Abstract
Follicle-stimulating hormone (FSH) is a gonadotrope-derived heterodimeric glycoprotein. Both the common α- and hormone-specific β subunits contain Asn-linked N-glycan chains. Recently, macro-heterogeneous FSH glycoforms consisting of β-subunits that differ in N-glycan number were identified in pituitaries of several species and subsequently the recombinant human FSH glycoforms biochemically characterized. Although chemical modification and in vitro site-directed mutagenesis studies defined the roles of N-glycans on gonadotropin subunits, in vivo functional analyses in a whole-animal setting are lacking. Here, we have generated transgenic mice with gonadotrope-specific expression of either an HFSHBWT transgene that encodes human FSHβ WT subunit or an HFSHBdgc transgene that encodes a human FSHβAsn7Δ 24Δ double N-glycosylation site mutant subunit, and separately introduced these transgenes onto Fshb null background using a genetic rescue strategy. We demonstrate that the human FSHβAsn7Δ 24Δ double N-glycosylation site mutant subunit, unlike human FSHβ WT subunit, inefficiently combines with the mouse α-subunit in pituitaries of Fshb null mice. FSH dimer containing this mutant FSHβ subunit is inefficiently secreted with very low levels detectable in serum. Fshb null male mice expressing HFSHBdgc transgene are fertile and exhibit testis tubule size and sperm number similar to those of Fshb null mice. Fshb null female mice expressing the mutant, but not WT human FSHβ subunit-containing FSH dimer are infertile, demonstrate no evidence of estrus cycles, and many of the FSH-responsive genes remain suppressed in their ovaries. Thus, HFSHBdgc unlike HFSHBWT transgene does not rescue Fshb null mice. Our genetic approach provides direct in vivo evidence that N-linked glycans on FSHβ subunit are essential for its efficient assembly with the α-subunit to form FSH heterodimer in pituitary. Our studies also reveal that N-glycans on FSHβ subunit are essential for FSH secretion and FSH in vivo bioactivity to regulate gonadal growth and physiology.
Keywords: Gonadotropin, Genetic rescue, N-glycosylation, Pituitary, Ovary, Testis
1. Introduction
FSH is a pituitary-derived heterodimeric glycoprotein hormone (Bousfield et al., 2006). It binds to G-protein coupled receptors on somatic cells of gonads and regulates both gametogenesis and steroidogenesis (Bousfield et al., 2006). FSH consists of a common α-subunit that is shared with two other glycoprotein hormones also produced within the pituitary, namely, LH and TSH (Bousfield et al., 2006). Additionally, the α-subunit is also present in human chorionic gonadotropin (hCG), a trophoblast-derived placental glycoprotein hormone (Bousfield et al., 2006). In specific cell types, the α-subunit is non-covalently associated with the specific β-subunit (FSHβ or LHβ or TSHβ or hCGβ) resulting in formation of a distinct heterodimeric hormone (FSH and LH in gonadotropes, TSH in thyrotropes and hCG in trophoblasts). A characteristic feature of this family of glycoprotein hormone subunits is the presence of N-linked oligosaccharides covalently attached to specific asparagine (Asn) residues (Bousfield et al., 2006; Baenziger and Green, 1988). Data obtained from several in vitro experiments involving both biochemical and site-directed mutagenesis approaches indicated that N-linked glycosylation of α- and β– subunits is critical for heterodimeric hormone assembly, rate of secretion, serum half -life and signal transduction in target cells (Bousfield et al., 2006; Baenziger and Green, 1988; Green et al., 1986; Matzuk and Boime, 1989).
Human (h) FSH consists of four co-translationally attached N-glycans, two each in the α– (at Аsn52 and Asn78) and β – (at Asn7 and Asn24) subunits (Bousfield et al., 2006). A series of α-subunit (Asn52 or Asn78 or at both Asn sites) and β-subunit (Asn7 or Asn24 or at both Asn sites) N-glycosylation mutations in hFSH were previously constructed (Bousfield et al., 2006; Bishop et al., 1994; Flack et al., 1994a,b). These mutants were expressed in CHO and COS7 cells and the recombinant proteins tested in various in vitro binding and signal transduction assays (Bousfield et al., 2006; Flack et al., 1994a,b). These studies documented the roles of N-glycosylation on FSH. First, individual mutation of N-glycosylation sites on either FSH α– or β -subunits enhanced FSH-receptor binding affinity when rat Sertoli cell homogenate was used as a receptor source (Bousfield et al., 2006; Flack et al., 1994a,b). Second, mutations in individual N-glycosylation sites on β–subunit resulted in reduced signal transduction in rat granulosa cell cultures (Bousfield et al., 2006; Flack et al., 1994a,b). Finally, the second N-glycosylation site at Asn24 in the FSHβ-subunit was found critical for both receptor binding and signal transduction (Bousfield et al., 2006; Flack et al., 1994a,b).
A variety of biochemical and structure-function studies indicated that the terminal sugars, such as sialic acid, contributes to micro-heterogeneity in FSH (Flack et al., 1994a,b; Ambao et al., 2009; Anobile et al., 1998; Campo et al., 2007; Creus et al., 2001; Creus et al., 1996; Crowe et al., 1998; Loreti et al., 2009; Padmanabhan et al., 1988; Padmanabhan et al., 1999; Padmanabhan et al., 1992; Timossi et al., 2000; West et al., 2002). The resulting charge variants, called FSH isoforms, are secreted from the anterior pituitary in an estrus/menstrual cycle stage-specific manner in different species. The micro-heterogeneous FSH isoforms differ in their net charge and their relative abundance in serum reflects the ovarian cycle stage (Anobile et al., 1998; Creus et al., 1996; Loreti et al., 2009; Padmanabhan et al., 1988, 1992; West et al., 2002). A fully glycosylated FSH (FSH24) has been the most abundant form expressed in mammalian expression systems in vitro. In more recent studies, Western blot analyses identified a novel macro-heterogeneity contributed by the absence of both (FSH15) or presence of either Asn24 (FSH18) or Asn7 (FSH21) N-glycan chains in FSHβ-subunit in pituitaries of several species (Bousfield et al., 2007; Davis et al., 2014; Walton et al., 2001). Based on the molecular mass of FSHβ subunits, each containing different number of N-glycan chains, different FSH glycoforms have been named. For example, FSH15 contains no Asn-linked glycans on the FSHβ-subunit with a molecular mass of 15 kDa; FSH18 and FSH21 each contain an FSHβ subunit with one N-linked glycan with molecular mass 18 KDa and 21 KDa, respectively. Collectively, these FSH glycoforms are referred to as hypo-glycosylated FSH (Bousfield et al., 2007; Davis et al., 2014). Thus, a FSH heterodimer consisting of a α-subunit with two N-glycans units and a non-glycosylated FSHβ-subunit lacking both the N-glycans (FSH15) was also identified (Bousfield et al., 2007; Davis et al., 2014; Walton et al., 2001).
Our group has recently reported the biochemical and cell biological properties of macro-heterogeneous FSH, hypoglycosylated FSH18/21 (mixture of both FSH18 and FSH21) and compared them in vitro to fully glycosylated FSH24 (Bousfield et al., 2014a,b). However, the biological roles of hypoglycosylated FSH15 glycoform are unknown. Here, we report targeted expression of a human FSHB mutant transgene encoding an Asn7Δ, 24Δ double N-glycosylation site (Thr to Ala mutation) mutant FSHβ subunit on an Fshb null genetic background. Using this genetic rescue strategy, we provide in vivo evidence that the double N-glycosylation site Asn7Δ, 24Δ mutant FSHβ subunit is heterodimer assembly-incompetent. We also demonstrate that the corresponding heterodimeric FSH15 glycoform is secreted in very low levels into serum and fails to rescue Fshb null mice.
2. Materials and methods
2.1. Mice
All mice were maintained under 12-h dark, 12-h light cycles with food and water supplied ad libitum. All studies with mice were carried out in accordance with the Guide for the Care and Use of Laboratory Animals per the NIH instructions as adopted by the University of Kansas Medical Center and approved institutional protocols.
2.2. Other methods
All other methods are described in detail in the Supplementary material.
2.3. Statistical analysis
All the data were represented as Mean ± SEM. Statistical analyses, including Student’s T-test and one-way ANOVA followed by Turkey’s post-hoc test, were performed using PRISM software (GraphPad Software, Inc. La Jolla, CA). A P value < 0.05 was considered statistically significant.
3. Results
3.1. Generation of Fshb null mice harboring an HFSHBdgc transgene
To test the biological roles of N-glycan chains on human FSHβ, we first generated transgenic mice expressing an HFSHBdgc mutant transgene (Fig. 1A) by pronuclear microinjections of fertilized one-cell mouse embryos. From a total of 22 viable pups produced from two independent microinjection experiments, we obtained 8 (8/22 = 36%) transgene positive mice as verified by genomic PCR assays on tail DNA samples (Fig. 1B) using specific primers in the oFshb promoter region as indicated (arrows in Fig. 1A). Five of the founders (3 males and 2 females) were mated to WT littermates from which we successfully established 3 independent lines (1 male and 1 female founder did not transmit the HFSHBdgc transgene) designated #30, #60 and #220. We performed almost all of the experiments described herein with two lines #30 and #60. Next, we intercrossed F2 mice from two independent lines (Line #30 and Line #60) onto Fshb null genetic background and finally generated Fshb−/− HFSHBdgc mice in a 2-step breeding scheme (Fig. 1C) similar to that described earlier for generating Fshb−/− HFSHBWT rescue mice (Kumar et al., 1998; Wang et al., 2014).
Fig. 1.
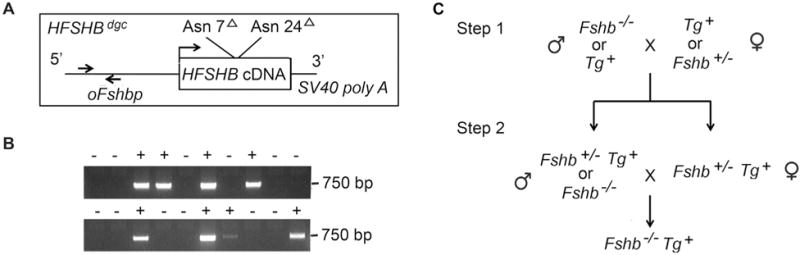
A transgene construct (A) consisting of a gonadotrope-specific ovine Fshb promoter driving the expression of a double N-glycosylation site mutant hFSHβ subunit-encoding cDNA-SV40 polyA sequences was engineered and microinjected into one-cell mouse embryos to produce transgenic mice. Genomic PCR assay of tail DNA samples (B) using oFshb promoter-specific primers (indicated by arrows in A) amplified a 750 bp fragment that identified and distinguished founder mice carrying the transgene (+) from control wild-type (WT), transgene-negative mice. A two-step genetic rescue scheme (C) was employed to produce the desired Fshb null mice carrying the HFSHBWT or HFSHBdgc transgene (Tg+).
3.2. Gonadotrope-specific expression of the HFSHBdgc transgene on Fshb−/− genetic background
The oFshb promoter (4.7 kb) has been extensively characterized previously and found to target various reporter genes to the pituitary gonadotrope lineage in transgenic mice (Huang et al., 2001; Pernasetti et al., 2003; Wang et al., 2016; Wu et al., 2004). To confirm that the HFSHBdgc transgene was appropriately targeted to the pituitary, we measured transgene mRNA with respect to an endogenous control Ppil1 mRNA in mouse pituitaries by Taqman real time qPCR assays using HFSHB-specific primers/probe combo. We found that HFSHBdgc mRNA expression in pituitaries of both lines of adult Fshb−/− HFSHBdgc female mice was nearly identical to and not significantly different from that of the HFSHBWT transgene encoded mRNA in pituitaries of Fshb−/− HFSHBWT mice (Fig. 2A; P > 0.05 by T-Test; n = 3 mice, each sample in triplicate).
Fig. 2.
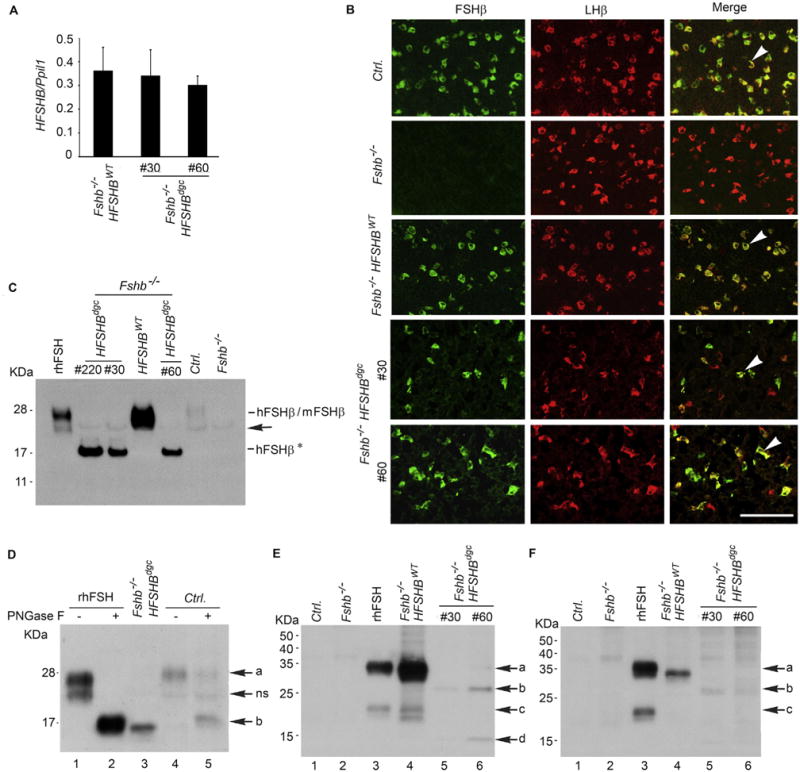
Real time qPCR assay (A) shows nearly identical expression of HFSHB mRNA in pituitaries of Fshb−/− mice expressing either a HFSHBWT or HFSHBdgc transgene. Two different lines #30 and #60, expressing the mutant transgene were developed. Dual label immunofluorescence visualization of pituitary sections (B) from adult mice confirmed gonadotrope-specific expression of HFSHBdgc transgene similar to that of HFSHBWT transgene (indicated by white arrowheads in merged images in the right panel in B). The absence of any green fluorescence in pituitary sections from Fshb−/− mice confirmed the specificity of the FSHβ antibody. The white bar in panel B represents 50 µm. Western blot analysis of proteins under denaturing conditions probed with a goat anti-human FSHβ-polyclonal antibody obtained from Santa Cruz Biotechnology, sc 7757 (C) shows a 15–17 KDa hFSHβAsn7Δ Asn24Δ non-glycosylated subunit (denoted as hFSHβ*) was abundantly expressed in pituitaries of three different lines of HFSHBdgc transgenic mice (line # 220, line #30 and line #60) on an Fshb null genetic background. Black arrow indicates a non-specific band. Recombinant human (r–h) FSHβ size-matched with that of HFSHBWT transgene-encoded hFSHβ subunit and weakly immunoreactive mouse (m) FSHβ subunit detected in normal control mouse (Ctrl.) pituitary. Proteins from Fshb−/− mouse pituitaries served as a negative control. PNGase F treatment followed by Western blot analysis of either r-hFSH or pituitary proteins from a normal control wild-type (Ctrl.) mouse showed an identical size de-glycosylated FSHβ subunit corresponding to that identified in an Fshb−/− HSFHBdgc mouse pituitary (D). Electrophoretic separation of proteins under non-denaturing conditions followed by Western blot analysis showed that in both male (E) and female (F) Fshb−/− HSFHBdgc mouse pituitaries, the non-glycosylated FSHβ subunit, despite high level expression (C) was present as an interspecies hybrid (mouse α + human FSHβ*) heterodimer (lanes 5 and 6 in panels E and F) at very low levels compared to the FSH heterodimer (mouse α + hFSHβ) in pituitaries of Fshb−/− HSFHBWT mice (lane 4 in panels E and F). Pituitary proteins from control and Fshb−/− mice (Lanes 1 and 2 in panels E and F) are used as negative controls and r-hFSH (Lane 3) as a positive control. Arrows in panel D denote: a = FSHβ subunit, ns = non-specific band, b = deglycosylated FSHβ subunit. Arrows in panel E and F indicate: a = FSHβ subunit, b = double N-glycosylation site mutant FSHβ subunit, c = uncombined/free FSHβ subunit, d = uncombined double N-glycosylation site mutant FSHβ subunit. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To further confirm that the HFSHBdgc transgene was expressed in gonadotropes, we performed dual immunofluorescence microscopy using specific antisera against FSHβ and LHβ on formalin-fixed pituitary tissue sections obtained from adult mice. As shown in Fig. 2B, many FSHβ+/LHβ+ dual – labeled gonadotropes were readily detectable in pituitary sections from Fshb−/− HFSHBdgc mice similar to those observed in sections from control normal (Ctrl.) and Fshb−/− HFSHBWT mice. As predicted, only LHβ+ gonadotropes were present in Fshb null mouse pituitary sections confirming the specificity of the FSHβ antiserum that was used. Dual-immunolabeling with FSHβ antiserum and antibodies against each of the other anterior pituitary cell markers (TSHβ, GH, PRL, ACTH) did not show any dual-labeled cells (data not shown) further indicating that the transgene is specifically targeted to only the gonadotrope lineage within the anterior pituitary.
The HFSHBdgc transgene encodes a double N-glycosylation site mutant human FSHβ subunit in which the N-glycosylation motif Thr9 and Thr26 residues were mutated to Ala residues by site directed mutagenesis (Fox et al., 2001). The predicted molecular weight of this double N-glycosylation site mutant human FSHβ subunit is 15–16 KDa. To determine the molecular mass of the HFSHBdgc transgene-encoded double N-glycosylation mutant human FSHβ subunit expressed in vivo, we performed Western blot analyses on pituitary extracts and probed the blots using an hFSHβ-specific goat polyclonal antibody. In three independent lines (#30, #60 and # 220) extracts of Fshb−/− HFSHBdgc mouse pituitaries showed an immunoreactive band, representing the 16 KDa hFSHβ subunit that was readily detectable (indicated as hFSHβ* in Fig. 2C). In contrast, in Fshb−/− HFSHBWT mouse pituitary, we detected a fully glycosylated hFSHβ 24 kDa subunit similar to recombinant hFSH (r-hFSH). A weakly immunoreactive band with a 24 kDa molecular mass was also detected in control normal mouse pituitary extract, consistent with limited cross-reactivity of the goat anti-human FSHβ antibody with the mouse FSHβ subunit (Fig. 2C). PNGase F treatment of the r-hFSH preparation and normal mouse pituitary extract followed by Western blot analysis revealed a 15–16 KDa immunoreactive de-glycosylated FSHβ subunit identical to that observed in Fshb−/− HFSHBdgc mouse pituitary extracts (Fig. 2D, arrow b). Although a non-specific immunoreactive band was apparent in all the samples tested (indicated with an arrow in Fig. 2, indicated as ns in panels C and D), the absence of an immunoreactive FSHβ 24 kDa band in Fshb−/− mouse pituitary further validated the specificity of the goat polyclonal antibody used for Western blot analyses.
Gonadotropin subunits are evolutionarily conserved and interspecies hybrid heterodimers, i.e α-subunit from one species and β-subunit from the other, could be assembled both in vitro (Licht et al., 1976) and in vivo (Kumar et al., 1998; Wang et al., 2014; Licht et al., 1976). Therefore, we next tested whether the HFSHBdgc transgene-encoded, double N-glycosylation site mutant human FSHβ subunit assembled with the endogenous mouse α-subunit and formed an interspecies hybrid heterodimer in pituitaries of Fshb−/− HFSHBdgc mice. We readily identified the interspecies hybrid FSH dimer (mouse α-subunit and hFSHβ wild-type subunit) in pituitary extracts of Fshb−/− HFSHBWT male and female mice prepared under non-denaturing conditions (Lane 4 in Fig. 2, panels E and F). In contrast, interspecies hybrid FSH dimers containing the mouse α-subunit and double N-glycosylation site mutant human FSHβ subunit were weakly detectable in pituitary extracts of both lines of male and female Fshb−/− HFSHBdgc mice (Lanes 5 and 6 in Fig. 2, panels E and F). Recombinant hFSH served as a positive control and showed an immunoreactive band similar to that seen in pituitary extracts obtained from Fshb−/− HFSHBWT male and female mice (Lane 3 in Fig. 2, panels E and F), whereas pituitary extracts from control normal and Fshb null mice served as negative controls and did not show any immunoreactive bands corresponding to heterodimer (Lanes 1 and 2 in Fig. 2, panels E and F).
Furthermore, FSH content in pituitary extracts measured by a 125I-hFSH radioimmunoassay was significantly higher in Fshb−/− HFSHBdgc mice as compared to Fshb−/− controls (4.9 ± 0.75 ng/pituitary vs. 0.6 ± 0.1 ng/pituitary; n = 6; P < 0.01 by T-test). Similarly, FSH bioactivity as determined by a radioreceptor assay using 125I-hFSH tracer and rat testicular membranes showed a trend towards higher value in pituitary extracts from Fshb−/− HFSHBdgc mice but was not significantly different compared to that in Fshb−/− mice (93 ± 13 ng/pituitary vs. 46 ± 12 ng/pituitary, n = 6; P > 0.05 by T-Test). In a separate assay, using CHO cells expressing hFSHRs, FSH content estimated was 1.4 ± 0.1 μg/pituitary in wild-type controls, 1.8 ± 0.4 μg/pituitary in case of Fshb−/− mice, 5.4 ± 0.5 mg/pituitary in Fshb−/− HFSHBWT mice and undetectable in case of HFSHBdgc mice. Collectively, all the above data indicate that the HFSHBdgc transgene was appropriately targeted to the pituitary and expressed in gonadotropes, similar to the HFSHBWT transgene. However, the double N-glycosylation site mutant hFSHβ subunit, unlike hFSHβ WT subunit, did not assemble well in vivo and formed inefficiently an interspecies hybrid heterodimer with the endogenous mouse α-subunit.
3.3. Double N-glycosylation mutant hFSHβ subunit containing FSH dimer is secretion incompetent both in vitro and in vivo
Despite the poor assembly with the mouse α-subunit, the double N-glycosylation site mutant hFSHβ containing interspecies hybrid FSH heterodimer was detectable in low abundance in the pituitary and thus it might be secreted in low levels into serum. To test this, we first evaluated secretion in vitro under short-term (6 h) and serum-free culture conditions using whole pituitaries from adult mice. Western blot analysis under denaturing conditions followed by densitometry quantification indicated that whereas ~10%–14% of FSHβ was readily detectable in medium compared to lysate (designated respectively as M and L in Fig. 3A) in case of control wild-type (WT) and Fshb−/− HFSHBWT mice, the FSHβ signal was undetectable in medium in the case of Fshb−/− HFSHBdgc mice (Fig. 3A). Next, we measured serum FSH levels using a rat/mouse FSH RIA that we had previously validated for detecting mouse α/human FSHβ interspecies hybrid FSH (Kumar et al., 1998; Wang et al., 2014). We found that serum FSH levels were significantly low in Fshb−/− HFSHBdgc mice compared to those in Fshb−/− HFSHBWT mice (Fig. 3, panels B and C) and were mostly undetectable as in serum samples from Fshb null mice.
Fig. 3.
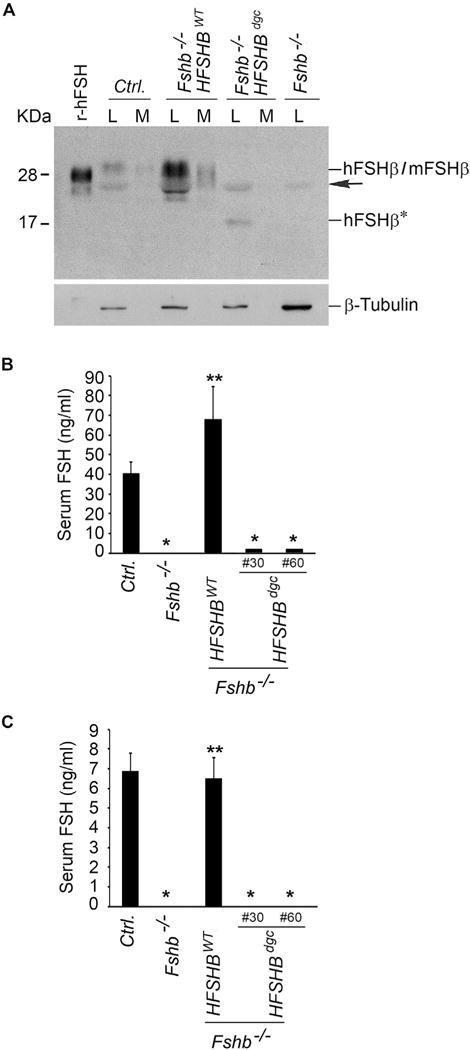
Western blot analysis (A) of proteins in lysates and medium fractions derived from whole pituitary organ cultures separated under non-reducing conditions and probed with 4B FSH monoclonal antibody, indicated that interspecies hybrid heterodimer containing the non-glycosylated hFSHβ mutant subunit (in Fshb−/− HSFHBdgc mouse pituitary), was barely secreted into medium compared to the hFSHβ WT subunit-containing interspecies hybrid heterodimer (in Fshb−/− HSFHBWT mouse pituitary) or mouse FSH heterodimer from control normal mouse pituitary. The black arrow indicates a non-specific band. Fshb−/− mouse pituitary lysate and r-hFSH served as negative and positive controls, respectively. Expression of β-tubulin was used as an internal control and is shown in the blot at the bottom of panel A. L: lysate; M: medium; hFSHβ*: non-glycosylated hFSHβAsn7Δ Asn24Δ double site mutant subunit. RIAs on serum samples from adult male (B) and female (C) mice revealed very low levels of non-glycosylated hFSHβAsn7Δ Asn24Δ double site mutant subunit-containing FSH dimer in two lines (Line #30 and Line #60) of Fshb−/− HSFHBdgc mice compared to that in normal control (Ctrl.) or Fshb−/− HSFHBWT mice expressing hFSHβ WT subunit. In panels B and C: * P < 0.01 compared to Ctrl. or Fshb−/− HSFHBWT groups; ** P < 0.01 compared to Fshb−/− group.
One reason for low-level secretion of double N-glycosylation site mutant FSHβ containing FSH dimer could be intracellular retention in the pituitary as a result of changes in N-glycosylation or defects in protein folding. Immunofluorescence followed by confocal microscopy analysis confirmed that the double N-glycosylation site mutant hFSHβ subunit in pituitary sections of Fshb−/− HFSHBdgc mice was readily detectable in gonadotropes by the human FSHβ-specific 4B monoclonal antibody. Moreover, when co-labeled with ER (Supplementary Fig. S1, panel A) and Golgi (Supplementary Fig. S1, panel B) resident protein markers, the mutant FSHβ showed no intracellular retention in these protein trafficking organelles. An identical labeling pattern of FSHβ and ER/Golgi markers was observed in pituitary sections from control and Fshb−/− HFSHBWT mice (Supplementary Fig. S1, panels A and B) that exhibited normal circulating serum levels of FSH. In addition, Taqman qPCR assays showed that expression of many of the gonadotrope-specific “hallmark” genes was not altered in pituitaries of Fshb−/− HFSHBdgc mice (Supplementary Fig. S2, panels A–F) and was not significantly different than that in pituitaries of control (Ctrl.) and Fshb−/− HFSHBWT mice (Fshb−/− HFSHBdgc mice vs. Ctrl. or Fshb−/− HFSHBWT mice, P > 0.05; ONE-WAY ANOVA followed by Turkey’s post-hoc test, n = 3 mice). Together, these data confirmed that an interspecies FSH heterodimer consisting of mouse α- and the double N-glycosylation site mutant hFSHβ subunit was secretion incompetent, not as a result of aberrant gonadotrope marker gene expression or ER/Golgi retention, and was detectable in very low levels in serum of Fshb−/− HFSHBdgc mice.
3.4. HFSHBdgc transgene does not rescue Fshb null male mice
Fshb null males demonstrate reduced testis size, accompanied by Sertoli and germ cell defects, but these null males are fertile (Wang et al., 2014; Kumar et al., 1997). Previously, we achieved genetic rescue of Fshb null mice by gonadotrope-targeted expression of a HFSHBWT transgene and characterized the male reproductive phenotypes of these rescue mice (Kumar et al., 1997). To determine whether low levels of double N-glycosylation site mutant hFSHβ subunit containing FSH dimer rescues Fshb null mice, we analyzed male reproductive phenotypes of Fshb−/− HFSHBdgc mice and compared them to those in Fshb−/− HFSHBWT mice. Gross testes weights were significantly lower in Fshb−/− HFSHBdgc adult male mice at 9 weeks of age compared to those in normal control and Fshb−/− HFSHBWT males (Fig. 4 A–B; P < 0.05; T-Test, n = 6) but were not different when compared to Fshb−/− mice (Fig. 4 A–B; P > 0.05; T-Test, n = 6). Furthermore, epididymal sperm number (Fig. 4C), histological analysis of PAS/hematoxylin stained testes sections (Fig. 4D) followed by quantification of tubule diameter (Fig. 4E), and real time qPCR assays on testes RNA (Fig. 4F and G) to measure FSH-responsive marker genes indicated that testes phenotypes in Fshb−/− HFSHBdgc mice were all similar to those in Fshb−/− male mice. But, these parameters were all significantly different when compared to normal control and Fshb−/− HFSHBWT male mice (Fig. 4 C–G). Thus, these data indicate that, whereas a HFSHBWT transgene efficiently rescued, the HFSHBdgc transgene failed to rescue Fshb−/− mice. Since Fshb null males were fertile, there were no differences, as expected, in fertility performance (number of litters produced and litter size, i.e. pups per litter) across all genotypes of mice tested over a 6-month period of time (Fig. 4 H and I).
Fig. 4.
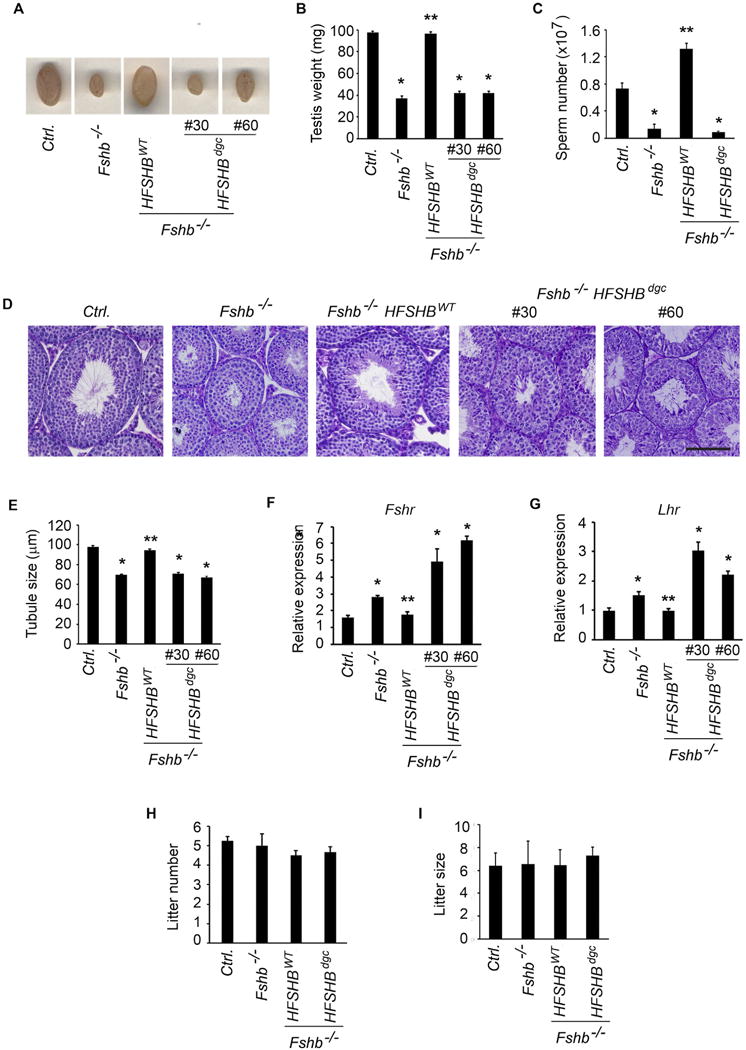
The HFSHBdgc transgene, in contrast to the HFSHBWT transgene, did not rescue Fshb null male mice as illustrated by gross testicular morphology (A) and size (B), quantitation of epididymal sperm (C), PAS/hematoxylin-stained testis histology (D), tubule diameter measurements (E) and FSH-responsive gene expression analysis by real time qPCR assays (F and G). Breeding performance over a period of 6 months was assessed in terms of number of litters produced (H) and litter size, i.e. number of pups per litter (I) is independent of FSH and Fshb genotype tested. *P < 0.01 versus Ctrl. or HFSHBWT groups; ** P < 0.05 versus Fshb−/− group. Fshr: follicle-stimulating hormone receptor, Lhr: luteinizing hormone receptor.
3.5. HFSHBdgc transgene does not rescue Fshb null female mice
The complete absence of FSH results in hypoplastic ovaries and uteri, and a pre-antral stage block in folliculogenesis leading to infertility in females (Wang et al., 2014; Kumar et al., 1997). Gonadotrope-targeted expression of a HFSHBWT transgene efficiently rescues and restores normal phenotypes in Fshb null female mice (Kumar et al., 1998; Wang et al., 2014). To determine if the HFSHBdgc transgene rescues Fshb null females, female reproductive tracts were analyzed morphologically and histologically (Fig. 5). Fshb null females expressing the HFSHBdgc transgene demonstrated hypoplastic uteri and ovaries similar to those observed in Fshb null females (Fig. 5A), and as reported earlier (Wang et al., 2014; Kumar et al., 1997). In contrast, uterine horn and ovarian morphology were indistinguishable in mice expressing the HFSHBWT transgene compared to normal control mice at 9 weeks of age (Fig. 5A). Histological analysis indicated all stages of folliculogenesis including many secondary follicles (SF) and the presence of corpora lutea (CL) in ovarian sections obtained from control and Fshb−/− HFSHBWT, but not Fshb−/− and Fshb−/− HFSHBdgc females at either 9 weeks (Fig. 5B) or 6 months of age (Supplementary Fig. S3), indicating anestrous and anovulatory phenotypes only in the latter two mouse genotypes. When expression of FSH-responsive genes was measured by Taqman real time qPCR assays, we found that these genes were suppressed in the absence of FSH-mediated signaling in ovaries of Fshb null mice (Fig. 6). Only the HFSHBWT but not the HFSHBdgc transgene rescued the expression of these FSH-responsive genes to those levels observed in normal control mouse ovaries (Fig. 6 A–F). Immunolabeling of ovarian sections with antibodies against markers downstream of FSH-mediated signaling such as phospho-CREB (Fig. 7A) and phospho-PKA substrate (Fig. 7B) similarly indicated no rescue in Fshb null female mice expressing the HFSHBdgc transgene compared to that in Fshb null female mice expressing the HFSHBWT transgene. However, there were no differences in the immunolabeling pattern for phospho-Histone H3, a general mitotic marker in ovarian sections across all genotypes (Fig. 7C). Consistent with all the above female reproductive phenotypes, mating trials over a period of 6-months indicated that Fshb null female mice expressing the HFSHBdgc transgene did not produce any litters, similar to Fshb null females (Fig. 7 D and E). In contrast, the breeding performance (i.e., litter number and size) of Fshb null female mice expressing the HFSHBWT transgene was indistinguishable from that of control females (Fig. 7 D and E). Thus, these data confirm that the HFSHBdgc transgene did not rescue Fshb null females similar to Fshb null males.
Fig. 5.
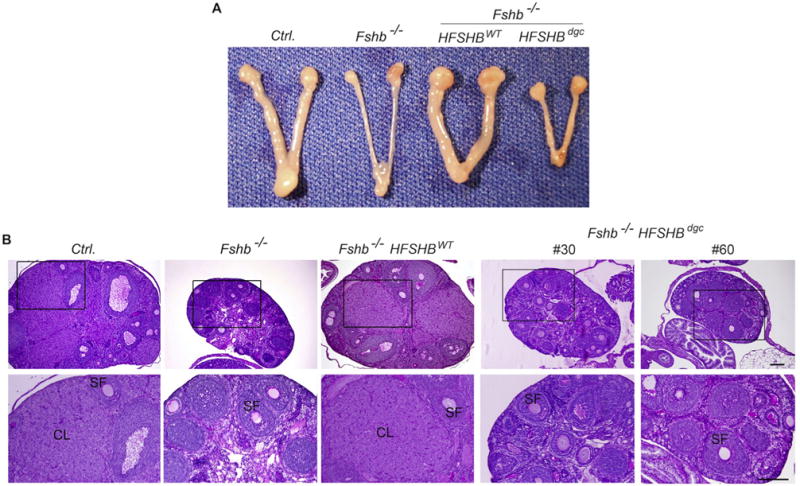
Gross morphology (A) and PAS-hematoxylin stained ovarian histology (B) showed that HFSHBdgc transgene unlike the HFSHBWT transgene did not rescue Fshb−/− females. Multiple developing secondary follicles and corpora lutea are readily seen in ovarian sections obtained from control (Ctrl.) and Fshb−/− HFSHBWT mice but not in those from Fshb−/− or Fshb−/− HSFHBdgc mice. Boxed areas in top panel in B are enlarged and shown below. SF: secondary follicle; CL: corpus luteum. The bar in panel B represents 200 μm.
Fig. 6.
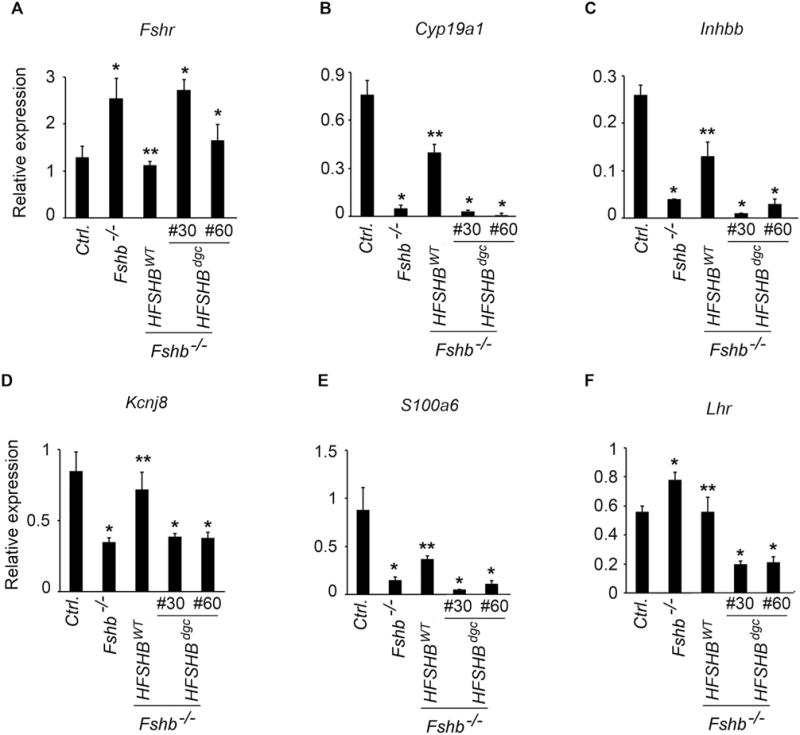
Ovarian gene expression measured by real time qPCR assays was indicated in panels A–F. The results showed many of the FSH-responsive genes suppressed in Fshb−/− mice remained the same in Fshb−/− HSFHBdgc mice. HFSHBWT transgene restored the expression levels of many genes similar to that observed in ovaries of control (Ctrl.) mice. Each assay was performed on four cDNA samples and each sample run in triplicate. * P < 0.01 versus Ctrl. or Fshb−/− HSFHBdgc groups; ** versus Fshb−/− group. qPCR assays to detect the expression of the following genes were used- Fshr: follicle-stimulating hormone-receptor, Cyp19a1: aromatase, Inhbb: activin beta b, Kcnj8: potassium inwardly-rectifying channel subfamily J member 8, S100a6: S100calcium binding protein A6 (calcyclin), Lhr: luteinizing hormone receptor.
Fig. 7.
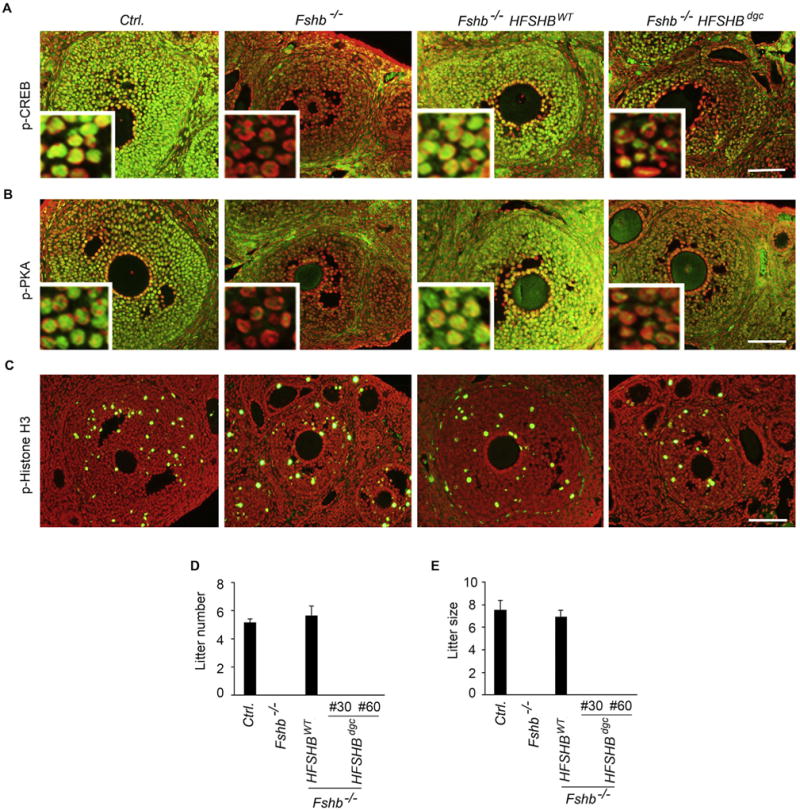
Immunolabeling of ovaries obtained from mice at 21d of age showed that phospho-CREB (A) and phospho-PKA substrate (B) levels did not differ between Fshb−/− and Fshb−/− HSFHBdgc mice indicating no rescue in contrast to that observed in Fshb−/− HSFHBWT mice. However, there were no differences in mitotically active phospho-Histone H3+ cells (C) in ovarian sections obtained from all genotypes of mice. Fshb−/− HSFHBdgc female mice, similar to Fshb−/− females were infertile as indicated by mating trials that resulted in production of no pups in a 6 month-period. In contrast, the breeding performance of Fshb−/− HSFHBWT mice was identical to that observed in control mice (D and E) indicating full-functional rescue by the HSFHBWT transgene.
4. Discussion
The biological significance of Asn-linked N-glycans in glycoprotein hormones was originally established by chemical modification methods using reagents such as hydrogen fluoride and trifluoromethane sulfonic acid, or enzymatic treatment using glycosidase mixtures on purified hormones (Kalyan and Bahl, 1983; Manjunath and Sairam, 1982; Manjunath et al., 1982; Sairam, 1982; Sairam and Jiang, 1992; Sairam et al., 1986; Thotakura et al., 1990). Such de-glycosylated hormone preparations were pharmacologically tested in vitro or injected into rodents to evaluate serum half-life and bioactivities (Kalyan and Bahl, 1983; Manjunath and Sairam, 1982; Manjunath et al., 1982; Sairam, 1982; Sairam and Jiang, 1992; Sairam et al., 1986; Thotakura et al., 1990). However, it was not possible to pinpoint the contributions of individual N-glycans selectively by these general deglycosylation methods. Hence, subsequent studies focused on site-directed mutagenesis and transfection approaches using mostly CHO cell expression systems and defined the biological roles of individual N-glycan chains on glycoprotein hormone subunits (Matzuk and Boime, 1989; Flack et al., 1994a,b). One disadvantage noted with this approach was when mutagenesis of Asn residues (and thus loss of N-glycan chains) affected dimer assembly and secretion, bioactivity assessment was not possible.
In this manuscript, we have circumvented the problems associated with chemical modification and in vitro mutagenesis experiments by undertaking an in vivo genetic rescue approach. We initially produced transgenic mice with gonadotrope-targeted expression of either HFSHBWT or HFSHBdgc mutant transgenes and introduced these separately onto an Fshb null genetic background by intercrossing. This strategy permitted us to directly test the roles of N-glycans on transgene-encoded hFSHβ subunit in the absence of endogenous mouse FSHβ subunit (Fig. 8). The use of a cDNA construct and thus the lack of all regulatory elements on the HFSHBdgc transgene was not an issue because full-length HFSHBWT transgene encompassing all elements was expressed at similar levels in the mouse pituitary (Fig. 2 A–C). This also suggests identical regulation of HFSHB and oFshb promoters in a mouse gonadotrope environment.
Fig. 8.
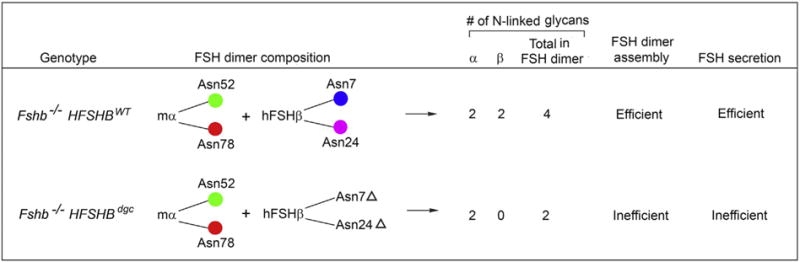
Comparison of Fshb−/− HSFHBWT and Fshb−/− HSFHBdgc mice, their interspecies hybrid FSH heterodimer composition, number of N-linked glycans, FSH dimer assembly and secretion characteristics. Genetic rescue of Fshb−/− mice was achieved with HSFHBWT, but not HSFHBdgc transgene, confirming that N-linked glycans of hFSHβ subunit are essential for FSH heterodimer assembly and secretion in vivo. The positions of Asn-linked N-glycans on FSH α (Asn52 and Asn78) – and β -subunits (Asn7 and Asn24) are indicated.
Although HFSHBdgc transgene-encoded, double N-glycosylation site mutant FSHβ subunit was expressed at high levels, it assembled poorly with the endogenous mouse α-subunit as assessed by non-denaturing electrophoresis followed by immunoblotting, and quantification of FSH content in pituitary lysates by human or mouse FSH RIAs. Thus, N-linked glycans covalently attached at Asn7 and Asn24 residues on hFSHβ subunit are essential in vivo for FSH dimer assembly. Our data are in agreement with biochemical studies using purified bovine glycoprotein hormone preparations in which differences in α-subunit glycosylation were similarly found and predicted to be important for dimer assembly (Arora et al., 1999; Arora et al., 2010). Moreover, recent molecular dynamics simulation studies have also indicated no evidence of misfolding of deglycosylated FSHβ subunit in combining with the alpha subunit (Meher et al., 2015). Our data, however, are contradictory to in vitro studies using a heterologous CHO cell expression system in which Asn7/Asn24 double mutant FSHβ subunit-containing heterodimer immunoactivity was readily detected in gel filtration chromatograms (Flack et al., 1994a,b).
Although, previous studies indicated that interspecies hybrid glycoprotein hormones between purified α- and β-subunits obtained from different species readily formed (Licht et al., 1976; Burke et al., 1979; Licht et al., 1978; Licht et al., 1983), co-expression of mutant FSHβ subunit with homologous α– subunit in vitro versus heterologous α– subunit in vivo could be one reason for the above discrepancy. An alternative approach to our strategy presented here would be to target in the future, expression of a mutant transgene encoding a single chain FSH analog in which double N-glycosylation site mutant human FSHβ encoding cDNA is genetically fused to that encoding the human α – subunit (Ben-Menahem and Boime, 1996; Garcia-Campayo and Boime, 2001). However, such a strategy will bypass the subunit/heterodimer assembly step. If the resulting single chain hormone were processed similar to FSH heterodimer and gets secreted from pituitary, then this strategy would allow us to test the in vivo biological function of this novel double N-glycosylation site mutant FSHβ – containing single chain FSH analog.
As a consequence of poor assembly, double N-glycosylation site mutant FSHβ subunit-containing FSH dimer was inefficiently secreted. We confirmed this by RIA measurements of both whole pituitary organ culture medium samples and mouse serum samples. Impaired secretion was not due to dysregulated gonadotrope-specific gene expression, intracellular retention or “trapping” of either the mutant hFSHβ subunit or FSH dimer containing this mutant subunit because both WT and mutant hFSHβ subunits displayed identical patterns of localization by confocal microscopy. Consistent with this intracellular behavior and impaired secretion, Fshb null male and female mice expressing double N-glycosylation site mutant hFSHβ-containing FSH dimer did not show any evidence of rescue. Testis and tubule size, as well as sperm number in Fshb−/− HFSHBdgc mutants were similar to those in Fshb null male mice. Similarly, female Fshb−/− HFSHBdgc mutants, like Fshb null mice were infertile, did not show any evidence of estrus cycles and their ovarian genes remained suppressed. Our previous (Kumar et al., 1998; Wang et al., 2014) and present studies also confirmed that Fshb−/− HFSHBWT transgene encoding human FSHβ subunit (with both N-glycosylation sites intact) fully rescues Fshb null mice. Both male and female Fshb−/− HFSHBWT mice exhibited phenotypes indistinguishable from those in control normal mice.
Micro-heterogeneity in glycoprotein hormones contributed by terminal sugar residues has been extensively studied (Creus et al., 2001, 1996; Loreti et al., 2013a,b). Over the past few years our group has identified and biochemically characterized several naturally occurring hypo-glycosylated FSH glycoforms resulting from macro-heterogeneity contributed by presence or absence of individual N-linked glycans on FSHβ subunit (Bousfield et al., 2007; Davis et al., 2014; Walton et al., 2001; Bousfield et al., 2014a,b; Butnev et al., 2015). Previous mass spectrometry results misleadingly detected only deglycosylated FSHβ in a FSHβ preparation derived from hFSH21 glycoform in which only the 21 KDa FSHβ band was detectable by Western blot (Walton et al., 2001). Thus, we initially believed hFSHβ N-glycosylation occurred in an all-or-none manner. We were confident in the mass spectrometry results because when applied to a FSHβ24 preparation possessing only the 24 KDa band in Western blot analysis, deglycosylated FSHβ was also present. This led to molecular dynamics studies of hFSH15 models bearing various N-glycans (Meher et al., 2015), an unsuccessful attempt to express deglycosylated FSHβ in bacteria, and the present manuscript to study transgenic FSH15 in vivo. Results reported herein provide the first evidence that loss of both N-glycans produced a 15 KDa FSHβ band on Western blots.
Interestingly, the FSH15 glycoform (consisting of the double N- glycosylation site mutant FSHβ subunit) was not the most abundant form identified in the above studies. This observation indicates that in addition to poor assembly, the double N-glycosylation site mutant hFSHβ subunit containing FSH dimer may also be highly unstable in human pituitaries. Attempts to express the recombinant human FSH15 glycoform in pituitary-derived GH3 cells also consistently failed to produce much FSH dimer owing to perhaps poor assembly and stability of the dimer or FSHβ subunit (Bousfield, G.R. et al., unpublished data). In contrast, hypo-glycosylated FSH21 (containing only one N-linked glycan chain on Asn7) was routinely detected in most human pituitaries (Bousfield et al., 2014a,b; Butnev et al., 2015), while FSH18 only became detectable in FSH21-enriched preparations. Both glycoforms were routinely expressed and have been functionally characterized in vitro (Bousfield et al., 2014a,b; Butnev et al., 2015). These recent data indicate that at least one N-linked glycan must be present on hFSHβ subunit for proper FSH dimer assembly and secretion.
Our experimental model of Fshb null mice engineered to express desired human FSHB transgenes in a gonadotrope-specific manner offers a well-established genetic rescue platform (Kumar et al., 1998; Wang et al., 2014). Thus, it is feasible for us to systematically engineer and express different human FSH glycoformencoding transgenes on an Fshb null genetic background. Such in vivo studies are ongoing in our laboratory that will ultimately allow us to define the physiological significance of the existence of multiple FSH glycoforms in the pituitary and decode the precise biological roles of each FSH glycoform in a whole animal setting.
Supplementary Material
Acknowledgments
We thank Dr. Bin Shuai and Mr. Ian Graham for technical assistance, Dr. James A. Dias for providing the HFSHBAsn7 Asn24 double mutant cDNA containing plasmid, Dr. Irving Boime for generously providing the recombinant human FSH, and antibodies against FSH, and Dr. A.F. Parlow, National Hormone and Pituitary Program, for supplying us with FSH RIA and RRA reagents. This research work was funded in part by the NIH grant P01AG 029531 (to T.R.K. and G.R.B.), CA166557 (to T.R.K.), and a K-INBRE Bridge Grant from the P20GM103418 (to T.R.K.). The Ligand Assay and Analysis Core Laboratory at the University of Virginia, Charlottesville was supported by a P50HD028934 grant.
Abbreviations
- ACTH
Adrenocorticotropin
- cAMP
cyclin adenosine 5′-monophosphate
- CHO
Chinese hamster ovary
- FITC
Fluorescein isothiocyanate
- FSH
Follicle-stimulating hormone
- GH
Growth hormone
- LH
Luteinizing hormone
- PNGase F
Peptide -N-Glycosidase F
- Ppil1
Peptidylprolyl isomerase-like 1
- PKA
protein kinase-A
- PVDF
Polyvinyl difluoride
- PAS
Periodic acid Schiff’s reagent
- PRL
Prolactin
- RIA
Radioimmuno assay
- RRA
Radioreceptor assay
- r-h
Recombinant human
- TSH
Thyroid-stimulating hormone
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.mce.2016.02.015.
Footnotes
Disclosure statement
The authors have nothing to disclose.
References
- Ambao V, Rulli SB, Carino MH, Console G, Ulloa-Aguirre A, Calandra RS, Campo S. Hormonal regulation of pituitary FSH sialylation in male rats. Mol Cell Endocrinol. 2009;309:39–47. doi: 10.1016/j.mce.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Anobile CJ, Talbot JA, McCann SJ, Padmanabhan V, Robertson WR. Glycoform composition of serum gonadotrophins through the normal menstrual cycle and in the post-menopausal state. Mol Hum Reprod. 1998;4:631–639. doi: 10.1093/molehr/4.7.631. [DOI] [PubMed] [Google Scholar]
- Arora T, Arundhati G, Muralidhar K. Occurrence of free alpha subunit of the thyroid stimulating hormone family in pituitaries of warer buffaloes (Bubalus bubalis) J Endocrinol Reprod. 1999;3:60–69. [Google Scholar]
- Arora T, Vashistha N, Chaudhary R, Muralidhar K. Pseudo-affinity chromatographic approach to probe heterogeneity in buffalo pituitary luteinizing hormone: probable pseudolectin-like behavior of immobilized Cibacron Blue 3GA. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878:2679–2684. doi: 10.1016/j.jchromb.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Baenziger JU, Green ED. Pituitary glycoprotein hormone oligosaccharides: structure, synthesis and function of the asparagine-linked oligosaccharides on lutropin, follitropin and thyrotropin. Biochim Biophys Acta. 1988;947:287–306. doi: 10.1016/0304-4157(88)90012-3. [DOI] [PubMed] [Google Scholar]
- Ben-Menahem D, Boime I. Converting heterodimeric gonadotropins to genetically linked single chains: new approaches to structure activity relationships and analogue design. Trends Endocrinol Metab. 1996;7:100–105. doi: 10.1016/1043-2760(96)88667-x. [DOI] [PubMed] [Google Scholar]
- Bishop LA, Robertson DM, Cahir N, Schofield PR. Specific roles for the asparagine-linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. Mol Endocrinol. 1994;8:722–731. doi: 10.1210/mend.8.6.7935488. [DOI] [PubMed] [Google Scholar]
- Bousfield GR, Jia L, Ward DN. Gonadotropins: chemistry and biosynthesis. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. Elsevier Press; New York: 2006. pp. 1581–1634. [Google Scholar]
- Bousfield GR, Butnev VY, Walton WJ, Nguyen VT, Huneidi J, Singh V, Kolli VS, Harvey DJ, Rance NE. All-or-none N-glycosylation in primate follicle-stimulating hormone beta-subunits. Mol Cell Endocrinol. 2007:260–262. doi: 10.1016/j.mce.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Bousfield GR, Butnev VY, Butnev VY, Hiromasa Y, Harvey DJ, May JV. Hypo-glycosylated human follicle-stimulating hormone (hFSH(21/18)) is much more active in vitro than fully-glycosylated hFSH (hFSH(24)) Mol Cell Endocrinol. 2014a;382:989–997. doi: 10.1016/j.mce.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousfield GR, Butnev VY, Rueda-Santos MA, Brown A, Hall AS, Harvey DJ. Macro- and Micro-heterogeneity in pituitary and urinary follicle-stimulating hormone glycosylation. J Glycom Lipidom. 2014b;4 doi: 10.4172/2153-0637.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke WH, Papkoff H, Licht P, Gallo AB. Preparation and properties of luteinizing hormone (LH) subunits from the turkey (Meleagris gallopavo) and their recombination with subunits of ovine LH. Gen Comp Endocrinol. 1979;37:501–507. doi: 10.1016/0016-6480(79)90032-7. [DOI] [PubMed] [Google Scholar]
- Butnev VY, Butnev VY, May JV, Shuai B, Tran P, White WK, Brown A, Smalter Hall A, Harvey DJ, Bousfield GR. Production, purification, and characterization of recombinant hFSH glycoforms for functional studies. Mol Cell Endocrinol. 2015;405:42–51. doi: 10.1016/j.mce.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo S, Ambao V, Creus S, Gottlieb S, Fernandez Vera G, Benencia H, Bergada C. Carbohydrate complexity and proportions of serum FSH isoforms in the male: lectin-based studies. Mol Cell Endocrinol. 2007:260–262. doi: 10.1016/j.mce.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Creus S, Pellizzari E, Cigorraga SB, Campo S. FSH isoforms: bio and immuno-activities in post-menopausal and normal menstruating women. Clin Endocrinol (Oxf) 1996;44:181–189. doi: 10.1046/j.1365-2265.1996.646467.x. [DOI] [PubMed] [Google Scholar]
- Creus S, Chaia Z, Pellizzari EH, Cigorraga SB, Ulloa-Aguirre A, Campo S. Human FSH isoforms: carbohydrate complexity as determinant of in-vitro bioactivity. Mol Cell Endocrinol. 2001;174:41–49. doi: 10.1016/s0303-7207(00)00453-6. [DOI] [PubMed] [Google Scholar]
- Crowe MA, Padmanabhan V, Mihm M, Beitins IZ, Roche JF. Resumption of follicular waves in beef cows is not associated with periparturient changes in follicle-stimulating hormone heterogeneity despite major changes in steroid and luteinizing hormone concentrations. Biol Reprod. 1998;58:1445–1450. doi: 10.1095/biolreprod58.6.1445. [DOI] [PubMed] [Google Scholar]
- Davis JS, Kumar TR, May JV, Bousfield GR. Naturally occurring follicle-stimulating hormone glycosylation variants. J Glycom Lipidom. 2014;4:e117. doi: 10.4172/2153-0637.1000e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack MR, Bennet AP, Froehlich J, Anasti JN, Nisula BC. Increased biological activity due to basic isoforms in recombinant human follicle-stimulating hormone produced in a human cell line. J Clin Endocrinol Metab. 1994a;79:756–760. doi: 10.1210/jcem.79.3.8077357. [DOI] [PubMed] [Google Scholar]
- Flack MR, Froehlich J, Bennet AP, Anasti J, Nisula BC. Site-directed mutagenesis defines the individual roles of the glycosylation sites on follicle-stimulating hormone. J Biol Chem. 1994b;269:14015–14020. [PubMed] [Google Scholar]
- Fox KM, Dias JA, Van Roey P. Three-dimensional structure of human follicle-stimulating hormone. Mol Endocrinol. 2001;15:378–389. doi: 10.1210/mend.15.3.0603. [DOI] [PubMed] [Google Scholar]
- Garcia-Campayo V, Boime I. Novel recombinant gonadotropins. Trends Endocrinol Metab. 2001;12:72–77. doi: 10.1016/s1043-2760(00)00338-6. [DOI] [PubMed] [Google Scholar]
- Green ED, Boime I, Baenziger JU. Differential processing of Asn-linked oligosaccharides on pituitary glycoprotein hormones: implications for biologic function. Mol Cell Biochem. 1986;72:81–100. doi: 10.1007/BF00230637. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Sebastian J, Strahl BD, Wu JC, Miller WL. The promoter for the ovine follicle-stimulating hormone-beta gene (FSHbeta) confers FSHbeta-like expression on luciferase in transgenic mice: regulatory studies in vivo and in vitro. Endocrinology. 2001;142:2260–2266. doi: 10.1210/endo.142.6.8202. [DOI] [PubMed] [Google Scholar]
- Kalyan NK, Bahl OP. Role of carbohydrate in human chorionic gonadotropin. Effect of deglycosylation on the subunit interaction and on its in vitro and in vivo biological properties. J Biol Chem. 1983;258:67–74. [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Low MJ, Matzuk MM. Genetic rescue of follicle-stimulating hormone beta-deficient mice. Endocrinology. 1998;139:3289–3295. doi: 10.1210/endo.139.7.6111. [DOI] [PubMed] [Google Scholar]
- Licht P, Papkoff H, Farmer SW, Muller CH, Tsui HW, Crews D. Evolution of gonadotropin structure and function. Recent Prog Horm Res. 1976;33:169–248. doi: 10.1016/b978-0-12-571133-3.50012-x. [DOI] [PubMed] [Google Scholar]
- Licht P, Farmer SW, Papkoff H. Biological activity of hybrid combinations of ovine and sea turtle LH subunits. Gen Comp Endocrinol. 1978;35:289–294. doi: 10.1016/0016-6480(78)90074-6. [DOI] [PubMed] [Google Scholar]
- Licht P, Papkoff H, Bona-Gallo A, Aggarwal BB. Subunits of an avian (ostrich) follicle-stimulating hormone and their hybridization with subunits of mammalian gonadotropins. Gen Comp Endocrinol. 1983;51:414–422. doi: 10.1016/0016-6480(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Loreti N, Ambao V, Juliato CT, Machado C, Bahamondes L, Campo S. Carbohydrate complexity and proportion of serum FSH isoforms reflect pituitary-ovarian activity in perimenopausal women and depot medroxyprogesterone acetate users. Clin Endocrinol (Oxf) 2009;71:558–565. doi: 10.1111/j.1365-2265.2009.03559.x. [DOI] [PubMed] [Google Scholar]
- Loreti N, Ambao V, Andreone L, Trigo R, Bussmann U, Campo S. Effect of sialylation and complexity of FSH oligosaccharides on inhibin production by granulosa cells. Reproduction. 2013a;145:127–135. doi: 10.1530/REP-12-0228. [DOI] [PubMed] [Google Scholar]
- Loreti N, Fresno C, Barrera D, Andreone L, Albarran SL, Fernandez EA, Larrea F, Campo S. The glycan structure in recombinant human FSH affects endocrine activity and global gene expression in human granulosa cells. Mol Cell Endocrinol. 2013b;366:68–80. doi: 10.1016/j.mce.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Manjunath P, Sairam MR. Biochemical, biological, and immunological properties of chemically deglycosylated human choriogonadotropin. J Biol Chem. 1982;257:7109–7115. [PubMed] [Google Scholar]
- Manjunath P, Sairam MR, Schiller PW. Chemical deglycosylation of ovine pituitary lutropin. A study of the reaction conditions and effects on biochemical, biophysical and biological properties of the hormone. Biochem J. 1982;207:11–19. doi: 10.1042/bj2070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Boime I. Mutagenesis and gene transfer define site-specific roles of the gonadotropin oligosaccharides. Biol Reprod. 1989;40:48–53. doi: 10.1095/biolreprod40.1.48. [DOI] [PubMed] [Google Scholar]
- Meher BR, Dixit A, Bousfield GR, Lushington GH. Glycosylation effects on FSH-FSHR interaction dynamics: a case study of different FSH glycoforms by molecular dynamics simulations. PLoS One. 2015;10:e0137897. doi: 10.1371/journal.pone.0137897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Lang LL, Sonstein J, Kelch RP, Beitins IZ. Modulation of serum follicle-stimulating hormone bioactivity and isoform distribution by estrogenic steroids in normal women and in gonadal dysgenesis. J Clin Endocrinol Metab. 1988;67:465–473. doi: 10.1210/jcem-67-3-465. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Mieher CD, Borondy M, I’Anson H, Wood RI, Landefeld TD, Foster DL, Beitins IZ. Circulating bioactive follicle-stimulating hormone and less acidic follicle-stimulating hormone isoforms increase during experimental induction of puberty in the female lamb. Endocrinology. 1992;131:213–220. doi: 10.1210/endo.131.1.1611999. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Lee JS, Beitins IZ. Follicle-stimulating isohormones: regulation and biological significance. J Reprod Fertil Suppl. 1999;54:87–99. [PubMed] [Google Scholar]
- Pernasetti F, Spady TJ, Hall SB, Rosenberg SB, Givens ML, Anderson S, Paulus M, Miller WL, Mellon PL. Pituitary tumorigenesis targeted by the ovine follicle-stimulating hormone beta-subunit gene regulatory region in transgenic mice. Mol Cell Endocrinol. 2003;203:169–183. doi: 10.1016/s0303-7207(02)00430-6. [DOI] [PubMed] [Google Scholar]
- Sairam MR. Effects of carbohydrate removal on the structure and activity of bovine lutropin. Biochim Biophys Acta. 1982;717:149–153. doi: 10.1016/0304-4165(82)90392-0. [DOI] [PubMed] [Google Scholar]
- Sairam MR, Jiang LG. Comparison of the biological and immunological properties of glycosylation deficient human chorionic gonadotropin variants produced by site directed mutagenesis and chemical deglycosylation. Mol Cell Endocrinol. 1992;85:227–235. doi: 10.1016/0303-7207(92)90261-4. [DOI] [PubMed] [Google Scholar]
- Sairam MR, Kato K, Mukhopadhyay AK, Bohnet HG. Preparation and properties of human chorionic gonadotropin antagonist for biological studies: antifertility effects in the female rat. Acta Endocrinol (Copenh) 1986;112:586–594. doi: 10.1530/acta.0.1120586. [DOI] [PubMed] [Google Scholar]
- Thotakura NR, Weintraub BD, Bahl OP. The role of carbohydrate in human choriogonadotropin (hCG) action. Effects of N-linked carbohydrate chains from hCG and other glycoproteins on hormonal activity. Mol Cell Endocrinol. 1990;70:263–272. doi: 10.1016/0303-7207(90)90217-v. [DOI] [PubMed] [Google Scholar]
- Timossi CM, Barrios-de-Tomasi J, Gonzalez-Suarez R, Arranz MC, Padmanabhan V, Conn PM, Ulloa-Aguirre A. Differential effects of the charge variants of human follicle-stimulating hormone. J Endocrinol. 2000;165:193–205. doi: 10.1677/joe.0.1650193. [DOI] [PubMed] [Google Scholar]
- Walton WJ, Nguyen VT, Butnev VY, Singh V, Moore WT, Bousfield GR. Characterization of human FSH isoforms reveals a nonglycosylated beta-subunit in addition to the conventional glycosylated beta-subunit. J Clin Endocrinol Metab. 2001;86:3675–3685. doi: 10.1210/jcem.86.8.7712. [DOI] [PubMed] [Google Scholar]
- Wang H, Larson M, Jablonka-Shariff A, Pearl CA, Miller WL, Conn PM, Boime I, Kumar TR. Redirecting intracellular trafficking and the secretion pattern of FSH dramatically enhances ovarian function in mice. Proc Natl Acad Sci U S A. 2014;111:5735–5740. doi: 10.1073/pnas.1321404111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hastings R, Miller WL, Kumar TR. Fshb-iCre mice are efficient and specific Cre deleters for the gonadotrope lineage. Mol Cell Endocrinol. 2016;419:124–138. doi: 10.1016/j.mce.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CR, Carlson NE, Lee JS, McNeilly AS, Sharma TP, Ye W, Padmanabhan V. Acidic mix of FSH isoforms are better facilitators of ovarian follicular maturation and E2 production than the less acidic. Endocrinology. 2002;143:107–116. doi: 10.1210/endo.143.1.8601. [DOI] [PubMed] [Google Scholar]
- Wu JC, Su P, Safwat NW, Sebastian J, Miller WL. Rapid, efficient isolation of murine gonadotropes and their use in revealing control of follicle-stimulating hormone by paracrine pituitary factors. Endocrinology. 2004;145:5832–5839. doi: 10.1210/en.2004-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


