Abstract
Major depressive disorder (MDD) is among the most prevalent neuropsychiatric disorders associated with HIV infection; however, its risks and neurobiologic correlates in diverse cultures are poorly understood. This study aimed to examine the frequency of MDD among HIV+ participants in southern Brazil. We hypothesized that the frequency and severity of MDD would be higher among individuals HIV+ compared with HIV−, and higher in HIV subtype B compared with C. Individuals with HIV (n=39) as well as seronegative controls (n=22) were enrolled in a cross-sectional, prospective, observational study. Current and lifetime history of MDD was diagnosed by MINI-Plus; symptom severity was assessed by BDI-II. Current and past episodes of MDD were significantly more frequent in the HIV+ versus HIV− group: current MDD, 15 (38.5%) vs. 0 (0%), p = 0.0004; past MDD, 24 (61.5%) vs. 3 (13.6%), p = 0.0004. The median BDI-II score in the HIV+ group was significantly higher than in the HIV− (13 [8–27.5] vs. 2.5 [1–5.5]; p < 0.0001). Current suicide risk, defined as during the last month, was found in 18% of participants in the HIV-positive and in none in the HIV-negative group. Neither current MDD frequency (8 (57.1%) vs. 6 (40%), p = 0.47) nor BDI-II score differed across subtypes B and C.
Conclusions
HIV+ group may be more likely to experience current MMD than HIV−. This was the first study to compare the frequency and severity of MDD in HIV subtype B and C; we found no difference between HIV subtypes B or C.
Keywords: MDD, HIV associated neurocognitive disorders (HAND), HIV-1, HIV-1 subtype B, HIV-1 subtype C, efavirenz
INTRODUCTION
Major depressive disorder (MDD) is among the most prevalent psychiatric disorders in HIV infection, but its risks and neurobiology are poorly understood. Previous reviews and meta-analysis have provided evidence that people who are HIV-positive have nearly twice the risk of developing MDD than people who are HIV− negative (Ciesla and Roberts, 2001; Wolff et al., 2010).
HIV-1 is characterized by extensive genetic diversity. HIV-1 subtype C is the most prevalent subtype, accounting for approximately 52% of infections in the entire world (Ariën et al., 2007). HIV-1 subtypes present many structural and functional differences, which may influence cellular tropism and organ involvement, including central nervous system (CNS) involvement and cognitive impairment. It was previously thought that different subtypes might account for variation in the incidence of cognitive impairment in distinct geographic regions of the world (Kanki et al., 1999; Kaleebu et al., 2002; Sacktor et al., 2009), although this hypothesis has not been supported by recent studies (de Almeida et al., 2013; Sacktor et al., 2014).
HIV-1 subtype C shows, in vitro, reduced chemoattractant activity of the trans-activator of transcription (tat) protein compared with subtype B, which may influence cellular trafficking and CNS inflammation neuropathogenesis (Satishchandra et al., 2000; Ranga et al., 2004). The defective tat chemokine activity of HIV-1 subtype C is due to loss of the “CC” group (cysteine replaced by a serine [CS, SC]) (Ranga et al., 2004). Previous studies have demonstrated that neuroinflammation is a risk factor for major depressive disorder. Because the Tat protein from HIV-1 subtype C lacks the chemokine motif seen in subtype B, the resulting reduced trafficking of inflammatory cells into the brain might be expected to lead to less frequent MDD.
The frequency of MDD could be, in the same way, influenced by structural and functional differences among HIV subtypes. No previous study has examined the frequency of MDD in individuals infected with subtypes B and C. The objective of this study was to determine the frequency of MDD and the risk of suicide among individuals who are HIV-positive, and compare the frequency of MDD among those infected with HIV subtype B vs. C. This study hypothesized that the proportion of individuals with MDD and the severity of depressive symptoms will be higher in people who are HIV-positive than HIV− negative, and higher in people diagnosed with HIV-1 subtype B than C. The objectives of this study were to determine the frequency of MDD and the risk of suicide among individuals who are HIV-positive, chiefly among those infected with HIV subtype B vs. C; and the prevalence of MDD among patients on ARV regimen including efavirenz (EFV). This is the first study evaluating the relation between HIV-1 subtype differences and MDD frequency.
DESIGN AND METHODS
A prospective cross-sectional study of MDD frequency and clinical characteristics among individuals who were HIV-positive and HIV-negative was performed in southern Brazil. HIV-positive research volunteers were recruited at the Hospital de Clínicas, Universidade Federal do Paraná (UFPR), Curitiba, Paraná, Brazil between 2007 and 2011. None had opportunistic infections in the CNS. All volunteers provided samples of blood and cerebrospinal fluid (CSF) under a study protocol approved by the Hospital de Clínicas-UFPR (Brazil) Institutional Review Board (IRB) and the Brazilian National IRB (CONEP). Participants provided written informed consent.
Subjects
As described previously (de Almeida et al., 2013); participants who were HIV-positive and HIV-negative/HCV-negative were enrolled from the same geographic region in Curitiba, southern Brazil. A total of 61 participants were studied. As seen in Table 1, the HIV+ and HIV− groups were matched for gender, age and years of education. All participants who were HIV-positive underwent serological testing to confirm their HIV status before enrollment, according to guidelines published by the Brazilian Ministry of Health (BRASIL, 2009). Cases with opportunistic CNS infections were excluded. HIV-negative control participants were recruited from the HC-UFPR blood bank, these participants tested serologically negative for HIV, HBV, HCV, HTLV-1 e 2, and syphilis, with no neurological co-morbidities.
Table 1.
Demographics, AIDS treatment, and co-morbidities in HIV-positive and HIV− negative groups
| HIV+ (n = 39) | HIV B (n = 14) | HIV C (n = 15) | HIV− (n = 22) | p (a) | p (b) | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age years – median (IQR) | 43 (34.5, 49.5) | 44.5 (35.5, 51.5) | 42 (35.5, 46) | 47 (34.5, 56.5) | 0.254 | 0.305 |
| Education, yrs–median (IQR) | 9.0 (5.5, 12) | 9.5 (4, 12.5) | 8 (5.5, 12) | 4.5 (4.0, 15) | 0.162 | 0.793 |
| Gender – N male (%) | 20 (51%) | 8 (57%) | 6 (40%) | 10 (45%) | 0.791 | 0.466 |
| Disease and Treatment | ||||||
| AIDS- N (%) | 34 (87%) | 13 (93%) | 12 (80%) | 0 | <0.0001 | 0.598 |
| Months of disease | 114 (34, 140) | 98.8 (64, 172) | 128 (23, 140) | - | - | 0.529 |
| Current CD4 – median (IQR) cells/mm3 | 372 (231, 576) | 479 (255; 852) | 372 (193; 469) | - | - | 0.085 |
| Nadir CD4 – median (IQR) cells/mm3 | 92 (38, 266) | 139 (25; 254) | 143 (29; 376) | - | - | 0.711 |
| Log Pl HIV RNA – median (IQR) | 1.68 (1.68, 2.87) | 1.68 (1.68; 1.68) | 2.24 (1.68; 4,38) | - | - | 0.026 |
| Log CSF HIV RNA – median (IQR) | 1.68 (1.68, 2.82) | 1.68 (1.68; 1.76) | 2.1 (1.68; 3.39) | - | - | 0.050 |
| on CART – N (%) | 32 (82%) | 12 (93%) | 9 (60%) | - | - | 0.080 |
| with EFV – N (%) of on CART | 07 (22%) | 3 (23%) | 1 (11%) | - | - | 0.616 |
| Adherence | 28 (88%) | 11 (92%) | 9 (100%) | - | - | 1.0 |
| CPE – median (IQR) | 8 (5, 9) | 8 (5, 9) | 7 (5.5, 9) | - | - | 0.867 |
| Co-morbidities | ||||||
| IHDS | 11 (10; 12) | 10.5 (8.25; 12) | 12 (12; 12) | 12 (11; 12) | 0.04 | 0.079 |
| HCV antibody positive* – N(%) | 6 (15%) | 2 (14%) | 1 (7%) | 0 | - | 0.588 |
Plasma VL (log10 c/mL); CART, combination antiretroviral therapy; EFV-efavirenz; CPE-CNS Penetration Effectiveness Rank for Antiretroviral
p HIV-positive vs. HIV-negative;
p HIV-1 subtype B vs. subtype C
Hepatitis C virus (HCV) serostatus was assessed using HCV antibody testing (Abbott-Architect); None of the HCV co-infected participants were on treatment with interferon-γ.
Risk factors for HIV infection
The risk factors for HIV infection, overall, were: heterosexual contact (n= 26; 67%), men who have sex with men (MSM; n=8; 21%), intravenous drug use (n= 3; 7.7%), and unknown (n=2; 5%). There was no difference in risk factors for HIV infection in the groups with and without MDD (p = 0.63). In the group with MDD, the risk factors were: heterosexuality (n= 10; 67%), MSM (n=2; 13%), intravenous drug use (n=2; 13%), and unknown (n=1; 7%). While in the group without MDD were: heterosexuality (n=16; 67%), MSM (n=6; 25%), intravenous drug use (n=1; 4%), and unknown (n=1; 4%).
Methods, described previously (Kamat et al, 2012; de Almeida et al., 2013).
Psychiatric assessments
Current and lifetime MDD were diagnosed by a trained psychiatrist using a Brazilian Portuguese version of the Mini-International Neuropsychiatric Interview (MINI-plus 5.0.0, 2002; Sheehan et al., 1998) and depression severity was assessed with the Portuguese Beck Depression Inventory-II (BDI-II). Participants completed the Beck Depression Inventory-II (BDI-II). The BDI-II is a 21-item self-report measure that rates severity of depressive symptoms during the past week, addressing somatic (e.g., weight loss, fatigue) and non-somatic (e.g., suicidal ideation, feelings of guilt) depressive symptoms. Higher scores indicate greater depressive symptomatology (Beck et al., 1996; Gorenstein and Andrade, 1996).
Neuromedical (NM) assessments
Participants underwent a comprehensive neuromedical assessment. This included a blood draw, lumbar puncture for the HIV+ group, neurological examination, and administration of the International HIV Dementia Scale (IHDS). ARV treatment adherence was evaluated with the AIDS clinical trial (ACTG) adherence questionnaire (4-day recall),
Examiner Training
There were separate training teams for the neurobehavioral, neuromedical, and psychiatric modules of the battery. The psychiatrist was trained by Dr. Amorin, author of the Brazilian Portuguese version of the MINI-plus.
Clinical laboratory measures
HIV RNA levels in serum and CSF were measured by branched DNA assay (Siemens) with a nominal limit of detection of 50 copies/mL. Current CD4 counts were quantified by flow cytometry (FACSCalibur-Multitest). Nadir CD4 levels was extracted from medical records.
HIV subtype was assigned for participants who had clinical resistance, using pol sequences. For the remainder of participants, subtype was determined by sequencing env from HIV DNA. HIV genotyping was performed at the Virology Laboratory, Hospital de Clínicas, UFPR, Brazil. Together these yielded 14 people with HIV subtype B, 15 people with HIV subtype C. Low frequency infections with subtype F (n = 1), recombinant form BF (n = 5), and CF (n = 1), were not included in the subtype analysis; subtype could not be identified for 3 additional subjects.
Statistical Analyses
Comparisons between groups were made using chi-square tests, Fisher’s exact test, and t-tests as appropriate. Values for subtypes B and C and the HIV-positive and HIV-negative groups were then compared with an independent-groups t-test (unadjusted analysis). The correlation between continuous variables was calculated with a Spearman correlation test. Statistical significance was evaluated at the 5% alpha level.
RESULTS
As shown in Table 1, individuals infected with HIV-1 subtypes B and C were similar in age, gender, and education and CD4 nadir. Participants infected with subtype C had longer estimated duration of HIV infection than subtype B. Participants infected with subtype B were more likely to be on ART, although this difference was not statistically significant (93% vs. 60%; p = 0.08), and those on ART were more likely to be virologically suppressed (plasma HIV RNA <50 copies/mL, 81% vs. 40%, p = 0.01). Among participants who were HIV-positive, hepatitis C serology was positive in 6 (15%).
1. MDD in HIV-positive and HIV-negative groups
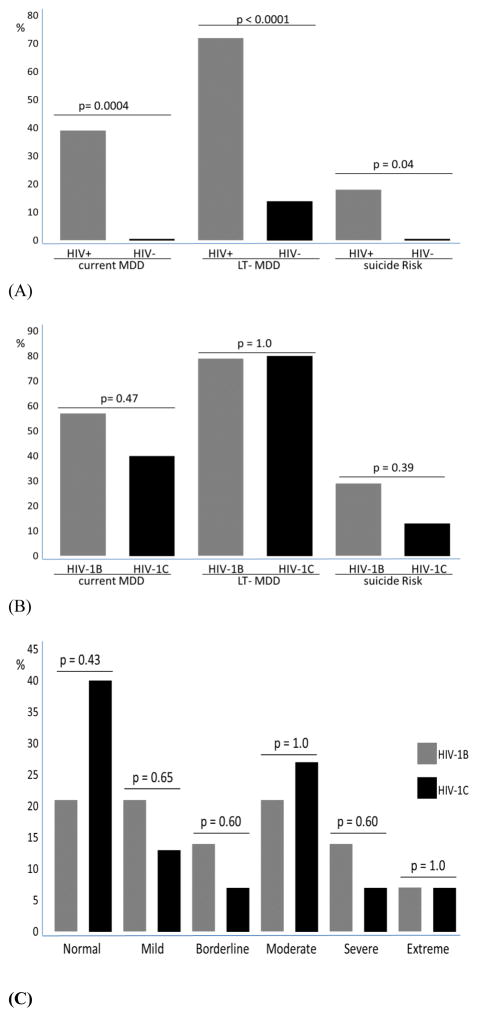
The severity of depressive symptoms (measured by the BDI-II score) and proportion of depression diagnosis (measured by the MINI-PLUS) were greater in the HIV+ group than in the HIV− group. Current MDD, defined as depression during last 2 weeks, was diagnosed in 15 (38.5%) of the participants in the HIV-positive group and in none in the HIV-negative group. The BDI-II score in the HIV-positive group (median, IQR) was 13 (8, 27.5) and 2.5 (1, 5.5) in the HIV-negative group, p < 0.0001 (Figure 1, Table supplementary). Past history of MDD, was diagnosed in 24 (62%) of the participants in the HIV-positive group and in 3 (14%) in the HIV-negative group (p= 0.0004).
Figure 1.
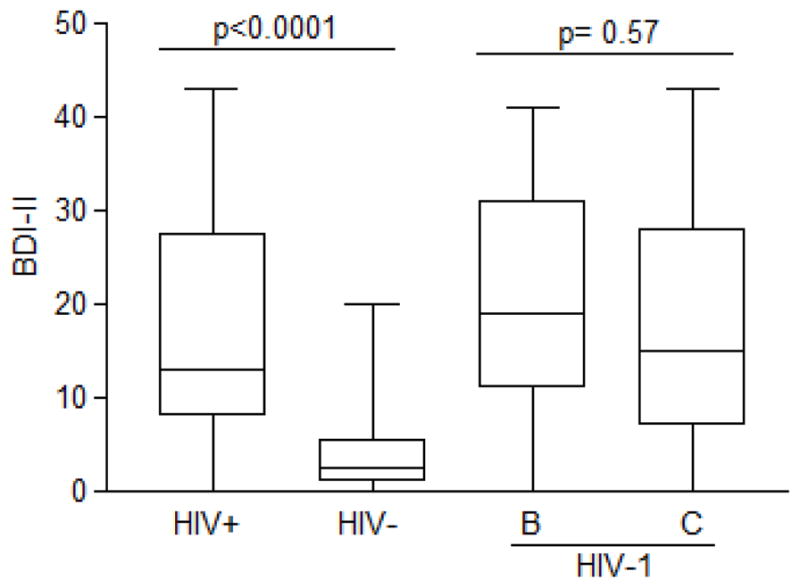
Depression severity according to BDI-II scores among participants in the HIV-positive and HIV-negative groups and by HIV-1 subtypes B and C
Current suicide risk, defined as suicide risk during the last month, was found in 7 (18%) participants in the HIV-positive group and in none in the HIV-negative group, p = 0.042 (Figure 2A). The severity of suicide risk was high in 5 (71%) of the participants with suicide risk and low in 2 (29%).
Figure 2.
Proportion of participants with syndromic depression, (A) measured by the MINI-PLUS in the HIV-positive and HIV-negative groups; (B) measured by the MINI-PLUS in the HIV-1 subtype B and C groups. (C) Depression severity as a function of BDI-II scores for participants with HIV-1 subtypes B and C: normal (0–10); mild mood disturbance (11–16); borderline clinical depression (17–20); moderate depression (21–30); severe depression (31–40); extreme depression (<40).
Other less frequent diagnosis from the MINI-PLUS in the HIV-positive and HIV-negative groups were: past history of manic episode was diagnosed in 1 (2.5%) vs. 0 respectively; current manic episode was not diagnosed in both groups; current hypomania was diagnosed in 4 (10%) vs. 1 (5%), p=0.645; past history of hypomania was diagnosed in 7 (18%) vs. 4 (18%), p=1.0; current and past history of bipolar type I disorder was diagnosed in 1 (2,5%) in the HIV-positive vs. none in the HIV-negative group; current bipolar type II disorder was present in 9 (23%) vs. 4 (18%), p=0.75; past history of bipolar II disorder was present in 6 (15%) vs. 3 (14%) respectively, p= 1.00. Alcohol dependence in the last 12 months and lifetime were higher in the HIV-positive than in the HIV-negative group (6 [15%] vs. 1 [5%], p= 0.405; and 8 [20.5%] vs. 1 [5%], p=0.138; respectively). Lifetime non-alcohol substance dependence, more frequent cocaine and crack cocaine, was also higher in the HIV-positive than in the HIV-negative (7 [18%] vs. 0, p = 0.04); and in the last 12 months it was present in 1 (2.5%) in the HIV-positive, vs. none in the HIV-negative group.
2. MDD in patients with HIV-1 subtypes B and C
The frequency of current MDD was in the HIV-1 subtype B group 8(57%) and in HIV-1 subtype C group 6(40%), p=0.47. There was no difference in the severity of depression measured by BDI-II scores for groups with HIV-1 subtypes B and C. The BDI-II score median (IQR) in the HIV+B group was 19 (11, 31) and in the HIV-1 subtype C group was 15 (7, 28), p = 0.57 (Figure 1). Current suicide risk was higher in HIV-1 subtype B (n = 4 [29%]) compared with HIV-1 subtype C (n = 2 [13%]), although this difference was not significant, p = 0.39 (Figure 2B). Figure 2C shows the depression severity as assessed using the BDI-II in the participants with HIV-1 subtypes B and C.
3. Comparison between HIV-positive participants with and without MDD
Demographics, AIDS treatment, and co-morbidities in participants who were HIV-positive with and without MDD are shown in Table 2. The group without MDD had more years of education, on an average, than the group with MDD, which is probably related to the greater understanding of HIV infection among more educated participants.
Table 2.
Demographics, AIDS treatment, and co-morbidities in groups that were HIV-positive with and without MDD
| With MDD (n = 15) | Without MDD (n = 24) | p | |
|---|---|---|---|
| Demographics | |||
| Age years – median (IQR) | 45 (43, 53) | 40 (34, 46) | 0.065 |
| Education, yrs–median (IQR) | 5 (4, 11) | 10.5 (7, 13.5) | 0.010 |
| Gender – N male (%) | 8 (53%) | 12 (50%) | 1.00 |
| Disease and Treatment | |||
| AIDS- N (%) | 13 (87%) | 21 (88%) | 1.00 |
| Months of disease | 128 (76, 166) | 87 (24, 139) | 0.383 |
| Current CD4 – median (IQR) cells/mm3 | 457 (260, 689) | 345 (211, 469) | 0.242 |
| Nadir CD4 – median (IQR) cells/mm3 | 186 (102, 303) | 63 (22, 201) | 0.126 |
| Log Pl HIV RNA – median (IQR) | 1.68 (1.68, 3.20) | 1.68 (1.68, 2.84) | 0.953 |
| Log CSF HIV RNA – median (IQR) | 1.80 (1.68, 3.08) | 1.68 (1.68, 2.45) | 0.182 |
| on CART – N (%) | 12 (80%) | 18 (75%) | 1.00 |
| with EFV – N (%) of on CART | 1 (8.3%) | 6 (33%) | 0.193 |
| Adherence | 12 (100%) | 16 (89%) | 0.503 |
| CPE – median (IQR) | 8 (5, 9) | 8 (5, 9) | 0.794 |
| Depression syndrome | |||
| BDI | 25 (17, 34) | 11 (4, 21) | 0.001 |
| Past MDD | 11 (73%) | 13 (54%) | 0.317 |
| Co-morbidities | |||
| IHDS | 11 (9.3, 12) | 11.5 (10.8, 12) | 0.098 |
| GDS | 0.9 (0.26, 1.26) | 0.53 (0.25, 1.0) | 0.304 |
| HCV antibody positive*-N (%) | 1(7%) | 5(21%) | 0.376 |
Plasma VL (log10 c/mL); CART, combination antiretroviral therapy; EFV-efavirenz;
Hepatitis C virus (HCV) serostatus was assessed using HCV antibody testing (Abbott-Architect). None of the HCV co-infected participants was on treatment with Interferon gamma.
In the group of participants who were HIV-positive, there was a weak negative correlation between BDI-II scores and years of education (Spearman’s r = −0.32; p = 0.05) and a weak positive correlation between BDI-II scores and CNS Penetration Effectiveness Rank for Antiretroviral-CPE (r = 0.39; p = 0.03). BDI-II scores were not significantly correlated with age, duration of infection, IHDS, nadir and current CD4 counts, plasma and CSF HIV-1 RNA levels, or number of peripheral blood platelets (Spearman’s r = 0.017, p = 0.92; r = 0.38, p = 0.10; r = −0.27, p = 0.09; r = 0.08, p = 0.63; r = 0.04, p = 0.79; r = 0.07, p = 0.67; r = 0.27, p = 0.10; r = −0.25, p = 0.13 respectively).
4. Prevalence of MDD among patients on an ARV regimen including efavirenz (EFV)
In this cohort, there was a high adherence of the participants to the CART. Among the 32 participants on ARV, 28 (88%) were adherent (Table 1). MDD was not related to non-adherence to CART in this population. All participants who were HIV+ with MDD were adherent to CART (Table 2).
Among participants who were HIV-positive and on CART, 7 (22%) had EFV in their regimen (Table 1). Among these, 1 (14%) had current MDD, 5 (71%) had a lifetime history of MDD (LT-MDD), and 1 (14%) had high suicide risk. Among the participants who were HIV+ and not receiving EFV in the ARV regimen, 12 (50%) had current MDD; 18 (75%) had LT-MDD and 5 (21%) had suicide risk (3 high risk and 2 low risk). Comparing both groups (p = 0.19), 1.0, 1.0 respectively). Scores on the BDI-II (median, IQR) in the groups with EFV and without EFV was 24 (12.5; 34.5) and 13 (6.5; 26.5), respectively, p = 0.28.
Discussion
To our knowledge, this is the first study to examine the frequency and severity of depression in HIV-1 subtypes B and C. Consistent with prior studies; we found that a greater proportion of HIV− positive participants met criteria for MDD compared to the seronegative group. Extending the literature, we found that there was no HIV-subtype related difference in the frequency of current or lifetime MDD.
Previous published studies, by the present group, investigating differences between HIV-1 subtypes B and C found no difference in the frequency of HIV-associated neurocognitive disorders evaluated by neuropsychological comprehensive testing (de Almeida et al., 2013). We did not find any molecular evidence to support the hypothesis of reduced intrathecal chemotaxis in subtype C than subtype B infections. Pleocytosis, a marker of cellular trafficking, was much more frequent in individuals who were HIV+ than those who were HIV−, but equally common in individuals who were infected with subtypes B and C (de Almeida et al., 2013). Chemokines and interleukins were frequently elevated in the CSF of subjects who were HIV-positive, but did not differ between subtypes B and C. MCP-1 was stimulated with the same intensity by subtype B and C (de Almeida et al., 2016, in press). The present findings, the frequency of MDD comparable in subtypes B and C provide more support for the belief that there is no difference in CNS HIV infection between these two HIV subtypes.
In this study Current MDD was diagnosed in 39% of the HIV-positive participants, similar to other studies in Brazil and other countries. Depression is the most frequent neuropsychiatric disorder among people living with HIV, with an estimated prevalence as high as 36% (Pieper and Treisman, 2005; Phillips, 2004; Wolff et al., 2010). Lifetime MDD prevalence rates are approximately 40% in North American cohorts (Atkinson et al., 1988; Bing et al., 2001; Ciesla and Roberts, 2001; Serafini et al., 2015). Other studies from Brazil have estimated the percentage of people infected with HIV who have depression as varying from 21% to 37%, across a broad range of cohorts who are HIV+, including patients on ART and those who were ART-naïve (Anastos et al., 2000; Mello et al., 2010; Silveira et al., 2012).
The frequency of MDD in this study was higher than in the general population. In Brazil, the annual prevalence of depression in the general population is considered to be between 3% and 11% (Jenkins et al., 1997; Kessler et al., 1994), and about 10% among patients in primary health care (Ustun and Sartorius, 1995). In a systematic review and meta-analysis of the prevalence of depression morbidity among Brazilian adults, the prevalence of depressive symptoms was 14% and the lifetime prevalence of MDD was 17% (Silva et al., 2014).
Depression has direct and indirect effects on the progression of HIV. MDD has a great impact on the lives of people who are HIV-positive. MDD impairs quality of life, and is associated with high-risk sexual behavior, which facilitates viral transmission, increasing mortality and morbidity. It is reported that MDD impairs adherence to CART (Schuster et al., 2012), although in our study, there was no relation between MDD and low adherence. CART adherence was 100% in the group with MDD, but the number of participants was relatively small. The higher educational level in the MDD group could also explain their good adherence to CART. The duration of infection and log CSF HIV RNA are higher in the MDD group, although this difference was not significant. In our study, there was no association between presence of MDD and cognitive impairment.
In this study, the risk of suicide among participants who are HIV-positive was very high, 18%; this is twice the risk of suicide in the general population in Brazil. Among 1,621 young adults not HIV-positive, the suicide risk was 8.6% (Rodrigues et al., 2012). A cross-sectional study was developed to evaluate suicide risk and associated factors in HIV/AIDS patients, interviewing 211 patients, the risk of suicide was 34% (Passos et al., 2014). In our study MSM was a risk factor for HIV infection in 21%, in a study in NYC was described increased risk of suicide attempts among black and latino lesbians, gay men, and bisexuals, lifetime suicide attempt was 17% (O’Donnell, 2011). According with HIV-1 subtypes, in our study, the risk of suicide was more than two times higher in people with HIV-1 subtype B than in HIV-1 subtype C (29% vs. 13%), although this difference was not significant. This is the first study describing risk of suicide according with HIV-1 subtypes.
Among the participants in this study with EFV antiretroviral regimen, current MDD was not frequent, and suicide risk was rare, although the number of participants receiving EFV was relatively low for a definitive conclusion. An association between EFV and increased risk for suicidal ideation or attempted or completed suicide has been reported. An EFV containing antiretroviral regimen was associated with a 2-fold increased hazard of suicidal ideation compared with a regimen without EFV (Mollan et al., 2014).
The risks and factors contributing to major depressive episodes in HIV infection remain unclear (Serafini et al., 2015; Slot et al., 2015). The immunologic response and incidence of depression in HIV need to be better evaluated. Evidence strongly suggests that cytokines have a role in the biology of depression (Loftis et al., 2008). There is extensive evidence that depression in HIV may also be associated with neurobiological changes related to persistent viral presence in the CNS. In our study, there was no correlation with CSF or plasma HIV RNA. In a longitudinal study, it was observed that persistent CSF HIV RNA, but not plasma HIV RNA, was associated with increased risk of new onset MDD (Hammond et al., 2014).
It is possible that in general, patients with a given chronic illness will have similar rates of depression. In chronic conditions, the emotional, familial, social, physical, and functional aspects of life are compromised. These factors are influenced by the type and degree, and period of the illness. Among inpatients with any physical illness, the prevalence of depression was found to be between 22% and 33% (World Psychiatric Association, 1997). The frequency of depression in other CNS infectious diseases is also higher than in the general population (Almeida and Gurjão, 2010; Forlenza et al., 1997; Srivastava et al., 2013). Immunological and inflammatory factors as Th1 and Th2 cytokines could be related with this increased depression frequency, as these cytokines are predominant in the immunological response to these infectious diseases (Aguilar-Rebolledo et al., 2001; Loftis et al., 2008) as in HIV.
In the adult brain, two types of CNS cells can be infected by HIV: cells derived from monocytes (microglia and macrophages) and astrocytes. These cells differ from other HIV-infected cells in some biological aspects, particularly in the way they express the products of the HIV. Neuronal injury in HIV infection results from an indirect mechanism due to the complex interaction of anions, inflammatory and neuronal injury proteins, and constitutional HIV proteins. Neurons do not have CD4 receptors, consequently, HIV does not infect neurons (Price, 2000; Tyler and McArthur, 2002). There is interplay between the various contributory factors during HIV infection and their possible role in the predisposition to depression. These factors include cytokinergic, inflammatory, and monoaminergic, as well as neurodegenerative mechanisms, and reduction of neurotrophic function induced by the virus (Brabers and Nottet, 2006). The chronic inflammatory state stimulated by viral infection leads to chronic sickness. Furthermore, HIV proteins are neurotoxic and impair monoaminergic function. Although immunological factors and neurotoxic viral effects on the brain could be related to the development of depression in HIV infection, the complex interaction with other factors such as genetic predisposition, or social and cultural factors are important (Del Guerra et al., 2013). HIV MDD can be classified into two different etiologic possibilities: one mostly due to psychological stress and the other due to HIV CNS infection leading to inflammation and degeneration. Thus, HIV depression may result from a combination of psychological stress, biochemical dysfunction (lower levels of neurotrophins and monoamines, glutamatergic disturbances, glucocorticoid receptor resistance, production of neurotoxic metabolites, and oxidative stress) due to chronic viral neuroinflammation, and neuronal death that surpasses neurogenesis (Castellon et al., 2006).
The positive points of this study were: The majority of published studies of MDD in HIV are in populations with HIV subtype B; this is the first study on MDD among participants with HIV-1 subtype C. Brazil accounts for the largest population of HIV+ persons in Latin America (OPAS, 2001); however, little is known about the psychiatric aspects of HIV infection in Brazil. All participants who were HIV-positive or HIV-negative were from the same geographic area, which allowed the study to avoid cultural confounding factors; and were interviewed and examined by the same investigators.
As with all cross-sectional studies, important limitations should be acknowledged. The small sample size limits the confidence in our findings. This is particularly true for the analyses pertaining to the relationship between MDD and EFV. From a statistical design standpoint, as this is an exploratory study, there were no adjustments made for multiple statistical analyses, although the groups B and C were similar in age, gender, education, CD4 nadir, duration of HIV infection, exposure to ART and CPE. Other issue is that we do not know the frequency of subtype C Tat C30C31S substitution in Brazil or in the samples studied, that is implied with the explanation of the difference on CNS complications on HIV-1 subtypes B and C. Future studies are needed to replicate and further examine the present findings.
To conclude, we found that individuals who are HIV+ may be more likely to experience current MMD than matched HIV− controls. Although we detected no difference between HIV subtypes B or C with regard to frequency of current MMD or depression severity, our power to detect an effect was limited. Additional research into neurobiological risks of MDD in HIV is warranted.
Supplementary Material
Table. Proportion of MDD, measured by the MINI-PLUS, and severity of depressive symptoms measured by the BDI-II score
Acknowledgments
Part of this work was presented at the International Neuropsychological Society’s conference, February 6–9, 2013, Waikoloa, Hawaii, USA.
This work was supported by the following grants: National Institute of Health, NIH R21 MH76651 (Ellis, Ronald J; Almeida, Sergio M.).
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH. The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Footnotes
Conflicts of interest:
SERGIO M. DE ALMEIDA, the author declare that have no conflict of interest.
FRANCISCO JAIME BARBOSA, the author declare that have no conflict of interest.
RUJVI KAMAT, the author declare that have no conflict of interest.
ANA PAULA DE PEREIRA, the author declare that have no conflict of interest.
SONIA M. RABONI, the author declare that have no conflict of interest.
INDIANARA ROTTA, the author declare that have no conflict of interest.
CLEA E. RIBEIRO, the author declare that have no conflict of interest.
MARIANA CHERNER, the author declare that have no conflict of interest.
RONALD J. ELLIS, the author declare that have no conflict of interest.
JOSEPH HAMPTON ATKINSON, the author declare that have no conflict of interest.
References
- Aguilar-Rebolledo F, Cedillo-Rivera R, Llaguno-Violante P, Torres-López J, Muñoz-Hernandez O, Enciso-Moreno JA. Interleukin levels in cerebrospinal fluid from children with neurocysticercosis. Am J Trop Med Hyg. 2001;64:35–40. doi: 10.4269/ajtmh.2001.64.35. [DOI] [PubMed] [Google Scholar]
- Almeida SM, Gurjão SA. Frequency of depression among patients with neurocysticercosis. Arq Neuropsiquiatr. 2010;68:76–80. doi: 10.1590/s0004-282x2010000100017. [DOI] [PubMed] [Google Scholar]
- Anastos K, Gange SJ, Lau B, Weiser B, Detels R, Giorgi JV, Melnick S. Association of race and gender with HIV-1 RNA levels and immunologic progression. J AIDS. 2000;24:218–226. doi: 10.1097/00126334-200007010-00004. [DOI] [PubMed] [Google Scholar]
- Ariën KK, Vanham G, Arts EJ. Is HIV-1 evolving to a less virulent form in humans? Nature Rev Microbiol. 2007;5:141–151. doi: 10.1038/nrmicro1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JH, Grant I, Kennedy CJ, Richman DD, Spector SA, McCutchan JA. Prevalence of psychiatric disorders among men infected with human immunodeficiency virus: a controlled study. Arch Gen Psych. 1988;45:859–864. doi: 10.1001/archpsyc.1988.01800330091011. [DOI] [PubMed] [Google Scholar]
- Atkinson JH, Heaton RK, Patterson TL, Wolfson T, Deutsch R, Brown SJ, Summers J, Sciolla A, Gutierrez R, Ellis RJ, Abramson I, Hesselink JR, McCutchan JA, Grant I. Two-year prospective study of major depressive disorder in HIV-infected men. J Affect Disord. 2008;108:225–34. doi: 10.1016/j.jad.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Manual for Beck Depression Inventory II (BDI-II) Psychology Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Vitiello B. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psych. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Brabers NACH, Nottet JSLM. Role Of The Pro-Inflamatory Cytokines TNF-alpha and IL-beta In HIV-associated dementia. Eur J Clin Investig. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Brasil. Ministério da Saúde. Departamento de DST, AIDS e Hepatites Virais (16/10/2009). Portaria SVS/MS - n° 151/2009
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, Grant I, Gupta JD, Satishchandra P, Gopukumar K, Wilkie F, Waldrop-Valverde D, Ellis R, Ownby R, Subbakrishna DK, Desai A, Kamat A, Ravi V, Rao BS, Satish KS, Kumar M. Neuropsychological deficits in human immunodeficiency virus type 1 clade C-seropositive adults from South India. J Neurovirol. 2007;13:195–202. doi: 10.1080/13550280701258407. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, Myers HF, Moore L. Components of depression in HIV-1 infection: their differential relationship to neurocognitive performance. J Clin Exp Neuropsychol. 2006;28:420–437. doi: 10.1080/13803390590935444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158:725–30. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- de Almeida SM, Ribeiro CE, de Pereira AP, Badiee J, Cherner M, Smith D, Maich I, Raboni SM, Rotta I, Barbosa FJ, Heaton RK, Umlauf A, Ellis RJ. Neurocognitive impairment in HIV-1 subtype C-versus B-infected individuals in Southern Brazil. J Neurovirol. 2013;19:550–556. doi: 10.1007/s13365-013-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Rotta I, Jiang Y, Li X, Raboni SM, Ribeiro CE, Smith D, Potter M, Vaida F, Letendre S, Ellis RJ. Biomarkers of Chemotaxis and Inflammation in Cerebrospinal Fluid and Serum in Individuals with HIV-1 Subtype C versus B. J Neurovirol. 2016 doi: 10.1007/s13365-016-0437-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Guerra FB, Fonseca JLI, Figueiredo VM, Ziff EB, Konkiewitz EC. Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. J Neurovirol. 2013;19:314–27. doi: 10.1007/s13365-013-0177-7. [DOI] [PubMed] [Google Scholar]
- Forlenza OV, Vieira AHG, Nobrega JPS, Machado LR, de Barros NG, de Camargo CH, da Silva MF. Psychiatric manifestations in neurocysticercosis: a study of 38 patients from a neurology clinic in Brazil. J Neurol Neurosurg Psych. 1997;62:612–616. doi: 10.1136/jnnp.62.6.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C, Andrade LHSG. Validation of a Portuguese version of the Beck Depression Inventory and the State-trait Anxiety Inventory in Brazilian subjects. Rev Bras Pesq Med Biol. 1996;29:453–57. [PubMed] [Google Scholar]
- Hammond ER, Crum RM, Treisman GJ, Mehta SH, Ellis RJ, Grant I, Letendre SL, Marra CM, Morgello S, McArthur JC. Persistent CSF, but not plasma HIV RNA is associated with increased risk of new onset depression. 21st Conference on Retrovirus and Opportunistic Infections (CROI 2014); Boston. March 3–6, 2014; 2014. Abstract 33. [Google Scholar]
- Jenkins R, Lewis G, Bebbington P, Brugha T, Farrell M, Gill B, Meltzer H. The National Psychiatric Morbidity Surveyof Great Britain – initial findings from the household surveys. Psychol Med. 1997;27:775–89. doi: 10.1017/s0033291797005308. [DOI] [PubMed] [Google Scholar]
- Kaleebu P, French N, Mahe C, Yirrell D, Waters C, Lyagoba F, Nakiyingi J, Rutebemberwa A, Morgan D, Weber J, Gilks C, Whitworth J. Effects ofhuman immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-postive persons in Uganda. J Infect Dis. 2002;185:1244–1250. doi: 10.1086/340130. [DOI] [PubMed] [Google Scholar]
- Kamat R, Morgan E, Marcotte TD, Badiee J, Maich I, Cherner M, de Almeida S, de Pereira AP, Ribeiro CE, Barbosa F, Atkinson JH, Ellis R. Implications of apathy and depression for everyday functioning in HIV/AIDS in Brazil. J Affect Disord. 2013;150:1069–75. doi: 10.1016/j.jad.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki PJ, Hamel DJ, Sankale JL, Hsieh C, Thior I, Barin F, Woodcock SA, Gueye-Ndiaye A, Zhang E, Montano M, Siby T, Marlink R, Ndoye I, Essex ME, Mboup S. Human immunodeficiency virus type 1 subtypes differ in disease progression. J Infect Dis. 1999;179:68–73. doi: 10.1086/314557. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-IIIR psychiatric disorders in the United States. Arch Gen Psych. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Huckans M, Ruimy S, Hinrichs DJ, Hauser P. Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1beta and tumor necrosis factor-alpha. Neurosci Lett. 2008;430:264–68. doi: 10.1016/j.neulet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello VA, Segurado AA, Malbergier A. Depression in women living with HIV, clinical and psychosocial correlates. Arch Women Ment Health. 2010;13:193–199. doi: 10.1007/s00737-009-0094-1. [DOI] [PubMed] [Google Scholar]
- Mini-International Neuropsychiatric Interview (MINI) 5.0.0, 2002 (MINI-plus 5.0.0, Brazilian version).
- Mollan KR, Smurzynski M, Eron JJ, Daar ES, Campbell TB, Sax PE, Gulick RM, Na L, O’Keefe L, Robertson KR, Tierney C. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med. 2014;161:1–10. doi: 10.7326/M14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell S, Meyer IH, Schwartz S. Increased Risk of Suicide Attempts Among Black and Latino Lesbians, Gay Men, and Bisexuals. Am J Public Health. 2011;101:1055–1059. doi: 10.2105/AJPH.2010.300032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPAS. VIH y SIDA en las Americas: una epidemia multifacetica. 2001 Retrieved from www.who.int/hiv/strategic/en/amr_map_01.pdf.
- Passos SM, Souza LD, Spessato BC. High prevalence of suicide risk in people living with HIV: who is at higher risk? AIDS Care. 2014;26:1379–82. doi: 10.1080/09540121.2014.913767. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Treisman G. Drug treatment of depression in HIV positive patients: safety considerations. Drug Saf. 2005;28:753–762. doi: 10.2165/00002018-200528090-00002. [DOI] [PubMed] [Google Scholar]
- Phillips K. Depression in HIV-infected patients: allopathic, complementary, and alternative treatments. J Psychosom Res. 2004;57:339–351. doi: 10.1016/j.jpsychores.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Price RW. The two faces of HIV infection of cerebrospinal fluid. Trends in Microbiol. 2000;8:387–390. doi: 10.1016/s0966-842x(00)01821-7. [DOI] [PubMed] [Google Scholar]
- Raboni SM, Almeida SM, Rotta I, Ribeiro CE, Rosario D, Vidal LR, Nogueira MB, Riedel M, da Winhescki MG, Ferreira KA, Ellis R. Molecular epidemiology of HIV-1 clades in Southern Brazil. Mem Inst Oswaldo Cruz. 2010;105(8):1044–9. doi: 10.1590/s0074-02762010000800015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranga U, Shankarappa R, Siddapa NB, Ramakrishna L, Nagendran R, Mahalingam M, Mahadevan A, Jayasuryan N, Satishchandra P, Shankar SK, Prasad VR. Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J Virology. 2004;78:2586–90. doi: 10.1128/JVI.78.5.2586-2590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MES, da Silveira TB, Jansen K, Cruzeiro ALS, Ores L, Pinheiro RT, da Silva RA, Tomasi E, Souza LDM. Suicide risk in young adults with anxiety disorders: population-based study. Psico-USF. 2012;17:53–62. [Google Scholar]
- Sacktor N, Nakasujja N, Skolasky RL, Rezapour M, Robertson K, Musisi S, Katabira E, Ronald A, Clifford DB, Laeyendecker O, Quinn TC. HIV subtype D is associated with dementia, compared with subtype A, in immunosuppressed individuals at risk of cognitive impairment in Kampala, Uganda. Clin Infect Dis. 2009;49:780–786. doi: 10.1086/605284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Nakasujja N, Redd AD, Manucci J, Laeyendecker O, Wendel SK, Porcella SF, Martens C, Bruno D, Skolasky RL, Okonkwo OC, Robertson K, Musisi S, Katabira E, Quinn TC. HIV subtype is not associated with dementia among individuals with moderate and advanced immunosuppression in Kampala, Uganda. Metab Brain Dis. 2014;29:261–268. doi: 10.1007/s11011-014-9498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satishchandra P, Nalini A, Gourie-Devi M, Khanna N, Santosh V, Rasvi V, Desai A, Chandramuki A, Jayakumar PN, Shankar SK. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, South India (1989–96) Indian J Med Res. 2000;111:14–23. [PubMed] [Google Scholar]
- Serafini G, Montebovi F, Lamis DA, Erbuto D, Girardi P, Amore M, Pompili M. Associations among depression, suicidal behavior, and quality of life in patients with human immunodeficiency vírus. World J Virol. 2015;12(4):303–312. doi: 10.5501/wjv.v4.i3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster R, Bornovalova M, Hunt E. The influence of depression on the progression of HIV: direct and indirect effects. Behav Modif. 2012;36:123–145. doi: 10.1177/0145445511425231. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Silva MT, Galvão TF, Martins SS, Pereira MG. Prevalence of depression morbidity among Brazilian adults: a systematic review and meta-analysis. Rev Bras Psiq. 2014;36:262–270. doi: 10.1590/1516-4446-2013-1294. [DOI] [PubMed] [Google Scholar]
- Silveira MPT, Guttier MC, Pinheiro CAT, Pereira TVS, Cruzeiro ALS, Moreira LB. Depressive symptoms in HIV-infected patients treated with highly active anti-retroviral therapy. Rev Bras Psiq. 2012;34:162–167. doi: 10.1590/s1516-44462012000200008. [DOI] [PubMed] [Google Scholar]
- Slot M, Sodemann M, Gabel C, Holmskov J, Laursen T, Rodkjaer L. Factors associated with risk of depression and relevant predictors of screening for depression in clinical practice: a cross-sectional study among HIV-infected individuals in Denmark. HIV Med. 2015;16:393–402. doi: 10.1111/hiv.12223. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Chadda RK, Bala K, Majumdar P. A study of neuropsychiatric manifestations in patients of neurocysticercosis. Indian J Psych. 2013;55:264–267. doi: 10.4103/0019-5545.117146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler KL, McArthur JC. Through a glass, darkly: cerebrospinal fluid viral load measurements and the pathogenesis of human immunodeficiency virus infection of the central nervous system. Arch Neurol. 2002;59:909–912. doi: 10.1001/archneur.59.6.909. [DOI] [PubMed] [Google Scholar]
- Ustun TB, Sartorius N, editors. Mental illness in primary care: international study. New York: John Wiley & Sons; 1995. [Google Scholar]
- Wolff LC, Alvarado MR, Wolff RM. Prevalencia, factores de riesgo y manejo de la depresión en pacientes con infección por VIH: revisión de la literatura. Rev Chi Infectol. 2010;27:65–74. [PubMed] [Google Scholar]
- World Psychiatric Association. Overview and fundamental aspects. New York: NCM; 1997. Educational program on depressive disorders. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table. Proportion of MDD, measured by the MINI-PLUS, and severity of depressive symptoms measured by the BDI-II score