Abstract
Objective
To evaluate differences in the inclusion of anesthesiologists in mobile extracorporeal membrane oxygenation (ECMO) teams between North American and European centers.
Design
A retrospective review of North American versus European mobile ECMO teams. The search terms used to identify relevant articles were the following: “extracorporeal membrane transport,” “mobile ECMO,” and “interhospital transport.”
Setting
MEDLINE review of articles.
Participants
None.
Interventions
None.
Results
Between 1986 and 2015, 25 articles were published that reported the personnel makeup of mobile ECMO teams in North America and Europe: 6 from North American centers and 19 from European centers. The included articles reported a total of 1,329 cases: 389 (29%) adult-only cohorts and 940 (71%) mixed-age cohorts. Among North American studies, 0 of 6 (0%) reported the presence of an anesthesiologist on the mobile ECMO team in contrast to European studies, in which 10 of 19 (53%) reported the inclusion of an anesthesiologist (Fisher exact p for difference = 0.05). In terms of number of cases, this discrepancy translated to 543 total cases in North America (all without an anesthesiologist) and 499 cases in Europe (37%) including an anesthesiologist on the team (Fisher exact p for difference <0.001).
Conclusions
This study demonstrated significant geographic discrepancies in the inclusion of anesthesiologists on mobile ECMO teams, with European centers more likely to incorporate an anesthesiologist into the mobile ECMO process compared with North American centers.
Keywords: extracorporeal membrane oxygenation, anesthesiology, mobile ECMO
EXTRACORPOREAL MEMBRANE OXYGENATION (ECMO) is a potentially lifesaving modality used in critically ill patients who experience severe cardiac and/or pulmonary failure, and its use has increased over the past 2 decades.1,2 Along with the rise in ECMO utilization, the ability to provide interhospital transfer to tertiary care centers with the assistance of ECMO support has led to the emergence of critical questions regarding the appropriate timing and execution of such transfers. Transport ECMO was first reported by Cornish et al in 1986,3 but standardization of this complex undertaking remains a potentially important quality improvement opportunity worthy of investigation.
Despite a relative paucity of data regarding transport ECMO, increasing numbers of primary and secondary care facilities are using mobile ECMO for interhospital transfer of critically ill patients to tertiary care centers.4 Appropriately equipped hospitals and other healthcare facilities around the world have put ECMO teams in place to carry out these transfers, but the makeup of these teams is not standardized across centers. Thus far, the largest systematic review of the mobile ECMO literature did not focus on the makeup of these teams across institutions or geographic regions.5
Accordingly, for this study, the authors analyzed differences in the personnel used during transport ECMO, with a particular focus on the inclusion of anesthesiologists in mobile ECMO teams as it differs between North American and European centers. Secondarily, the authors sought to perform a qualitative review of the complications encountered during the mobile ECMO experience between North American and European centers.
METHODS
Search Criteria
The authors conducted a PubMed database search to identify literature that reported experiences with interhospital transfer of patients undergoing ECMO. The search terms used for identification of relevant articles were the following: “extracorporeal membrane transport,” “mobile ECMO,” and “interhospital transport.”
Analytic Plan
After gathering descriptive statistics on mobile ECMO teams between North American and European centers, the authors compared the proportion of studies from each continent that reported the inclusion of anesthesiologists in its mobile ECMO teams and the number of cases these studies represented. This difference was analyzed using Fisher exact test, with a 2-sided p value of <0.05 considered significant. The types of complications encountered among transport ECMO teams also were examined. Because these data were not standardized and frequently omitted across the studies analyzed, the authors did not attempt to perform a quantitative analysis of the incidence of complications. Complications were grouped by type in accordance with the descriptions contained in the relevant references. The type of ECMO used (ie, venoarterial [VA] v venovenous [VV]) also was reported (Table 14–28). Finally, data on transport distance were gathered and are summarized herein as ranges. Because many studies did not include full descriptions of the distributions of distance traveled, only ranges are reported because weighted means, which would have accounted for each study's sample size, were not possible to calculate.
Table 1.
A Systematic Review of Articles Reporting Interhospital Transport of Patients on ECMO
| Reference | Year Ranges | Anesthesiologist on ECMO Transport Tea | Non-anesthesiologist Intensivist on Team | No. of Patients Transported (n = 1,329) | Patient Population | Distance Range or Percent (km) | En-Route Complications (No. of Incidents Reported) | ECMO Type |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VV | VA | VVA | VV-VA | VA-VV | ||||||||
| North America | ||||||||||||
| Biscotti et al6 | — | No | — | 100 | Adult | 4–1,1400 | NR | 79 | 19 | 2 | ||
| Bryner et al5 | 1990–2012 | No | Critical care surgeon and fellow | 221 | Neo/Ped/Adult | — | Patient related (9): arterial catheter rupture, cardiac arrest, death | 107 | 114 | |||
| Missing item (23): specific cannula sizes, stretcher, sterile water bath | ||||||||||||
| Electrical/mechanical problem (39): ambulance battery outage, portable laboratory device not working, battery loss requiring hand-cranking of pump, water heater failure | ||||||||||||
| Transportation mishap (8): landing at wrong airport, ambulance engine failure | ||||||||||||
| Circuit issue (20): circuit depriming, oxygenator clotting | ||||||||||||
| Clement et al7 | 1990–2008 | No | Intensivecare physician, not specified | 112 | Neo/Ped | — | NR | 1 | 111 | |||
| Coppola et al8 | 1985–2007 | No | — | 68 | Neo/Ped | 13–12,070 | Patient related (2): mild hypothermia | 1 | 67 | |||
| Circuit issue (2): membrane oxygenator failure thrombosis | ||||||||||||
| Electrical/mechanical problem (4): power supply failure, blood warmer leakage, roller pump failure with subsequent circuit tubing rupture while hand-cranking pump | ||||||||||||
| Gebremichael et al9 | 1994–1996 | No | Intensive care physician, not specified | 36 | Ped/Adult | 4.83–740.3 | Patient related (2): transient hypotension (1 died while being moved from the sending hospital bed to the transport gurney; the death was sudden and believed to be consistent with an acute pulmonary embolus) | — | — | — | — | — |
| Horne et al10 | 2004–2009 | No | — | 6 | Neo/Ped | — | Patient related (2): cannula site bleeding, infection | 1 | 4 | 1 | ||
| Circuit issue (1): cannula site thrombosis | ||||||||||||
| Europe | ||||||||||||
| Broman et al4 | 2010–2013 | Yes | 322 | Neo/Ped/Adult | 6.9–13,447 | Patient related (66): loss of tidal volume, flooding of the lung, bleeding, hypovolemia, hypothermia, bradycardia, loss of arterial line, thrombocytosis, cardiac stun, electrolyte imbalance, arousal, leg ischemia, vasovagal reflex/secretions, circulatory instability, cannulation problems | — | — | — | — | — | |
| Equipment/technical (18): clotting of ECMO system, cannula clot, oxygenator clot, broken laboratory device, syringe pump failure, broken heater/hose, broken oxygen hose, broken ventilator hose, loss of power supply to pump; ECMO system forgotten, pump head forgotten | ||||||||||||
| Transportation mishap (7): wrong ambulance, ambulance traffic accident, colliding with wildlife, no electricity, change in destination, no transport after delivery | ||||||||||||
| Chenaitia et al11 | 2009–2010 | Yes | 43 | Adult | 7–135 | NR | 11 | 32 | ||||
| Ciapetti et al12 | 1998–2004 | No | Intensive care physician, not specified | 4 | Adult | 35–520 | NR | 4 | ||||
| D'Ancona et al13 | 2009–2010 | Yes | 8 | Adult | — | Electrical/mechanical problem (1): pump arrest secondary to an electrical failure of the pump battery | 7 | — | — | — | — | |
| Delnoij et al14 | 2009–2013 | No | Intensive care physician, not specified | 10 | Adult | 27–126 | NR | 10 | ||||
| Gariboldi et al15 | 2006–2008 | Yes | 38 | Ped/Adult | 1–230 | NR | 6 | 32 | ||||
| Haneya et al16 | 2001–2008 | Yes | 9 | Adult | Equipment (1): oxygen supply was insufficient | 9 | ||||||
| Isgrò et al17 | 2004–2009 | No | Intensive care physician, not specified | 12 | Ped/Adult | — | Electrical/mechanical (3): battery pack was unable to maintain the charge and the ventilator switched off during ICU ambulance transfer, touch screen malfunctioned, magnetic decoupling of the centrifugal pump head due to street roughness | 12 | ||||
| Linden et al18 | 1996–2000 | No | — | 29 | Neo/Ped/Adult | 4–1,500 | Transportation (1): ambulance malfunctioned, disabling shock absorbers | — | — | — | — | — |
| Electrical (2): electric supply circuits went down | ||||||||||||
| Lucchini et al19 | 2004–2012 | No | Intensive care physician, not specified | 29 | Ped/Adult | 9–1,044 | Electrical (1): battery failure of ventilator during transport from the ICU to the ambulance | 28 | 1 | |||
| Circuit issue (1): difficulty in obtaining an acceptable extracorporeal flow due to the patient's position | ||||||||||||
| Lunz et al20 | — | Yes | 6 | Adult | 66–178 | NR | 6 | |||||
| Philipp et al21 | 2010–2010 | Yes | 6 | Adult | 80–5,850 | Patient related (1): systemic pressure drop | 5 | 1 | ||||
| Raspé et al22 | 2010–2013 | Yes | 36 | Ped/Adult | NR | 36 | ||||||
| Roch et al23 | 2009–2013 | No | Intensive care physician, not specified | 85 | Adult | — | NR | 77 | 8 | |||
| Roncon-Albuquerque et al24 | 2009–2011 | No | Intensive care physician, not specified | 10 | Adult | 116–133 | Patient related (2): unexplained respiratory deterioration | 9 | 1 | |||
| Rossaint et al25 | 1993–1995 | Yes | 8 | Ped/Adult | — | Mechanical (1): breakage of a stopcock on top of membrane lung | 8 | |||||
| Starck et al26 | 2009–2011 | No | – | 6 | Adult | 12–55 | NR | 6 | ||||
| Vaja et al27 | 2010–2014 | No | Intensive care physician, not specified | 102 | Adult | 3.6–980 | Patient related (1): ventricular tachycardia | 95 | 7 | |||
| Wagner et al28 | 1992–2008 | Yes | 23 | Neo/Ped/Adult | — | NR | 6 | 13 | 3 | 1 | ||
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; Neo, neonatal; NR, none reported; Ped, pediatric; VA, venoarterial; VV, venovenous; VVA, veno-veno-arterial.
RESULTS
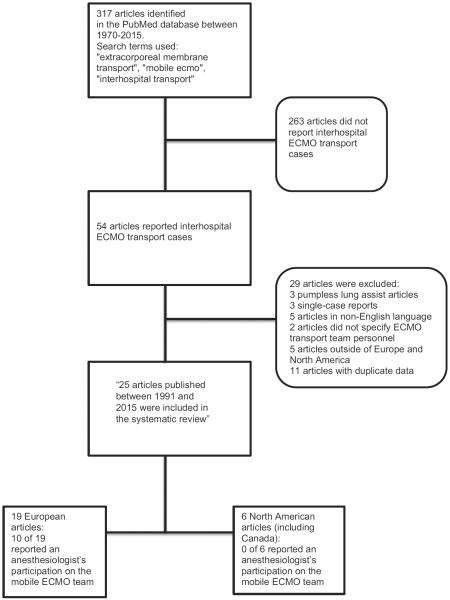
A total of 317 articles were identified for screening using the aforementioned search terms in PubMed. Identified articles were published between 1986 and 2015, of which 54 were specifically about mobile ECMO (see Fig 1). Of these 54 articles, the following were excluded: pumpless extracorporeal lung-assist cases (3), single-case reports (3), articles not available in the English language (5), articles not specifying an ECMO team (2), articles from institutions outside of Europe and North America (5), and articles that included overlapping, duplicate data from the same institution (11), leaving a total of 25 included articles for this analysis (see Fig 1)—6 from North American centers and 19 from European centers. In sum, the included articles reported a total of 1,329 cases: 389 (29%) adult-only cohorts and 940 (71%) mixed-age cohorts.
Fig 1.
Diagram of article selection and analysis.
Contrasting North American with European practice, a notable discrepancy was found in the proportion of studies that included an anesthesiologist on the ECMO transport team. Among North American studies, 0 of 6 (0%) reported the presence of an anesthesiologist on the mobile ECMO team in contrast to 10 of 19 (53%) studies from Europe reporting the inclusion of an anesthesiologist on the transport team. (Fisher exact p for difference between proportions by studies was 0.05). In terms of the number of cases, this discrepancy translated to 543 total cases in North America (all without an anesthesiologist) and 499 cases in Europe (37%) incorporating an anesthesiologist on the team (Fisher exact p for difference between proportions by cases was <0.001 [see Table 1]). The inclusions of surgeons, nurses, and perfusionists on the transport team were similar between centers on the 2 continents; they were all reported in 50% or more of the studies. A few studies reported intensivists, but their specialties were unspecified. A complete list of mobile ECMO team members by study is listed in Table 24–28.
Table 2.
Detailed List of ECMO Transport Personnel by Study
| Reference | ECMO Team Personnel |
|---|---|
| North America | |
| Biscotti et al6 | 2 perfusionists, 2 critical care paramedics, 1 cardiothoracic surgeon, and, since 2013, 1 surgical fellow |
| Bryner et al5 | 2 medical flight nurses, 2 ECMO specialists, 1 critical care surgeon, 1 critical care fellow |
| Clement et al7 | 1 ECMO coordinator, 1 pediatric cardiac surgeon, 1 surgical assistant, 1 intensive care physician |
| Coppola et al8 | 1 pediatric cardiologist, 1 surgeon, 2 circuit/child nurse, 1 respiratory therapist, 1 ECMO director, 1 ECMO coordinator, technicians and trainees |
| Gebremichael et al9 | 1 critical care physician, 1 practicing critical care nurse, 1 respiratory therapist |
| Horne et al10 | Adult cardiac surgeon/pediatric general surgeon/pediatric cardiologist and perfusionists |
| Europe | |
| Broman et al4 | 1 ECMO physician (anesthesiologist and transport team leader), 1 ECMO specialist (ICU nurse), 1 cannulating surgeon |
| Chenaitia et al11 | 1 cardiac surgeon, 1 resident surgeon, 1 perfusionist, 1 anesthesiologist |
| Ciapetti et al12 | Intensivist, cardiac surgeon, cardiologist, perfusionist, and nurses |
| D'Ancona et al13 | 1 anesthesiologist, 1 cardiac surgeon, 1 perfusionist |
| Delnoij et al14 | 2 intensivists, 1 intensive care nurse, 1 perfusionist |
| Gariboldi et al15 | 1 cardiac surgeon, 1 anesthesiologist, 1 perfusionist |
| Haneya et al16 | 1 anesthesiologist experienced in cardiopulmonary bypass, 1 perfusionist, 1 nurse or paramedic, 1 cardiac surgeon |
| Isgrò et al17 | 2 ICU physicians, 1 ICU nurse, and 1 ECMO specialist, plus trainees (1 ICU physician and 1 ICU nurse) |
| Linden et al18 | 1 ECMO physician, 1 ECMO coordinator, 1 cannulating surgeon |
| Lucchini et al19 | 2 intensivists, 1 ICU nurse, 1 perfusionist |
| Lunz et al20 | 1 cardiac anesthesiologist, 1 clinical perfusionist |
| Philipp et al21 | 1 cardiac anesthesiologist, 1 cardiac surgeon, 1 pump technician |
| Raspé et al22 | 1 cardiac anesthesiologist, 1 cardiac surgeon, 1 clinical perfusionist |
| Roch et al23 | 1 ICU physician, 1 cardiac surgeon, 1 perfusionist |
| Roncon-Albuquerque et al24 | 2 intensive care physicians, 1 nurse, 1 perfusionist |
| Rossaint et al25 | 2 anesthesiologists, 1 nurse |
| Starck et al26 | 1 cardiac surgeon, 1 perfusionist |
| Vaja et al27 | Someone trained in cannulation for ECMO, transport and intensive care, perfusion, and the ECMO circuit; and an ECMO specialist nurse |
| Wagner et al28 | 1 cardiothoracic surgeon, 1 anesthesiologist, 1 perfusionist, 1 ICU nurse |
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Other notable characteristics between the North American and European experience included similar ranges of transport distance (4–12,070 km for North American v 1–13,447 km for European cohorts). There was nearly 100% survival during the transport process, with only 1 reported death en route.
In relation to the type of ECMO used in the transported patients, North American studies reported 189 cases of VA ECMO versus 315 cases of VV ECMO, whereas the European studies reported 389 and 94 cases, respectively. It is notable that one of the biggest studies performed in Europe did not report the type of ECMO used in its transported patients.5
Regarding complications, due to inconsistent reporting between and within studies, a quantitative representation of the incidence of complications was not possible. This was exemplified by some overlapping studies that reported mutually inconsistent complications. Nevertheless, it still was informative to review the types of complications reported as a qualitative representation of the range of issues encountered during transport ECMO. Although reporting was inconsistent, complications included death, cardiac arrest, arrhythmia, cardiac stun, bleeding, loss of tidal volume, hypothermia, hypotension, bradycardia, equipment malfunction/failure, overinfusion of intravenous drugs, and transportation mishaps such as an airplane landing at the wrong airport (see Table 1). Interestingly, just as critical care patient complications were, as expected, a dominant theme within this qualitative review, electrical and mechanical malfunctions also were highly prevalent among those reported.
DISCUSSION
This study demonstrated significant geographic discrepancies in the inclusion of anesthesiologists in mobile ECMO teams, with European centers much more likely to incorporate an anesthesiologist in the mobile ECMO process compared with centers in North America.
Patients with critical, life-threatening cardiopulmonary conditions refractory to medical therapy require specialized assistance by a team of clinicians in a multispecialty environment.29 Particularly relevant to this analysis, several of the complications reported in the literature are ones that commonly are encountered in the perioperative environment and for which anesthesiologists are trained to provide lifesaving interventions, including the treatment of hypotension, hypothermia, arrhythmias, tidal volume/airway management, pressor support, equipment failure, and appropriate sedation.
Limitations
The difference in historic practice patterns may not have any relationship to outcomes and simply may reflect the differing role of anesthesiologists between these areas, with the role of anesthesiologist-intensivists much more prominent historically in Europe than in North America.30 This difference in the role of anesthesiologist-intensivists was reflected in a 2000 study by Angus et al, in which it was reported that anesthesiologist-intensivists in the United States made up 6% of the critical care workforce, and the supply for these specialty-trained individuals was expected to remain stagnant.31
Even though some studies in the review presented here reported the experiences in relation to the type of ECMO used, the specificity of reporting was insufficient to determine the related complications stratified by type of ECMO.20 In addition, the reasons as to why VA versus VV ECMO use differed in proportion between the 2 continents are worthy of further investigation. Furthermore, due to the limitations in this study, whether or not an anesthesiologist should be included to improve patient outcomes is a subject that warrants further prospective studies. The authors hope that future studies of transport ECMO will report these findings in detail to get a better idea of what role ECMO personnel may play in improving patient outcomes.
Despite the limitations posed by a lack of uniformity among ECMO transport teams, the need for an anesthesiologist may be warranted. For example, according to Day et al, “transport teams should be thoroughly familiar with the pathophysiology of cardiac and respiratory failure. They should be equipped to continue the monitoring and treatment initiated at the referring center, to maintain that level of care during transfer, and to treat complications of the diseases or of the therapy itself.”32 Studies showed that significant life-threatening cardiopulmonary changes can occur during patient transport, and cardiac or critical-care-trained anesthesiologists deal with these issues as a part of their daily clinical responsibility.4 Thus, it may be prudent to use their expertise during interhospital transport of ECMO patients. The second aspect of this qualitative review of complications worthy of note was the variety of equipment malfunctions that were described. From electrical failures to loss of equipment, these failures were present across studies and may indicate that a key avenue for quality improvement going forward will include checklists designed to ensure that equipment is functioning properly, that critical backup equipment is immediately available (including battery supplies and surplus oxygen), and that ECMO transport teams are trained in how to recognize and respond to common equipment failures. Such checklists also might serve to prevent logistics-related complications like the complication experienced by one unfortunate patient whose plane landed at the wrong airport.
A final consideration worthy of further study is the costs associated with mobile ECMO.33 Although mobile ECMO provides crucial support to hemodynamically unstable patients during inter-hospital transfers, Coppola et al have reported costs for mobile ECMO of up to $160,000.8,13,34 In contrast to the authors' suggestions regarding the role of anesthesiologists in mobile ECMO, Schwartz et al referenced costs in concluding that the role of a physician is better used for clinical decisions than on-the-scene responses.35
In addition to the aforementioned practical limitations, the study presented here was, by necessity, only able to include transport ECMO experience that was included in peer-reviewed articles indexed in MEDLINE. There may be more variation in practice among centers that have not published their experience, and the literature may be skewed toward centers with limited complications. This publication bias naturally would bias these results to make transport ECMO appear to be more feasible and safer than it actually may be. Despite these limitations, this analysis of the literature documents what is likely to be an important difference in practice between North American and European centers.
In conclusion, as opposed to the North American experience, most European ECMO teams have recruited anesthesiologists to provide critical treatment during transfers. Whether recruiting anesthesiologists to North American ECMO transport teams may lead to better outcomes during and after transport is a subject worthy of further investigation. If improvements in outcome are demonstrated, the workforce and economic considerations may be paramount in enabling such a change in North American practice.
Acknowledgments
This work was supported in part by NIGMS Grant T32 GM086287 (P.I. Niklason) from the National Institutes of Health.
REFERENCES
- 1.Stretch R, Sauer CM, Yuh DD, et al. National trends in the utilization of short-term mechanical circulatory support: Incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407e–1415. doi: 10.1016/j.jacc.2014.07.958. [DOI] [PubMed] [Google Scholar]
- 2.Maxwell BG, Powers AJ, Sheikh AY, et al. Resource use trends in extracorporeal membrane oxygenation in adults: An analysis of the Nationwide Inpatient Sample 1998–2009. J Thorac Cardiovasc Surg. 2014;148:416e–421. doi: 10.1016/j.jtcvs.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Cornish JD, Gerstmann DR, Begnaud MJ, et al. Inflight use of extracorporeal membrane oxygenation for severe neonatal respiratory failure. Perfusion. 1986;1:281–287. [Google Scholar]
- 4.Broman LM, Holzgraefe B, Palmér K, et al. The Stockholm experience: Interhospital transports on extracorporeal membrane oxygenation. Crit Care. 2015;19:278. doi: 10.1186/s13054-015-0994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryner B, Cooley E, Copenhaver W, et al. Two decades' experience with interfacility transport on extracorporeal membrane oxygenation. Ann Thorac Surg. 2014;98:1363–1370. doi: 10.1016/j.athoracsur.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Biscotti M, Agerstrand C, Abrams D, et al. One hundred transports on extracorporeal support to an extracorporeal membrane oxygenation center. Ann Thorac Surg. 2015;100:34–39. doi: 10.1016/j.athoracsur.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 7.Clement KC, Fiser RT, Fiser WP, et al. Single-institution experience with interhospital extracorporeal membrane oxygenation transport: A descriptive study. Pediatr Crit Care Med. 2010;11:509–513. doi: 10.1097/PCC.0b013e3181c515ca. [DOI] [PubMed] [Google Scholar]
- 8.Coppola CP, Tyree M, Larry K, et al. 22-year experience in global transport extracorporeal membrane oxygenation. J Pediatr Surg. 2008;43:46–52. doi: 10.1016/j.jpedsurg.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Gebremichael M, Borg U, Habashi NM, et al. Interhospital transport of the extremely ill patient: The mobile intensive care unit. Crit Care Med. 2000;28:79–85. doi: 10.1097/00003246-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Horne D, Lee JJ, Maas M, et al. Air transported pediatric rescue extracorporeal membrane oxygenation: A single institutional review. World J Pediatr Congenit Heart Surg. 2012;3:236–240. doi: 10.1177/2150135111428627. [DOI] [PubMed] [Google Scholar]
- 11.Chenaitia H, Massa H, Toesca R, et al. Mobile cardio-respiratory support in prehospital emergency medicine. Eur J Emerg Med. 2011;18:99–101. doi: 10.1097/MEJ.0b013e3283402249. [DOI] [PubMed] [Google Scholar]
- 12.Ciapetti M, Cianchi G, Zagli G, et al. Feasibility of inter-hospital transportation using extra-corporeal membrane oxygenation (ECMO) support of patients affected by severe swine-flu(H1N1)-related ARDS. Scand J Trauma Resusc Emerg Med. 2011;19:32. doi: 10.1186/1757-7241-19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Ancona G, Capitanio G, Chiaramonte G, et al. Extracorporeal membrane oxygenator rescue and airborne transportation of patients with influenza A (H1N1) acute respiratory distress syndrome in a Mediterranean underserved area. Interactive Cardiovasc Thorac Surg. 2011;12:935–937. doi: 10.1510/icvts.2010.260448. [DOI] [PubMed] [Google Scholar]
- 14.Delnoij TS, Veldhuijzen G, Strauch U, et al. Mobile respiratory rescue support by off-centre initiation of extracorporeal membrane oxygenation. Perfusion. 2015;30:255–259. doi: 10.1177/0267659114540735. [DOI] [PubMed] [Google Scholar]
- 15.Gariboldi V, Grisoli D, Tarmiz A, et al. Mobile extracorporeal membrane oxygenation unit expands cardiac assist surgical programs. Ann Thorac Surg. 2010;90:1548–1552. doi: 10.1016/j.athoracsur.2010.06.091. [DOI] [PubMed] [Google Scholar]
- 16.Haneya A, Philipp A, Foltan M, et al. Extracorporeal circulatory systems in the interhospital transfer of critically ill patients: Experience of a single institution. Ann Saudi Med. 2009;29:110–114. doi: 10.4103/0256-4947.51792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isgrò S, Patroniti N, Bombino M, et al. Extracorporeal membrane oxygenation for interhospital transfer of severe acute respiratory distress syndrome patients: 5-year experience. Int J Artif Organs. 2011;34:1052–1060. doi: 10.5301/ijao.5000011. [DOI] [PubMed] [Google Scholar]
- 18.Lindén V, Palmér K, Reinhard J, et al. Inter-hospital transportation of patients with severe acute respiratory failure on extracorporeal membrane oxygenation—national and international experience. Intensive Care Med. 2001;27:1643–1648. doi: 10.1007/s001340101060. [DOI] [PubMed] [Google Scholar]
- 19.Lucchini A, De Felippis C, Elli S, et al. Mobile ECMO team for inter-hospital transportation of patients with ARDS: A retrospective study. Heart Lung Vessel. 2014;6:262–273. [PMC free article] [PubMed] [Google Scholar]
- 20.Lunz D, Philipp A, Judemann K, et al. First experience with the deltastream® DP3 in venovenous extracorporeal membrane oxygenation and air-supported inter-hospital transport. Interact Cardiovasc Thorac Surg. 2013;17:773–777. doi: 10.1093/icvts/ivt320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philipp A, Arlt M, Amann M, et al. First experience with the ultra compact mobile extracorporeal membrane oxygenation system Cardiohelp in interhospital transport. Interactive Cardiovasc Thorac Surg. 2011;12:978–981. doi: 10.1510/icvts.2010.264630. [DOI] [PubMed] [Google Scholar]
- 22.Raspé C, Rückert F, Metz D, et al. Inter-hospital transfer of ECMO-assisted patients with a portable miniaturized ECMO device: 4 years of experience. Perfusion. 2015;30:52–59. doi: 10.1177/0267659114531611. [DOI] [PubMed] [Google Scholar]
- 23.Roch A, Hraiech S, Masson E, et al. Outcome of acute respiratory distress syndrome patients treated with extracorporeal membrane oxygenation and brought to a referral center. Intensive Care Med. 2014;40:74–83. doi: 10.1007/s00134-013-3135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roncon-Albuquerque R, Jr, Basílio C, Silva S, et al. Portable miniaturized extracorporeal membrane oxygenation systems for H1N1-related severe acute respiratory distress syndrome: A case series. J Crit Care. 2012;27:454–463. doi: 10.1016/j.jcrc.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Rossaint R, Pappert D, Gerlach H, et al. Extracorporeal membrane oxygenation for transport of hypoxaemic patients with severe ARDS. Br J Anaesth. 1997;78:241–246. doi: 10.1093/bja/78.3.241. [DOI] [PubMed] [Google Scholar]
- 26.Starck CT, Hasenclever P, Falk V, et al. Interhospital transfer of seriously sick ARDS patients using veno-venous extracorporeal membrane oxygenation (ECMO): Concept of an ECMO transport team. Int J Crit Ill Inj Sci. 2013;3:46–50. doi: 10.4103/2229-5151.109420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaja R, Chauhan I, Joshi V, et al. Five-year experience with mobile adult extracorporeal membrane oxygenation in a tertiary referral center. J Crit Care. 2015;30:1195–1198. doi: 10.1016/j.jcrc.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Wagner K, Sangolt GK, Risnes I, et al. Transportation of critically ill patients on extracorporeal membrane oxygenation. Perfusion. 2008;23:101–106. doi: 10.1177/0267659108096261. [DOI] [PubMed] [Google Scholar]
- 29.Beckmann A, Benk C, Beyersdorf F, et al. ECLS Working Group. Position article for the use of extracorporeal life support in adult patients. Eur J Cardiothorac Surg. 2011;40:676–680. doi: 10.1016/j.ejcts.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Hanson C, Durbin C, Maccioli A, et al. The anesthesiologist in critical care medicine: Past, present, and future. Anesthesiology. 2001;95:781–788. doi: 10.1097/00000542-200109000-00034. [DOI] [PubMed] [Google Scholar]
- 31.Angus DC, Kelley MA, Schmitz RJ, et al. Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: Can we meet the requirements of an aging population? JAMA. 2000;284:2762–2770. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 32.Day SE, Chapman RA. Transport of critically ill patients in need of extracorporeal life support. Crit Care Clin. 1992;8:581–596. [PubMed] [Google Scholar]
- 33.Schonberger RB. The importance of cost as an outcome in anesthesiology research. J Cardiothorac Vasc Anesth. 2016;30:10–11. doi: 10.1053/j.jvca.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Foley DS, Pranikoff T, Younger JG, et al. A review of 100 patients transported on extracorporeal life support. ASAIO J. 2002;48:612–619. doi: 10.1097/00002480-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz RJ, Jacobs LM, Lee M. The role of the physician in a helicopter emergency medical service. Prehosp Disaster Med. 1990;5:31–39. [Google Scholar]