Abstract
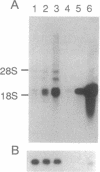
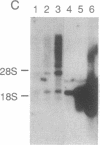
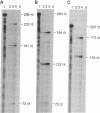
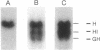
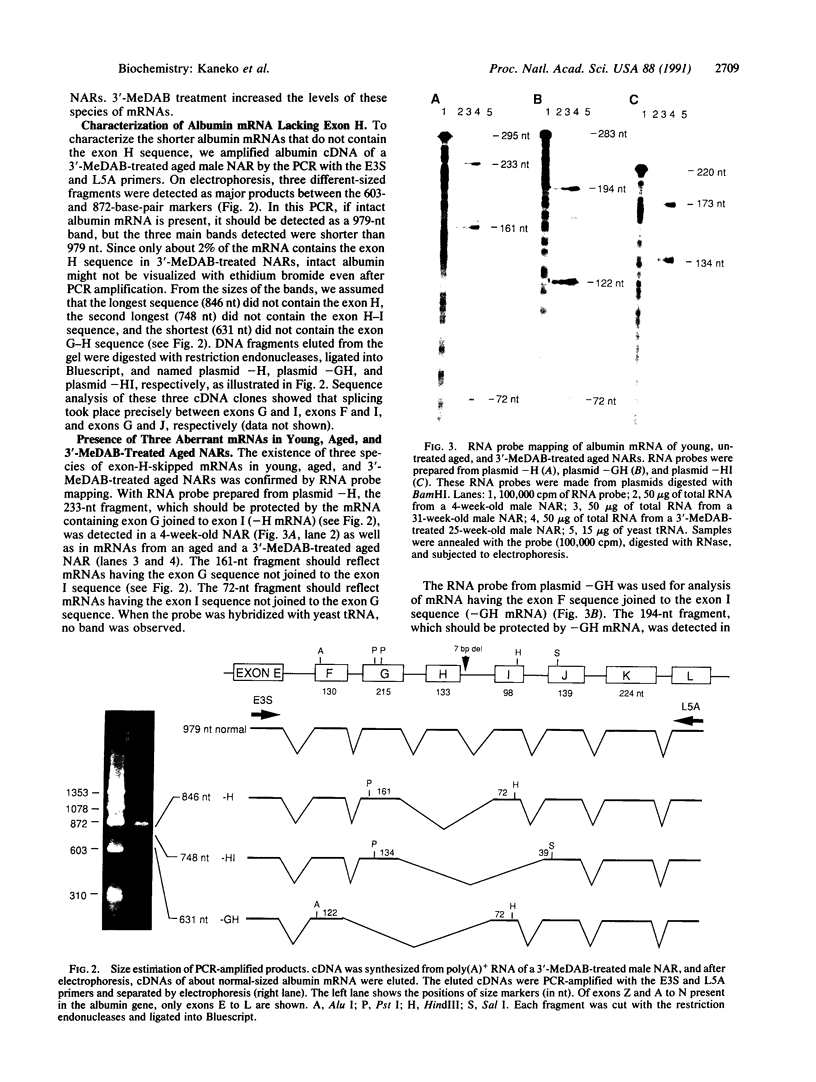
Nagase analbuminemic rats (NARs) have a 7-base-pair deletion at the 5' splice site of the HI intron of the albumin gene. The level of immunohistochemically albumin-positive hepatocytes is about 1 per 10(5) cells in neonatal NARs, increases with age, and further increases with chronic oral treatment with 3'-methyl-4-dimethylaminoazobenzene (3'-MeDAB). The mechanisms involved in the increase in albumin-positive hepatocytes during aging of NARs and their treatment with 3'-MeDAB were analyzed. NARs were found to have four species of albumin mRNA: intact mRNA and those lacking the regions corresponding to exon H, exon G-H, and exon H-I. In 4-week-old NARs, the level of intact albumin mRNA was about 1/4000 of that in normal rats and mRNA lacking the exon H sequence was the major species. In aged and 3'-MeDAB-treated aged NARs, all four species of mRNA increased and the relative proportion of mRNAs lacking two exon sequences to mRNAs lacking one exon sequence was greatly increased, suggesting that aging is associated with changes of the splicing pattern and that 3'-MeDAB treatment enhanced these changes. In aged NARs and 3'-MeDAB-treated aged NARs, there was an increase in the amount of aberrant 60-kDa albumin. The 60-kDa protein could be a translation product of mRNAs lacking two exons, the amount of which increases in aged NARs and 3'-MeDAB-treated NARs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Esumi H., Takahashi Y., Sato S., Nagase S., Sugimura T. A seven-base-pair deletion in an intron of the albumin gene of analbuminemic rats. Proc Natl Acad Sci U S A. 1983 Jan;80(1):95–99. doi: 10.1073/pnas.80.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi H., Takahashi Y., Sekiya T., Sato S., Nagase S., Sugimura T. Presence of albumin mRNA precursors in nuclei of analbuminemic rat liver lacking cytoplasmic albumin mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(3):734–738. doi: 10.1073/pnas.79.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., de Rooij F., Beaumont C., Wilson P., Deybach J. C., Nordmann Y. A point mutation G----A in exon 12 of the porphobilinogen deaminase gene results in exon skipping and is responsible for acute intermittent porphyria. Nucleic Acids Res. 1989 Aug 25;17(16):6637–6649. doi: 10.1093/nar/17.16.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbach G. J., Princen H. M., Van der Kroef M., Van Bezooijen C. F., Yap S. H. Changes in the sequence content of albumin mRNA and in its translational activity in the rat liver with age. Biochim Biophys Acta. 1984 Oct 5;783(1):60–66. doi: 10.1016/0167-4781(84)90078-2. [DOI] [PubMed] [Google Scholar]
- Humphries R. K., Ley T. J., Anagnou N. P., Baur A. W., Nienhuis A. W. Beta O-39 thalassemia gene: a premature termination codon causes beta-mRNA deficiency without affecting cytoplasmic beta-mRNA stability. Blood. 1984 Jul;64(1):23–32. [PubMed] [Google Scholar]
- Krainer A. R., Conway G. C., Kozak D. The essential pre-mRNA splicing factor SF2 influences 5' splice site selection by activating proximal sites. Cell. 1990 Jul 13;62(1):35–42. doi: 10.1016/0092-8674(90)90237-9. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T., Ruskin B., Green M. R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984 Apr;36(4):993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makino R., Esumi H., Sato S., Takahashi Y., Nagase S., Sugimura T. Elevation of serum albumin concentration in analbuminemic rats by administration of 3'-methyl-4-dimethylaminoazobenzene. Biochem Biophys Res Commun. 1982 Jun 15;106(3):863–870. doi: 10.1016/0006-291x(82)91790-9. [DOI] [PubMed] [Google Scholar]
- Makino R., Sato S., Esumi H., Negishi C., Takano M., Sugimura T., Nagase S., Tanaka H. Presence of albumin-positive cells in the liver of analbuminemic rats and their increase on treatment with hepatocarcinogens. Jpn J Cancer Res. 1986 Feb;77(2):153–159. [PubMed] [Google Scholar]
- Marvit J., DiLella A. G., Brayton K., Ledley F. D., Robson K. J., Woo S. L. GT to AT transition at a splice donor site causes skipping of the preceding exon in phenylketonuria. Nucleic Acids Res. 1987 Jul 24;15(14):5613–5628. doi: 10.1093/nar/15.14.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Urlaub G., Chasin L. Spontaneous splicing mutations at the dihydrofolate reductase locus in Chinese hamster ovary cells. Mol Cell Biol. 1986 Jun;6(6):1926–1935. doi: 10.1128/mcb.6.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase S., Shimamune K., Shumiya S. Albumin-deficient rat mutant. Science. 1979 Aug 10;205(4406):590–591. doi: 10.1126/science.451621. [DOI] [PubMed] [Google Scholar]
- Robberson B. L., Cote G. J., Berget S. M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol. 1990 Jan;10(1):84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Yang M., Bonner J. Nucleotide sequence of cloned rat serum albumin messenger RNA. Proc Natl Acad Sci U S A. 1981 Jan;78(1):243–246. doi: 10.1073/pnas.78.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F., Shafritz D. A. Exon skipping during splicing of albumin mRNA precursors in Nagase analbuminemic rats. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2652–2656. doi: 10.1073/pnas.87.7.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Miwa M., Slamon D. J., Chen I. S., Hoshino H., Takano M., Fujino M., Sugimura T. Identification of new gene products coded from X regions of human T-cell leukemia viruses. Proc Natl Acad Sci U S A. 1985 Jan;82(2):302–306. doi: 10.1073/pnas.82.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K., Forget B. G., Scarpa A., Benz E. J., Jr Intranuclear defect in beta-globin mRNA accumulation due to a premature translation termination codon. Blood. 1984 Jul;64(1):13–22. [PubMed] [Google Scholar]
- Tanaka H., Matsuhisa A., Nagamori S., Shimizu K., Niiya M., Ohno T., Nagase S. Characteristics and significance of albumin-positive hepatocytes in analbuminemic rats. Eur J Cell Biol. 1990 Dec;53(2):267–274. [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Weil D., D'Alessio M., Ramirez F., de Wet W., Cole W. G., Chan D., Bateman J. F. A base substitution in the exon of a collagen gene causes alternative splicing and generates a structurally abnormal polypeptide in a patient with Ehlers-Danlos syndrome type VII. EMBO J. 1989 Jun;8(6):1705–1710. doi: 10.1002/j.1460-2075.1989.tb03562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa B., Meyer F., Reiser J., Weissmann C. Unusual splice sites revealed by mutagenic inactivation of an authentic splice site of the rabbit beta-globin gene. Nature. 1983 Jan 6;301(5895):38–43. doi: 10.1038/301038a0. [DOI] [PubMed] [Google Scholar]
- Yuo C. Y., Weiner A. M. A U1 small nuclear ribonucleoprotein particle with altered specificity induces alternative splicing of an adenovirus E1A mRNA precursor. Mol Cell Biol. 1989 Aug;9(8):3429–3437. doi: 10.1128/mcb.9.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]