Abstract
Extracellular vesicles, such as exosomes, are newly recognized intercellular conveyors of functional molecular mechanisms. Notably, they transfer RNAs and proteins between cells in general, that then can participate, as described herein, in the complex pathogenesis of allergic and related hypersensitivity responses and disease mechanisms. This review highlights this important new appreciation of the in vivo participation of such extracellular vesicles in the interactions between allergy-mediating cells, taking into account paracrine epigenetic exchanges mediated by surrounding stromal cells and the endocrine receipt of exosomes from distant cells via the circulation.
Exosomes are natural ancient nanoparticles of life. They are made by all cells and in some form by all species down to fungi and bacteria, and are present in all fluids. Besides a new focus on their role in the transmission of genetic regulation, exosome transfer of allergens was recently shown to induce allergic inflammation. Importantly, regulatory and tolerogenic exosomes can potently inhibit allergy and hypersensitivity responses, usually acting non-specifically, but also can proceed in an antigen-specific manner due to coating of the exosome surface with antibodies.
Deep analysis of processes mediated by exosomes should result in development of early diagnostic biomarkers, as well as allergen-specific, preventive and therapeutic strategies. These likely will significantly diminish the risks of current allergen specific parenteral desensitization procedures, and of the use of systemic immunosuppressive drugs. Since extracellular vesicles are physiological, they can be fashioned for specific delivery of therapeutic molecular instructions through easily tolerated, non-invasive routes, such as oral ingestion, nasal administration, and perhaps even inhalation.
Keywords: extracellular vesicles, exosomes, allergy, asthma, delayed-type hypersensitivity, contact hypersensitivity, contact dermatitis, immunosuppression, immunoregulation
Introduction
Exosomes are exciting newly recognized extracellular vesicles that transfer a variety of bioactive compounds, including RNA molecules, proteins and lipids, between nearby cells in a paracrine manner, such as at synapses. This has been exquisitely shown at the central immunological synapse between T cells and antigen (Ag) presenting cells (APC) [1]. Furthermore, locally secreted exosomes can then enter the vasculature to act systemically on distant cells in an endocrine manner. We have demonstrated this recently for splenic and lymph node-derived T suppressor (Ts) cell secreted suppressive exosomes regulating the interface of APC and effector T cells at the peripheral tissue site of an immune inflammatory response [2]. Morphologically, exosomes are spherical vesicles with a bilamellar membrane that are 50–200 nanometers in diameter depending on the source and activation state of the donor cell [2]. They are formed by budding from the wall of terminal endosomes to then accumulate at the intracellular periphery in the multivesicular body (MVB). When the MVB undergoes exocytosis, there is the release of the contained bunches of exosomes into the extracellular space [3,4]. Alternatively, microvesicles bud individually from the cell surface, are larger (200–1000 nanometers) and mediate similar mechanisms with great overlap in the characteristics of exosomes. Together they are called extracellular vesicles. Study of exosomes is a new field concerned with very large numbers of very tiny vesicles, for which there are yet no specially designed machines for their particular analysis. According to current experimental observations, there seems to be billions of exosomes per milliliter of blood, that is a thousand times greater than the number of white blood cells. Moreover, there is a great variability of vesicle types with many subtypes in exosome preparations [5]. There also is a significant variability in properties between individual exosomes, like their membrane content [6], their contained proteome and types of RNAs [7–9], with yet further variability due to the stage of maturity and activation of the cells generating the exosomes [2,10,11]. The proteome and RNAome of exosomes differ markedly from those from their donor cells, indicating active mechanisms of their production, sorting and loading as cargo.
Exosomes are natural and physiological ancient nanoparticles of life, since they are made by all cells, are present in all body fluids and are made in some form by all species down to and through fungi [4] and bacteria [12,13]. Accordingly, bacteria are well known to produce at their surface the microbial extracellular vesicles (mEV), formerly termed outer membrane vesicles (OMV), that enables transfer of contents consisting of enzymes and toxins [14] to regulate other bacteria. Further, mEV, like exosomes, are now known to contain RNAs [15,16]. Importantly, mEV can crucially influence host allergic immune responses. They have the ability to drive clinical diseases such as in atopic dermatitis, via the mEV from skin surface microorganisms commonly associated with this allergic disease, such as staphylococci [17] and fungi [18].
Exosomes offer cell-free therapy without the transfer of possibly oncogenic DNA, compared to whole nucleated cells. However, DNA as fragments has been recently demonstrated in exosomes from cancer cells [19–21]. Thus, in some instances the functions of cells can be replaced by their produced exosomes. A prominent example is that mesenchymal stem cell healing functions often can be completely replaced by their exosomes [22–25], which in some cases was shown to be due to identified exosome miRNA cargo [26,27]. A very important difference from cells is that exosomes are resistant to harsh conditions, like a pH of 3–4 [28,29], or even pH = 1 [30] encountered in the gastrointestinal (GI) tract, as well as hyperosmolarity due to expression of the water channel called aquaporin-1 [31], and hyperoxia [32] or hypoxia [33], when the exosome donor cells are stimulated under those conditions. Therefore, extracellular vesicles, such as exosomes from various sources, play a newly recognized, important and diverse role in physiological processes and pathological conditions relevant to diseases, such as those of allergy and hypersensitivities.
Exosome Transfer of Signaling Proteins and Genetically Active RNAs Pertinent to Allergy and Hypersensitivity
There is a well-recognized role of exosomes in allergy [34]. The outstanding new property that exosomes bring to allergic and other responses is the transfer between cells of functional genetic material and signaling proteins. Transferred regulatory RNAs can then epigenetically alter gene function in the acceptor cells [35–41], while transferred signaling proteins, like wnt, can alter their intracellular functions [42–45]. Accordingly, such transfers of genetic information have been shown for immune [46–50], hypersensitivity [2], IgE [51] and allergic asthmatic [52] responses. Such intercellular communication and consequent regulation at the genetic level is unprecedented in human physiology and disease processes. Therefore, this greatly widens the possibilities of a more complete understanding and more precise interventions in allergy and related immune mediated diseases.
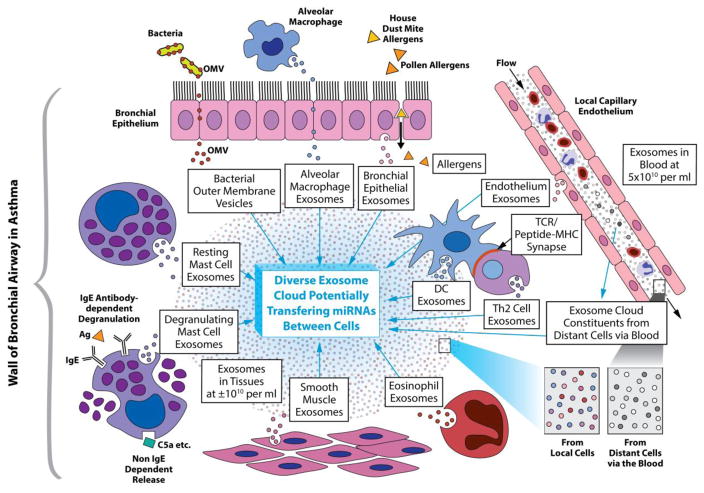
As noted, there is a great variability between exosomes, even those produced by the same cell [6], in their proteome [53] and RNAome. Importantly, functional mRNA and miRNAs can be a small percentage of the total exosome RNA [2,54,55]. However, functions of many of the miRNAs still have to be delineated. The remaining RNA cargo is usually made up of other classes of RNA, including long non-coding RNAs of unknown significance, along with numerous ribosomal RNA fragments and tRNAs, also of unknown functions [2,54,55]. Therefore, exosome exchange of molecules between cells is a sophisticated and potentially quite heterogeneous process of great complexity, with diverse functional consequences for mediation and modulation of cellular functions in immunity, allergy and hypersensitivity. On a wider level, the potential for exosome transfer of functional RNAs and signaling proteins from neighboring and distant cells of other systems, that have been thought to be independent of allergy and immunity, now should be seen as likely interacting with or affecting the cells mediating allergic and immunological responses. For instance, exosomes derived from various cell types of the microenvironment influence the established immune cell-cell interactions in allergy and immunity, and thus are now considered as important in actual in vivo circumstances. Fig. 1 shows how this might apply to interactions of the various immune and stromal tissue cell populations in the airways of asthma patients. The intercellular transfer of RNAs mediating epigenetic changes and exchange of signaling molecules, including transcription factors [56], or their regulators [57], makes this a powerful new source for a fuller understanding of allergy and hypersensitivity. Therefore, this should provide new diagnostic opportunities and therapeutic maneuvers to potentially intervene in allergic and immunological disease processes at entirely new levels.
Fig. 1. Postulated allergic exosome cloud in the airway of an asthmatic patient.
Proposed details of a postulated exosome cloud in the airways of asthmatic patients and other relevant tissues like the nasal mucosa. The tissue cloud consists of diverse exosomes derived from various cell sources that have a variety of cargos. The exosomes and other related extracellular vesicles in this cloud are postulated to be at a concentration of about 1010 vesicles per milliliter in the interstitial fluids between the various cells, shown as tiny spheres of different colors expanded at lower right. The extracellular vesicles from donor cells are able to transfer miRNAs, other RNAs, proteins etc. to other cells via the fluid between them to potentially alter the functions of the acceptor cells epigenetically.
These diverse intercellular transfers of genetic information can be mediated by exosome-derived miRNAs and is potentially able to alter the development, maturation, activation and importantly function of other cells of various types. Some of these exosomes of the local cloud likely leave this tissue site to enter another cloud in the draining lymph to affect distant cells, such as those in the bone marrow or the cells of other organs like immune cells in the spleen or lymph nodes, via entering another cloud in the systemic circulation. Those in the blood are a mixture from all or most of the cells in the body to potentially serve as accessible clinical markers of disease. The circulating exosomes are headed for all possible sites, including those from elsewhere to the bronchial airway in asthma, shown in the figure at the right, along with exosomes from the endothelium, (expanded at lower right) to enter this site to join the local cloud to potentially exert effects on its constituent cells, thus in an endocrine manner.
Note that the local cellular interactions not only involve the usual cross talk between lymphoid T and B cells and antigen presenting DC (some in immunological synapse, mid central right of the figure) and macrophages, with other myeloid-derived cells like eosinophils and mast cells. Very importantly, these immune and myeloid cells interact via mutual released exosomes with other local cells of the microenvironment, like bronchial epithelium, smooth muscle cells, as well as fibroblasts and other cells of the stroma that lie between all of the above cells. Further note that constituent cells are sending out exosomes at base line for physiological interactions and then other “activtated” exosomes when the cells are stimulated like the mast cells (at the left) responding to inhaled allergen (top right) or C5a, or cells responding to microbial extracellular vesicles from bacteria in the airways (top left). These newly recognized intercellular genetic interactions mediated by diverse exosomes in an intercellular cloud are present in addition to the already known exchanges based on conventional cytokines and their receptors, along with “micro-mediators”, like histamine, leukotrienes and C5a.
Effects of Exosome Intercellular Interactions on Allergy and Immunity
According to the above considerations, exosomes have recently been shown to exhibit great effects on immunological activities, as mediators involved in many stages of immune and inflammatory responses, such as induction, orchestration, elicitation, resolution and regulation. As an important example, T regulatory (Treg) Foxp3pos cells produce exosomes that suppress Th1 cell proliferation and cytokine production via gene silencing due to transferred Treg cell-derived miRNAs [58]. Further, thymic epithelial cells produce exosomes carrying tissue-restricted Ag that guide development of the Treg cells [59,60].
Signaling pathways also can be altered by either exosome transferred miRNA influencing translation by mRNA inhibition in the targeted cell, the transfer of mRNA to alter production of specific protein in the targeted cells, or exosome transfer of the proteins themselves. For instance, the signaling molecule wnt can be gained [44,45] or lost by cells via exosomes [42]. This protein can be expressed on the exosome surface and, thus, may potentially be transferred between cells in such a form [43]. Importantly, inflammation and immunity can also be influenced by transfer of exosome contained cytokines [61–64], their receptors [65], and signaling molecules [65], by stimulation of cytokine production via exosome-derived TLR agonists [61], or finally by cells activated by exosomes. This includes T cell vesicles activating mast cells [66,67] as well as exosome transfer of mRNA encoding mast cell cytokines [38].
Furthermore, endogenously induced, natural exosomes, or those modified ex vivo, can carry and transfer immunoregulation via miRNAs that might be altered for application in therapy of diverse allergic diseases, better control of dysregulated immune responses, as well as for influencing hypersensitivity mechanisms that underlie clinical disease processes. With the growing prevalence of allergies, hypersensitivities and diseases with immune mediated manifestations, this newly recognized function mediated by previously unknown exosome intercellular transfer of epigenetic regulation joins the currently unraveled genetic and molecular mechanisms underlying their complex pathogenesis. Many other aspects of these disorders remain to be clarified by further and deeper investigation and then translation to clinical understanding and new therapies, and these also seem to depend on exosome transfer of gene regulation and cell signaling. This current review particularly highlights the involvement of these processes in allergic and immunological diseases, that have recently have shown increasing prevalence and severity. A revealing example relevant to allergy concerns CD23pos (Fc-epsilonR-2pos) B cells that capture immune complexes consisting of IgE and allergen as Ag to then produce exosomes (B cell exosomes called “bexosomes”) carrying CD23, IgE and MHC class II, that are transferred to dendritic cells (DC) that in turn stimulate Ag-specific T cells [51]. This suggests that “bexosomes” can provide the essential transfer mechanism for IgE-Ag complexes from B cells to DC for subsequent activation of allergen-specific T cells. In this case, anti-IgE or rituximab (anti-CD20) therapy should block this Other examples of exosome-mediated intercellular influences in immunity, hypersensitivity and allergy, include: a. helper T cell augmentation of B cell production of HLApos exosomes [11], b. Ts [2] and Treg [58] cell-derived exosome inhibition of effector Th1 cells, c. thymic epithelial cell-derived, Ag carrying exosome mediated maturation of Treg cells [59,60], d. DC activation of other DC and also B cells [50], e. T cell activation of APC and DC [49], and, the opposite, f. DC-derived exosomes stimulating CD4pos T cells [68]. Further, DC can be guided by exosomes with MHC class II and co-stimulatory molecules present in human BALF [69], and by mast cell-derived exosomes inducing phenotypic and functional maturation of DC to elicit specific immune responses in vivo [70].
Exosome mediated intercellular interactions involving mast cells are particularly relevant to allergy. These include: T cell exosome activation of mast cells [66,67], and mast cell activation of T and B cells [71,72], mast cell exosome-mediated phenotypic and functional maturation of DC [61,62] and endothelial cells [73], or other mast cells or progenitor stem cells [74]. These effects can be induced via exosome transmitted cytokines [66,75] or mast cell exosome transfer of mRNA encoding the cytokines [37], and by affecting cytokine signaling [75] or triggering cytokine production by bronchial cells [76] or by airway smooth muscle cells [77]. Finally, mast cell exosomes induce phenotypic and functional maturation of DC enabling them to elicit specific immune responses in vivo [78], they activate endothelial cells to secrete clotting factors [73] and can activate T and B cells [79,80].
Taken together, such intercellular exchanges via exosomes occur in established processes dominant in classical allergic mechanisms, for instance in the release of traditional mast cell mediators, like histamine, bioactive peptides, leukotrienes and even cytokines. These traditional pro-allergic mediators affect surrounding cells in classical Type I immediate hypersensitivity. Additionally, target cells are likely modified by miRNAs and other elements transferred by the mast cell-derived exosomes to mediate epigenetic changes in these cells. Further, the releasing mast cells themselves are now seen as being affected by exosomes from surrounding and even distant cells.
Of additional relevance to mechanisms of allergic asthma, these patients have increased numbers of airway exosomes expressing MHC and co-stimulatory molecules that may play a similar role as APC [69,81]. Further, among the increased numbers of exosomes in airways of mice with a model of asthma, the bronchoepithelial cell-derived exosomes stimulated by IL-13 induce activation of macrophages [77], and exosomes play a role in Th2 cell activation of auxiliary cells [77,78], acting via specific cell surface cytokine receptors, such as IL-4 receptor. This seems to influence triggering of established intracellular signaling pathways that regulate gene expression of Th2 responses. Eosinophil-derived exosomes are also suspected to play a role in asthma pathogenesis [78]. Thus, it now must be considered that established allergic mast cell activation for mediator release, as well as conventional intercellular cytokine dominated pathways, are proceeding in parallel with, or more likely interact with donation and receipt of a variety of intracellular communications arising locally or from distant cells via the blood. Simultaneously they are triggered by epigenetic effects of exosome-derived miRNAs and mRNAs, or proteins transferred between these cells [Fig. 1]. These newly recognized exosome influence described as an intercellular cloud of diverse exosomes from immune, inflammatory and tissue cells results in modulation of the established mechanisms of allergic and hypersensitivity responses and thus likely plays an important role allergic and immunological diseases.
Mast Cell Exosomes Act in Allergen Ag Presentation
It was previously shown that classical APC, like macrophages and particularly DC, pulsed with whole native Ag release immunogenic exosomes that have surface complexes of the Ag-peptides in MHC molecules. These exosomes can act as “mini-APC-like” substitutes, that can bind to T cell surface Ag-MHC-specific TCR receptors to induce T cell signaling for effector functions [79–81], such as activation of CD4pos T cells [68]. As above, sensitization for allergic atopic Type I responses involves allergen engulfment and then intracellular processing by APC, principally DC, but also macrophages. The subsequent cell surface Ag presentation of DC-digested Ag peptides derived from allergen proteins, to the effector Th2 lymphocytes stimulate their release of helper cytokines, like IL-4 and IL-13. These act on IgE positive B cells that produce allergen-specific IgE Ab and on asthma-associated tissue cells, like goblet cells, to produce eosinophil-recruiting chemokines.
New findings pertinent to this review show that besides DC and macrophages, mast cells can function similarly as APC in Th2 responses, also by taking up allergens to possibly generate similar Ag-presenting exosomes. However, it remains possible that these are taken up by DC or B cells [82] to then truly present Ag [83]. Such transfer of mini-APC may thus account for transferred functions at a mast cell and DC synapses [1,84–86], and are connected with demonstrated ability of mast cell exosomes to induce maturation of DC that allows for their conventional Ag presentation to T cells [70]. IgE antibodies (Ab) may also play a role in mast cell function in Ag presentation. Thus, mast cell-released exosomes express FcεRI receptors that may be involved in the reuptake of IgE complexed with allergen, not only by mast cells but in humans also by conventional APC also expressing FcεRI, that in turn likely mediate allergen presentation [87].
At such newly described mast cell synapses formed with other cells, like recently shown at the central immunological synapse of T cells and APC or B cells [1], there is intercellular passage of RNAs and proteins from the released mast cell exosomes. Thus, during the effector phase of allergic responses, apart from classical allergen-specific IgE-mediated degranulation of mast cells to release diverse pro-allergy and inflammatory mediators, there is an additional layer of molecular mediator release due to exocytosis of MVB-derived exosomes that may transfer regulatory RNAs and proteins to neighboring cells [70,71]. Note that the first description of exosome-mediated intercellular exchange of functional RNA was shown in mast cell lines, with the appearance of donor cell proteins in the acceptor cells due to the transferred mRNA, and interestingly some of mRNAs were hardly expressed in the donor cells [38].
The release of Ag/MHC coated exosomes by traditional APC and mast cells, represents an entirely new aspect of Ag presentation. Not only does the process proceed at the immunological synapses between APC and effector T cells, but likely also during the interaction of APC exosomes with T cells, which can be envisioned as a cloud of “mini-Ag-presenting” exosomes around T cells. This would greatly change the stoichiometry of the APC phenomenon, since existence of such Ag-presenting exosome cloud would greatly enlarge this essential and central immune cell interaction. This therefore will not merely depend on the membrane to membrane co-localization of the T cell and APC for formation of the conventional synapse between the surfaces of rare DC acting as APC, with passing rare Ag-specific T cells. In an analogous fashion, it seems that there also is a potential cloud of effector T cell-derived exosomes expressing membrane TCR and CD3 that are capable of binding with Ag/MHC complexes on the cell membranes of neighboring Ag-presenting B cells to trigger their function [1], and conversely a mini αβTCR/CD3 T cell exosome cloud back-stimulating the B cell or DC. Thus, a molecular exosome RNA-mediated epigenetic effect of the T cells on the APC [36,37] or similarly on B cells [1], and, conversely, of the APC on the T cells [60], and even stimulated DC activating other DC, are newly recognized aspects of central immunological processes. Thereby, this opens possible new levels for understanding and treating allergic and hypersensitivity diseases. Therefore, this novel exosomal Ag presentation process can be exploited for new modes of allergen desensitization, that could perhaps be reduced from the use of native allergens to just treating patients with APC-derived exosomes, as is currently done for vaccination against infectious agents [88,89] and cancer [90–92].
Exosome Involvement in Allergic Rhinitis and Possibly in Non-Allergic Rhinitis
Intriguingly, there is a relevant study that extends the concepts described above about APC exosome allergen-presentation. Cultured B cells from patients allergic to birch pollen, including those with allergic rhinitis, that have B cells expressing surface immunoglobulin receptors (BCR) of the same allergen specificity as their produced Ab, released Ag-specific exosomes with BCR on their surface. After in vitro loading with a birch pollen-derived allergenic peptide Bet v 1, these anti-birch pollen specific B cell exosomes were used as mini-APC to stimulate patient T cells. This new exosome mediated B and T cell collaboration caused in vitro T cell proliferation and secretion of the Th2-dependent cytokines IL-5 and IL-13, that likely could participate in the allergic rhinitis and asthma [93].
It was shown recently that there is great diversity of miRNA expression in tissues of nasal mucosal biopsies from patients with persistent and also non-persistent asthma in comparison to healthy volunteers [94]. In another relevant study, analysis of the miRNA profile in extracellular vesicles obtained from nasal mucus of patients with allergic rhinitis, when compared to healthy individuals, revealed differences, implying that nanovesicle-transmitted RNAs likely may also be involved in the pathogenesis of this most common Type I allergic disease [95]. There is an association of mRNA levels in nasal polyps with the release of eosinophil mediators, like RANTES and eotaxin [96], which raises the possibility of treating nasal polyps with intranasally administered exosomes containing RNA antagonist of the reverse sequence to the polyp-associated mRNAs or miRNAs. Finally, isolation of nasal mucosal and epithelial samples from patients with chronic obstruction due to atypical allergic rhinitis, for their further examination using immune gold electron microscopy, showed that the resulting exosomes carried relevant nasal allergens, like Staphylococcal enterotoxin B and house dust mite (HDM)-derived Derp1 Ag. These exosome mini-allergen-APC were claimed to induce naive CD3pos T cells to differentiate into CD8pos T cells. Further, on exposure to specific Ag, the CD8pos T cells released granzyme B and perforin, and more than 30% of the Ag-specific CD8pos T cells proliferated [97]. An interpretation of these data suggests that this could be an example of functioning in vivo allergen positive APC-derived exosomes that are able to stimulate T cells in the pathogenesis of chronic atypical rhinitis. Exosomes collected from human nasal lavage fluid were shown to induce the migration of innate immune cells, that may play an important role in the defense against pathogens and allergens. On the other hand, the decreased expression of antimicrobial proteins in nasal exosomes from patients with airway diseases likely contributes to an increased susceptibility to infections and to disease progression [98].
Thus, it could be postulated that similar clinical syndromes seeming to be independent of IgE Ab might be caused by pathogenic exosomes from local and/or distant cells, that perhaps act in an Ag-specific manner. Alternatively, the mechanism underlying these clinical symptoms could be due to a combined action of such mini-APC exosomes and very low levels of IgE Ag-specific antibodies that do not cause the elicitation of macroscopically positive skin tests or other in vitro tests. This recalls our demonstration in mice that very low amounts of IgE (1 ng per mouse equal to 2 pg per person) failed to elicit macroscopic immediate skin responses to allergen challenge, but still mediated microscopic Type I vasoactive responses that in fact can allow for local recruitment of hypersensitivity effector T cells into the tissues [99]. Another postulate involves cross kingdom considerations pertinent to exosomes; like action of food-derived miRNAs carried from the intestine by host exosomes to influence a human patient with allergy [100]. In this regard, it is postulated that extracellular vesicle-delivered enzymes or RNAs produced by the allergy-inducing plants, or even released from their pollens [101], or in allergy to house mites, where insects are known to release exosomes from their MVB [44,45], may play a role in some of these atypical clinical conditions [102].
Accordingly, exosomes derived from DC of patients exposed to cats, carrying the major peptide determinant of cat allergen (Fel d 1), recently were shown to potently stimulate cytokine production by Th2 lymphocytes in cat allergen sensitive individuals [103]. On the contrary, particular miRNAs from exosomes of nasal epithelial cells from this atypical allergic rhinitis microenvironment stimulate IL-10 production by monocytes to inhibit nasal allergy [104]. Thus, this sort of allergen induced intercellular communication might be responsible for the seemingly anomalous finding that, although cat dander is very sensitizing and produces strong symptoms in many atopic individuals, exposure to multiple cats curtails this powerful allergic sensitivity. This could be due to a “modified Th2 response;” characterized by an emerging role of antigen-specific Treg cells and associated IgG4 Ab responses, that prevent the specific allergic reaction in allergic individuals, and of clinical practice interest may constitute the results of clinical desensitization with high doses of cat allergen [105] as is true of desensitization with stinging insects [105a].
Additionally, tissue iNKT cells may be involved in such exosome communications, since exosomes express not only MHC but also can express the minor histocompatibility CD1d molecules known to bind glycolipid Ag, like alpha-galactosylceramide (alpha-gal-cer) the canonical glycolipid Ag activating semi-invariant αβTCR on iNKT cells [106]. Such mini-APC exosomes with surface CD1d may stimulate tissue iNKT cells to release allergy-promoting Th2 cytokines, like IL-4 and IL-13, after interaction with host, plant or mite glycolipids. As a result, these CD1d-dependent iNKT cells may induce allergic responses against glycolipid Ag with the release of IL-4, as we showed previously in contact hypersensitivity [107], or IL-13 reacting against polysaccharides from bacteria, as we showed in a murine model of pneumococcal pneumonia [108]; or the plant pollens [101], as a requirement for eventual effector Th1 cell participation.
Very interestingly, plant allergens, that play a major role in atopic allergic rhinitis and asthma, have been known for a long time to be associated with their pollens, and recently were shown to be released from pollens in exosome-like vesicles called “pollensomes” [109]. Regarding such interactions in food allergy, extracellular MHC class II positive exosomes released by epithelial cells of the GI tract, that have mini-APC abilities, may play an important role in transmission of food allergens from the intestinal lumen to the local lymphatic system [110]. This intercellular exosome communication may thus allow for Ag-presentation by DC to specific T cells. Such a T cell response to the dietary Ag of these mini-APC exosomes may initiate and then aggravate the responses to food allergens. Distinctly, these conditions may be represented by patients with food allergy induced by iNKT cells reacting to the APC-derived exosomes pulsed with the plant Ag, and, therefore, producing clinically significant symptoms, but lacking the positive result of immediate skin tests to the food allergens. Moreover, exosomes were recently shown to transfer haptenated proteins to possibly activate allergy to beta-lactams in a model of clinical allergy to amoxicillin [111]. Host cell derived exosomes and cross kingdom allergens presented on mini-APC exosomes, should now be considered to possibly be involved in several other atopic disease imitators, that apparently are not due to classical IgE/mast cell mechanisms.
Bacterial Extracellular Vesicles (mEV) Promote Atopic Dermatitis
Remarkably, allergens can also be transmitted by extracellular vesicles originating from the microbiome, that can significantly drive clinical allergic diseases. As an example, Staphylococcus aureus is known to be strongly associated with the pathogenesis and progression of atopic dermatitis. Accordingly, anti-staphylococcal antibiotic therapy and/or daily immersion in Clorox mixed in a bath are routinely prescribed for patients with this disease. It has been known for a number of years that bacteria secrete mEV, that now are seen as related to exosomes. mEV are formed at the cell surface from budding off of a very thin tissue cell layer over the tough peptidoglycan outer calix that surrounds the bacterial cell membrane for maintaining shape and protecting against challenging environments and enemies.
The mEV are subsequently extruded as vesicles containing signaling molecules for quorum sensing by other bacteria, leading to promotion of microbial growth and spreading. They also contain enzymes and toxins for fighting enemies, and importantly RNAs, as recently demonstrated [15,16]. These extracellular vesicles enable the bacteria to mediate long-distance delivery of toxic cargo with minimized dilution or degradation in transit to then optimally affect other organisms. Of clinical interest, these properties allow for the transmission of bacterial factors mediating invasion and virulence to act at the host-pathogen interface, and to participate in some allergic diseases, like atopic dermatitis [17,18].
Accordingly, application of mEV from Staphylococcus aureus to mouse skin, previously stimulated by local tape stripping and then Ag application to express a murine model of atopic dermatitis, results in augmented development of the characteristic eosinophil-rich inflamed lesions along with the increased local levels of Th2 cytokines [17]. Further, these staphylococcal mEV are able to stimulate dermal fibroblasts to release stromal-derived pro-allergic inflammatory mediators, like TSLP, IL-6, MIP-1a and eotaxin, and also to induce IgE responses to Ag, particularly found in the mEV, compared to the staphylococci [17].
In a related study, a large group of patients with atopic dermatitis was found to be reactive to Malassezia sympodialis fungal allergens, that were shown to be carried by mEV released by this yeast [18]. Like staphylococci, this yeast is considered as a part of the physiological cutaneous flora. These allergen-bearing fungal exosome-like vesicles isolated from the supernatant of the co-culture of Malassezia sympodialis and monocyte-derived dendritic cells collected from patients with atopic dermatitis, are able to stimulate the in vitro production of IL-4 by cultured peripheral blood mononuclear cells (PBMC) from these patients [18]. In an analogous manner, the mEV of the nasal microbiome may participate in allergic rhinitis to promote this most common Type I allergic disease. Accordingly, molecular evidence based on bacterial 16s-ribosomal sequencing shows a possible relationship between bacterial type and clinical outcome, and S. aureus dominance is being associated with development of chronic rhinosinusitis [112]. Taken together, these data suggest that infectious agent-derived mEV transport microbial allergens and likely deliver functional RNA and proteins that are involved in the pathogenesis of atopic allergic diseases.
Helminthic Worm Exosome miRNAs Inhibit Host Th2 Immunity To Mediate Molecular Parasitism
Related to the role of exosomes in allergy is recent work showing that the mucus oily surface secretion of an intestinal helminth, called “worm spit”, known to be associated with its pathogenicity, contains exosomes with pro-parasitic and anti-allergic miRNAs [113–115]. It is known that such worm-derived exosomes are taken up and internalized by cells of the parasitized host [114]. The recently uncovered mechanism of the molecular pathogenic role of these parasite miRNAs is to specifically bind to the 3’ untranslated region (UTR) of targeted specific mRNAs in the host, to antagonize them or cause their degradation. Thereby they alter gene translation epigenetically to then affect host cell function by decreasing production of targeted gene encoded proteins [113]. This provides a newly recognized exosome mediated molecular mechanism of parasitism; acting in this case by inhibiting translation of Th2-associated genes, like those encoding IL-33, which is an IL-1 related cytokine that normally enhances Th2 immune responses. This contributes to down regulation of innate Type II immune responses that are known to be associated with repelling worm infestation, and simultaneously promoting anti-worm Th2 responses [113]. Therefore, it has been proposed that extracellular vesicles of helminth origin, that are internalized by host immune cells, transfer inhibitory miRNAs for genetic down regulation of Th2 associated host-protective proteins. Clinically, the possible eventual use of these particular miRNAs, perhaps delivered in therapeutic exosomes, could be applied to treat Type I allergies in patients, employing natural and physiological mechanisms.
Other related work also demonstrates the role of exosomes in the evolution of parasitism. It was shown that Leishmania constitutively secrete exosomes in the gut of the sand fly vector to become a part of the inoculum transferred by the fly into the host during the insect’s bite [116]. The co-transferred exosomes influence the infectious process by inducing host inflammation, particularly via IL-17a. The Leishmania-derived exosomes are an integral part of the parasite’s infectious life cycle, acting as newly recognized virulence factor vesicles that are associated with this vector-transmitted infection [117]. Prior work has demonstrated that Leishmania parasitism is augmented by production of exosomes modulating host innate and adaptive immune responses through their effects on monocytes and DC [117]. Conversely to helminth exosomes that block host Th2 responses, vesicles shed by Trypanosoma cruzi parasites increase parasitism in heart and generate an intense inflammatory response due to their induction of Th2 cell production of IL-4 and IL-10 [118]. Finally, it was shown that Trichomonas vaginalis-derived exosomes also deliver factors to the host that favor their infectivity [119].
Role of Exosomes and Contained miRNAs in Interactions Between Airway Tissue Cells and Infiltrating Asthma Pathogenic Immune and Inflammatory Cells
As noted above, asthma can be augmented due to allergen presenting exosomes. The disease can progress in sensitized individuals due to chronic airway exposure to inhaled aerosol allergens. This consists of either limited seasonal contact with allergens of outdoor plants or year round contact with indoor allergens from house dust mites and the dander of mice and pets. Beyond the acute clinical bronchospastic phase, asthma is associated with “late phase”, eosinophil rich airway inflammation. These chronic manifestations can lead to airway tissue remodeling with fibrosis and airway muscle sensitization that induces easily provoked bronchoconstriction; all significantly impairing the life and comfort of patients.
These phases of asthma are classically noted to be mediated by IgE-dependent, early immediate hypereactivity, and subsequent late phase inflammation resulting from IgE and Th2 cell-dependent mechanisms. This latter process, that is critical to the generation of chronic asthma, includes crucial interactions between local tissue cells of the airway microenvironment and the infiltrating immune inflammatory constituents. These interactions are now recognized to be mediated, at least in part, by released extracellular vesicles that transfer their miRNA and protein content between the involved cells [Fig. 1]. This likely includes exosomes exchanging information between the intrinsic lung tissue cells of the local airway microenvironment, and extracellular vesicles derived from infiltrating immune and inflammatory cells emigrating to the lungs from the bone marrow and lymphoid tissues. The local microenvironmental exosome sources include cells of airway smooth muscles, vasculature, and epithelium, as well as mast cells, goblet cells, DC and alveolar macrophages.
We postulate that these wider than previously anticipated interactions produced by exosome mediated intercellular exchanges between cells of the tissue microenvironment and the infiltrating immune and inflammatory cells are important in the development of chronic airway remodeling. This is analogous to the newly expanded ideas of tissue stromal cell responses to cancer cells spreading metastasis [120,121]. Interestingly, this can depend on chronic exposure to tumor cell-derived exosomes delivering proteins or RNAs, that act on the tissue cells. It was demonstrated that exosomes from particular cancers act at a distance to fuse specifically with resident cells of the preferred organ to generate a pre-metastatic niche. This appears to be due to specific, Darwinian selected exosome surface integrins. These could be considered analogous to bar codes for specific recognition affinity of particular other cells [122]. By analogy, cells within an allergic tissue, such as in the late or especially remodeling phase of asthmatic airway inflammation, could generate a bronchial microenvironmental multicellular-derived cloud of exosomes with specific affinities for various local, or distant, targets driving the allergic tissue response [Fig. 1]. Thus, the local airway tissue cells are often the targets of immune and inflammatory cell-derived cytokines inducing production of chemokines and enzymes, like in chronic obstructive pulmonary disease (COPD) [123], and further often can be the targets of exosomes [81,124,125].
BALF Analysis Suggests Participation of Exosome Intercellular Exchanges in the Pathogenesis of Allergic Asthma and Perhaps its Late Sequela
Exosomes derived from the BALF of healthy individuals differed from those of patients with even mild asthma, that expressed higher levels of exosome-associated markers, such as the tetraspanins CD63 and CD81, and the scavenger receptor CD36 [126]. Although there were no major differences between BALF exosomes from asthmatics before and after birch pollen allergen provocation, they contained enzymes for leukotriene biosynthesis that were able to promote LTC4 release, as well as synthesis and release of the neutrophil-recruiting IL-8. Importantly, a leukotriene receptor antagonist (montelukast) reduced exosome-induced secretion of this pro-inflammatory cytokine that behaves like a neutrophil chemokine [126].
Furthermore, in a mouse model of asthma, exosomes isolated from the BALF of asthmatic versus control mice had increased levels of exosome-associated proteins. The asthma featured enhanced secretion of exosomes by bronchial epithelial cells, but not macrophages. Stimulation with IL-13 to imitate the function of specific allergen-pulsed Th2 cells, which is common in asthma, enhanced asthma severity [76]. This resulted in the release of exosomes from the bronchial epithelial cells that induced proliferation and chemotaxis of macrophages. These effects were suppressed by treatment with the ceramide blocker GW4869 [76], an inhibitor of neutral sphingomyelinase-2 that is essential for exosome formation. Thus, it is known to inhibit exosome transfers [127–129], and indeed, it alleviated allergic airway inflammation in these asthmatic mice [76]. Interestingly, isolated wild type mast cells stimulated with Ag for typical mediator release were strongly inhibited in sphingomyelinase deficient mice [130]. However, a possible role of an activating exosome exchange between mast cell subtypes for mediator release was not yet investigated.
Pertinent to possible exosome-derived miRNA regulation of asthma are experiments showing that allergic disease resulting from in vivo activation of TLR4 by house dust mite Ag leads to expression of an unique subset of miRNAs. Remarkably, selective blockade of miRNA-126 alone with specific antagonists inhibited the asthmatic phenotype. This resulted in diminished Th2 inflammation, airways hyperresponsiveness, eosinophil recruitment, and mucus hypersecretion [131]. The data suggest that targeting miRNA in the airways may lead to anti-inflammatory treatments for allergic asthma. In a follow up study using the ovalbumin (OVA)-induced asthma model, specific inhibition of prominently involved miRNA-155 with antagomiR treatment failed to alter the disease phenotype, leading to the conclusion that the level of a particular miRNA may not indicate its importance and that blocking is needed to ascertain the functional significance of a particular miRNA from exosomes [132].
Interestingly, there was the variable efficacy of the ant-miRNA treatment across different immune cell types. Thus, effective targeting of myeloid cells but not lymphocytes indicated the possible need for cell type specific targeting of such therapy. Taken together, these data show that exosomes produced by various cells in the airways have an important and previously unrecognized role in the pathogenesis of the acute early phase of atopic allergic asthma. Since exosome effects are often mediated by intercellular transfer of miRNAs, these findings raise the possibility of new therapies at an entirely new level; namely blocking epigenetic effects of miRNAs with appropriate polynucleotide antagonists, as we demonstrated in CHS [2,7].
Potential Role of Exosomes in Airway Remodeling During Late Phase of Asthma Inflammation
Exosomes produced by eosinophils may possibly contribute to tissue remodeling during late phase of asthma [78,96,133–135]. Eosinophils collected from blood of asthmatic patients that were stimulated in vitro with cytokines, had increased release of tetraspanin CD63pos exosomes [135]. Potential augmenting of the number of eosinophil-derived exosomes can alter and even cause rapid necrosis of structural lung cells, that not only can exacerbate allergic asthma, but especially can lead to airway remodeling [133]. These effects may be larger than expected, since it is possible that some of the granules thought to be produced by eosinophils may be in fact exosomes.
In an analogous manner bronchial epithelial cells seem to produce proinflammatory exosomes that activate macrophages in the inflammatory phase via the transfer of miRNA. Th2 cell-derived IL-13 stimulates airway epithelial cells to release exosomes that transfer Let-7 miRNA to activate macrophages in the lungs during asthmatic inflammation [136]. During the inflammation neutrophils may also contribute to subsequent remodeling. Here, the LTB4-induced neutrophil chemotaxis, their released exosomes contain LTB4 and its synthesizing enzymes [137], and can activate neighboring resting neutrophils in a LTB4 receptor-dependent manner [138]. Thus, the exosomal pool of LTB4 acts in an autocrine fashion to sensitize neutrophils towards a primary chemoattractant, and then in a paracrine fashion to mediate recruitment of neighboring neutrophils to augment inflammation. Therefore, this forms an exosome intercellular mediator cascade effect, augmenting inflammation pertinent to the late phase of asthma and perhaps contributing to the progress of remodeling [139]. Accordingly, use of drugs to inhibit exosome production might interfere with this deleterious augmenting cascade. Further, airway administration of LPS causes acute injury with recruitment of neutrophils into lung and BALF [140]. Finally, neutrophils stimulated with LPS produce exosomes that alter proliferative properties of airway smooth muscle cells [125]. In an analogous manner, LPS activates bronchial epithelial cells of asthmatic patients to release exosomes that contain overexpressed pro-angiogenic tissue factor stimulated by compressive stress, that possibly also contributes to airway remodeling during chronic asthma [124].
Role of Bacterial mEV of Dust in Airway Remodeling during Atopic Allergic Asthma
Inhalation of allergens and other entities in dusts is a common exposure contributing to the pathogenesis of asthma in allergic individuals. Indoor dust contains not only house dust mite Ag as the dominant allergens, but also mEV components from Gram negative bacteria derived from the GI tract of humans and pets. Such an occurrence contributes to an expansion of the hygiene hypothesis. This proposes that our changed relationships to infectious agents are a part of processes that have led to a great increase in some diseases of Western Civilization, like allergies, and particularly asthma.
In homes that are a part of dairy farms, the dust contains mEV LPS from Gram negative bacteria of the cattle. The LPS containing mEV are likely involved in an asthma-protective effect of chronic low dose exposure to the farm dust. This can happen in children growing up on a dairy farm near the animals, which protects them from allergic rhinitis and asthma [141]. The protective effect depends on mEV LPS induction of the ubiquitin-modifying enzyme A20 in lung epithelium [142]. Thus, the farming environment protects from allergy by modifying nanovesicle intercellular communication between barrier lung epithelial cells and then DC through the induction of A20 by LPS in mEV. Such a chronic exposure to a very low dose of mEV LPS in farm dust protects experimental mice from developing asthma in a model induced by house dust mite Ag. The mEV-protected mouse airway epithelial cells produced less cytokines to activate DC, thus suppressing Th2 reactivity to house dust mites.
In contrast, repeated airway administration of LPS mEV isolated from indoor house dust to mice led to neutrophilic pulmonary inflammation with infiltration of Th1 and Th17 cells [143]. The vesicles were internalized by airway epithelial cells and alveolar macrophages. This process was blocked by treatment with polymyxin B antagonist of LPS in the mEV of Gram negative bacteria [143]. In this study, serum levels of IgG1 Ab reactive with the dust vesicles were significantly higher in atopic children with asthma, than in healthy children and those with rhinitis or dermatitis [143]. Thus, nanovesicle intercellular communication, like that mediated by mEV LPS in environmental dust, can be crucial in tipping the balance between development of Th2 versus Th17 dominated asthma [144]. Importantly, IL-17 is known to recruit and activate neutrophils and is an important mediator of chronic inflammation, so in the present context, it is another contributor to potential asthmatic airway remodeling.
Interestingly, sphingosine-1-phosphate inhibits murine endotoxin-induced inflammatory lung injury. This likely is because of formation of exosomes and involves inhibitory G protein-coupled sphingosine-1-phosphate receptors [145] that regulate exosomal maturation in MVB [146]. As mentioned above, the release of exosomes depends on ceramide biosynthesis regulated by neutral sphingomyelinase-2 and thus can be inhibited by its inhibitor GW4869; both in vitro and in vivo [76,147], as already employed in a study on the role of exosomes in allergic airway inflammation in a murine model [76].
Yet another possibly was brought by the recent demonstration that mEV contain RNA [14,15], and can release this RNA [16] to possibly function or trigger TLR4 stimulation in mEV-induced airway remodeling in asthma. Accordingly, we predict that bacteria-derived mEV containing LPS and epigenetic effects of their delivered RNAs will be found to be an important causative agent for asthma remodeling. Of related interest, Staphylococcus aureus-derived mEV, that play a significant role in the pathogenesis of atopic dermatitis [17], also have been shown to induce neutrophilic pulmonary inflammation via both Th1 and Th17 cell dependent pathways [148,149]. This could be important in chronic phases of allergic asthma and in some forms of non-atopic asthma, with subsequent remodeling.
Taken together, these findings suggest that exosomes derived from endogenous airway stromal cells and infiltrating inflammatory cells of late phase asthmatic inflammation, and or together with mEV, and possibly bacteria toxins, stimulating release of lung tissue-derived exosomes, can participate in the sensitization for airway hyperresponsiveness and tissue remodeling changes in allergen driven chronic asthma. Such a remodeling is particularly difficult to treat clinically, especially using inappropriate anti-mediator and anti-inflammatory agents aimed at earlier phases. Thus, the new realization of a potential role for exosome delivery of miRNAs in remodeling opens a new pathway that might possibly lead to entirely new and likely more effective therapies aimed at impairing the consequent epigenetic mechanisms of exosome transferred miRNAs. Also, documentation of participation of mEV, and possibly TLR agonists, in the evolution of remodeling in allergic asthma might lead to more vigorous use of antibiotics, perhaps administered to the lung topically in an aerosol, or specific bacterial vaccination, perhaps with mEV, to possibly impede this process, again by analogy with atopic dermatitis.
Exosome RNA Biomarkers for Predicting and Following Allergic Asthma
The use of miRNAs as biomarkers that are carried in extracellular vesicles from the blood [149], BALF [150] and airway epithelium [151] of asthmatic patients were recently described, and similarly in serum derived exosomes from mice with asthmatic airway inflammation [152]. In addition, this biomarker information of respiratory tract conditions was collected non-invasively, and very innovatively, by assaying the miRNAs of the exosomes from exhaled breath condensate from the patients. Samples were compared from patients with Type I allergic asthma, COPD, pulmonary tuberculosis, and from normal individuals [153,154]. The data showed potential distinctive alterations of miRNA profiles in exhaled exosomes from asthma patients; and very importantly, even those with mild or early disease. Thus, miRNA profiles of exosomes from BALF, airway exhalate and blood might serve as excellent asthma biomarkers, especially useful in possible early detection of components determined to be predictors of remodeling. If particular exosome miRNAs were known to be such predictors, then perhaps airway aerosol treatments with hybridizing polynucleotides of particular specific anti-miRNA sequence might prevent the process.
It is established that extracellular miRNAs in the blood are carried in exosomes. However, some of the blood miRNAs are transported not by exosomes, but by chaperone hydrophobic proteins, like argonautes and lipoproteins, for similar protection from RNases [155–158]. We found that these non-exosomal miRNAs act to suppress in vivo immune inflammatory responses by associating with exosomes produced by neighboring cells to then target effector cells, and, like those in exosomes [2], were susceptible to anti-miR treatment [7].
In summary, the use of exosomes for development of useful clinical biomarkers that would aid early detection of susceptible individuals prone to develop more significant asthma and late tissue remodeling, and the use of entirely new modes of specific therapy based on inhibition of particular miRNAs with specific antagonists, would be revolutionary. Such new procedures may prevent the progression of airway remodeling from its current non-reversible state and possibly reduce the need for extensive chronic treatment with anti-inflammatory steroids and bronchodilators.
Exosomes in Immune Tolerance and Suppression of Asthma
Since miRNAs usually down regulate mRNA translation they often mediate suppression of immune responses. Therefore, exosomes delivering miRNAs play a particularly prominent role in immunoregulation, mostly in tolerance and suppression. As an example, BALF exosomes (called “tolerosomes”) obtained from donor mice tolerized to olive pollen peptide by repeated intranasal administration of high dose of Ag significantly suppressed allergic airway reactions when administered to mouse recipients with a model of asthma [159]. The tolerogenic exosomes decreased allergic eosinophil-rich bronchial tissue inflammation and reduced production of IgE Ab and Th2 cytokines. The observed suppression likely was Ag-non-specific, since mice pretreated by intranasal administration of BALF-derived exosomes from donors tolerized to the allergen-derived olive pollen peptide were protected from development of airway allergic inflammation after subsequent sensitization with another allergen, i.e. birch pollen peptide. However, the Ag cross-reactivity between both of the pollen Ag could possibly occur. In the analogous system of Ag high dose tolerance in a Th2-type response described above, treatment of recipient mice before sensitization with intestinal epithelial cell-derived tolerosomes found in serum of mice orally tolerized to OVA [160] induced oral tolerance. Further, as a possible example of a relationship between bacterial mEV and asthma tolerance, neonatal exposure to the enterotoxin superantigen of Staphylococcus aureus augmented subsequent induction of oral tolerance to OVA in a mouse model of asthma [161]. In contrast, exosomes from an epithelial cell line cultured in the presence of an OVA hydrolysate of peptides with IFNγ were not tolerogenic, but instead activated the humoral Ab immune response to OVA [162]. This suggested that such exosomes can tilt a tolerogenic versus immunogenic response by pre-incubation of donor cells with specific cytokines. Additionally, cytokines can be transported between cells by nanovesicles. In a neurological system, extracellular vesicles from neural stem cells were shown to be able to transfer IFNγ bound to its receptor, in order to activate Stat1 signaling in the targeted cells [65].
Exosomes of Breast Milk In Allergy and Immunity
Breast milk contains a rich pool of exosomes carrying numerous immune-related miRNAs that are considered to be involved in the development of immunity and allergic disease responses [163,164]. Since miRNAs mediate inhibition of mRNA translation, they often are involved in immunosuppression, as is thought true for those present in exosomes of breast milk. By definition the miRNAs in these exosomes are protected from the GI tract RNases and other degradative conditions [165]. As mentioned, exosomes, as ancient particles of life, resist many harsh conditions that cells do not survive, such as the acidic pH of the stomach [28,29], even down to pH = 1 [30]. Thus, the miRNAs in breast milk exosomes can survive normal neonatal ingestion to then mediate immunoregulation in the neonate that is related to the microbiome being acquired and the possible development of allergies. It is established that colostrum delivered from mother to neonate just after birth is rich in IgA Ab protective against GI tract and skin infectious agents. This provides the infant with the mother’s prior humoral Ab experience. Colostrum is also known to contain exosomes [166,167] that can transfer different immune information, likely regarding cellular immunity that is delivered to the neonate during breast feeding. This is postulated to be related to cell-mediated responses aiding neonatal regulation of the microbiome that the child is acquiring and sharing with the mother. Accordingly, mother’s milk transfers various factors of immunosuppressive functions that likely protect the neonate from over reactivity to the great load of highly stimulatory foreign Ag of the microbiome they are acquiring. Furthermore, breast milk exosomes also seem to provide protection that is needed for control of unnecessary and deleterious allergic responses to foreign Ag. This likely pertains particularly to food Ag that are also being encountered for the first time. If not controlled, responses to new microbial Ag and food allergen exposures might lead to clinical hypersensitivity or allergy later in life.
Functional analysis has revealed that the mother’s milk exosomes can inhibit T cell production of IL-2 and IFNγ following MHC stimulation [168]. In addition, incubation of the milk vesicle preparations with peripheral blood mononuclear cells (PBMC) increased the number of suppressive Foxp3pos Treg cells. In studies extending these findings, breast milk exosomes were shown to contain functional inhibitory TGF-beta [169], along with miRNA that can promote thymic Treg cell maturation [170], and that their immunosuppressive action likely inhibits innate immune functions of macrophages that may be acting as APC [171]. Further, mother’s milk exosomes contain miRNA-17, part of the miRNA-17–19 cluster, which is important for the activity of myeloid suppressor cells, as well as miRNA-181a, a modulator of TCR sensitivity to Ag. This acts partly through down regulation of phosphatases, which leads to elevated phosphorylation of intermediate signaling proteins and a consequent reduction of the TCR signaling threshold [172].
In addition, the miRNAs transferred by milk exosomes include miRNA-155 that is a distinctive regulator of T- and B-cell maturation and of the innate immune response [173]. miRNA-155 mediates inhibitory function in several other aspects of immune responses, including allergies. Such inhibition is in part due to the miRNA-155 control of the expression of the specific Treg transcription factor FoxP3, and consequent IL-4 signaling, together with immunoglobulin class switching to IgE and FcγRI expression [170]. These findings confirm that a dominant function of breast milk exosomes is to genetically control neonatal immune reactivity, inflammation and likely allergy via intercellular transfer of miRNAs focused on epigenetic modulation of cellular immunity relevant to the newly colonizing microbiota of the GI tract and exposure to food allergens. Therefore, isolation and characterization of inhibitory mother’s milk-derived exosome-derived miRNAs could then be developed into natural clinical candidate agents mediating completely new therapies for down regulation of atopic sensitization and Th2 effector immune responses associated with production of IL-4, IL-5 and IL-13.
Accordingly, natural mother’s milk miRNAs may induce pivotal immunoregulatory and epigenetic modifications required for long-term central thymic Treg cell lineage commitment explaining the atopy-protective effect of mother’s milk. Since exosomes are poorly immunogenic and miRNAs are universal, this applies to raw allogeneic cow’s milk consumption as well [174]. Further, bovine milk components may also target APC in the neonate, since its exosomes have been shown to influence macrophages [171], and this might affect food allergy to milk too. These ideas offer a new option for the prevention of atopic diseases, for example by the addition of physiological amounts of miRNA-155-enriched exosomes into infant formula for mothers that are incapable of breastfeeding. In addition, bovine milk extracellular vesicles may show therapeutic effects since they were found to affect the course of mouse arthritis [175].
However, some studies with breast milk have pointed out that exosomes carrying allergen can in some circumstances augment rather than suppress immune responses, and thus may contribute to the development of allergy in infants. This may explain the higher incidence of neonatal allergy transmission from the mother than the father. Notably, the composition of exosomes in human breast milk differs greatly according to the mother’s sensitization status and lifestyle, that then can influence the risk of allergy development in breast milk-nourished children [174].
The recent rise in allergic diseases in infants of developed countries may in part be due to the reduced or different microbial exposure during early life and consequent alteration of gut microbiota-derived mEV [14], which is relevant to the “hygiene hypothesis”. Since the microbiota content of the infant’s GI tract likely plays a critical role in the maturation and development of the immune system, mEV from these “self microorganisms” may possibly contribute to the risk of allergic and immune diseases. Therefore, breast milk feeding may provide important exosomes that can either directly modify the immune response to and composition of the intestinal microflora or influence the host to reduce the development of allergic diseases. Further, mEV of the microbiome may be involved in its important role in the development of the neonatal immune system as well as aspects of the endocrine and nervous systems. Indeed, it was recently demonstrated that exosomes delivered in breast milk can promote the colonization of the newborn intestine with physiological microbial flora, that in turn seems to reduce the risk of allergy development and thus is crucially involved in the effects of the microbiome by the release of mEV or affection of intestinal production of host exosomes that transmit protective miRNAs [176]. Of further interest, it was recently proposed that polymorphisms in susceptibility genes in humans promote inflammatory bowel disease development causing impaired sensing of protective signals derived by the mEV, which confirms the significant role of microbiome-derived vesicles in the maintenance of immune homeostasis in digestive system [177].
The Role of Exosomes in Delayed-Type and Contact Hypersensitivity That Can Replace APC Function With Simpler “Mini-APC” vesicles
Delayed-type hypersensitivity (DTH) reactions exemplify mechanistic steps underlying Th1-mediated immune diseases and also defenses against pathogenic intracellular bacteria, fungi, parasites and some viruses, that must include intercellular actions of exosomes [89]. Major examples of exosome involvement in cell-mediated immunity are represented by DC that were pulsed with native Ag of infectious agents [88,178], to then be used as non-cellular potent vaccines substituting for DC. Therefore, DC-derived nanovesicles competent to present peptide/MHC complexes, and thereby acting like parental DC, were used to induce immune responses against tumor cells [90–92], and also inhibition of inflammatory responses to LPS [179]. We are not yet aware of the use of allergen pulsed DC or other APC to produce Ag-presenting exosomes to desensitize against allergens, as is traditionally done by administration of ascending doses of soluble Ag in buffer for induction of tolerance.
However, this can definitely be expected. Such a mini-APC approach should be far safer, since allergen peptides are being given in a form that should not readily trigger IgE/mast cell responses that are more directed at the specificity of IgE to conformational determinants of native allergens. Further, such a mini-APC vesicle method likely will be far more effective due to the direct facilitation of Ag presentation not requiring the processing of native allergen. Accordingly, exosomes derived from OVA-pulsed mouse bone marrow DC express OVA-peptide complexed in MHC. Administration of this exosome peptide-MHC complex leads to the polarization of immune response towards Th1 phenotype [82], and thus may be effective clinically in treating Th2-mediated allergic diseases.
As noted above, exosome-like mEV can influence cell-mediated immune responses as agents of the microbiome that shape and modulate the immune response. This approach takes advantage of the fact that mEV can be manipulated for their immunogenic contents for utilization as potent pathogen-free vaccines for immunizing humans and animals against infectious agents. Accordingly, DC, that are pulsed with Toxoplasma gondii [180], release to the culture supernatant immunostimulatory exosomes that can replace DC. Additionally, Escherichia coli release mEV [181], which, after injection into mice, are readily taken up by APC to efficiently stimulate specific Th1-dependent immune response preventing bacterial infections, and likely allergy development as well.
On the other hand, DC can be modified to produce immunosuppressive exosomes. Thus, administration of exosomes released by mouse DC genetically modified to express inhibitory Fas Ligand resulted in suppression of DTH elicited in mice immunized by intradermal injection of OVA or keyhole limpet hemocyanin (KLH) Ag emulsified with Freund’s complete adjuvant [182]. Similarly, exosomes derived from cultured DC stimulated with IL-4 [183] or IL-10 [184] inhibited DTH induced by KLH with adjuvant. This effect was found to be dependent on expression of the co-stimulatory molecule B7 (CD80/87) on the cytokine treated DC [184]. It was concluded that B7, but not PD-L1/L2, on the surface of IL-10-treated DC and their derived exosomes play a critical role in the observed immunosuppressive functions.
In related in vivo studies, systemic injection of IL-10-containing DC-derived exosomes suppressed the onset of murine collagen induced arthritis (CIA) and reduced the severity of already established arthritis [185]. Taken together, these data suggest that DC secrete exosomes that can be tailored to suppress inflammatory and autoimmune responses. In further study, indoleamine 2,3-dioxygenase (IDO), that is a tryptophan-degrading enzyme important for immune regulation and tolerance maintenance, was expressed in DC and their exosomes inhibited DTH-effector T cells by depleting them of essential tryptophan and/or by producing toxic metabolites, as well as by generating Treg cells [186]. Accordingly, exosomes derived from these IDO-positive DC suppressed DTH and CIA. The suppressive effects were partially dependent on B7 co-stimulatory molecules. In addition, gene transfer of CTLA-4 immunoglobulin to DC resulted in induction of IDO in the DC releasing exosomes that were able to reduce inflammation in an IDO-dependent manner [186]. These results demonstrate that both types of IDO-expressing DC-derived exosomes are immunosuppressive and anti-inflammatory, and consequently are able to reverse established arthritis. Therefore, exosomes from IDO-positive DC may represent a novel therapy for rheumatoid arthritis, and highlight studies showing that altered exosomes from in vitro manipulated DC can significantly influence DTH and related clinical disease models, like CIA.
As noted above, rats fed with OVA develop a tolerogenic activity in serum that can transfer tolerance to OVA and suppression when given at the time of induction of an immune response, and more over can abolish an established DTH response in the recipients, as well as their humoral IgG responses. The mechanism possibly involves inhibitory exosomes (“tolerosomes”) acting through CD25pos Treg cells and produced by GI epithelial cells from rats undergoing induction of oral tolerance to OVA. Further, this exosome induced tolerance seems to be MHC-restricted, and probably Ag non-specific, since tolerosomes from animals fed with OVA suppress DTH induced with OVA or with human serum albumin [187,188]. In contrast, exosomes from the culture supernatant of a tumor cell line manipulated genetically to express OVA, when injected at the time of DTH elicitation, were able to suppress mouse OVA-specific DTH induced as above, but not DTH induced by a KLH-adjuvant mixture, and thus acted in an Ag-specific manner [189]. As noted, tumor cell-derived exosomes usually contain tumor Ag and have been used as mini-APC to stimulate anti-tumor immune responses [90–92]. However, it is unclear if the tumor-derived exosomes can actually facilitate tumor immune evasion Ag specifically. Here, the tumor-derived OVA-expressing exosomes were internalized by CD11cpos DC and transported to the draining lymph nodes to likely induce TGF-β 1 and IL-4 producing Ts cells that seemed to modulate the APC to express Ag-specific inhibitory function [189]. Further, exosomes isolated from plasma of mice immunized to KLH, but not from naive mice nor OVA-immunized mice, could suppress KLH and not OVA-specific DTH, with the effect mediated by MHC class IIpos, Fas Ligandpos, CD11b pos, but CD11cneg plasma exosomes and, in part, dependent on the presence of Fas Ligand on the exosomes and Fas on the recipient mouse cells [190]. These results suggest that plasma exosomes likely produced by APC and expressing CD11b and MHC class II complexed with tumor cell-derived peptides can suppress immune response in a peptide Ag/MHC-specific manner partly through a Fas/FasL interactions.
CD8pos Suppressor T Cell Exosomes Ag-Specifically Suppress CHS and DTH by Delivering Inhibitory miRNA-150
Current research of our laboratories aims to investigate the detailed mechanisms of Ag-specific suppression of mouse hapten-induced cutaneous contact hypersensitivity (CHS), as well as DTH that is induced by intradermal injection of protein Ag without an adjuvant. CHS is considered as a model that is relevant to human allergic contact dermatitis. Both are also considered to depend on mechanisms underlying cell mediated immune responses to proteins in a variety of diseases, such as asthma, atopic dermatitis and various autoimmune conditions. We have re-investigated previously observed suppression of CHS by an enigmatic Ag-specific factor secreted into culture supernatant by Ts lymphocytes harvested from mice tolerized by intravenous injection of high doses of Ag. This led to the discovery of suppressive exosomes carrying inhibitory miRNA-150 that are surface coated with Ab light chains responsible for their Ag-specificity [2,191]. Thus, we demonstrated that this enigmatic Ag-specific suppressor factor from mice rendered Ag-specifically tolerant by intravenous administration of a high dose of hapten Ag conjugated to autologous red blood cells, and subsequently immunized for CHS induced by the same hapten, was produced by CD8pos Ts cells as exosomes. These exosomes act by carrying and subsequently delivering inhibitory miRNA-150 Ag-specifically, due to their surface coating of Ab light chains, and interestingly not heavy chains nor whole Ab [2]. The suppressive exosomes could be isolated from either plasma of the tolerized animals or from culture supernatant of the Ts cells harvested from tolerized mouse spleen and peripheral lymph nodes [2,191]. Critically, inhibitory activity of the miRNA-150 was blocked by pre-incubation of the suppressive exosomes with an antagonist of miRNA-150, a polynucleotide of reverse sequence, i.e. anti-miR-150, compared to several controls [2].
The most important proofs were brought by experiments with miRNA-150−/− mice. Although normal appearing and able to be fully sensitized for elicitation of CHS, these animals could not be tolerized, and exosomes from these unsuccessfully tolerized mice were lacking suppressive function in vivo in CHS and in vitro in IL-2 receptivity of T cell line [2]. Also very crucially, in vitro supplementation of these non-suppressive exosomes from the miRNA-150−/− mice tolerized with high dose of hapten Ag, with synthetic miRNA-150 agonist rendered them suppressive [2,7]. Taken together, these findings show definitively that miRNA-150 delivered by the Ts cell-derived exosomes is the crucial mediator of the suppression and that surface Ab light chains are responsible for the Ag-specificity of their suppression. The CD8pos Ts cells producing the inhibitory exosomes are Foxp3neg [2], and thus are not conventional Treg cells, but instead originate from a separate lineage of regulatory cells that act to suppress CHS and DTH responses in vivo and in vitro [2].
A Special Small Subset of B Cells Produces the Ag-Specific Ab Light Chains That Coat the Suppressive Exosomes
The Ts cell-derived exosomes are able to act as a preventive as well as therapeutic agent by inhibiting, respectively, sensitization and elicitation of CHS, and by alleviating of its symptoms [192]. However the Ts cell exosomes are not non-specifically pan-suppressive, since, while suppressing the Th1 effector cells mediating the classical late phase of CHS and DTH, they do not inhibit the required early phase mediated by B1a cells. These B1a cells are stimulated by IL-4 released rapidly after sensitization (by only18 minutes) by hepatic iNKT cells [107] that apparently are activated by glycolipid Ag swiftly released at the cutaneous sensitization site to then apparently migrate to the liver to preferentially target hepatic iNKT cells [193]. As stated above, miRNA-150 loaded exosomes act in a strictly Ag-specific manner. Definitive dual reciprocal testing showed that exosomes obtained from mice tolerized to the trinitrophenyl (TNP) hapten suppressed only TNP-induced CHS, but not oxazolone (OX)-induced CHS effector cells, and conversely, exosomes from mice tolerized to OX suppressed only CHS effector cells induced with the OX hapten, but not with TNP [2]. This unusual Ag-specificity has been unraveled by the discovery that the Ts cell-derived exosomes have a surface coating of hapten Ag-specific Ab light chains derived from B1a cells accompanying the CD8pos Ts cells. These B1a cells are activated by hapten-self complexes induced by application of the relevant hapten on mouse skin, which completes the tolerogenic procedure [2]. The strict Ag-specificity of the participating B1a cell subpopulation is due to activation induced deaminase (AID), a nuclear mutating enzyme, that acted on the DNA of these cells prior to immunization to cause mutations in the immunoglobulin V-region genes, thus encoding the Ag-specific combining sites of Ab heavy and light chains [108,194,195].
This compares to the usual strictly germ line V-region DNA sequences without mutations in the majority of typical B1a cells that are not acted on by AID, and produce so-called natural Ab. However, recent single cell sequencing of immunoglobulin V-regions of developing B1a cells has shown that many of these cells, more than expected by prior information, are acted on by AID [196]. However, the processes stimulating the occurrence of AID-dependent mutations before immunization require further investigation. They do not involve prior sensitizing interactions with the microbiome, as they occur in germ free animals. Thus, the presumed Ag activation, that stimulated this subpopulation of B1a cells, did not come from the standard external bacterial, viral or parasite-derived foreign Ag, but likely from unknown endogenous Ag [196].
Characteristically, the special B1a cells, having only a few immunoglobulin V-region mutations mediated by AID that account for their strict Ag-specificity, are able to respond within minutes of hapten-self primary skin immunization, documented to occur in separate systems by just 18 minutes [107], 30 minutes [108] or one hour [197] after the original skin sensitization. As a consequence, the exosome sensitizing Ab light chains with few mutations are of low affinity for Ag, compared to their associated isolated heavy chains or the parental whole Ab. However, arrangement as multiple adjacent on the exosome surface likely results in their greater Ag-avidity, sufficient to manifest the observed strict dual reciprocal Ag-specificity of the suppressive exosomes [2]. Altogether, the suppressive exosomes that inhibit allergic cutaneous CHS are an unusual and unique product of components from both T cells (the miRNA-150 containing exosomes) and B cells (the exosome coating Ab light chains). The crucial experiment determining the role of Ab light chains coating the suppressive exosomes attempted to induce typical Ag high dose tolerization in pan-immunoglobulin deficient JH−/− mice. These mice lack Ab and, therefore, Ab light chains to coat the exosomes. Thus, cutaneous immunization for CHS in JH−/− mice previously tolerized with high dose of Ag was not suppressed and their exosomes were not active [2]. Very importantly, suppression was restored by coating of these non-suppressive exosomes from tolerized JH−/− mice with monoclonal Ab light chains [2]. Of related translational clinical significance, in our prior work on immediate hypersensitivity, on models of CHS [198] and asthma [199], induced by cutaneous sensitization with hapten or protein [200], the rapid activation of the small subpopulation of Ag-specific special B1a cells induced early after immunization was observed in all of these instances. Their activation resulted in production of Ag-specific IgM Ab and the Ag-specific Ab light chains that coat the Ts cell-derived exosomes and that have the additional ability to sensitize cells; so far principally mast cells by surface coating for mediator release when Ag is added, in an analogous manner to IgE Ab [201].
Regarding the suppressor exosomes, Ag-specific Ab free light chains [2] are produced by previously activated B1a cells and have low numbers of AID-mediated V-region mutations [108,195], and bind to the surface of CD8pos Ts cell-derived exosomes in a manner that keeps their combining sites available and allows quite Ag-specific function [2]. Note that tests in Fc receptor deficient mice showed that these receptors are not involved in Ab light chain binding [201], as expected since they have no Fc portion. Instead, this binding may depend on surface lipid changes in the membrane of “activated” exosomes [2,7], or by other unknown processes that are induced by tolerization or immunization. In contrast, exosomes from spleen and lymph nodes of non-immunized mice do not have the ability to bind free Ab light chains. It is important to note that in related clinical work, it has been shown that various human leukocytes, like T cells, B cells and monocytes, can bind free Ab light chains [202,203], and it was shown that this depends on cell surface lipids [203,204]. Therefore, this subpopulation of B1a cells, stimulated by hapten contact skin application as the final step in the tolerance procedure or for induction of CHS, or the analogous maneuver of intradermal injection of OVA protein Ag to produce tolerance to this protein, is responsible for the generation of hapten or protein Ag-specific Ab for coating of Ts cell-derived “activated” exosomes in mice undergoing Ag high dose tolerization.
Free miRNAs That Are Not In Exosomes Also Suppress Ag-Specifically by Final Association With Ag-Specific Exosomes
In other studies we defined an alternate pathway of suppression mediated by miRNA-150 present in a mixture of miRNAs that are free of exosomes. This mixture of extracellular RNAs (exRNA) was obtained by phenol chloroform extraction of the exosomes derived from the Ag-tolerized mice. miRNAs free of exosomes are also protected from RNases, by complexing with a hydrophobic chaperone, like an argonaute protein [7,155–158].
Interestingly, miRNA-150 among the vast excess of other free RNAs and miRNAs in the exRNA mixture that are Ag-non-specific, nonetheless inhibited CHS effector cells Ag-specifically, as Ts cell derived exosomes [2]. This unusual finding of exRNAs acting Ag-specifically in the absence of exosomes was unraveled by demonstrating that the miRNAs became associated with Ag-specific exosomes produced by the Ag-specific B1a cells present in the mixture of CHS effector cells that made exosomes expressing surface Ab-like B cell Ag receptors (BCR). These mixed effector cell population also contained the Ag-specific CHS effector T cells and APC. These then were suppressed by the Ag-specific B1a cell-derived exosomes that had become associated with the miRNA [7].
These findings led to speculation that this represents an alternate pathway of Ag-specific targeting by freely circulating functional miRNA not contained in exosomes to regulate CHS effector T cells. In this case the miRNA among the exososme-free exRNA can be delivered to function in allergic CHS and other responses by its association with such B1a cell-derived, Ag-specific, originally non-suppressive exosomes. These exosomes were originally shown not to carry miRNA-150, but then were induced to gained it from the associating mixture of free exRNAs. Ag-specificity of the exosome-associated miRNA suppressive activity results from the presence of BCR on the B1a cell-derived exosome surface, allowing for their specific delivery to affect Ag-specific T cells in the CHS-effector cell mixture. The major miRNA fraction in the human circulation is free of exosomes, and instead is also protected from numerous RNases by complexing with chaperones, like argonautes and lipoproteins. This suggests that these miRNAs may similarly act by this alternative pathway of associating with or transfecting exosomes released by either cells neighboring the target cell population, or possibly even by the target cells themselves.
Overall, this is the first demonstration that miRNA free of exosomes can be functional and gain Ag-specific cell targeting to affect genetic function. This alternate pathway appears to be the only natural mechanism yet described for passing functional free extracellular miRNA between cells. This pathway of free exRNA intercellular transfer contrasts with classical donor cell release of RNA-containing and cell-targeting exosomes. Since significant amounts of circulating exRNA are not in exosomes, these findings likely have important biological and immunological significance, and may be relevant to both pathogenesis and treatment of diseases.
Determination That miRNA-150 Was Responsible for Exosome Suppression by Exploiting Their Ag-Specificity
The discovery that coating of the suppressive exosomes with Ag-specific Ab light chains was responsible for their Ag-specificity then led to discovery of miRNA-150 as the crucial delivered inhibitory molecule. The Ag-specific Ab light chains were judged to enable Ag-specific binding of the suppressive exosomes. This allowed for their fractionation using Ag-affinity column chromatography [2]. The Ag-binding exosomes had all the suppressive activity [2]. Processing of these Ag-binding exosomes, versus the Ag non-binding exosomes, to mRNA, with further conversion to cDNA, and then DNA deep sequencing with subsequent bioinformatic analysis suggested consideration of miRNA-150 as the important inhibitory molecule in the Ag-specific suppressive exosomes. This was verified by blocking of exosome suppression with the anti-sense polynucleotide of miRNA-150 (anti-miR-150), compared to numerous controls. Final definitive confirmation was shown by experiments in Ag-tolerized miRNA-150−/− mice. The crucial experiment showed that association of the non-suppressive exosomes from the unsuccessfully tolerized miRNA-150−/− mice with miRNA-150, rendered them suppressive, in comparison to several controls as described above. This constituted a definitive proof that miRNA-150 is the crucial inhibitory molecule carried by the exosomes that is responsible for the suppression of allergic cutaneous CHS.
APC Are the Target of the Ag-Specific Suppressive Exosomes
Further experiments showed that the inhibited CHS effector T cells are not the direct target of the suppressive exosomes, but rather their companion Ag-presenting cells, represented in our initial experiments by Ag-carrying macrophages. These APC are altered to become suppressive by delivery of exosome miRNA-150 to then be able to Ag-specifically suppress companion Ag-specific CHS-effector T cells that are recognizing Ag/MHC complexes on the APC [205]. Finally, as confirmation of the above formulation, depletion of macrophages from the total CHS-effector cell population, prior to in vitro incubation with the suppressive exosomes, that precedes the adoptive cell transfer of CHS, abolishes the suppressive action of the Ts cell exosome-delivered miRNA-150 on the CHS effector cell mixture. This observation confirms that Ag-presenting macrophages are essential for transferring the subsequent suppressive signal of yet unknown nature [205].
In current follow-up studies employing DTH induced with OVA without an adjuvant, we have observed similar tolerance, allowing us to attempt to confirm the hypothesis that this mechanism requires the binding of OVA peptides on the APC surface by the Ab light chains on the suppressive exosomes. Accordingly, our preliminary evidence shows that the Ts cell exosomes inhibition of CHS involves the binding of Ag peptide determinants in the peptide/MHC complex on the surface of the APC by the Ag-specific Ab light chains on the exosome surface. This specific binding likely allows for internalization of the suppressive exosomes for delivery of their miRNA-150 into the Ag-presenting macrophages to tilt their gene function towards a suppressive pathway to inhibit the CHS effector T cells. Further preliminary data suggests that the Ag-presenting macrophages that received the Ts cell exosomes may suppress the CHS effector T cells via the release of secondary suppressive exosomes. These secondary vesicles as a part of suppressive exosome cascade finally target the CHS effector T lymphocytes, leading to inhibition of their function. Currently, the exact mechanism of the Ag-specific, miRNA-150-mediated suppression by the CD8pos Ts cell-derived exosomes affecting Ag-presenting macrophages to inhibit effector T cells is the focus of our research. In this regard, in a helper T cell system, pretreatment of Ag-primed macrophages with the Ts cell-derived, Ag-specific exosomes, as in the CHS suppression system above, results in the reduction of helper T cell dependent, macrophage-mediated induction of B cell Ab responses to corpuscular Ag [205]. Additionally, treatment of macrophages with suppressive exosomes causes the enhancement of the generation of reactive oxygen intermediates [206]. On the other hand, the mechanism of action of the suppressive exosome miRNA-150 cargo may depend on cytokine responsiveness of the final T cell targets. Accordingly, the suppressive exosomes or the mixed miRNA in their phenol chloroform extracts, or even miRNA-150 alone, inhibit the in vitro responsiveness of HT-2 cells, a T cell line, to IL-2 [2,7]. In contrast, exosomes derived from lymphoid cells of unsuccessfully tolerized miRNA-150−/− mice are inactive in this model and their suppressive activity is restored by addition of miRNA-150 itself [2]. These additional findings suggest that the mechanisms enumerated above, acting alone or together, may play a role in the effector T cell suppression begun by the CD8pos Ts cell-derived primary exosomes delivering inhibitory miRNA-150 [2,7].
Additional preliminary data show that we are able to similarly induce DTH by intradermal injection of soluble casein Ag from milk, and activate similar Ag-specific suppression mediated by exosomal miRNA-150. Note that clinical observations suggest that hypersensitivity to casein is an aspect of food allergy to milk products, that likely consists of a mixture of a Type I allergic response mediated by food Ag-specific IgE Ab, and an additional possible DTH-like component. Therefore, this new technology of established procedures for dissection of biological properties of functional miRNA carried by suppressive exosomes may enable us to examine and perhaps alter immunoregulatory pathways in food allergy to milk products in a new molecular way.
Thus overall, our data have significant translational potential for greater understanding and potential construction of new therapies for diverse clinical allergic and immunological diseases based on the newly recognized, diverse and complex intercellular communications mediated by extracellular exosomes released by the involved cells, delivering RNAs and proteins to alter functions of acceptor cells involved in allergies and hypersensitivities.
Concluding Remarks
The pathogenesis of allergic and hypersensitivity responses relevant to human diseases appears to be significantly influenced by various exosome-dependent mechanisms. This principally involves exchange of functional RNA between cells that are nearby or at a distance. This leads us to the following conclusions.
Firstly, appreciation of novel near or distant exosome release to mediate functional exchanges, along with established intercellular communication pathways via cytokines, provides new reasons to more strongly consider allergy and hypersensitivity responses in vivo in preference to in vitro; i.e. in vivo veritas. This leads to a preference for in vivo and ex vivo experiments in order to more fully appreciate the undoubtedly exosome mediated interactions between immune and allergy mediating cells, and the various stromal cells in their microenvironment, as well as other cells at a distance, that also are sending RNA genetic instructions via the circulation [Fig. 1].
Secondly, it seems fairly certain that there will be new types of diagnostic approaches to allergic and hypersensitivity diseases involving the analysis of the RNAome and proteome of extracellular vesicles, by which genetic instructions are delivered to other cells. These instructive RNAs either may be or may not be carried in exosomes in the peripheral circulation, or body fluids, like the BALF, and interestingly may even be present in the breath exhalate.
Thirdly, understanding of allergic and hypersensitivity diseases will undoubtedly be better when integrating these newly recognized exosome intercellular exchange processes into prior findings and constructs. Thus, this new knowledge should lead to development of new preventive and therapeutic strategies beyond conventional modes of regulating and attenuating the immune response to specific allergens and Ag.
Fourthly, our and other studies have determined that therapeutic exosomes are biologically active after delivery via different routes, that were not considered previously. Since exosomes are natural and physiological, they can resist hostile environments, can have long lasting effects and can penetrate tissue barriers that do not permit transit of cells. These possibilities include therapeutic approaches far more tolerable to the patient than routine allergy shots. Such potential exosome therapies involve essentially cell free, non-invasive approaches, with new routes of administration, such as oral, nasal or inhaled. For the first time there is a real prospect of being able to specifically alter cell functions genetically in a physiological manner. This would be a huge and significant advance with profound clinical consequences.
Acknowledgments
This work was supported by NIH grants to P.W.A.: AI-59801, AI-07174, AI-76366-02, AI-1053786 and U54 DA036134, and by grants from the Polish National Scientific Centre number 2013/09/N/NZ6/00753 to K.N. and 2013/11/B/NZ6/02041 to K.B.
Abbreviations
- Ab
antibody
- Ag
antigen
- APC
antigen presenting cells
- BALF
bronchoalveolar lavage fluid
- BCR
B cell surface receptor for Ag
- CHS
contact hypersensitivity
- DC
dendritic cells
- DTH
delayed-type hypersensitivity
- PBMC
peripheral blood mononuclear cells
- KLH
keyhole limpet hemocyanin
- mEV
microbial extracellular vesicles
- TNP
trinitrophenyl
- Ts
T suppressor cells
References
- 1.Choudhuri K, Llodrá J, Roth EW, Tsai J, Gordo S, Wucherpfennig KW, Kam LC, Stokes DL, Dustin ML. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507:118–123. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryniarski K, Ptak W, Jayakumar A, Pullmann K, Caplan MJ, Chairoungdua A, Lu J, Adams BD, Sikora E, Nazimek K, Marquez S, Kleinstein SH, Sangwung P, Iwakiri Y, Delgato E, Redegeld F, Blokhuis BR, Wojcikowski J, Daniel AW, Groot Kormelink T, Askenase PW. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J Allergy Clin Immunol. 2013;132:170–181. e9. doi: 10.1016/j.jaci.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1:18397. doi: 10.3402/jev.v1i0.18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith ZJ, Lee C, Rojalin T, Carney RP, Hazari S, Knudson A, Lam K, Saari H, Ibañez EL, Viitala T, Laaksonen T, Yliperttula M, Wachsmann-Hogiu S. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J Extracell Vesicles. 2015;4:28533. doi: 10.3402/jev.v4.28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryniarski K, Ptak W, Martin E, Nazimek K, Szczepanik M, Sanak M, Askenase PW. Free extracellular miRNA functionally targets cells by transfecting exosomes from their companion cells. PLoS One. 2015;10:e0122991. doi: 10.1371/journal.pone.0122991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conde-Vancells J, Rodriguez-Suarez E, Embade N, Gil D, Matthiesen R, Valle M, Elortza F, Lu SC, Mato JM, Falcon-Perez JM. Characterization and Comprehensive Proteome Profiling of Exosomes Secreted by Hepatocytes. J Proteome Res. 2008;7:5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa Y, Taketomi Y, Murakami M, Tsujimoto M, Yanoshita R. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol Pharm Bull. 2013;36:66–75. doi: 10.1248/bpb.b12-00607. [DOI] [PubMed] [Google Scholar]
- 10.Arita S, Baba E, Shibata Y, Niiro H, Shimoda S, Isobe T, Kusaba H, Nakano S, Harada M. B cell activation regulates exosomal HLA production. Eur J Immunol. 2008;38:1423–1434. doi: 10.1002/eji.200737694. [DOI] [PubMed] [Google Scholar]
- 11.Mfunyi CM, Vaillancourt M, Vitry J, Nsimba Batomene TR, Posvandzic A, Lambert AA, Gilbert C. Exosome release following activation of the dendritic cell immunoreceptor: A potential role in HIV-1 pathogenesis. Virology. 2015;484:103–112. doi: 10.1016/j.virol.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyrer PC, Frizelle FA, Keenan JI. Escherichia coli-derived outer membrane vesicles are genotoxic to human enterocyte-like cells. Infect Agent Cancer. 2014;10:2. doi: 10.1186/1750-9378-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosal A, Upadhyaya BB, Fritz JV, Heintz-Buschart A, Desai MS, Yusuf D, Huang D, Baumuratov A, Wang K, Galas D, Wilmes P. The extracellular RNA complement of Escherichia coli. Microbiologyopen. 2015 doi: 10.1002/mbo3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sjöström AE, Sandblad L, Uhlin BE, Wai SN. Membrane vesicle-mediated release of bacterial RNA. Sci Rep. 2015;5:15329. doi: 10.1038/srep15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong SW, Kim MR, Lee EY, Kim JH, Kim YS, Jeon SG, Yang JM, Lee BJ, Pyun BY, Gho YS, Kim YK. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy. 2011;66:351–359. doi: 10.1111/j.1398-9995.2010.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, Gabrielsson S, Scheynius A. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses - novel mechanisms for host-microbe interactions in atopic eczema. PLoS One. 2011;6:e21480. doi: 10.1371/journal.pone.0021480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R. Identification of Double Stranded Genomic DNA Spanning all Chromosomes with Mutated KRAS and p53 DNA in the Serum Exosomes of Patients with Pancreatic Cancer. J Biol Chem. 2014;289:3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.San Lucas FA, Allenson K, Bernard V, Castillo J, Kim D, Ellis K, Ehli EA, Davies GE, Petersen JL, Li D, Wolff R, Katz M, Varadhachary G, Wistuba I, Maitra A, Alvarez H. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann Oncol. 2015 doi: 10.1093/annonc/mdv604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, Williams C, Rodriguez-Barrueco R, Silva JM, Zhang W, Hearn S, Elemento O, Paknejad N, Manova-Todorova K, Welte K, Bromberg J, Peinado H, Lyden D. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;41:283–287. doi: 10.1042/BST20120192. [DOI] [PubMed] [Google Scholar]
- 23.Camussi G, Deregibus MC, Tetta C. Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens. 2010;19:7–12. doi: 10.1097/MNH.0b013e328332fb6f. [DOI] [PubMed] [Google Scholar]
- 24.Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. 2016:51. doi: 10.1016/j.jpedsurg.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b Promotes Neural Plasticity and Functional Recovery after Treatment of Stroke with Multipotent Mesenchymal Stromal Cells in Rats Via Transfer of Exosome-Enriched Extracellular Particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ban JJ, Lee M, Im W, Kim M. Low pH increases the yield of exosome isolation. Biochem Biophys Res Commun. 2015;461:76–79. doi: 10.1016/j.bbrc.2015.03.172. [DOI] [PubMed] [Google Scholar]
- 29.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Lessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. 2010;1:1–8. doi: 10.1186/1758-907X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanc L, Liu J, Vidal M, Chasis JA, An X, Mohandas N. The water channel aquaporin-1 partitions into exosomes during reticulocyte maturation: implication for the regulation of cell volume. Blood. 2009;114:3928–3934. doi: 10.1182/blood-2009-06-230086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamzah M, Braun R, Eldridge M. Exosomes secreted by mesenchymal stem cells prevent hyperoxia-induced lung injury in newborn rat. Critical Care Med. 2014;42(Supplement 1):A1531. [Google Scholar]
- 33.King HW, Michael MZ, Gleadl JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Admyre C, Telemo E, Almqvist N, Lotvall J, Lahesmaa R, Scheynius A, Gabrielsson S. Exosomes - nanovesicles with possible roles in allergic inflammation. Allergy. 2008;63:404–408. doi: 10.1111/j.1398-9995.2007.01600.x. [DOI] [PubMed] [Google Scholar]
- 35.Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1:98–110. [PMC free article] [PubMed] [Google Scholar]
- 36.Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Turco DD, Starmann J, Macas J, Karpova D, Devraj K, Depboylu C, Landfried B, Arnold B, Plate KH, Höglinger G, Sültmann H, Altevogt P, Momma S. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12:e1001874. doi: 10.1371/journal.pbio.1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoorvogel W. Functional transfer of microRNA by exosomes. Blood. 2012;119:646–648. doi: 10.1182/blood-2011-11-389478. [DOI] [PubMed] [Google Scholar]
- 38.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–672. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 39.van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, Eijndhoven MA, Pegtel DM, Stoorvogel W, Würdinger T, Verhaar MC. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 40.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of β-catenin: a novel mechanism that antagonizes signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 44.Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, Budnik V. Mechanism of evenness interrupted (evi)-exosome release at synaptic moutons. J Biol Chem. 2012;287:16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal A, Fanelli G, Letizia M, Tung SL, Boardman D, Lechler R, Lombardi G, Smyth LA. Regulatory T cell-derived exosomes: Possible therapeutic and diagnostic tools in transplantation. Front Immunol. 2014;5:555. doi: 10.3389/fimmu.2014.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 48.Fernández-Messina L, Gutiérrez-Vázquez C, Rivas-García E, Sánchez-Madrid F, de la Fuente H. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol Cell. 2015;107:61–77. doi: 10.1111/boc.201400081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, Bernad A, Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin RK, Badrooks KB, Henningsson F, Heyman B, Conrad DH. Antigen transfer from exosomes to dendritic cells as an explanation for the immune enhancement seen by IgE immune complexes. PLoS One. 2014;9:e110609. doi: 10.1371/journal.pone.0110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujita Y, Yoshioka Y, Ito S, Araya J, Kuwano K, Ochiya T. Intercellular communication by extracellular vesicles and their microRNAs in asthma. Clin Ther. 2014;36:873–881. doi: 10.1016/j.clinthera.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural Heterogeneity and Protein Composition of Exosome-Like Vesicles (Prostasomes) in Human Semen. Prostate. 2009;69:159–167. doi: 10.1002/pros.20860. [DOI] [PubMed] [Google Scholar]
- 54.Jenjaroenpun P, Kremenska Y, Nair VM, Kremenskoy M, Joseph B, Kurochkin IV. Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. Peer J. 2013;1:e201. doi: 10.7717/peerj.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nolte-’t Hoen ENM, Buermans HPJ, Waasdorp M, Stoorvogel W, Wauben MHM, ’tHoen PAC. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou H, Cheruvanky A, Hu X, Matsumoto T, Hiramatsu N, Cho ME, Berger A, Leelahavanichkul A, Doi K, Chawla LS, Illei GG, Kopp JB, Balow JE, Austin HA, III, Yuen PST, Star RA. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008;74:613–621. doi: 10.1038/ki.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ung TH, Madsen HJ, Hellwinkel JE, Lencioni AM, Graner MW. Exosome proteomics reveals transcriptional regulator proteins with potential to mediate downstream pathways. Cancer Sci. 2014;105:1384–1392. doi: 10.1111/cas.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skogberg G, Lundberg V, Berglund M, Gudmundsdottir J, Telemo E, Lindgren S, Ekwall O. Human thymic epithelial primary cells produce exosomes carrying tissue-restricted antigens. Immunol Cell Biol. 2015;93:727–734. doi: 10.1038/icb.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang GJ, Liu Y, Qin A, Shah SV, Deng Z-b, Xiang X, Cheng Z, Liu C, Wang J, Zhang L, Grizzle WE, Zhang HG. Thymus Exosomes-Like Particles Induce Regulatory T Cells. J Immunol. 2008;181:5242–5248. doi: 10.4049/jimmunol.181.8.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bretz NP, Ridinger J, Rupp A-K, Rimbach K, Keller S, Rupp C, Marme F, Umansky L, Umansky V, Eigenbrod T, Sammar M, Altevogt P. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via TLR signaling. J Biol Chem. 2013;288:36691–36702. doi: 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson K, Chu J, Huang M, PowelL M, Villinger F, Bond V. Innate cytokines associate with exosomes in plasma of HIV-1+ individuals (P6205) J Immunol. 2013;190(Meeting Abstract Supplement):118.26. [Google Scholar]
- 63.Konadu KA, Chu J, Huang MB, Amancha PK, Armstrong W, Powell MD, Villinger F, Bond VC. Association of Cytokines With Exosomes in the Plasma of HIV-1-Seropositive Individuals. J Infect Dis. 2015;211:1712–1716. doi: 10.1093/infdis/jiu676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sampey GC, Saifuddin M, Schwab A, Barclay R, Punya S, Chung M-C, Hakami RM, Asad Zadeh M, Lepene B, Klase ZA, El-Hage N, Young M, Iordanskiy S, Kashanchi F. Exosomes from HIV-1-infected Cells Stimulate Production of Pro-inflammatory Cytokines through Trans-activating Response (TAR) RNA. J Biol Chem. 2016;291:1251–1266. doi: 10.1074/jbc.M115.662171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cossetti C, Iraci N, Mercer TR, Leonardi T, Alpi E, Drago D, Alfaro-Cervello C, Saini HK, Davis MP, Schaeffer J, Vega B, Stefanini M, Zhao CJ, Muller W, Garcia-Verdugo JM, Mathivanan S, Bachi A, Enright AJ, Mattick JS, Pluchino S. Extracellular Vesicles from Neural Stem Cells Transfer IFN-γ via Ifngr1 to Activate Stat1 Signaling in Target Cells. Mol Cell. 2014;56:193–204. doi: 10.1016/j.molcel.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shefler I, Pasmanik-Chor M, Kidron D, Mekori YA, Hershko AY. T cell derived microvesicles induce mast cell production of IL-24: Relevance to inflammatory skin diseases. J Allergy Clin Immunol. 2014;133:217–224. e3. doi: 10.1016/j.jaci.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 67.Shefler I, Salamon P, Reshef T, Mor A, Mekori YA. T Cell-Induced Mast Cell Activation: A Role for Microparticles Released from Activated T Cells. J Immunol. 2010;85:4206–4212. doi: 10.4049/jimmunol.1000409. [DOI] [PubMed] [Google Scholar]
- 68.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 69.Admyre C, Grunewald J, Thyberg J, Gripenback S, Tornling G, Eklund A, Scheynius A, Gabrielsson S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J. 2003;22:578–583. doi: 10.1183/09031936.03.00041703. [DOI] [PubMed] [Google Scholar]
- 70.Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mécheri S. Mast Cell-Derived Exosomes Induce Phenotypic and Functional Maturation of Dendritic Cells and Elicit Specific Immune Responses In Vivo. J Immunol. 2003;170:3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 71.Skokos D, Le Panse S, Villa I, Rousselle JC, Peronet R, David B, Namane A, Mécheri S. Mast Cell-Dependent B and T Lymphocyte Activation Is Mediated by the Secretion of Immunologically Active Exosomes. J Immunol. 2001;166:868–876. doi: 10.4049/jimmunol.166.2.868. [DOI] [PubMed] [Google Scholar]
- 72.Skokos D, Le Panse S, Villa I, Rousselle JC, Peronet R, Namane A, David B, Mécheri S. Nonspecific B and T cell-stimulatory activity mediated by mast cells is associated with exosomes. Int Arch Allergy Immunol. 2001;124:133–136. doi: 10.1159/000053691. [DOI] [PubMed] [Google Scholar]
- 73.Al-Nedawi K, Szemraj J, Cierniewski CS. Mast cell-derived exosomes activate endothelial cells to secrete plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2005;25:1744–1749. doi: 10.1161/01.ATV.0000172007.86541.76. [DOI] [PubMed] [Google Scholar]
- 74.Ekström K, Valadi H, Sjöstrand M, Malmhäll C, Bossios A, Eldh M, Lötvall J. Characterization of mRNA and microRNA in human mast cell-derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. J Extracell Vesicles. 2012;16:1. doi: 10.3402/jev.v1i0.18389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wahlgren J, de Karlson TL, Glader P, Telemo E, Valadi H. Activated Human T Cells Secrete Exosomes That Participate in IL-2 Mediated Immune Response Signaling. PLoS One. 2012;7:e49723. doi: 10.1371/journal.pone.0049723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kulshreshtha A, Ahmad T, Agrawal A, Ghosh B. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J Allergy Clin Immunol. 2012;131:1194–1203. e14. doi: 10.1016/j.jaci.2012.12.1565. [DOI] [PubMed] [Google Scholar]
- 77.Xia YC, Harris T, Stewart AG, Mackay GA. Secreted factors from human mast cells trigger inflammatory cytokine production by human airway smooth muscle cells. Int Arch Allergy Immunol. 2013;160:75–85. doi: 10.1159/000339697. [DOI] [PubMed] [Google Scholar]
- 78.Mazzeo C, Canas JA, Zafra MP, Rojas Marco A, Fernandez-Nieto M, Sanz V, Mittelbrunn M, Izquierdo M, Baixaulli F, Sastre J, Del Pozo V. Exosome secretion by eosinophils: A possible role in asthma pathogenesis. J Allergy Clin Immunol. 2015;135:1603–1613. doi: 10.1016/j.jaci.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 79.Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA, Newman GR, Jasani B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods. 2001;247:163–174. doi: 10.1016/s0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- 80.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sobo-Vujanovic A, Munich S, Vujanovic NL. Dendritic-cell exosomes cross-present Toll-like receptor-ligands and activate bystander dendritic cells. Cell Immunol. 2014;289:119–127. doi: 10.1016/j.cellimm.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qazi KR, Gehrmann U, Domange Jordo E, Karlsson MC, Gabrielsson S. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood. 2009;113:2673–2683. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- 83.Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, Bonnerot C. Exosomes bearing HLA DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14:713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- 84.Carroll-Portillo A, Surviladze Z, Cambi A, Lidke DS, Wilson BS. Mast cell synapses and exosomes: membrane contacts for information exchange. Front Immunol. 2012;3:46. doi: 10.3389/fimmu.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaudenzio N, Espagnolle N, Mars LT, Liblau R, Valitutti S, Espinosa E. Cell-cell cooperation at the T helper cell/mast cell immunological synapse. Blood. 2009;114:4979–4988. doi: 10.1182/blood-2009-02-202648. [DOI] [PubMed] [Google Scholar]
- 86.Ramachandran S, Palanisamy V. Horizontal transfer of RNAs: exosomes as mediators of intercellular communication. Wiley Interdiscip Rev RNA. 2012;3:286–293. doi: 10.1002/wrna.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gong J, Yang NS, Croft M, Weng IC, Sun L, Liu FT, Chen SS. The antigen presentation function of bone marrow-derived mast cells is spatiotemporally restricted to a subset expressing high levels of cell surface FcεRI and MHC II. BMC Immunol. 2010;11:34. doi: 10.1186/1471-2172-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beauvillain C, Juste MO, Dion S, Pierre J, Dimier-Poisson I. Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine. 2009;27:1750–1757. doi: 10.1016/j.vaccine.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 89.Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16:24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pitt JM, Charrier M, Viaud S, André F, Besse B, Chaput N, Zitvogel L. Dendritic Cell-Derived Exosomes as Immunotherapies in the Fight against Cancer. J Immunol. 2014;193:1006–1011. doi: 10.4049/jimmunol.1400703. [DOI] [PubMed] [Google Scholar]
- 91.Vermeij R, Leffers N, van der Burg SH, Melief CJ, Daemen T, Nijman HW. Immunological and Clinical Effects of Vaccines Targeting p53-Overexpressing Malignancies. J Biomed Biotechnol. 2011;2011:702146. doi: 10.1155/2011/702146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Viaud S, Théry C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N. Dendritic cell-derived exosomes for cancer immunotherapy: what’s next? Cancer Res. 2010;70:1281–1285. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 93.Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, Gabrielsson S. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007;120:1418–1424. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 94.Suojalehto H, Lindström I, Majuri ML, Mitts C, Karjalainen J, Wolff H, Alenius H. Altered microRNA expression of nasal mucosa in long-term asthma and allergic rhinitis. Int Arch Allergy Immunol. 2014;163:168–178. doi: 10.1159/000358486. [DOI] [PubMed] [Google Scholar]
- 95.Wu G, Yang G, Zhang R, Xu G, Zhang L, Wen W, Lu J, Liu J, Yu Y. Altered microRNA Expression Profiles of Extracellular Vesicles in Nasal Mucus From Patients With Allergic Rhinitis. Allergy Asthma Immunol Res. 2015;7:449–457. doi: 10.4168/aair.2015.7.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shin SH, Park JY, Jeon CH, Choi JK, Lee SH. Quantitative analysis of eotaxin and RANTES messenger RNA in nasal polyps: association of tissue and nasal eosinophils. Laryngoscope. 2000;110:1353–1357. doi: 10.1097/00005537-200008000-00025. [DOI] [PubMed] [Google Scholar]
- 97.Qiu S, Du Y, Duan X, Geng X, Xie J, Gao H, Yang PC. Cytotoxic T lymphocytes mediate chronic inflammation of the nasal mucosa of patients with atypical allergic rhinitis. N Am J Med Sci. 2011;3:378–383. doi: 10.4297/najms.2011.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lässer C, O’Neil SE, Shelke GV, Sihlbom C, Hansson SF, Gho YS, Lundbäck B, Lötvall J. Exosomes in the nose induce immune cell trafficking and harbour an altered protein cargo in chronic airway inflammation. J Transl Med. 2016;14:181. doi: 10.1186/s12967-016-0927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ptak W, Geba GP, Askenase PW. Initiation of delayed-type hypersensitivity by low doses of monoclonal IgE antibody. Mediation by serotonin and inhibition by histamine. J Immunol. 1991;146:3929–3936. [PubMed] [Google Scholar]
- 100.Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Y, Li J, Bian Z, Liang X, Cai X, Yin Y, Wang C, Zhang T, Zhu D, Zhang D, Xu J, Chen Q, Ba Y, Liu J, Wang Q, Chen J, Wang J, Wang M, Zhang Q, Zhang J, Zen K, Zhang CY. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22:107–126. doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prado N, de Alché JD, Casado-Vela J, Mas S, Villalba M, Rodríguez R, Batanero E. Nanovesicles Are Secreted during Pollen Germination and Pollen Tube Growth: A Possible Role in Fertilization. Mol Plant. 2014;7:573–577. doi: 10.1093/mp/sst153. [DOI] [PubMed] [Google Scholar]
- 102.Wei T, Hibino H, Omura T. Release of Rice dwarf virus from insect vector cells involves secretory exosomes derived from multivesicular bodies. Commun Integr Biol. 2009;2:324–326. doi: 10.4161/cib.2.4.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vallhov H, Gutzeit C, Hultenby K, Valenta R, Gronlund H, Scheynius A. Dendritic cell-derived exosomes carry the major cat allergen Fel d 1 and induce an allergic immune response. Allergy. 2015;70:1651–1655. doi: 10.1111/all.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luo X, Han M, Liu J, Wang Y, Luo X, Zheng J, Wang S, Liu Z, Liu D, Yang PC, Li H. Epithelial cell-derived micro RNA-146a generates interleukin-10-producing monocytes to inhibit nasal allergy. Sci Rep. 2015;5:15937. doi: 10.1038/srep15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Platts-Mills TA, Vaughan JW, Blumenthal K, Woodfolk JA, Sporik RB. Decreased prevalence of asthma among children with high exposure to cat allergen: relevance of the modified Th2 response. Mediators Inflamm. 2001;10:288–291. doi: 10.1080/09629350152700902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105a.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015;8(1):17. doi: 10.1186/s40413-015-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gehrmann U, Hiltbrunner S, Georgoudaki AM, Karlsson MC, Näslund TI, Gabrielsson S. Synergistic induction of adaptive antitumor immunity by co-delivery of antigen with α-galactosylceramide on exosomes. Cancer Res. 2013;73:3865–3876. doi: 10.1158/0008-5472.CAN-12-3918. [DOI] [PubMed] [Google Scholar]
- 107.Campos RA, Szczepanik M, Itakura A, Akahira-Azuma M, Sidobre S, Kronenberg M, Askenase PW. Cutaneous immunization rapidly activates liver invariant Vα14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198:1785–1796. doi: 10.1084/jem.20021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamamoto N, Kerfoot SM, Hutchinson AT, Dela Cruz CS, Nakazawa N, Szczepanik M, Majewska-Szczepanik M, Nazimek K, Ohana N, Bryniarski K, Mori T, Muramatsu M, Kanemitsu K, Askenase PW. Expression of activation-induced cytidine deaminase enhances the clearance of pneumococcal pneumonia: evidence of a subpopulation of protective anti-pneumococcal B1a cells. Immunology. 2016;147:97113. doi: 10.1111/imm.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prado N, De Linares C, Sanz ML, Gamboa P, Villalba M, Rodríguez R, Batanero E. Pollensomes as Natural Vehicles for Pollen Allergens. J Immunol. 2015;195:445–449. doi: 10.4049/jimmunol.1500452. [DOI] [PubMed] [Google Scholar]
- 110.Gol J, Van Niel G, Lebreton C, Lepelletier Y, Candalh C, Dugave C, Heath JK, Raposo G, Cerf-Bensussan N, Heyman M. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology. 2007;132:1866–1876. doi: 10.1053/j.gastro.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 111.Sánchez-Gómez FJ, González-Morena JM, Vida Y, Pérez-Inestrosa E, Blanca M, Torres MJ, Pérez-Sala D. Amoxicillin haptenates intracellular proteins that can be transported in exosomes to target cells. Allergy. 2016 doi: 10.1111/all.12958. in press. [DOI] [PubMed] [Google Scholar]
- 112.Choi EB, Hong SW, Kim DK, Jeon SG, Kim KR, Cho SH, Gho YS, Jee YK, Kim YK. Decreased diversity of nasal microbiota and their secreted extracellular vesicles in patients with chronic rhinosinusitis based on a metagenomic analysis. Allergy. 2014;69:517–526. doi: 10.1111/all.12374. [DOI] [PubMed] [Google Scholar]
- 113.Buck AH, Coakley G, Simbari F, McSorley HJ, Quintana JF, Le Bihan T, Kumar S, Abreu-Goodger C, Lear M, Harcus Y, Ceroni A, Babayan SA, Blaxter M, Ivens A, Maizels RM. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun. 2014;5:5488. doi: 10.1038/ncomms6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marcilla A, Trelis M, Cortés A, Sotillo J, Cantalapiedra F, Minguez MT, Valero ML, Sánchez del Pino MM, Muñoz-Antoli C, Toledo R, Bernal D. Extracellular Vesicles from Parasitic Helminths Contain Specific Excretory/Secretory Proteins and Are Internalized in Intestinal Host Cells. PLoS One. 2012;7:e45974. doi: 10.1371/journal.pone.0045974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Siles-Lucas M, Morchon R, Simon F, Manzano-Roman R. Exosome-transported microRNAs of helminth origin: new tools for allergic and autoimmune diseases therapy? Parasite Immunol. 2015;37:208–214. doi: 10.1111/pim.12182. [DOI] [PubMed] [Google Scholar]
- 116.Atayde VD, Aslan H, Townsend S, Hassani K, Kamhawi S, Olivier M. Exosome secretion by the parasitic protozoan Leishmania within the sand fly midgut. Cell Rep. 2015;13:957–967. doi: 10.1016/j.celrep.2015.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, Reiner NE. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol. 2010;185:5011–5022. doi: 10.4049/jimmunol.1000541. [DOI] [PubMed] [Google Scholar]
- 118.Trocoli Torrecilhas AC, Tonelli RR, Pavanelli WR, da Silva JS, Schumacher RI, de Souza W, Silva ENC, de Almeida Abrahamsohn I, Colli W, Manso Alves MJ. Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 2009;11:29–39. doi: 10.1016/j.micinf.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 119.Twu O, de Miguel N, Lustig G, Stevens GC, Vashisht AA, Wohlschlegel JA, Johnson PJ. Trichomonas vaginalis Exosomes Deliver Cargo to Host Cells and Mediate Host:Parasite Interactions. PLoS Pathog. 2013;9:e1003482. doi: 10.1371/journal.ppat.1003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chung KF. Cytokines as targets in chronic obstructive pulmonary disease. Curr Drug Targets. 2006;7:675–681. doi: 10.2174/138945006777435263. [DOI] [PubMed] [Google Scholar]
- 124.Park JA, Sharif AS, Tschumperlin DJ, Lau L, Limbrey R, Howarth P, Drazen JM. Tissue factor-bearing exosome secretion from human mechanically stimulated bronchial epithelial cells in vitro and in vivo. J Allergy Clin Immunol. 2012;130:1375–1383. doi: 10.1016/j.jaci.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vargas A, Lavoie J-P. Neutrophil-Derived Exosomes: A New Mechanism Possibly Contributing To Airway Smooth Muscle Remodeling. Am J Respir Crit Care Med. 2015;191:A6475. doi: 10.1165/rcmb.2016-0033OC. [DOI] [PubMed] [Google Scholar]
- 126.Torregrosa Paredes P, Esser J, Admyre C, Nord M, Rahman QK, Lukic A, Radmark O, Gronneberg R, Grunewald J, Eklund A, Scheynius A, Gabrielsson S. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy. 2012;67:911–919. doi: 10.1111/j.1398-9995.2012.02835.x. [DOI] [PubMed] [Google Scholar]
- 127.Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral Sphingomyelinase 2 (nSMase2)-dependent Exosomal Transfer of Angiogenic MicroRNAs Regulate Cancer Cell Metastasis. J Biol Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Singh R, Pochampally R, Watabe K, Lu Z, Mo YY. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13:256. doi: 10.1186/1476-4598-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, Furukawa J, Fujitani N, Shinohara Y, Igarashi Y. Decreased Amyloid-β Pathologies by Intracerebral Loading of Glycosphingolipid-enriched Exosomes in Alzheimer Model Mice. J Biol Chem. 2014;289:24488–24498. doi: 10.1074/jbc.M114.577213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang W, Schmid E, Nurbaeva MK, Szteyn K, Leibrock C, Yan J, Schaller M, Gulbins E, Shumilina E, Lang F. Role of acid sphingomyelinase in the regulation of mast cell function. Clin Exp Allergy. 2014;44:79–90. doi: 10.1111/cea.12229. [DOI] [PubMed] [Google Scholar]
- 131.Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci USA. 2009;4:18704–18709. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Plank MW, Maltby S, Tay HL, Stewart J, Eyers F, Hansbro PM, Foster PS. MicroRNA Expression Is Altered in an Ovalbumin-Induced Asthma Model and Targeting miR-155 with Antagomirs Reveals Cellular Specificity. PLoS One. 2015;10:e0144810. doi: 10.1371/journal.pone.0144810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Canas JA, Mazzeo C, Guerra A, Barranco P, Quirce S, Ruiz Hornillos J, Izquierdo M, Sastre J, del Pozo V. Exosomes from eosinophils of asthmatic patients produce functional alterations on structural lung cell. EAACI Online Library. 2015:98145. [Google Scholar]
- 134.Del Pozo V, Mazzeo C, Rodriguez Marco A, Fernandez-Nieto MDM, Paz Zafra M, Sanz V, Sastre J. Exosomes Secretion By Eosinophils: A Possible Role In Asthma Pathogenesis. J Allergy Clin Immunol. 2014;133(Supplement):AB58. doi: 10.1016/j.jaci.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 135.Mahmudi-Azer S, Downey GP, Moqbel R. Translocation of the tetraspanin CD63 in association with human eosinophil mediator release. Blood. 2002;99:4039–4047. doi: 10.1182/blood.v99.11.4039. [DOI] [PubMed] [Google Scholar]
- 136.Kumar M, Ahmad T, Sharma A, Mabalirajan U, Kulshreshtha A, Agrawal A, Ghosh B. Let-7 microRNA-mediated regulation of IL-13 and allergic airway inflammation. J Allergy Clin Immunol. 2011;128:1077–1085. e10. doi: 10.1016/j.jaci.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 137.Esser J, Gehrmann U, D’Alexandri FL, Hidalgo-Estevez AM, Wheelock CE, Scheynius A, Gabrielsson S, Radmark O. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J Allergy Clin Immunol. 2010;126:1032–1040. doi: 10.1016/j.jaci.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 138.Majumdar R, Tavakoli Tameh A, Parent CA. Exosomes Mediate LTB4 Release during Neutrophil Chemotaxis. Plos Biol. 2016;14:e1002336. doi: 10.1371/journal.pbio.1002336. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139.Sadik CD, Luster AD. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J Leukoc Biol. 2012;91:207–215. doi: 10.1189/jlb.0811402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289:L807–815. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- 141.Peters M, Kauth M, Schwarze J, Körner-Rettberg C, Riedler J, Nowak D, Braun-Fahrländer C, von Mutius E, Bufe A, Holst O. Inhalation of stable dust extract prevents allergen induced airway inflammation and hyperresponsiveness. Thorax. 2006;61:134–139. doi: 10.1136/thx.2005.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, Madeira FB, Beyaert R, van Loo G, Bracher F, von Mutius E, Chanez P, Lambrecht BN, Hammad H. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349:1106–1110. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 143.Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY, Park J, Jee YK, Zhu Z, Koh YY, Gho YS, Kim YK. Extracellular vesicles, especially derived from Gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin Exp Allergy. 2013;43:443–454. doi: 10.1111/cea.12085. [DOI] [PubMed] [Google Scholar]
- 144.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, Vannella KM, Butler CA, Hargadon B, Sciurba JC, Gieseck RL, Thompson RW, White S, Abbas AR, Jackman J, Wu LC, Egen JG, Heaney LG, Ramalingam TR, Arron JR, Wynn TA, Bradding P. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7:301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 145.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JGN. Protective Effects of Sphingosine 1-Phosphate in Murine Endotoxin-induced Inflammatory Lung Injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 146.Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013;4:2712. doi: 10.1038/ncomms3712. [DOI] [PubMed] [Google Scholar]
- 147.Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging. 2014;35:1792–1800. doi: 10.1016/j.neurobiolaging.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim MR, Hong SW, Choi EB, Lee WH, Kim YS, Jeon SG, Jang MH, Gho YS, Kim YK. Staphylococcus aureus-derived extracellular vesicles induce neutrophilic pulmonary inflammation via both Th1 and Th17 cell responses. Allergy. 2012;67:1271–1281. doi: 10.1111/all.12001. [DOI] [PubMed] [Google Scholar]
- 149.Rebane A, Akdis CA. MicroRNAs in allergy and asthma. Curr Allergy Asthma Rep. 2014;14:424. doi: 10.1007/s11882-014-0424-x. [DOI] [PubMed] [Google Scholar]
- 150.Levanen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JL, Skold CM, Svartengren M, Grunewald J, Gabrielsson S, Eklund A, Larsson BM, Woodruff PG, Erle DJ, Wheelock AM. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013;131:894–903. doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M, Nguyen C, Barczak AJ, Zlock LT, Blagev DP, Finkbeiner WE, Ansel KM, Arron JR, Erle DJ, Woodruff PG. Airway epithelial miRNA expression is altered in asthma. Am J Respir Crit Care Med. 2012;186:965–974. doi: 10.1164/rccm.201201-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maruoka S, Gon Y, Shikano S, Shintani Y, Koyama D, Sekiyama T, Hiranuma H, Inoue T, Takeshita I, Tsuboi E, Soda K, Hashimoto S. Exosomal MicroRNAs In The Serum Are Potential Real-Time Biomarkers For Allergic Inflammation In The Airway Of Mice. ATS 2014 Meeting Abstracts; p. A4211. [Google Scholar]
- 153.Pinkerton M, Chinchilli V, Banta E, Craig T, August A, Bascom R, Cantorna M, Harvill E, Ishmael FT. Differential expression of microRNAs in exhaled breath condensates of patients with asthma, patients with chronic obstructive pulmonary disease, and healthy adults. J Allergy Clin Immunol. 2013;132:217–219. doi: 10.1016/j.jaci.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 154.Sinha A, Yadav AK, Chakraborty S, Kabra SK, Lodha R, Kumar M, Kulshreshtha A, Sethi T, Pandey R, Malik G, Laddha S, Mukhopadhyay A, Dash D, Ghosh B, Agrawal A. Exosome-enclosed microRNAs in exhaled breath hold potential for biomarker discovery in patients with pulmonary diseases. J Allergy Clin Immunol. 2013;132:219–222. doi: 10.1016/j.jaci.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 155.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kurchinovich A, Burwinkel B. Distinct AGO1 and AGO2 associated miRNA profiles in human cells and blood plasma. RNA Biol. 2012;9:1066–1075. doi: 10.4161/rna.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li L, Zhu D, Huang L, Zhang J, Bian Z, Chen X, Liu Y, Zhang CY, Zen K. Argonaute 2 complexes selectively protect the circulating microRNAs in cell-secreted microvesicles. PLoS One. 2012;7:e46957. doi: 10.1371/journal.pone.0046957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Prado N, Marazuela EG, Segura E, Fernandez-Garcia H, Villalba M, Thery C, Rodriguez R, Batanero E. Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J Immunol. 2008;181:1519–1525. doi: 10.4049/jimmunol.181.2.1519. [DOI] [PubMed] [Google Scholar]
- 160.Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, Telemo E. “Tolerosomes” are produced by intestinal epithelial cells. Eur J Immunol. 2001;31:2892–2900. doi: 10.1002/1521-4141(2001010)31:10<2892::aid-immu2892>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 161.Lonnqvist A, Ostman S, Almqvist N, Hultkrantz S, Telemo E, Wold AE, Rask C. Neonatal exposure to staphylococcal superantigen improves induction of oral tolerance in a mouse model of airway allergy. Eur J Immunol. 2009;39:447–456. doi: 10.1002/eji.200838418. [DOI] [PubMed] [Google Scholar]
- 162.Van Niel G, Mallegol J, Bevilacqua C, Candalh C, Brugière S, Tomaskovic-Crook E, Heath JK, Cerf-Bensussan N, Heyman M. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut. 2003;52:1690–1697. doi: 10.1136/gut.52.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. J Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 164.Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, Zhou X, Wang X, Gao X, Li X. Immune-related MicroRNAs are Abundant in Breast Milk Exosomes. Int J Biol Sci. 2012;8:118–123. doi: 10.7150/ijbs.8.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J Dairy Sci. 2012;95:4831–4841. doi: 10.3168/jds.2012-5489. [DOI] [PubMed] [Google Scholar]
- 166.Gu Y, Li M, Wang T, Liang Y, Zhong Z, Wang X, Zhou Q, Chen L, Lang Q, He Z, Chen X, Gong J, Gao X, Li X, Lv X. Lactation-Related MicroRNA Expression Profiles of Porcine Breast Milk Exosomes. PLoS One. 2012;7:e43691. doi: 10.1371/journal.pone.0043691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sun Q, Chen X, Yu J, Zen K, Zhang CY, Li L. Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein Cell. 2013;4:197–210. doi: 10.1007/s13238-013-2119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Parigi SM, Eldh M, Larssen P, Gabrielsson S, Villablanca EJ. Breast Milk and Solid Food Shaping Intestinal Immunity. Front Immunol. 2015;6:415. doi: 10.3389/fimmu.2015.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pieters BC, Arntz OJ, Bennink MB, Broeren MG, van Caam AP, Koenders MI, van Lent PL, van den Berg WB, de Vries M, van der Kraan PM, van de Loo FA. Commercial Cow Milk Contains Physically Stable Extracellular Vesicles Expressing Immunoregulatory TGF-β. PLoS One. 2015;10:e0121123. doi: 10.1371/journal.pone.0121123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Melnik BC, John SM, Schmitz G. Milk: An exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy? J Transl Med. 2014;12:43. doi: 10.1186/1479-5876-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Izumi H, Tsuda M, Sato Y, Kosaka N, Ochiya T, Iwamoto H, Namba K, Takeda Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J Dairy Sci. 2015;98:2920–2933. doi: 10.3168/jds.2014-9076. [DOI] [PubMed] [Google Scholar]
- 172.Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ. miR-181a Is an Intrinsic Modulator of T Cell Sensitivity and Selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 173.Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine. 2014;10:1517–1527. doi: 10.1016/j.nano.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Torregrosa Paredes P, Gutzeit C, Johansson S, Admyre C, Stenius F, Alm J, Scheynius A, Gabrielsson S. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy. 2014;69:463–471. doi: 10.1111/all.12357. [DOI] [PubMed] [Google Scholar]
- 175.Arntz OJ, Pieters BC, Oliveira MC, Broeren MG, Bennink MB, de Vries M, van Lent PL, Koenders MI, van den Berg WB, van der Kraan PM, van de Loo FA. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol Nutr Food Res. 2015;59:1701–1712. doi: 10.1002/mnfr.201500222. [DOI] [PubMed] [Google Scholar]
- 176.Kim JH, Jeun EJ, Hong CP, Kim SH, Jang MS, Lee EJ, Moon SJ, Yun CH, Im SH, Jeong SG, Park BY, Kim KT, Seoh JY, Kim YK, Oh SJ, Ham JS, Yang BG, Jang MH. Extracellular vesicle-derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. J Allergy Clin Immunol. 2016;137:507–516. e8. doi: 10.1016/j.jaci.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 177.Chu H, Khosravi A, Kusumawardhani IP, Kwon AH, Vasconcelos AC, Cunha LD, Mayer AE, Shen Y, Wu WL, Kambal A, Targan SR, Xavier RJ, Ernst PB, Green DR, McGovern DP, Virgin HW, Mazmanian SK. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116–1120. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cheng Y, Schorey JS. Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur J Immunol. 2013;43:3279–3290. doi: 10.1002/eji.201343727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM, O’Connell RM. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immunol. 2004;72:4127–4137. doi: 10.1128/IAI.72.7.4127-4137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim OY, Hong BS, Park KS, Yoon YJ, Choi SJ, Lee WH, Roh TY, Lötvall J, Kim YK, Gho YS. Immunization with Escherichia coli Outer Membrane Vesicles Protects Bacteria-Induced Lethality via Th1 and Th17 Cell Responses. J Immunol. 2013;190:4092–4102. doi: 10.4049/jimmunol.1200742. [DOI] [PubMed] [Google Scholar]
- 182.Kim SH, Bianco N, Menon R, Lechman ER, Shufesky WJ, Morelli AE, Robbins PD. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol Ther. 2006;13:289–300. doi: 10.1016/j.ymthe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 183.Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J Immunol. 2007;179:2242–2249. doi: 10.4049/jimmunol.179.4.2242. [DOI] [PubMed] [Google Scholar]
- 184.Ruffner MA, Kim SH, Bianco NR, Francisco LM, Sharpe AH, Robbins PD. B7–1/2, but not PD-L1/2 molecules, are required on IL-10-treated tolerogenic DC and DC-derived exosomes for in vivo function. Eur J Immunol. 2009;39:3084–3090. doi: 10.1002/eji.200939407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 186.Bianco NR, Kim SH, Ruffner MA, Robbins PD. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009;60:380–389. doi: 10.1002/art.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Karlsson MR, Kahu H, Hanson LA, Telemo E, Dahlgren UI. An established immune response against ovalbumin is suppressed by a transferable serum factor produced after ovalbumin feeding: a role of CD25+ regulatory cells. Scand J Immunol. 2002;55:470–477. doi: 10.1046/j.1365-3083.2002.t01-1-01079.x. [DOI] [PubMed] [Google Scholar]
- 188.Karlsson MR, Kahu H, Hanson LA, Telemo E, Dahlgren UI. Tolerance and bystander suppression, with involvement of CD25-positive cells, is induced in rats receiving serum from ovalbumin-fed donors. Immunology. 2000;100:326–333. doi: 10.1046/j.1365-2567.2000.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang C, Kim SH, Bianco NR, Robbins PD. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One. 2011;6:e22517. doi: 10.1371/journal.pone.0022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. MHC class II+ exosomes in plasma suppress inflammation in an antigen-specific and Fas ligand/Fas-dependent manner. J Immunol. 2007;179:2235–2241. doi: 10.4049/jimmunol.179.4.2235. [DOI] [PubMed] [Google Scholar]
- 191.Ptak W, Nazimek K, Askenase PW, Bryniarski K. From mysterious supernatant entity to miRNA-150 in antigen-specific exosomes: a history of hapten-specific T suppressor factor. Arch Immunol Ther Exp. 2015;63:345–356. doi: 10.1007/s00005-015-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bryniarski K, Nazimek K, Martin E, Ptak M, Askenase PW, Ptak W. CD8+ T cell-derived exosomal miRNA-150 suppresses induction and effector phases of murine contact sensitivity as well as symptoms of active allergy. Centr Eur J Immunol. 2014;39(Supplement 1):20. [Google Scholar]
- 193.Dey N, Szczepanik M, Lau K, Majewska M, Askenase P. Stimulatory lipids accumulate in the mouse liver within 30 min of contact sensitization to facilitate the activation of naive iNKT cells in a CD1d-dependent fashion. Scand J Immunol. 2011;74:52–61. doi: 10.1111/j.1365-3083.2011.02540.x. [DOI] [PubMed] [Google Scholar]
- 194.Askenase PW, Bryniarski K, Paliwal V, Redegeld F, Groot Kormelink T, Kerfoot S, Hutchinson AT, van Loveren H, Campos R, Itakura A, Majewska-Szczepanik M, Yamamoto N, Nazimek K, Szczepanik M, Ptak W. A subset of AID-dependent B-1a cells initiates hypersensitivity and pneumococcal pneumonia resistance. Ann N Y Acad Sci. 2015;1362:200–214. doi: 10.1111/nyas.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kerfoot S, Szczepanik M, Tung J, Askenase P. Identification of initiator B-cells, a novel subset of activation induced deaminase-dependent B-1-like cells that mediate initiation of contact sensitivity. J Immunol. 2008;181:1717–1727. doi: 10.4049/jimmunol.181.3.1717. [DOI] [PubMed] [Google Scholar]
- 196.Yang Y, Wang C, Yang Q, Kantor AB, Chu H, Ghosn EE, Qin G, Mazmanian SK, Han J, Herzenberg LA. Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. eLife. 2015;4:e09083. doi: 10.7554/eLife.09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Itakura A, Szczepanik M, Campos RA, Paliwal V, Majewska M, Matsuda H, Takatsu K, Askenase PW. An hour after immunization peritoneal B-1 cells are activated to migrate to lymphoid organs where within 1 day they produce IgM antibodies that initiate elicitation of contact sensitivity. J Immunol. 2005;175:7170–7178. doi: 10.4049/jimmunol.175.11.7170. [DOI] [PubMed] [Google Scholar]
- 198.Askenase PW, Rosenstein RW, Ptak W. T cells produce an antigen binding factor with in vivo activity analogous to IgE antibody. J Exp Med. 1983;157:862–873. doi: 10.1084/jem.157.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Geba GP, Wegner CD, Wolyniec WW, Li Y, Askenase PW. Nonatopic asthma: in vivo airway hyperreactivity adoptively transferred to naive mice by THY-1(+) and B220(+) antigen-specific cells that lack surface expression of CD3. J Clin Invest. 1997;100:629–638. doi: 10.1172/JCI119574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Szczepanik M, Akahira-Azuma M, Bryniarski K, Tsuji RF, Kawikova I, Ptak W, Kiener C, Campos RA, Askenase PW. B-1 B cells mediate required early T cell recruitment to elicit protein-induced delayed-type hypersensitivity. J Immunol. 2003;171:6225–6235. doi: 10.4049/jimmunol.171.11.6225. [DOI] [PubMed] [Google Scholar]
- 201.Redegeld FA, van der Heijden MW, Kool M, Heijdra BM, Garssen J, Kraneveld AD, Van Loveren H, Roholl P, Saito T, Verbeek JS, Claassens J, Koster AS, Nijkamp FP. Immunoglobulin-free light chains elicit immediate hypersensitivity-like responses. Nat Med. 2002;8:694–701. doi: 10.1038/nm722. [DOI] [PubMed] [Google Scholar]
- 202.Hutchinson AT, Jones DR, Raison RL. The ability to interact with cell membranes suggests possible biological roles for free light chain. Immunol Lett. 2012;142:75–77. doi: 10.1016/j.imlet.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 203.Hutchinson AT, Ramsland PA, Jones DR, Agostino M, Lund ME, Jennings CV, Bockhorni V, Yuriev E, Edmundson AB, Raison RL. Free Ig light chains interact with sphingomyelin and are found on the surface of myeloma plasma cells in an aggregated form. J Immunol. 2010;185:4179–4188. doi: 10.4049/jimmunol.1001956. [DOI] [PubMed] [Google Scholar]
- 204.Hutchinson AT, Malik A, Berkahn MB, Agostino M, To J, Tacchi JL, Djordjevic SP, Turnbull L, Whitchurch CB, Edmundson AB, Jones DR, Raison RL, Ramsland PA. Formation of assemblies on cell membranes by secreted proteins: molecular studies of free λ light chain aggregates found on the surface of myeloma cells. Biochem J. 2013;454:479–489. doi: 10.1042/BJ20130575. [DOI] [PubMed] [Google Scholar]
- 205.Nazimek K, Ptak W, Nowak B, Ptak M, Askenase PW, Bryniarski K. Macrophages play an essential role in antigen-specific immune suppression mediated by T CD8+ cell-derived exosomes. Immunology. 2015;146:23–32. doi: 10.1111/imm.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nazimek K, Nowak B, Marcinkiewicz J, Ptak M, Ptak W, Bryniarski K. Enhanced generation of reactive oxygen intermediates by suppressor T cell-derived exosome-treated macrophages. Folia Med Cracov. 2014;54:37–52. [PubMed] [Google Scholar]