Abstract
Background
Obstructive sleep apnea (OSA) associated with obesity is known to improve after bariatric surgery, but little is known about early changes in this condition following surgery.
Objectives
To study the clinical course of OSA after bariatric surgery.
Setting
Children's hospital in the United States.
Methods
Adolescents and young adults with obstructive sleep apnea undergoing vertical sleeve gastrectomy (n=6) or gastric bypass (n=1) were enrolled in this prospective study. Participants underwent formal polysomnography before and at 3 and 5 weeks following bariatric surgery. Anthropometric measurements and assay for orexin and leptin were also performed at study visits. Thirty-one adolescents who underwent 2 polysomnography studies that were 4 weeks apart served as control subjects.
Results
Baseline mean (range) age of participants was 17.8 (15.4-20.7) years, 71% were male, with body mass index of 55.2 (41.3-61.6) kg/m2 and had a median apnea hypopnea index (AHI) of 15.8 (7.1-23.8) events/hour. Differences in least-square means from longitudinal analysis did not show significant differences in AHI in the control group but showed significant post-operative decline in AHI relative to baseline. AHI declined post-operatively from baseline by 9.2 events/hour (95% CI: 3.8 to 14.5) at 3 weeks (p=0.002) and 9.1 events/hour (95% CI: 3.8 to 14.5) at 5 weeks (p=0.002); there was no significant change from 3 to 5 weeks in AHI. Leptin decreased and orexin levels increased significantly by 3 weeks postoperatively.
Conclusions
These observations suggest that OSA responds early and out of proportion to weight loss following metabolic/bariatric surgery, thus weight independent factors may at least in part be responsible for early improvement in OSA postoperatively.
Keywords: sleeve gastrectomy, sleep apnea, orexin, leptin, polysomnography
Introduction
In severely obese adults with body mass index (BMI) ≥ 40 kg/m2, the prevalence of obstructive sleep apnea (OSA) ranges from 64% to 98% (1) (2) . OSA is also a major problem for younger age groups. In a large series of severely obese adolescents undergoing bariatric surgery in the Teen-LABS consortium (3), OSA was the second most prevalent comorbidity at baseline, with 57% diagnosed with OSA at baseline (4). Prior studies have documented OSA improvement in the majority of adults and even resolution in adolescents when assessed at 3-12 months following bariatric surgery (5), and the majority of evidence for improvement in OSA comes from studies of Roux-en-Y gastric bypass patients. Little is known about specific diagnostic characteristics of OSA after vertical sleeve gastrectomy (SG). In addition, the factors that contribute to the resolution of OSA after bariatric surgery besides weight loss are yet to be defined. Testing for change in OSA severity after major bariatric weight loss has occurred does not permit the examination of possible early, weight independent neurohormonal effects of surgery that might contribute to OSA resolution.
The objectives of this study were to describe changes in OSA at 3 and 5 weeks after bariatric surgery, and explore links to physiologic changes occurring postoperatively. We hypothesized that in adolescents with documented OSA, the severity of OSA would decrease within as early as 2 weeks after surgery. We further hypothesized that change in OSA status is associated with a decrease in serum leptin and an increase in plasma orexin, a hormone that is known to regulate wakefulness, and respiratory control, and a decrease in leptin.
Materials and Methods
Adolescents and young adults undergoing bariatric surgery were enrolled in this prospective observational study. Patients who were 18 years of age or older underwent written informed consent for the study while those <18 years of age provided verbal assent and their parent or legally authorized representative gave written permission for their participation. After enrollment, subjects underwent anthropometric assessments, and a formal overnight polysomnogram in the sleep laboratory at visit 1. These assessments were repeated at visit 2 (range 2-35d, median 20 days postoperatively), and visit 3 (range 30-44, median 39 days postoperatively). Polysomnography was performed using a computerized system and the following parameters were recorded during the study: electroencephalogram, right and left electrooculogram, submental, tibial and intercostal electromyogram, electrocardiography, nasal/oral airflow through nasal pressure sensor, end-tidal CO2 measured at the nose by infrared capnometry, oxygen saturation by pulse oximeter, oximeter pulse waveform, video monitoring using an infrared video camera and recorded on a videotape. Rib cage and abdominal volume changes were recorded with a computer-assisted respiratory inductance plethysmograph. OSA severity was measured by apnea hypopnea index (AHI). Phlebotomy was also performed at baseline, and visits 2 and 3; and plasma orexin and leptin were measured. BMI was measured by weighing participants in light clothing on a digital scale and height by stationary stadiometer. To minimize confounding, the first postoperative evaluation was performed after discontinuation of narcotic medication usage. Patients’ dietary intake during the first 3 months was approximately 500-700 Cal per day. The diet is composed of 40% carbohydrate, 30% protein, and 30% fat. In order to determine the night-to-night variability in adolescents with obstructive sleep apnea, we enrolled 31 subjects who underwent two polysomnograms that were four weeks apart.
Linear mixed effects models for repeated measures were used to assess changes from baseline for each follow-up time and also post-operatively between weeks 3 and 5, with respect to each outcome of interest in the OSA group. Differences over time were estimated using least-square means; the 95% CI is reported for each estimated difference. Tukey adjustment for multiple comparisons was used to obtain p-values, which were considered statistically significant if p<0.05. Analyses were implemented using SAS 9.3 (SAS Institute, Cary, NC). The intraclass correlation coefficient (ICC) was obtained to estimate the night-to-night variability in the control group.
Results
Seven adolescents with OSAS (71% males) with mean (range) age of 17.8 (15.4-20.7) years participated in the study (Table 1). Additional baseline comorbid conditions are shown in Table 1. Six subjects underwent sleeve gastrectomy and one underwent Roux-en-Y gastric bypass. Mean BMI at the first visit (baseline) was 55.2 (range, 41.3-61.6) kg/m2 (weight 164.6 [126.5-198]). There were no statistically significant longitudinal changes in BMI, which decreased by 3.2 (95% CI: −10.9 to 4.6) kg/m2 by week 3; from week 3 to week 5, the decrease was 2.4 (95% CI: −10.1 to −5.4) kg/m2. Similarly, weight loss over the post-operative period occurred but changes did not meet statistical significance in this sample (decrease from baseline to week 3: 9.5 kg, 95% CI: −36.7 to 17.7; decrease from week 3 to week 5: 7.2 kg, 95% CI: − 34.4 to 20.0).
Table 1.
| Subject | Age (yrs) | Sex | Race | Baseline Comorbidities | Operation |
|---|---|---|---|---|---|
| 1 | 16.2 | M | Black | Dyslipidemia, OSAS | RYGB |
| 2 | 20.7 | F | Black | OSAS | SG |
| 3 | 15.7 | F | Biracial | OSAS, GERD, NASH | SG |
| 4 | 18.5 | M | Cauc | OSAS | SG |
| 5 | 18.5 | M | Black | HTN, Dyslipidemia, OSAS | SG |
| 6 | 15.4 | M | Cauc | OSAS | SG |
| 7 | 19.4 | M | Cauc | Type 1 DM, Dyslipidemia, OSAS | SG |
Cauc, Caucasian; OSAS, obstructive sleep apnea syndrome; GERD, gastroesophageal reflux disease; DM, diabetes mellitus; HTN, hypertension requiring medication; dyslipidemia, abnormally elevated of LDL cholesterol or triglycerides for age, or abnormally low HDL cholesterol for age; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy
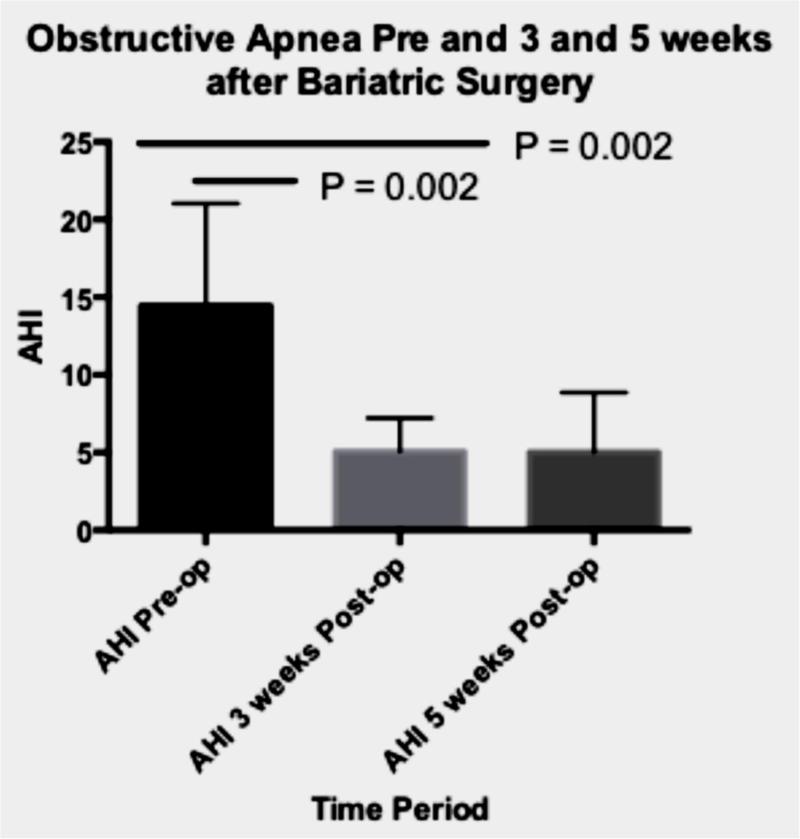
The apnea hypopnea index (AHI) at baseline in the surgical group was 15.8 (median) or 14.6 (mean) events/hour with a range of 7.1 to 23.8. There was a significant decrease in AHI postoperatively (Table 2; Figure 1). From baseline to week 3, AHI decreased by 9.2 events/hour (95% CI: 3.8 to 14.5). Although the mean decrease in AHI was not significant from week 3 to week 5 (decrease: −0.01, 95% CI: −5.4 to 5.3), the AHI at week 5 was significantly lower than the pre-operative (baseline) level (decrease: 9.1 events/hour; 95% CI: 3.8 to 14.5).
Table 2.
Polysomnography data from subjects who underwent bariatric surgery
| PSG 1 | PSG 2 | PSG 3 | |
|---|---|---|---|
| Total sleep time (TST) | 393 ± 22 | 358 ± 36 | 361 ± 68 |
| Sleep efficiency | 80 ± 7 | 74 ± 9 | 70 ± 11 |
| Arousal index | 15.7 ± 5.8 | 12 ± 2.5 | 12.4 ± 4.7 |
| AHI | 13 ± 6.9¶§ | 4.5 ± 2.5 | 5 ± 4.3 |
| SAO2 REM | 96 ± 1 | 96 ± 2.1 | 97 ± 1.3 |
| SAO2 NREM | 97 ± 1.4 | 96 ± 2.3 | 97 ± 1.21 |
| SAO2 nadir | 84 ± 6 | 88 ± 5 | 89 ± 6 |
| End tidal CO2 | 41 ± 3.9 | 41 ± 2.3 | 40 ± 5 |
| Maximum CO2 | 50 ± 2.6 | 48 ± 2.3 | 47 ± 5 |
| % TST in supine | 61 ± 33 | 70 ± 39 | 58 ± 42 |
AHI, apnea hypopnea index; SAO2 REM, average oxygen saturation during REM sleep; SAO2 NREM, average oxygen saturation during NREM sleep. The table describes the polysomnogaphic findings prior to surgery (PSG 1), 3 weeks (PSG 2) and 5 weeks (PSG3) after surgery.
PG1 vs PG2 P < 0.05
PG1 vs PG3 (P < 0.05)
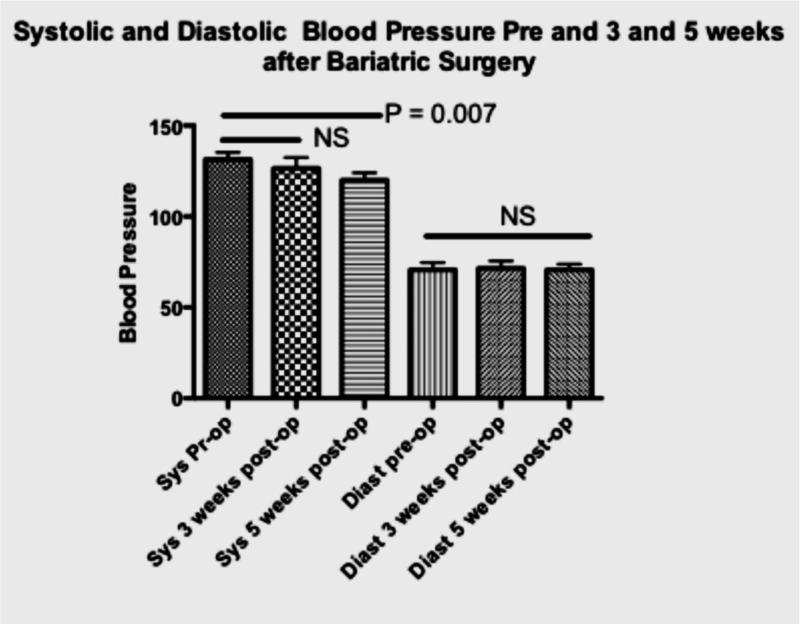
Figure 1. Severity of sleep apnea and changes in blood pressure before and after surgery.
Panel A
AHI= apnea hypopnea index
AHI at baseline, 3 and 5 weeks after bariatric surgery.
P-values obtained by repeated measures analysis with Tukey adjustment for multiple comparisons.
Panel B
Systolic and Diastolic blood pressure at baseline, 3 and 5 weeks after bariatric surgery. P-values obtained by repeated measures analysis with Tukey adjustment for multiple comparisons.
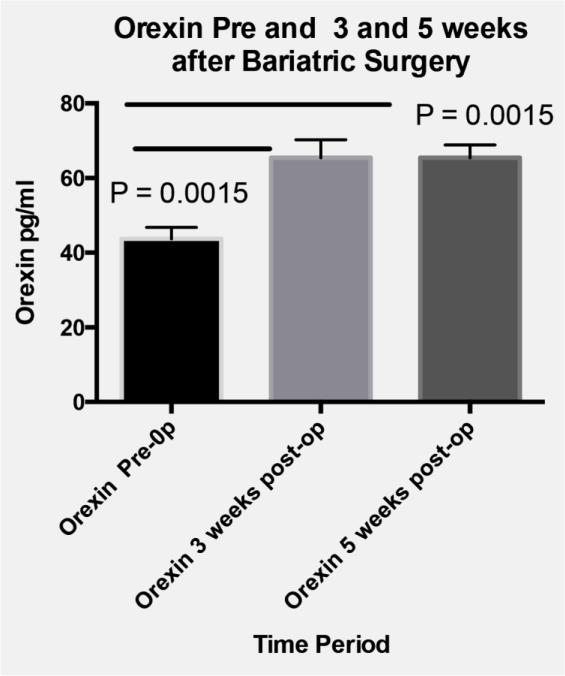
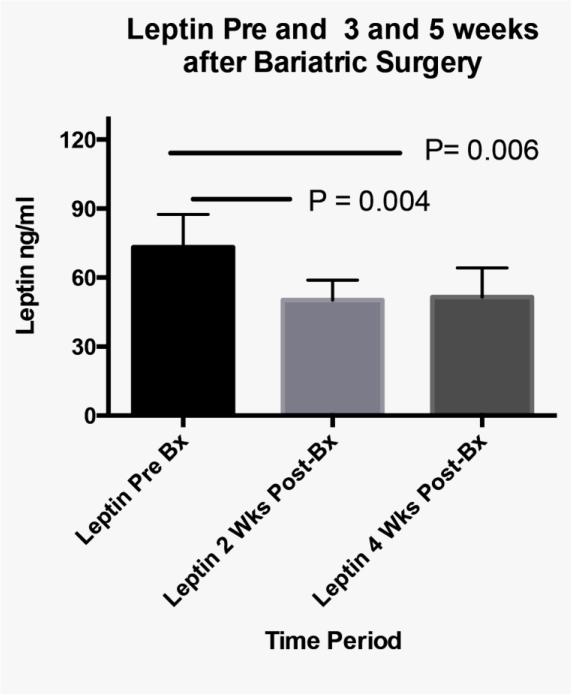
In addition to changes in severity of OSAS, a significant decrease in systolic but not diastolic blood pressure was measured between the baseline and the third visit (Figure 1; median decrease 9%). Finally, compared to baseline levels, serum leptin decreased and orexin increased from baseline to both postoperatively time points in the five participants with biochemical data available (Figure 2).
Figure 2. Orexin and Leptin changes.
Panel A
Plasma Orexin at baseline, 3 and 5 weeks after bariatric surgery. P-values obtained by repeated measures analysis with Tukey adjustment for multiple comparisons.
Panel B
Serum Leptin at baseline, 3 and 5 weeks after bariatric surgery. P-values obtained by repeated measures analysis with Tukey adjustment for multiple comparisons.
The control group consisted of 31 subjects (58% males) with a mean (range) age of 15 (13-17) years and mean BMI of 36.90 ± 9.09. The night-to night variability in AHI and oxygen saturation did not vary between the two studies (Table 3). However, sleep duration, efficiency and arousal index were significantly different between the two studies. The ICC for AHI between the studies is 0.78.
Table 3.
Polysomnography data from control group
| PSG 1 | PSG 2 | P | |
|---|---|---|---|
| Total sleep time (TST) | 391 ±57 | 429 ± 49 | 0.0005 |
| Sleep efficiency | 77 ± 12 | 84 ± 9 | 0.003 |
| Arousal index | 19 ± 15 | 16 ±14 | 0.03 |
| AHI | 17 ± 20 | 16 ± 25 | 0.17 |
| SAO2 REM | 97 ± 1 | 97± 1 | 0.5 |
| SAO2 NREM | 97 ± 1 | 97± 1 | 0.6 |
| % TST in supine | 58 ± 28 | 48± 28 | 0.04 |
Polysomongraphy data from subjects with obstructive apnea who underwent 2 studies that were 4 weeks apart (Control group)
Discussion
To our knowledge, this is the first demonstration of early and profound postoperative improvement in OSA following bariatric surgery. Similar to the early, weight-independent metabolic effects of surgery on type 2 diabetes mellitus, the 60% decline we observed in AHI by 3 weeks was of far greater magnitude than the 5-6% weight loss in this same period of time. Furthermore, the decline in AHI could not be explained by a difference in sleep duration or sleep position. The inclusion of a control group demonstrates that the mean change in AHI observed in subjects who underwent bariatric surgery is extremely unlikely to be due to night-to night variability in the severity of OSA.
Based on the concomitant rise in orexin and decrease in leptin, it is apparent that metabolic changes are also occurring in this same time span and thus it is plausible that physiologic rather than anatomic changes may underlie the clinically significant improvement in OSA as early as 11 days following surgery.
Orexin-producing neurons located in the brain (6, 7) intestines and pancreas (7) are strong candidates for mediators which may provide a critical link between nutritional energy balance and the central mechanisms that coordinate glucose homeostasis, breathing, and wakefulness (8-13). The fact that 1) orexin neurons are involved in sensing the body's external and internal environments (14-16), 2) orexin neurons have a role in stimulating upper airway (17)and central respiratory neurons (12), and 3) our findings showing increased orexin levels after bariatric surgery are all facts that support our speculation that neurophysiologic mechanisms may be responsible for the observed changes in OSA.
A significant decrease in serum leptin was observed at both the second and third visits after surgery. Leptin is a protein synthesized in adipose tissue that increases with advancing obesity. It acts in the hypothalamus to inhibit appetite (18) and is also a respiratory stimulant (19). There are multiple potential explanations of the decrease in serum leptin so early postoperatively. The obvious suspects that might be related to a modest decline in leptin would be the decrease in insulin resistance, or the modest weight loss that occurred. However, a more attractive hypothesis in the context of these data would be that changes in the frequency of intermittent hypoxia, a well-described mechanism affecting leptin levels (20) (21) might have contributed to the decline in serum leptin.
Finally, it is plausible that the special diet prescribed after bariatric surgery contribute the changes in the levels of orexin and leptin.
Limited data are available on the hormonal changes that might take place days or weeks after the administration of general anesthesia or after laparoscopic surgery. During the intraoperative and perioperative periods, anesthesia with propofol or sevoflurane is associated with a decrease in leptin (22) while laparoscopic cholecystectomy is associated with an increase in leptin for the first 48 hours after surgery (23). Studies, which examined the changes in Orexin A following anesthesia or surgical procedures, showed an increase in plasma Orexin during the first 2 hours after the induction of anesthesia and as patients emerge from anesthesia (24). It is believed that increased Orexin signaling is essential to the emergence from general anesthesia (25). However, there is no evidence that the acute hormonal changes during and immediately after anesthesia are sustained for weeks.
Limitations of this study include the small sample size, precluding adjustment for important covariates. Despite observed mean differences in both BMI and weight after surgery, the magnitude of the between-subject variability, coupled with the small number of subjects, limited our ability to detect statistical significance in these outcomes. In addition, without a control group of individuals with similar baseline severity of OSA undergoing an elective non-bariatric laparoscopic procedure, we cannot formally exclude the possibility that a unique feature of the postoperative state influenced the outcomes of interest in this study. However, there is no obvious reason to speculate that either general anesthesia or laparoscopy alone would result in significant improvement in OSA.
Finally, while we cannot exclude the possibility that changes in fat mass around the airway might account for early changes in AHI, the magnitude of weight loss by 3 weeks would suggest weight loss alone is not a major contributor.
Conclusions
In conclusion, these observations extend our initial observations (26-29) of resolution of OSA in adolescents following Roux-en-Y gastric bypass further suggest that the response of OSA is quite early following gastric surgery, and occurs with or without gastroduodenal bypass. These data firmly support the inclusion of OSA as an indication for use of weight loss surgery for severely obese adolescents. Larger scale study of early changes in OSA following bariatric surgery, including a broader age range, controlling for weight loss, and with measurement of key covariates is warranted.
Acknowledgments
Funding: This study was supported by the Division of Pulmonary Medicine and by a cooperative agreement with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), through grant: U01DK072493 (PI, Dr. Thomas Inge, CCHMC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors have indicated they have no relationships relevant to this article to disclose. Thomas H. Inge has received bariatric research grant funding from Ethicon Endosurgery and has served as consultant for Sanofi Corporation, both unrelated to this project.
Author Contributions
Study concept and design: Amin, Inge.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Amin.
Critical revision of the manuscript for important intellectual content: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Szczesniak.
Obtained funding: Amin, Inge.
Administrative, technical, or material support: Amin.
Disclosure Statement
All authors have indicated they have no relationships relevant to this article to disclose.
References
- 1.Daltro C, Gregorio PB, Alves E, et al. Prevalence and severity of sleep apnea in a group of morbidly obese patients. Obesity surgery. 2007;17(6):809–814. doi: 10.1007/s11695-007-9147-6. [DOI] [PubMed] [Google Scholar]
- 2.Carneiro G, Florio RT, Zanella MT, et al. Is mandatory screening for obstructive sleep apnea with polysomnography in all severely obese patients indicated? Sleep Breath. 2012;16(1):163–168. doi: 10.1007/s11325-010-0468-7. [DOI] [PubMed] [Google Scholar]
- 3.Michalsky MP, Inge TH, Teich S, et al. Adolescent bariatric surgery program characteristics: the Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study experience. Semin Pediatr Surg. 2014;23(1):5–10. doi: 10.1053/j.sempedsurg.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeller MH, Inge TH, Modi AC, et al. Severe obesity and comorbid condition impact on the weight-related quality of life of the adolescent patient. J Pediatr. 2015;166(3):651–659. e654. doi: 10.1016/j.jpeds.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122(6):535–542. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Badami VM, Rice CD, Lois JH, Madrecha J, Yates BJ. Distribution of hypothalamic neurons with orexin (hypocretin) or melanin concentrating hormone (MCH) immunoreactivity and multisynaptic connections with diaphragm motoneurons. Brain Res. 2010;1323:119–126. doi: 10.1016/j.brainres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchgessner AL. Orexins in the brain-gut axis. Endocr Rev. 2002;23(1):1–15. doi: 10.1210/edrv.23.1.0454. [DOI] [PubMed] [Google Scholar]
- 8.Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol. 2008;29(1):70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai T. Roles of orexins and orexin receptors in central regulation of feeding behavior and energy homeostasis. CNS Neurol Disord Drug Targets. 2006;5(3):313–325. doi: 10.2174/187152706777452218. [DOI] [PubMed] [Google Scholar]
- 10.Sakurai T. Reverse pharmacology of orexin: from an orphan GPCR to integrative physiology. Regul Pept. 2005;126(1-2):3–10. doi: 10.1016/j.regpep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Shahid IZ, Rahman AA, Pilowsky PM. Intrathecal orexin A increases sympathetic outflow and respiratory drive, enhances baroreflex sensitivity and blocks the somato-sympathetic reflex. Br J Pharmacol. 2011;162(4):961–973. doi: 10.1111/j.1476-5381.2010.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarenko RM, Stornetta RL, Bayliss DA, Guyenet PG. Orexin A activates retrotrapezoid neurons in mice. Respiratory physiology & neurobiology. 2011;175(2):283–287. doi: 10.1016/j.resp.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore MW, Akladious A, Hu Y, Azzam S, Feng P, Strohl KP. Effects of orexin 2 receptor activation on apnea in the C57BL/6J mouse. Respir Physiol Neurobiol. 2014;200:118–125. doi: 10.1016/j.resp.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venner A, Karnani MM, Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Orexin neurons as conditional glucosensors: paradoxical regulation of sugar sensing by intracellular fuels. J Physiol. 2011;589(Pt 23):5701–5708. doi: 10.1113/jphysiol.2011.217000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Metabolism-independent sugar sensing in central orexin neurons. Diabetes. 2008;57(10):2569–2576. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahoney CE, Brewer JM, Bittman EL. Central control of circadian phase in arousal-promoting neurons. PLoS One. 2013;8(6):e67173. doi: 10.1371/journal.pone.0067173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang GH, Liu ZL, Zhang BJ, et al. Orexin A activates hypoglossal motoneurons and enhances genioglossus muscle activity in rats. Br J Pharmacol. 2014;171(18):4233–4246. doi: 10.1111/bph.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein S, Coppack SW, Mohamed-Ali V, Landt M. Adipose tissue leptin production and plasma leptin kinetics in humans. Diabetes. 1996;45(7):984–987. doi: 10.2337/diab.45.7.984. [DOI] [PubMed] [Google Scholar]
- 19.Pho H, Hernandez AB, Arias RS, et al. The effect of leptin replacement on sleep disordered breathing in the leptin-deficient ob/ob mouse. Journal of applied physiology. 2015:jap 00494 02015. doi: 10.1152/japplphysiol.00494.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu C, Jiang L, Zhu F, et al. Chronic intermittent hypoxia leads to insulin resistance and impaired glucose tolerance through dysregulation of adipokines in non-obese rats. Sleep Breath. 2015;19(4):1467–1473. doi: 10.1007/s11325-015-1144-8. [DOI] [PubMed] [Google Scholar]
- 21.He Q, Yang QC, Zhou Q, et al. Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines and adipokines in rats and 3T3-L1 adipocytes. PLoS One. 2014;9(1):e86326. doi: 10.1371/journal.pone.0086326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marana E, Scambia G, Colicci S, et al. Leptin and perioperative neuroendocrine stress response with two different anaesthetic techniques. Acta Anaesthesiol Scand. 2008;52(4):541–546. doi: 10.1111/j.1399-6576.2008.01589.x. [DOI] [PubMed] [Google Scholar]
- 23.Di Vita G, Patti R, Fama F, Balistreri CR, Candore G, Caruso C. Changes of inflammatory mediators in obese patients after laparoscopic cholecystectomy. World J Surg. 2010;34(9):2045–2050. doi: 10.1007/s00268-010-0616-z. [DOI] [PubMed] [Google Scholar]
- 24.Kushikata T, Yoshida H, Kudo M, Kudo T, Hirota K. Plasma orexin A increases at emergence from sevoflurane-fentanyl anesthesia in patients undergoing ophthalmologic surgery. Neurosci Lett. 2010;482(3):212–215. doi: 10.1016/j.neulet.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 25.Kelz MB, Sun Y, Chen J, et al. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105(4):1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalra M, Inge T. Effect of bariatric surgery on obstructive sleep apnoea in adolescents. Paediatr Respir Rev. 2006;7(4):260–267. doi: 10.1016/j.prrv.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Kalra M, Mannaa M, Fitz K, et al. Effect of surgical weight loss on sleep architecture in adolescents with severe obesity. Obesity surgery. 2008;18(6):675–679. doi: 10.1007/s11695-008-9472-4. [DOI] [PubMed] [Google Scholar]
- 28.Miyano G, Kalra M, Inge TH. Adolescent paraplegia, morbid obesity, and pickwickian syndrome: outcome of gastric bypass surgery. J Pediatr Surg. 2009;44(3):e41–44. doi: 10.1016/j.jpedsurg.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Kalra M, Inge T, Garcia V, et al. Obstructive sleep apnea in extremely overweight adolescents undergoing bariatric surgery. Obes Res. 2005;13(7):1175–1179. doi: 10.1038/oby.2005.139. [DOI] [PubMed] [Google Scholar]