Abstract
The occurrence of microorganisms from the Vibrio genus in saline lakes from northern Chile had been evidenced using Numerical Taxonomy decades before and, more recently, by phylogenetic analyses of environmental samples and isolates. Most of the knowledge about this genus came from marine isolates and showed temperature and salinity to be integral agents in shaping the niche of the Vibrio populations. The stress tolerance phenotypes of Vibrio sp. Teb5a1 isolated from Salar de Atacama was investigated. It was able to grow without NaCl and tolerated up to 100 g/L of the salt. Furthermore, it grew between 17° and 49°C (optimum 30°C) in the absence of NaCl, and the range was expanded into cold temperature (4–49°C) in the presence of the salt. Other additional adaptive strategies were observed in response to the osmotic stress: pigment production, identified as the known antibacterial prodigiosin, swimming and swarming motility and synthesis of a polar flagellum. It is possible to infer that environmental congruence might explain the cellular phenotypes observed in Vibrio sp. considering that coupling between temperature and salinity tolerance, the production of antibacterial agents at higher temperatures, flagellation and motility increase the chance of Vibrio sp. to survive in salty environments with high daily temperature swings and UV radiation.
Keywords: Vibrio, osmotic-stress, halotolerant, psychrotolerant, prodigiosin
Introduction
Extremophiles are considered microorganisms that require, for optimal growth, conditions that are not conducive to human life, like low or high pH, extreme temperatures, chemical oxidizing agents, hypersalinity or certain types of ultraviolet light (Sandle and Skinner, 2013). Among extremophiles, there are some microorganisms that are known as salt-tolerating (halotolerant) because they are able to grow in presence of relatively high salt concentrations and in absence of salt; and others are known as salt-loving (halophilic) because halophiles require a saline environment for growth (Ventosa et al., 1998; Oren, 2013).
In previous works, some halophilic microorganisms from the Vibrionaceae family have been studied, like Photobacterium, Listonella, Moritella, Salinivibrio, and Vibrio genus (Kamekura and Kushner, 1984; Garcia et al., 1987; Shieh, 2003; Borić et al., 2011; Lucena et al., 2012). They are facultative anaerobic, Gram-negative, and rod-shaped bacteria; these organisms are ever-present in estuarine, coastal, oceanic water, and marine sediments (Shieh, 2003; Borić et al., 2011; Lucena et al., 2012). Few studies have been reported about microorganisms isolated from Chilean salt lakes (Campos, 1993; Monteoliva-Sánchez et al., 2002; Demergasso et al., 2004).
Salinivibrio costicola and Vibrio ruber are the most studied halophilic eubacteria. They can grow in a salinity range from 0.5 to 12% and from 0.05 to 17% of salinity, respectively, and in a range of temperature from 5 to 45 and from 10 to 44°C, respectively (Supplementary Table 1). The adaptation strategies developed by those microorganisms to survive environmental extremes, and perturbations of salinity and temperature have been extensively studied and can be summarized as follows: salinity, (i) balance their cytoplasm with the osmotic pressure of the external medium by accumulation of osmoprotectants (like sugars, polyols, amino acids, and their derivatives) either by uptake from the environment or by their de novo synthesis (Ventosa et al., 1998; Danevcic et al., 2005; Zhu et al., 2008; Danevcic, 2014); (ii) modification of the lipid composition by changing of both polar head groups and acyl chains, the amount of unsaturated fatty acids and the occurrence of hydroxyl fatty acids (Hanna, 1984; Adams and Russell, 1992; Ventosa et al., 1998; Danevcic, 2014); (iii) changes in the membrane reaction potential of V. ruber (Danevcic, 2014); (iv) activity of ion pumps in S. costicola membranes represented by two alternative mechanisms, a Na+/H+ antiport and the presence of a primary respiration-driven Na+ pump (Ventosa et al., 1998; Müller and Oren, 2003); (v) changes in the composition and activity of ribosomal proteins, on the protein turnover, in the composition and activity of glycolytic and electron transport active proteins involved in central metabolic pathways, like pyruvate kinase and dehydrogenase (de Médicis and Rossignol, 1979; Ventosa et al., 1998; Danevčič and Stopar, 2011); (vi) increase of the secondary metabolite production – prodigiosin- by V. ruber with known capacity as a transmembrane chloride anion carrier among other properties (Danevcic, 2014; Seganish and Davis, 2005); and temperature, (vii) changes in the prodigiosin production by V. ruber with an optimum at 28°C (Danevcic, 2014); (viii) change in the polar lipid head groups composition, in saturation of the phospholipid fatty acyl composition and in the mean acyl chain length of S. costicola (Adams and Russell, 1992). The ecophysiological response to other relevant environmental factors which determines the growth and survival of those microorganisms like nutrients availability, viscosity, and UV radiation have been also reported (Danevcic, 2014).
According to some authors, Vibrio presents a red pigment known as prodigiosin (representative of the prodiginines family), which is a secondary metabolite that was first characterized from Serratia marcescens and it was later found to be produced by other bacteria, mostly members of the proteobacteria (Suryawanshi et al., 2015). Several bacteria that produce prodigiosin were characterized as inhabiting open habitats with high microbial diversity, and therefore there was intense competition between the community members (Suryawanshi et al., 2015). In addition, some environmental factors can influence prodigiosin production, like temperature, pH, media composition, and salinity, among others (Venil, 2009). For instance, one of the major requirements for effective pigment production and for the growth of isolated marine Vibrio sp. is NaCl (Kirishna et al., 2014). Moreover, some other research groups reported that NaCl is required for growth and pigmentation in Serratia marcescens (Silverman and Munoz, 1973; Allen et al., 1983). Besides the impact of ion concentrations, the effect of the temperature on the activation of enzymes involved in prodigiosin biosynthetic pathway (Williams et al., 1971; Casullo de Araújo et al., 2010; Starič et al., 2010) has also been reported. However, most of the studies were related to room temperature or optimal temperature.
Protective or metabolic roles, among others, have been attributed to prodigiosin; some of these roles are related to energy spilling reaction, air dispersal of bacteria, light storage (energy), UV survival, anion exchange and antimicrobial activity (Burger and Bennett, 1985; Ryazantseva et al., 1995; Seganish and Davis, 2005; Haddix et al., 2008; Venil, 2009; Starič et al., 2010; Borić et al., 2011; Danevcic, 2014). Besides, algicidal activity was also reported and proposed to be used in the control of bloom-forming red-tide phytoplanktons (Kim et al., 2008). Furthermore, prodigiosin 1, parent of compounds isolated from Serratia marcescens as described by Fürstner (2003), transports chloride ions into the cell across phospholipid vesicles by functioning as H+/Cl- symporters. Then, this pigment operates as chloride anion carrier, but it is also able to exchange chloride for nitrate anions without any change in the internal pH during transmembrane transport by functioning as antiporter (Seganish and Davis, 2005; Díaz et al., 2007).
In addition, some biotechnological roles of prodigiosin were already reported to have immunosuppressive, antiproliferative, antimalarial, bactericidal, UV protection, antioxidant, and antitumor properties (Burger and Bennett, 1985; Venil, 2009; Borić et al., 2012; Arivizhivendhan et al., 2015; Darshan and Manonmani, 2015; Lapenda et al., 2015).
An isolated strain of Vibrio sp. (Supplementary Figure 1) from Salar de Atacama (Supplementary Table 2) showed an ample range of saline as well as temperature tolerance. Both salinity and temperature affected morphology (flagellation and aggregation), growth, motility and pigment production (prodigiosin). The cellular phenotypes observed were described and the ecological significance was discussed.
Materials and Methods
Strains and Culture Conditions
All chemical used in these experiments were purchased from Merck and Difco.
A strain of Vibrio genus (Vibrio sp. Teb5a1) isolated from Laguna de Tebenquiche in Salar de Atacama was used to work during these experiments. Initially, the microorganism Vibrio sp. was grown at 30°C in an orbital shaker at 100 rpm in a seawater medium (salinity 3% approximately), with yeast extract and peptone. The isolations were achieved in plates with the same medium and bacteriologic agar. Subsequently, modifications of this medium were performed to control the growing conditions (“defined medium”). This new medium was prepared according to (Shieh, 2003); it comprised peptone (6 g/L), yeast extract (2 g/L), MgSO4x7H2O (3 g/L), CaCl2 (0.01 g/L), KCl (0.6 g/L) and variable NaCl concentrations. Incubation conditions were 30°C in an orbital shaker (100 rpm). Additionally, Vibrio sp. was isolated in plates with the same defined medium and bacteriologic agar.
Immediate Adaptation of Vibrio sp. to Salt
To perform the immediate adaptation assays, the microorganism was grown in the defined medium without NaCl until its exponential phase was reached at 30°C and 100 rpm orbital shaking. Successively, this culture was used to inoculate media with different NaCl concentrations (from 0 to 150 g/L NaCl) and incubated at the same temperature (30°C). Three biological replicates were used to perform the adaptation of Vibrio sp.
Identification of Optimum Growth Conditions for Vibrio sp.
The defined medium was used to identify the optimum conditions for Vibrio sp. growing, as described previously. The growth experiments were performed to identify the optimum temperature and the optimum NaCl concentration, which were achieved in a range from 4 to 49°C and from 0 to 150 g/L of NaCl, respectively. The growth of each culture was checked using a Neubauer camera of 0.01 mm depth (Labolan) in an optical microscope (Olympus). Additionally, the growth of Vibrio sp. was checked by optical density at 600 nm wavelength in a UV-visible spectrophotometer (Perkin Elmer). Three biological replicates were used to identify the optimum growth conditions (temperature and salinity) for Vibrio sp.
Extraction of Red Pigment in Vibrio sp.
Considering that Vibrio sp. produced a red pigment, three methods (Cang et al., 2000; Song et al., 2006; Alihosseini et al., 2008) for pigment extraction with minor modifications were used to identify the pigment by UV/Vis absorption and to compare it with published results. Method 1 (Song et al., 2006): 25 mL of supernatant from cultures of Vibrio sp. with 25 mL of 95% Ethanol pH 3 (Merck) were mixed in a separatory funnel during 30 s. After 15 min, 25 mL of chloroform (Merck) were added to the previous solution and mixed again for 30 s. The lower phase (polar phase) was stored at room temperature in a dark flask until it was used again. Method 2 (Cang et al., 2000): 25 mL of supernatant from Vibrio sp. cultures with 25 mL of Acetone (Merck) were mixed in a separatory funnel during 30 s. After 10 min, 25 mL of chloroform pH 3 (Merck) were added and mixed. Again, the polar phase was stored at room temperature in darkness until it was used. Method 3 (Alihosseini et al., 2008): 25 mL of supernatant from Vibrio sp. cultures with 25 mL of chloroform (Merck) were mixed in a separatory funnel during 30 s. After 1 min, the polar phase was collected and stored at room temperature in a dark flask. In addition, absorption of UV/Visible light (UV/Vis Lambda EZ 301 spectrometer, PerkinElmer) of all tree collected samples (from methods 1, 2, and 3) was performed, considering as blank chloroform.
Purification of Extracted Red Pigment from Vibrio sp.
The cellular pellet from 6 L of Vibrio sp. was obtained by centrifugation (Centrifuge Eppendorf 5804R) during 10 min at 9000 rpm. This pellet was dried at 80°C for 18 h in a sterile petri dish. Two chromatographic columns were prepared: “first column”, 5 g of dried pellet of Vibrio sp. plus 10 g of silica gel were loaded into 100 g of pure silica gel column chromatography (Silica gel 60, 0.063–0.200 mm, Merck). Samples collected from this first column were analyzed by UV/vis absorbance spectroscopy. “Second column”, 389 mg of purified (and dried) pellet from the first column plus 5 g of silica gel were loaded into 60 g of pure silica gel column chromatography (Silica gel 60, 0.063–0.200 mm, Merck). Samples collected from this second column were used for mass spectrometry analysis. To remove air bubbles, 1 L of n-hexane were loaded into both columns. Polarity of solvents were changed as follow: 10:0, 9:1, 7:3, 6:4, 1:1, 4:6, 3:7, 2:8, 1:9 n-hexane:chloroform. Along with polarity changes, fractions were collected and an aliquot was loaded in a 5 cm × 5 cm silica gel TLC plate (Silica gel 60 F254, Merck) to check the isolation of new spots. The elution of fractions in the TLC plates was done with n-hexane:Ethyl acetate in a proportion 95:5. Once different compounds were appearing, the polarity was increased. Visualization of spots was done under UV/Vis light, and five methods were used as Supplementary Table 3 shows.
Identification of Red Pigment by Mass Spectrometry (MS)
In order to determine whether red pigment corresponds to the prodiginine family members, samples were analyzed by mass spectrometry. Fractions from the second column (as it was mentioned before) were analyzed by Mass spectrometry through an HPLC system Agilent 1100 (Agilent Technologies Inc., Folsom, CA, USA) online coupled to ESI-ITMS (Esquire 4000) at the mass spectrometry facility of Universidad de Chile. Separation of the sample was performed through a C18 column (Luna 150 mm × 4.6 mm, 5 μm, 100 Å; Phenomenex, Inc.) during 60 min gradient from 0 to 98% buffer B (details are shown in Supplementary Table 4). Ionization was at 325°C, 30 psi and with nitrogen as the gas carrier. Data analysis was performed with Data Analysis 3.2 (Bruker Daltonik GmbH, Germany).
Effect of NaCl Concentration in the Motility of Vibrio sp.
Two types of motility assays were done (Roeßler et al., 2000) to observe the swarming and swimming motility. For swarming phenomenon, the microorganism was grown in the solidly defined medium with 0.5% of bacteriologic agar and the cultures were inoculated into the agar. For swimming phenomenon, Vibrio sp. was grown in the solidly defined medium with 0.3% of bacteriologic agar and the cultures were inoculated with a drop over the agars. Both assays were done in the absence (0 g/L NaCl) and in the presence of NaCl (25 and 100 g/L NaCl), as well as at low temperature (17°C). Three biological replicates were used to study the effect of salinity in the motility of Vibrio sp.
Transmission Electron Microscopy (TEM)
The morphology and the presence of flagellum, in presence and absence of NaCl, were analyzed as previously reported (Gonzalez and Jensen, 1998). The cells were grown until its lag phase with 0, 25, 50, and 100 g/L of NaCl; then cells were washed with ultra-pure water. Later, the cells were resuspended to 0.5 of optical density at A600, and aliquots of the cellular suspensions of 10 μL were taken and air-dried on coated grids. Finally, cells were examined under a transmission electron microscope (Philips Tecnai 12) operating in the scanning transmission mode at 80 kV (Pontificia Universidad Católica de Chile).
Effect of Prodigiosin in the DNA Cleavage
DNA cleavage experiments were performed with 0.4 μg plasmid DNA obtained from an isolate from the salt flat environment using the QIAprep Spin Miniprep (QIAGEN) and the QIAquick PCR purification kits for extraction and purification, respectively. DNA was quantified using a NanoDrop UV-Vis Spectrophotometer.
The plasmid DNA was incubated, either in the absence or presence of 4% v/v of the purified extract and 120 μM Cu II, in 10 mM 3-(N-Morpholino)-propanesulfonic Acid-Acetate buffer (MOPS buffer) (pH 7.4), 75 M NaCl, 10% v/v Acetonitrile at 37°C for 0, 30, 60, 90, and 120 min. After incubation, DNA samples were run on horizontal agarose gels (0.8%) containing ethidium bromide (0.2 μg/mL) in 0.5 Tris-acetate-EDTA (1x TAE) buffer for 1 h at 85 V.
Results
Effect of Salt Concentration on Growth of Vibrio sp.
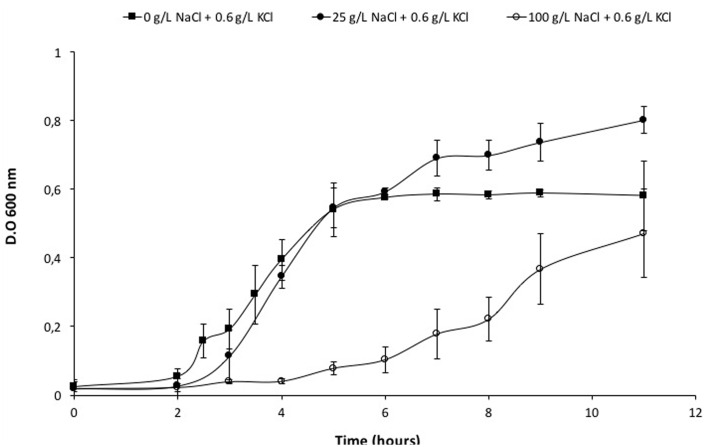
Initially, Vibrio sp. was grown in a saline medium (seawater). To control the growth conditions, the microorganism was grown successfully in a defined medium containing 25 g/L of NaCl, and the same characteristics (pigment generation and short duplication time) were observed. Afterward, Vibrio sp. was adapted to grow in the absence of NaCl as shown in Figure 1 and Supplementary Figure 2. In absence of salt and in a medium with up to 25 g/L NaCl, the culture grew immediately; besides, at 50 g/L of NaCl, a longer lag phase evidenced a slower adaptation. Experiments with higher salt concentration (150 g/L NaCl) did not yield positive growth results (data not shown). When the microorganism was already adapted, the optimum conditions of growth were identified. For instance, Vibrio sp. was able to grow in absence of NaCl, with a generation time less than 2 h and could tolerate up to 100 g/L NaCl, with a generation time five folds longer than in the absence of salt (Supplementary Figure 3; Supplementary Table 5B).
FIGURE 1.
Adaptation growth curves of Vibrio sp. in presence of NaCl.
Vibrio sp. cells were adapted previously to grow in presence of 0 g/L NaCl and KCl ( ), 25 g/L NaCl (
), 25 g/L NaCl ( ) and 100 g/L NaCl (
) and 100 g/L NaCl ( ). New cultures were prepared from these cells and used to perform their respective curves.
). New cultures were prepared from these cells and used to perform their respective curves.
Effect of Temperature on Growth of Vibrio sp.
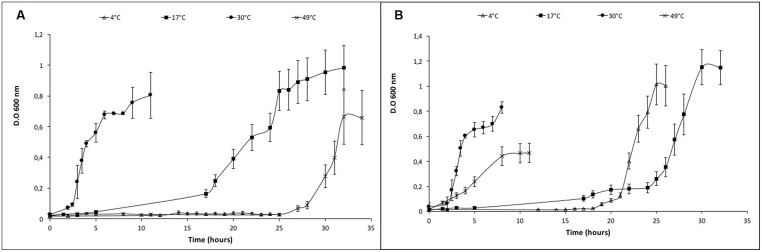
After the identification of the optimum salt concentration for growing at 30°C, the microorganism was grown in a range of temperature (from 4 to 49°C) in the absence of NaCl and with 25 g/L NaCl. In the absence of salt, it was able to grow between 17 and 49°C with different lag phases (Figure 2A; Supplementary Figure 4A). The range of temperature for growth was extended when Vibrio sp. grew with 25 g/L NaCl from 4 (with a generation time of 2, as shown in Supplementary Table 5A) to 49°C. In addition, the lag phases at temperatures below and above the optimum were reduced (Figure 2B; Supplementary Figure 4B). The maximal accumulation of the red pigment was evidenced during the growth of Vibrio sp. between 26 and 40°C when NaCl was amended. It was reduced below 17°C and there was no pigmentation at 4°C (Supplementary Figure 5).
FIGURE 2.
Growth curves of Vibrio sp. at different temperatures in presence and absence of NaCl. (A) Vibrio sp. cells grown in absence of salt (0 g/L NaCl) (B) Vibrio sp. cells grown in presence of salt (25 g/L NaCl). The temperature was changed from 4° to 49°C under both growth conditions.
Extraction, Purification, and Identification of Red Pigment in Vibrio sp.
Solvent extraction of the red pigment through the previously reported methods (Cang et al., 2000; Song et al., 2006; Alihosseini et al., 2008) showed that the maximal absorbance for methods 1 and 3 was 539 nm, whereas, for method 2, the maximal absorbance was 534 nm wavelength (Supplementary Figure 6). Both values for absorbance correlate with prodigiosin or prodigiosin-like compounds (Gerber, 1969).
Purification of the red pigment using the “first column” showed three different compounds related to Prodiginines. According to TLC plates, collected fractions from 336 to 394 correspond to the first compound mixtures, collected fractions from 395 to 423 correspond to the second compound mixtures and collected fractions from 424 to 487 correspond to the third compound mixtures (Supplementary Figure 7). All these fractions were collected in gradients 7:3 (for first and second compound mixtures) and 6:4 (for third compound mixtures) n-hexane:Ethyl acetate, respectively. UV/Vis spectroscopy confirmed the presence of three different compounds, and the maximal absorbance was observed at 535, 538, and 533 nm (Supplementary Figure 8). Absorbance below 400 nm corresponds to different compounds or contamination, which were contained in the previous and later prodiginine-containing fractions (as shown in Supplementary Figure 8).
Identification of prodiginines by LC-MS/MS confirmed the presence of more than fifteenth different compounds. Among these compounds, undecylprodiginine, methyldodecylprodiginine, cycloprodigiosin, and prodigiosin were identified, which are the most often reported (Supplementary Table 6). The identification was done comparing the observed fragmentation spectra (in our samples) with those reported by several authors, as it is cited in Supplementary Table 6. However, there were some fragmentation spectra that could not be assigned because there are not reports about that. Therefore, to verify if these MS/MS spectra represent new compounds, additional experiments, like 13C NMR and 1H NMR, would be necessary.
Effect of Salinity in Prodigiosin Production of Vibrio sp.
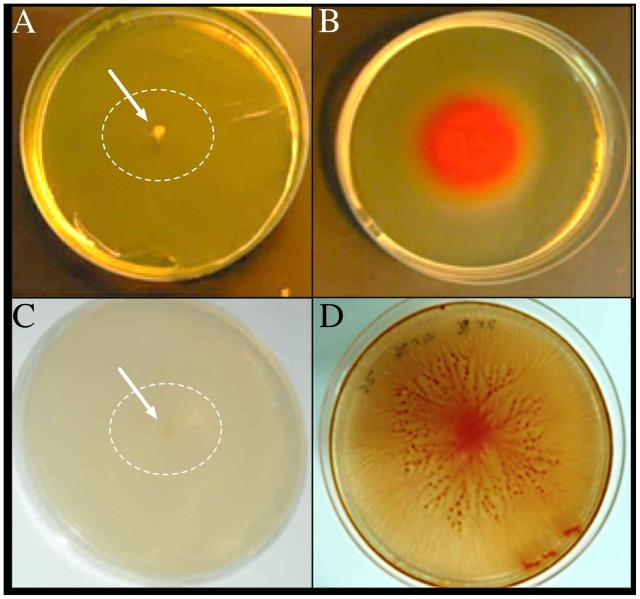
It was observed the production of a salt-dependent pigment in each experiment. This pigment is responsible for the red color and it is known as prodiginines. The pigment production showed a direct effect of the salt concentration of the growth medium (Figure 3). This pigment production was increased when the NaCl concentration reached up to 100 g/L of NaCl. Assays with higher salt concentration (medium with up to 150 g/L of NaCl) were done but the microorganism did not grow (data not shown).
FIGURE 3.

Effect of salinity on the pigmentation of Vibrio sp. Cells were previously adapted to grow in presence of different salt concentration and temperatures, showing non-accumulation of pigment in absence of NaCl.
Effect of Salinity on Motility of Vibrio sp.
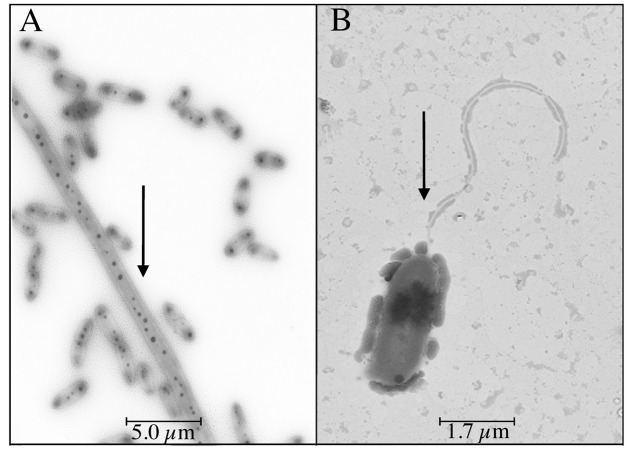
Vibrio sp. showed swimming and swarming motility, which were salt concentration dependent, as shown in Figure 4. Both swimming and swarming motility were observed in cultures amended with NaCl from 0 to 100 g/L NaCl; however, the highest motility radio was observed at 25 g/L of NaCl. Motility at higher NaCl concentration (150 g/L of NaCl) was not seen and a filament structure was detected (data not shown). Finally, TEM image revealed that Vibrio sp. cells grown in the presence of NaCl presented a single polar flagellum, and cells grown in absence of NaCl evidenced the presence of dense granules, presumably polyphosphate granules (Seufferheld et al., 2008) (Figure 5; Supplementary Figure 9). In addition, preliminary information suggested that motility and prodigiosin production might be specifically chloride-dependent (Supplementary Table 7).
FIGURE 4.
Swimming and swarming motilities of Vibrio sp. The microorganism was adapted to grow with 0 g/L NaCl (A,C) and 25 g/L NaCl (B,D). Cells were inoculated in their respective salts concentration in the swim (A,B) and swarm (C,D) plates and photographed after 72 h of incubation at 30°C. The arrows and white circles highlight the single colonies in absence of NaCl.
FIGURE 5.
NaCl-dependent flagellum in Vibrio sp. Cells were grown in 0 g/L (A) and 25 g/L (B) NaCl and analyzed by transmission electron microscopy. Arrows indicate the presence of dense granules and flagella, respectively.
Effect of Prodigiosin in the DNA Cleavage
As can be seen from the agarose gel depicted in Supplementary Figure 10, neither the pigment extract per se (lane 6) nor Cu II alone (lane 7) damage purified double-stranded plasmid DNA of the isolate (lane 3). In contrast, a combination of both is very effective. The progress of strand cleavage caused by the complex with increasing incubation time is depicted in lanes 8 ± 12. It is clearly visible that the most relaxed form, which is not evidenced in the controls, constantly gains intensity at the expense of the supercoiled form (band 5).
Discussion
The characterization of the physiology and morphology changes of Vibrio sp. isolated from Salar de Atacama are reported, especially physiological and morphological changes induced by osmotic stress. Very little is known about the presence of microorganisms from the genus Vibrio in Chilean salt lakes (Prado et al., 1991). For instance, Prado et al. (1991) isolated 161 moderately halophilic Gram-negative bacteria (including V. costicola). Our microorganism Vibrio sp. has 97% 16S rRNA similarity with V. ruber DSM 14379 (Danevcic, 2014) and 98% with V. ruber VR1 (Shieh, 2003). Supplementary Figure 1 shows the phylogeny of Vibrio sp. (Teb5a1 strain) and suggest that it is a novel species of the genus Vibrio with 97–98% of 16S rRNA gene sequence similarity with the other members. All three (Vibrio sp. Teb5a1, V. ruber DSM 14379 and V. ruber VR1) are able to grow in a broad range of NaCl concentrations. However, Vibrio sp. (Teb5a1 strain) can also grow in the absence of NaCl; as a consequence, it must be characterized as halotolerant. Vibrio sp. (Teb5a1 strain) was also adapted and able to grow in a wider range of temperature (Supplementary Table 1; Supplementary Figure 4; Figure 2), additionally indicating that it is a psychrotolerant strain.
Furthermore, a relationship between temperature and salinity stress parameters (Figure 2; Supplementary Figure 4) was observed, resembling the relationship that has been described in several marine bacterial species within the genus Vibrio and Salinivibrio (Adams and Russell, 1992; Ventosa et al., 1998). This resemblance could explain a linking between physiological, ecological and evolutionary aspects in determining their niche shapes (Materna et al., 2012). Moreover, the coupling between temperature and salinity tolerance might be explained by the effect of chaotropic ions (like chloride) that may counter the macromolecular rigidification induced by low temperature (Chin et al., 2010). Then, it can be assumed that the effect produced by chaotropic agents (like chloride) increases the chance of Vibrio sp. to survive in salty environments with high daily temperature swings (Supplementary Table 5). In addition, the relationship between optimum salt concentration and growth temperature in S. costicola has been proposed to be due to their effects on membrane lipid phase stability at temperatures below the optimum (Adams and Russell, 1992).
Moreover, a red color pigment was observed in Vibrio sp. as the result of prodiginine (prodigiosin) accumulation when the medium was amended with 25 g/L of NaCl (Figure 3). Purification and characterization of the red pigment by LC-MS/MS confirmed that it corresponds to prodigiosin and prodigiosin-like compounds (more than 15 different species were identified, as shown in Supplementary Table 6). Unlike to our strain Vibrio sp., fewer compounds of prodigiosin or its derivatives have been identified in a single microorganism in previous reports. For instance, Kim et al. (2008) reported in three main constituents in Hahella chejuensis that were identified by LC-MS/MS: prodigiosin, norprodigiosin, and undecylprodiginine. Besides, they also reported four prodigiosin analogs but in small quantities: 2-methyl-3-propyl-prodiginine, 2-methyl-3-butyl-prodiginine, 2-methyl-3-hexyl-prodiginine, and 2-methyl-3-heptyl-prodiginine (Kim et al., 2008). Tsao et al. (1985) identified some derivatives of prodigiosin by UV, MS, and HNMR in Streptomyces coelicolor A3(2), like undecylprodigiosin, butylcycloheptylprodiginine, and metacycloprodigiosin.
Additionally, prodiginine accumulation in Vibrio sp. showed temperature dependency, being the optimum temperature 25°C. However, the ideal temperature for the microbial production of this particular pigment cannot be generalized. For instance, Starič et al. (2010) reported that V. ruber DSM 14379 did not produce pigmentation at low (<15°C) and high temperatures (>43°C). Casullo de Araújo et al. (2010) reported that high temperatures (over 30°C) affected the prodigiosin production in S. marcescens UCP 1549, and the ideal temperature for pigment production was 28°C. They reported that the activity of one or more enzymes involved in prodigiosin synthesis is affected at high temperatures (Casullo de Araújo et al., 2010). Williams et al. (1971) defined 27°C as the optimum temperature for prodigiosin production in S. marcescens, and that the pigment is synthesized during the stationary phase of growth. Further, they determined protein production and reported that, when bacteria were incubated at 38°C, prodigiosin was not produced, and fewer proteins were formed during the incubation period (Williams et al., 1971). Thus, similar to these previous reports, the prodigiosin biosynthesis in Vibrio sp. is affected by high or low temperatures. At 17°C and over 40°C the pigmentation production is already affected. However, in this study, it was not determined which enzyme or protein involved in the biosynthetic pathway is inactivated or perhaps degraded at different growth conditions. Five proteins comprise the prodigiosin biosynthetic pathway: PigA, G, H, I, and J (Garneau-Tsodikova et al., 2006). Quantification of these proteins at different growth temperatures would give a notion of the changes produced by temperature in the prodigiosin synthesis.
Furthermore, preliminary results suggest that the prodigiosin production might be specifically chloride-dependent in Vibrio sp. (Supplementary Table 7). Strict chloride dependence of growth or significant stimulation was first reported for H. halophilus (Claus et al., 1983; Roeßler and Muller, 1998) and then it has also been described in other microorganisms including V. fischeri (Roeßler et al., 2003), at high salt concentrations. Interestingly, germination of endospores, activation of compatible solute transporters, flagella production and motility are among the specific physiological processes described as chloride-dependent (Dohrmann and Muller, 1999; Roeßler et al., 2000; Averhoff and Müller, 2010). Further, Roeßler and Muller (2002) found that chloride ions stimulate fliC gene expression, which encodes the major subunit of the flagellum, and regulates the protein FliC synthesis in H. halophilus. In addition, the accumulation of both chloride and compatible solutes observed in the moderately halophilic chloride-dependent H. halophilus to cope with elevated salinities has been proposed as a hybrid strategy that would represent an intermediate step in the evolution of salt adaptation (Saum et al., 2013). Those reports reinforce the suggestion that Vibrio sp. grown only in NaCl-based medium (not in NaBr or Na2SO4) produces the pigment as has been preliminary observed (Supplementary Table 7).
Besides, the morphology of Vibrio sp. was also affected by NaCl concentration (Figure 5). Flagellation dependent on NaCl has been previously reported by Rubiano-Labrador et al. (2014, 2015), and Flagellin protein (among others) was identified by mass spectrometry in Tislia consotensis grown in 4% of NaCl, but it was not observed at 0.5% NaCl. This protein is the evidence of bacterial flagella and it forms helical chains around the hollow core of the flagellar filament (Rubiano-Labrador et al., 2014, 2015).
In addition, the motility of Vibrio sp. was also affected by the presence of NaCl (Figure 4) and, perhaps, specifically by chloride ions (data not shown). Halang et al. (2013) reported that in V. cholerae chloride ions act as chaotropic agents and enhance cell motility. For instance, the nano-machine flagellum is composed of several subunits, MotA and MotB in H+-dependent, or PomA and PomB in Na+-dependent flagella (Halang et al., 2013). Chloride ions disrupt hydrogen bonds between water molecules that interact with PomB protein, specifically with D23 and S26 residues. This disruption facilitates the access of sodium ions to PomA and PomB proteins and, then, the flagellum can rotate and the bacteria present motility (Halang et al., 2013). Furthermore, it has been reported that the Vibrio motility greatly influences the infectivity of V. cholera (Butler and Camilli, 2004) and, on the contrary, reduced motility is induced by starvation in V. vulnificus (Chen and Chen, 2014). In both cases, the change means a competitive advantage.
The preliminary confirmation of the ability of the pigment produced by Vibrio sp. to damage double-stranded DNA in the presence of Cu II cations has allowed us to infer the ecological significance of this adaptation. The oxidative cleavage of dsDNA to ssDNA by prodigiosin in presence of copper has been reported before (Melvin et al., 2000, 2002). The exact mechanism of this oxidative cleavage is still not clear, but it is known that the cytotoxic activity is mediated by oxidation of the electron-rich polypyrrole molecule (Melvin et al., 2002). Moreover, Darshan and Manonmani (2015) reviewed that Cu-promoted strand cleavage by prodigiosin (CuProd) induced apoptosis of cancer cells, since they presented higher concentration of Cu(II) than normal cells. Taking into account that the copper concentration available in the ecological niche (Supplementary Table 2) of Vibrio sp. might fulfill the level required, it can be hypothesized that the double-strand cleavage of DNA by prodigiosin would be an additional mechanism to survive and compete in a diverse microbial community. In addition, the mode of action of prodigiosin as antimicrobial in Vibrio sp. might be also related to its hydrophobicity as has been already reported (Suryawanshi et al., 2015). In that scenario, the knowledge that was obtained about the conditions that promote the prodiginine accumulation by the isolate will be very useful, and more studies would be required to describe in detail the ecological reason of this intriguing characteristic.
The appearance of a red pigmentation was observed only in Site 2 (Supplementary Table 2) of Laguna Tebenquiche in the same sampling campaign when Vibrio sp. was isolated. The comparison of the physicochemical parameters of the pigmented and non-pigmented sites at Laguna Tebenquiche, at the same and different campaigns (Supplementary Table 5) evidenced that the chloride concentration, the temperature and the pH of the pigmented brine were in the prodigiosin production range observed and/or reported before (Wei et al., 2005). The occurrence of Vibrio in athalasohaline lakes represents another example of phylogenetically close and metabolically similar microorganisms that can be found in rather different environments (Tamames et al., 2016).
The results allow us to conclude that environmental congruence might explain the cellular phenotypes observed in the halo- and psychro-tolerant Vibrio sp. (Teb5a1 strain): (i) the environmental conditions where the strain was found agreed well with the strain temperature and salinity tolerance; (ii) the notable temperature range for the growth and the coupling between temperature and salinity tolerance increases the chance of Vibrio sp. to survive in an extreme environment with high daily temperature swings encountered at Salar de Atacama (average minimum 2.3°C to average maximum 35°C); (iii) the chloride and temperature dependent production of prodigiosin would be also a mechanism used by Vibrio sp. to compete and to survive inside a microbial community in an extreme environment characterized by a high UV radiation as well; (iv) flagellation and motility would further enhance the competitive of Vibrio sp. over other members of the microbial community. Finally, considering the diverse biotechnological potential of prodigiosin reported in several microorganisms, like photoprotectant in sunscreens, immunosuppressive activity, antibacterial, antimalarial and anticancerous properties, food colorant, and as colorant for polyolefines (Darshan and Manonmani, 2015), our study of the microbial ecology of this pigment in Vibrio sp. can be a first step to explore its diverse biotechnological potential in the future.
Author Contributions
KG, FR, JC, and CD designed the research experiments; KG and JC conducted the experiments; LE performed the phylogenetic analysis of the isolates; KG and CD analyzed the results and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We also acknowledge M. V. Coalova for language improvements.
Abbreviations
- 13C NMR
carbon-13 nuclear magnetic resonance
- 1H NMR
proton nuclear magnetic resonance
- ESI-ITMS
electrospray ionization-ion trap mass spectrometer
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- TEM
transmission electron microscopy
- TLC
thin layer chromatography.
Footnotes
Funding. We gratefully acknowledge financial support from Proyecto DGIP of Universidad Católica del Norte, from Proyecto CONADI FPCR.005-2007, Proyecto PBCT Bicentenario IPC-81, from Minera Escondida research project and from the scholarship to KG from Conicyt, Chile.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01943/full#supplementary-material
References
- Adams R. L., Russell N. J. (1992). Interactive effects of salt concentration and temperature on growth and lipid composition in the moderately halophilic bacterium Vibrio costicola. Can. J. Microbiol. 38 823–827. 10.1139/m92-134 [DOI] [PubMed] [Google Scholar]
- Alihosseini F., Ju K.-S., Lango J., Hammock B. D., Sun G. (2008). Antibacterial colorants: characterization of prodiginines and their applications on textile materials. Biotechnol. Prog. 24 742–747. 10.1021/bp070481r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. R., Reichelt J. L., Gray P. P. (1983). Influence of environmental factors and medium composition on Vibrio gazogenes growth and prodigiosin production. Appl. Environ. Microbiol. 45 1727–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arivizhivendhan K. V., Mahesh M., Regina Mary R., Sekaran G. (2015). Bioactive prodigiosin isolated from Serratia marcescens using solid state fermenter and its bactericidal activity compared with conventional antibiotics. J. Microb. Biochem. Technol. 7 305–312. 10.4172/1948-5948.1000230 [DOI] [Google Scholar]
- Averhoff B., Müller V. (2010). Exploring research frontiers in microbiology: recent advances in halophilic and thermophilic extremophiles. Res. Microbiol. 161 506–514. 10.1016/j.resmic.2010.05.006 [DOI] [PubMed] [Google Scholar]
- Borić M., Danevćić T., Stopar D. (2011). Prodigiosin from Vibrio sp. DSM 14379; a new UV-protective pigment. Microb. Ecol. 62 528–536. 10.1007/s00248-011-9857-0 [DOI] [PubMed] [Google Scholar]
- Borić M., Danevćić T., Stopar D. (2012). Viscosity dictates metabolic activity of Vibrio ruber. Front. Microbiol. 3:255 10.3389/fmicb.2012.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger S. R., Bennett J. W. (1985). Droplet enrichment factors of pigmented and nonpigmented Serratia marcescens: possible selective function for prodigiosin. Appl. Environ. Microbiol. 50 487–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler S. M., Camilli A. (2004). Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 101 5018–5023. 10.1073/pnas.0308052101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos V. (1993). Microorganismos de Ambientes Extremos Salar de Atacama. Chile. in II Simposio Internacional de Estudios Altiplánicos. Available at: http://mazinger.sisib.uchile.cl/repositorio/lb/ciencias_veterinarias_y_pecuarias/simposio1993/03areaecosistema.html/27.html [Google Scholar]
- Cang S., Sanada M., Johdo O., Ohta S., Nagamatsu Y., Yoshimoto A. (2000). High production of prodigiosin by Serratia marcescens grown on ethanol. Biotechnol. Lett. 22 1761–1765. 10.1023/A:1005646102723 [DOI] [Google Scholar]
- Casullo de Araújo H. W., Fukushima K., Takaki G. M. C. (2010). Prodigiosin production by Serratia marcescens UCP 1549 using renewable-resources as a low cost substrate. Molecules 15 6931–6940. 10.3390/molecules15106931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Chen C.-Y. (2014). Starvation induces phenotypic diversification and convergent evolution in Vibrio vulnificus. PLoS ONE 9:e88658 10.1371/journal.pone.0088658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. P., Megaw J., Magill C. L., Nowotarski K., Williams J. P., Bhaganna P., et al. (2010). Solutes determine the temperature windows for microbial survival and growth. Proc. Natl. Acad. Sci.U.S.A. 107 7835–7840. 10.1073/pnas.1000557107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus D., Fahmy F., Rolf H. J., Tosunoglu N. (1983). Sporosarcina halophila sp. nov., an obligate, slightly halophilic bacterium from salt marsh soils. Syst. Appl. Microbiol. 4 496–506. 10.1016/S0723-2020(83)80007-1 [DOI] [PubMed] [Google Scholar]
- Danevcic T., Borić M. (2014). Microbial ecophysiology of Vibrio ruber. Food Technol. Biotechnol. 9862 198–203. [Google Scholar]
- Danevcic T., Rilfors L., Strancar J., Lindblom G., Stopar D. (2005). Effects of lipid composition on the membrane activity and lipid phase behaviour of Vibrio sp. DSM14379 cells grown at various NaCl concentrations. Biochim. Biophys. Acta 1712 1–8. 10.1016/j.bbamem.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Danevčič T., Stopar D. (2011). Asymmetric response of carbon metabolism at high and low salt stress in Vibrio sp. DSM14379. Microb. Ecol. 62 198–204. 10.1007/s00248-011-9870-3 [DOI] [PubMed] [Google Scholar]
- Darshan N., Manonmani H. K. (2015). Prodigiosin and its potential applications. J. Food Sci. Technol. 52 5393–5407. 10.1007/s13197-015-1740-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Médicis E., Rossignol B. (1979). The halophilic properties of pyruvate kinase from Vibrio costicola, a moderate halophile. Experientia 35 1546–1548. 10.1007/BF01953183 [DOI] [PubMed] [Google Scholar]
- Demergasso C., Casamayor E. O., Chong G., Galleguillos P., Escudero L., Pedrós-Alió C. (2004). Distribution of prokaryotic genetic diversity in athalassohaline lakes of the Atacama Desert, Northern Chile. FEMS Microbiol. Ecol. 48 57–69. 10.1016/j.femsec.2003.12.013 [DOI] [PubMed] [Google Scholar]
- Díaz R. I. S., Regourd J., Santacroce P. V., Davis J. T., Jakeman D. L., Thompson A. (2007). Chloride anion transport and copper-mediated DNA cleavage by C-ring functionalized prodigiosenes. Chem. Commun. 26 2701–2703. 10.1039/B701919J [DOI] [PubMed] [Google Scholar]
- Dohrmann A. B., Muller V. V. (1999). Chloride dependence of endospore germination in Halobacillus halophilus. Arch. Microbiol. 172 264–267. 10.1007/s002030050769 [DOI] [PubMed] [Google Scholar]
- Fürstner A. (2003). Chemistry and biology of Roseophilin and the prodigiosin alkaloids: a survey of the Last 2500 years. Angew. Chem. Int. Ed. Engl. 42 3582–3603. 10.1002/anie.200300582 [DOI] [PubMed] [Google Scholar]
- Garcia M. T., Ventosa A., Ruiz-Berraquero F., Kocur M. (1987). Taxonomic study and amended description of Vibrio costicola. Int. J. Syst. Bacteriol. 37 251–256. 10.1099/00207713-37-3-251 [DOI] [Google Scholar]
- Garneau-Tsodikova S., Dorrestein P. C., Kelleher N. L., Walsh C. T. (2006). Protein assembly line components in prodigiosin biosynthesis: characterization of PigA,G,H,I,J. J. Am. Chem. Soc. 128 12600–12601. 10.1021/ja063611l [DOI] [PubMed] [Google Scholar]
- Gerber N. N. (1969). Prodigiosin-like pigments from Actinomadura (Nocardia) pelletieri and Actinomadura madurae. Appl. Microbiol. 18 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H., Jensen T. E. (1998). Nickel sequestering by polyphosphate bodies in Staphylococcus aureus. Microbios 93 179–185. [PubMed] [Google Scholar]
- Haddix P. L., Jones S., Patel P., Burnham S., Knights K., Powell J. N., et al. (2008). Kinetic analysis of growth rate, ATP, and pigmentation suggests an energy-spilling function for the pigment prodigiosin of Serratia marcescens. J. Bacteriol. 190 7453–7463. 10.1128/JB.00909-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halang P., Leptihn S., Meier T., Vorburger T., Steuber J. (2013). The function of the Na+-driven flagellum of Vibrio cholerae is determined by osmolality and pH. J. Bacteriol. 195 4888–4899. 10.1128/JB.00353-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna K. I. M. (1984). The effect of salt concentration on the phospholipid and fatty acid composition of the moderate halophile Vibrio costicola. Can. J. Microbiol. 30 669–675. 10.1139/m84-100 [DOI] [Google Scholar]
- Kamekura M., Kushner D. J. (1984). In vitro protein synthesis by the moderate halophile Vibrio costicola: site of action of Cl- ions. J. Bacteriol 160 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Kim J. F., Yim J. H., Kwon S.-K., Lee C. H., Lee H. K. (2008). Red to red - the marine bacterium Hahella chejuensis and its product prodigiosin for mitigation of harmful algal blooms. J. Microbiol. Biotechnol 18 1621–1629. [PubMed] [Google Scholar]
- Kirishna S., Kumar B. S., Singaracharya M. A., Prakasham R. S. (2014). Process optimization of red pigment production from Vibrio sp isolated from marine source. Octa J. Biosci. 2 79–85. [Google Scholar]
- Lapenda J. C., Silva P. A., Vicalvi M. C., Sena K. X. F. R., Nascimento S. C. (2015). Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World J. Microbiol. Biotechnol. 31 399–406. 10.1007/s11274-014-1793-y [DOI] [PubMed] [Google Scholar]
- Lucena T., Ruvira M. A., Arahal D. R., Macián M. C., Pujalte M. J. (2012). Vibrio aestivus sp. nov. and Vibrio quintilis sp. nov., related to Marisflavi and Gazogenes clades, respectively. Syst. Appl. Microbiol. 35 427–431. 10.1016/j.syapm.2012.08.002 [DOI] [PubMed] [Google Scholar]
- Materna A. C., Friedman J., Bauer C., David C., Chen S., Huang I. B., et al. (2012). Shape and evolution of the fundamental niche in marine Vibrio. ISME J. 6 2168–2177. 10.1038/ismej.2012.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin M. S., Calcutt M. W., Noftle R. E., Manderville R. A. (2002). Influence of the a-ring on the redox and nuclease properties of the prodigiosins: importance of the bipyrrole moiety in oxidative DNA cleavage. Chem. Res. Toxicol. 15 742–748. 10.1021/tx025508p [DOI] [PubMed] [Google Scholar]
- Melvin M. S., Tomlinson J. T., Saluta G. R., Kucera G. L., Lindquist N., Manderville R. A., et al. (2000). Double-strand DNA cleavage by copper Prodigiosin. J. Am. Chem. Soc. 122 6333–6334. 10.1021/ja0000798 [DOI] [Google Scholar]
- Monteoliva-Sánchez M., Aguilera M., Ramos-Cormenzana A., Suárez-García A., Campos V., Roselló-Mora R., et al. (2002). Halorubrum tebenquichense sp. nov., a novel halophilic archaeon isolated from the atacama saltern. Chile. Int. J. Syst. Evol. Microbiol. 52 149–155. 10.1099/00207713-52-1-149 [DOI] [PubMed] [Google Scholar]
- Müller V., Oren A. (2003). Metabolism of chloride in halophilic prokaryotes. Extremophiles 7 261–266. 10.1007/s00792-003-0332-9 [DOI] [PubMed] [Google Scholar]
- Oren A. (2013). “Life at high salt concentrations,” in The Prokaryotes, eds Rosenberg E., E. F. DeLong, S. Lory, E. Stackebrandt, and F. Thompson (Berlin: Springer; ), 10.1007/978-3-642-30123-0 [DOI] [Google Scholar]
- Prado B., Del Moral A., Quesada E., Ríos R., Monteoliva-Sanchez M., Campos V., et al. (1991). Numerical taxonomy of moderately halophilic gram-negative rods isolated from the salar de atacama, chile. Syst. Appl. Microbiol. 14 275–281. 10.1016/S0723-2020(11)80381-4 [DOI] [Google Scholar]
- Roeßler M., Muller V. V. (1998). Quantitative and physiological analyses of chloride dependence of growth of halobacillus halophilus. Appl. Environ. Microbiol. 64 3813–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeßler M., Muller V. (2002). Chloride, a new environmental signal molecule involved in gene regulation in a moderately halophilic bacterium, halobacillus halophilus. J. Bacteriol. 184 6207–6215. 10.1128/JB.184.22.6207-6215.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeßler M., Sewald X., Müller V. (2003). Chloride dependence of growth in bacteria. FEMS Microbiol. Lett. 225 161–165. 10.1016/S0378-1097(03)00509-3 [DOI] [PubMed] [Google Scholar]
- Roeßler M., Wanner G., Müller V. (2000). Motility and flagellum synthesis in Halobacillus halophilus are chloride dependent. J. Bacteriol. 182 532–535. 10.1128/JB.182.2.532-535.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubiano-Labrador C., Bland C., Miotello G., Armengaud J., Baena S. (2015). Salt stress induced changes in the exoproteome of the halotolerant bacterium tistlia consotensis deciphered by proteogenomics. PLoS ONE 10:e0135065 10.1371/journal.pone.0135065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubiano-Labrador C., Bland C., Miotello G., Guérin P., Pible O., Baena S., et al. (2014). Proteogenomic insights into salt tolerance by a halotolerant alpha-proteobacterium isolated from an Andean saline spring. J. Proteomics 97 36–47. 10.1016/j.jprot.2013.05.020 [DOI] [PubMed] [Google Scholar]
- Ryazantseva I. N., Andreyeva I. N., Klementyeva G. S., Ogorodnikova T. I., Petrov V. Y. (1995). Pigment-dependent light influence on the energetics of Serratia marcescens. Thermochim. Acta 251 63–67. 10.1016/0040-6031(94)02148-H [DOI] [Google Scholar]
- Sandle T., Skinner K. (2013). Study of psychrophilic and psychrotolerant micro-organisms isolated in cold rooms used for pharmaceutical processing. J. Appl. Microbiol. 114 1166–1174. 10.1111/jam.12101 [DOI] [PubMed] [Google Scholar]
- Saum S. H., Pfeiffer F., Palm P., Rampp M., Schuster S. C., Müller V., et al. (2013). Chloride and organic osmolytes: a hybrid strategy to cope with elevated salinities by the moderately halophilic, chloride-dependent bacterium Halobacillus halophilus. Environ. Microbiol. 15 1619–1633. 10.1111/j.1462-2920.2012.02770.x [DOI] [PubMed] [Google Scholar]
- Seganish J. L., Davis J. T. (2005). Prodigiosin is a chloride carrier that can function as an anion exchanger. Chem. Commun. 46 5781–5783. 10.1039/b511847f [DOI] [PubMed] [Google Scholar]
- Seufferheld M. J., Alvarez H. M., Farias M. E. (2008). Role of polyphosphates in microbial adaptation to extreme environments. Appl. Environ. Microbiol. 74 5867–5874. 10.1128/AEM.00501-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh W. Y. (2003). Vibrio ruber sp. nov., a red, facultatively anaerobic, marine bacterium isolated from sea water. Int. J. Syst. Evol. Microbiol. 53 479–484. 10.1099/ijs.0.02307-0 [DOI] [PubMed] [Google Scholar]
- Silverman M. P., Munoz E. F. (1973). Effect of iron and salt on prodigiosin synthesis in Serratia marcescens. J. Bacteriol. 114 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.-J., Bae J., Lee D.-S., Kim C.-H., Kim J.-S., Kim S.-W., et al. (2006). Purification and characterization of prodigiosin produced by integrated bioreactor from Serratia sp. KH-95. J. Biosci. Bioeng. 101 157–161. 10.1263/jbb.101.157 [DOI] [PubMed] [Google Scholar]
- Starič N., Danevčič T., Stopar D. (2010). Vibrio sp. DSM 14379 pigment production–A competitive advantage in the environment? Microb. Ecol. 60 592–598. 10.1007/s00248-010-9671-0 [DOI] [PubMed] [Google Scholar]
- Suryawanshi R. K., Patil C. D., Borase H. P., Narkhede C. P., Stevenson A., Hallsworth J. E., et al. (2015). Towards an understanding of bacterial metabolites prodigiosin and violacein and their potential for use in commercial sunscreens. Int. J. Cosmet. Sci. 37 98–107. 10.1111/ics.12175 [DOI] [PubMed] [Google Scholar]
- Tamames J., Sánchez P. D., Nikel P. I., Pedrós-Alió C. (2016). Quantifying the relative importance of phylogeny and environmental preferences as drivers of gene content in prokaryotic microorganisms. Front. Microbiol. 7:433 10.3389/fmicb.2016.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao S. W., Rudd B. A., He X. G., Chang C. J., Floss H. G. (1985). Identification of a red pigment from Streptomyces coelicolor A3(2) as a mixture of prodigiosin derivatives. J. Antibiot. (Tokyo). 38 128–131. 10.7164/antibiotics.38.128 [DOI] [PubMed] [Google Scholar]
- Venil C. K. (2009). An insightful overview on microbial pigment. Prodigiosin. Electron. J. Biol. 5 49–61. [Google Scholar]
- Ventosa A., Nieto J. J., Oren A. (1998). Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 62 504–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.-H., Yu W.-J., Chen W.-C. (2005). Enhanced undecylprodigiosin production from Serratia marcescens SS-1 by medium formulation and amino-acid supplementation. J. Biosci. Bioeng. 100 466–471. 10.1263/jbb.100.466 [DOI] [PubMed] [Google Scholar]
- Williams R. P., Gott C. L., Qadri S. M., Scott R. H. (1971). Influence of temperature of incubation and type of growth medium on pigmentation in Serratia marcescens. J. Bacteriol. 106 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Cui S., Nagata S. (2008). Isolation and characterization of salt-sensitive mutants of the moderately halophilic bacterium Salinivibrio costicola subsp. yaniae. Biosci. Biotechnol. Biochem. 72 1977–1982. 10.1271/bbb.70652 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.