown in Figure 2. The animals were challenged in both flanks with 105 CFU S. aureus LAC mixed with cytodex beads. After 48 h, the animals were euthanized, and the abscesses were excised, weighed and homogenized; quantitative cultures were performed to determine the bacterial burden. (A) Log CFU S. aureus per abscess. (B) Weight in grams of excised abscesses. Horizontal lines represent group medians.
Protective efficacy of AT62 in a necrotic skin lesion model
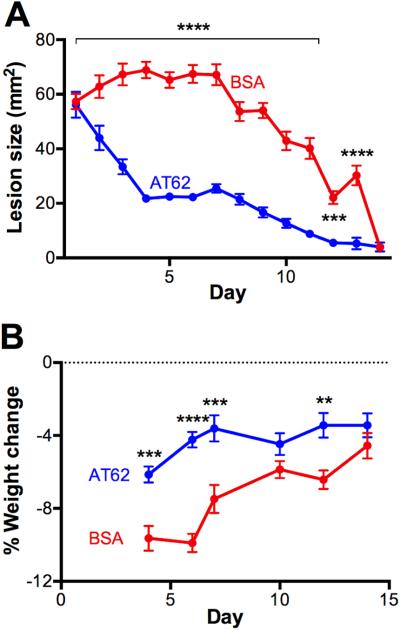
The mouse model of skin infection and dermonecrosis is particularly well suited to studies focused on the pathogenesis and prevention of infections provoked by S. aureus USA300 [12]. To determine whether AT62 was effective in protecting the host in this necrotic SSTI model, immunized mice were challenged on the flank with 107 CFU LAC in a 50-μl volume delivered SC (no cytodex beads). The mice were monitored for lesion size and weight change for 14 days. Mice immunized with AT62 + SAS developed lesions that were significantly smaller than those of control animals given BSA + SAS (Fig. 5A). Moreover, weight loss experienced by mice immunized with AT62 was reduced compared to control animals (Fig. 5B).
FIG 5.
Protective efficacy of AT62 in a necrotic lesion model of SSTI. Mice were actively immunized with 20 μg AT62 or BSA before challenge with 107 CFU of S. aureus LAC. Lesion size (A) and weight loss (B) were measured over 14 days. The data are representative of two independent experiments and were analyzed by two-way ANOVA with multiple comparisons corrected for repeated measures. **, P <0.01; ***, P <0.001; ****, P <0.0001.
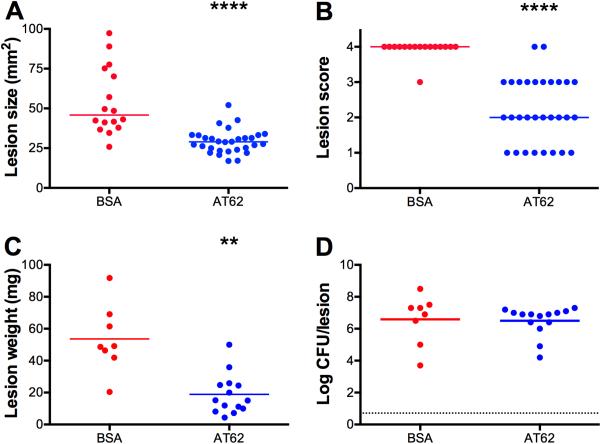
We also investigated whether active immunization would have a significant impact on the bacterial burden in the necrotic lesion staphylococcal infection model. Groups of 8 to 16 mice were immunized as described above with either 20 μg AT62 or BSA. Three weeks after the third immunization, the mice were challenged by the SC route with 4.25 × 107 CFU strain LAC. On day four after bacterial challenge, the lesion sizes were measured and scored for necrosis. The skin lesions were then excised, weighed, homogenized, and cultured quantitatively. Animals immunized with AT62 had smaller lesions (Fig. 6A) and fewer necrotic lesions (Fig. 6B) than mice given BSA. The excised abscesses were significantly smaller (in weight) in the AT62-immunized mice (Fig. 6C), but the bacterial burdens within the lesions were similar for both groups (Fig. 6D).
FIG 6.
Protective efficacy of AT62 measured on day four of the necrotic skin infection model. Mice actively immunized with 20 μg of AT62 or BSA were challenged with 4 × 107 CFU of S. aureus LAC. On day four, lesion size was measured (A) and tissue necrosis (B) was scored for severity as 0, no lesion; 1, small bump or lesion; 2, inflamed bump or lesion; 3, open lesion with crusty scab; 4, necrotic, crusty lesion. The lesions were excised and weighed (C). Quantitative cultures were performed on the homogenized tissues to determine the bacterial burden (D). Horizontal lines represent group medians, and the results were compared by the Mann Whitney test. **, P <0.01; ****, P <0.0001.
DISCUSSION
Vaccine development targeting staphylococcal infections faces many challenges. S. aureus strains are geographically diverse, and virulence factors can vary markedly from one strain to another. There are multiple manifestations of staphylococcal infections, ranging from superficial skin and soft tissue infections to biofilm formation on implanted devices to invasive diseases such as bacteremia, endocarditis, bacteremia, and pneumonia. Moreover, the correlates of protective immunity against S. aureus infection are unknown. Development of a successful vaccine has remained elusive, but in all likelihood will comprise multiple components, including surface polysaccharides and proteins, as well as detoxified exotoxins. Each vaccine component should contribute protective efficacy against some manifestation of S. aureus infection.
The results reported by multiple research groups indicate that detoxified Hla is a good target for the prevention of pathology associated with staphylococcal disease [2-8]. Moreover, Adhikari et al. reported that among patients with invasive S. aureus infections, the risk of sepsis was significantly lower in those patients with higher levels of IgG against specific staphylococcal exotoxins, including Hla [16]. Whereas most vaccine studies have utilized HlaH35L as the vaccine, a potent toxin with a single point mutation may not be deemed safe for human use [10, 11]. Ragle et al. prepared a nontoxic peptide (GST-Hla1-50) that included the first 50 amino acids of native Hla. Immunization with GST-Hla1-50 mixed with Freund's adjuvant reduced lethality in a murine model of S. aureus pneumonia [17].
More recently, Adhikari et al. prepared a novel recombinant subunit vaccine AT62 that represents an Hla structural domain that is critically involved in toxin oligomerization [8]. Antibodies to AT62 prevented oligomerization of the Hla subunits, preventing pore formation. In a comparative study, AT62 was shown to be more immunogenic and protective against murine sepsis and lethal pneumonia than GST-Hla1-50 when both vaccines were formulated with Glucopyranosyl Lipid Adjuvant-Stable Emulsion [8]. AT62 antibodies neutralized the cytolytic activity of native Hla and protected mice against sepsis, bacteremia, and bacterial dissemination to kidneys, liver, spleen, and lung.
In this study we evaluated the protective efficacy of AT62 formulated with SAS in murine models of surgical wound infection and SSTI. We challenged the mice with strain LAC, since most SSTIs are caused by USA300 strains. In the surgical wound infection model, mice immunized with AT62 showed a significantly reduced S. aureus burden in the infected tissues compared to animals given BSA. Likewise, mice passively immunized with rabbit IgG to AT62 showed reduced surgical site infection, indicating that the protection observed was primarily antibody mediated. Of note, AT62 is the first vaccine antigen that has shown protective efficacy in the surgical wound infection model. Tissue sections from mice given AT62 antibodies showed somewhat less inflammation and tissue necrosis than those from mice given normal IgG, although the differences did not reach significance.
In a skin necrosis infection model, immunization with the AT62 vaccine resulted in smaller lesions and reduced mouse weight loss compared to controls given BSA. Although AT62 immunization reduced tissue necrosis associated with the lesions, it did not reduce the bacterial burdens in the lesions compared to controls. This finding is consistent with our results in the SC abscess model in which the S. aureus inoculum was administered SC with cytodex beads. The AT62 vaccine was not effective in reducing the bacterial burden in either abscess model, although the lesions were smaller in animals immunized with AT62.
Our quantitative culture results are in contrast to those of Brady et al. [6] who immunized mice by the SC route with 20 μg HlaH35L formulated with 200 μg Alhydrogel® and 15 μg CpG. These investigators demonstrated a reduction in the tissue bacterial burden of a USA300 strain in a mouse ear model of SSTI and in a systemic renal abscess model. In contrast, no protection was achieved by the HlaH35L vaccine in a prosthetic implant model of chronic biofilm infection. Bubeck Wardenburg et al. observed an ~1-log reduction in the lung bacterial burden of mice immunized with 20 μg HlaH35L and Freund's adjuvant and challenged with S. aureus Newman [3]. Whether these differences in reducing the bacterial load in the infected tissues can be attributed to the use of different adjuvants or are tissue-specific remains to be resolved. Nonetheless, our data indicate that AT62 may be a valuable component of a multivalent vaccine against S. aureus due to its safety and protective efficacy in models of wound infection and SSTI
Highlights.
An AT62 vaccine reduced S. aureus infection in a surgical wound infection model.
AT62 is the first vaccine to show protection in the surgical wound infection model
The AT62 vaccine did not protect against S. aureus subcutaneous abscess formation.
The AT62 vaccine reduced S. aureus necrotic skin lesions and weight loss in mice.
AT62 merits consideration as a component of a multivalent S. aureus vaccine.
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number 1R43AI098232. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Tom Kort for production and purification of the AT62 protein and Meghan Dowd and Kelly Shields Lapierre for technical assistance. R. P. Adhikari and M. J. Aman are employees of Integrated Biotherapeutics, Inc. J. C. Lee has consulted in the past for Sanofi Pasteur, Pfizer, and Crucell.
Abbreviations
- Hla
alpha hemolysin
- SAS
Sigma Adjuvant System
- BSA
bovine serum albumin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
C. D. Thompson reports no potential conflicts.
References
- 1.Tomita T, Kamio Y. Molecular biology of the pore-forming cytolysins from Staphylococcus aureus, alpha- and gamma-hemolysins and leukocidin. Biosci Biotechnol Biochem. 1997;61:565–72. doi: 10.1271/bbb.61.565. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–8. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wardenburg JB, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–94. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauch S, DeDent AC, Kim HK, Bubeck Wardenburg J, Missiakas DM, Schneewind O. Abscess formation and alpha-hemolysin induced toxicity in a mouse model of Staphylococcus aureus peritoneal infection. Infect Immun. 2012;80:3721–32. doi: 10.1128/IAI.00442-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wacker M, Wang L, Kowarik M, Dowd M, Lipowsky G, Faridmoayer A, et al. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J Infect Dis. 2014;209:1551–61. doi: 10.1093/infdis/jit800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady RA, Mocca CP, Prabhakara R, Plaut RD, Shirtliff ME, Merkel TJ, et al. Evaluation of genetically inactivated alpha toxin for protection in multiple mouse models of Staphylococcus aureus infection. PLoS One. 2013;8:e63040. doi: 10.1371/journal.pone.0063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagnoli F, Fontana MR, Soldaini E, Mishra RP, Fiaschi L, Cartocci E, et al. Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. Proc Natl Acad Sci U S A. 2015;112:3680–5. doi: 10.1073/pnas.1424924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adhikari RP, Karauzum H, Sarwar J, Abaandou L, Mahmoudieh M, Boroun AR, et al. Novel structurally designed vaccine for S. aureus alpha-hemolysin: protection against bacteremia and pneumonia. PLoS One. 2012;7:e38567. doi: 10.1371/journal.pone.0038567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fattom A, Matalon A, Buerkert J, Taylor K, Damaso S, Boutriau D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum Vaccin Immunother. 2015;11:632–41. doi: 10.4161/hv.34414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panchal RG, Bayley H. Interactions between residues in staphylococcal alpha-hemolysin revealed by reversion mutagenesis. J Biol Chem. 1995;270:23072–6. doi: 10.1074/jbc.270.39.23072. [DOI] [PubMed] [Google Scholar]
- 11.Liang X, Yan M, Ji Y. The H35A mutated alpha-toxin interferes with cytotoxicity of staphylococcal alpha-toxin. Infect Immun. 2009;77:977–83. doi: 10.1128/IAI.00920-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–70. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 13.McLoughlin RM, Solinga RM, Rich J, Zaleski KJ, Cocchiaro JL, Risley A, et al. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc Natl Acad Sci U S A. 2006;103:10408–13. doi: 10.1073/pnas.0508961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portoles M, Kiser KB, Bhasin N, Chan KHN, Lee JC. Staphylococcus aureus Cap5O has UDP ManNAc dehydrogenase activity and is essential for capsule expression. Infect Immun. 2001;69:917–23. doi: 10.1128/IAI.69.2.917-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Y, Onodera Y, Lee JC, Hooper DC. NorB, an efflux pump in Staphylococcus aureus strain MW2, contributes to bacterial fitness in abscesses. J Bacteriol. 2008;190:7123–9. doi: 10.1128/JB.00655-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adhikari RP, Ajao AO, Aman MJ, Karauzum H, Sarwar J, Lydecker AD, et al. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis. 2012;206:915–23. doi: 10.1093/infdis/jis462. [DOI] [PubMed] [Google Scholar]
- 17.Ragle BE, Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun. 2009;77:2712–8. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]