Abstract
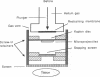
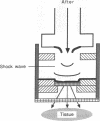
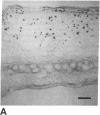
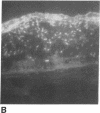
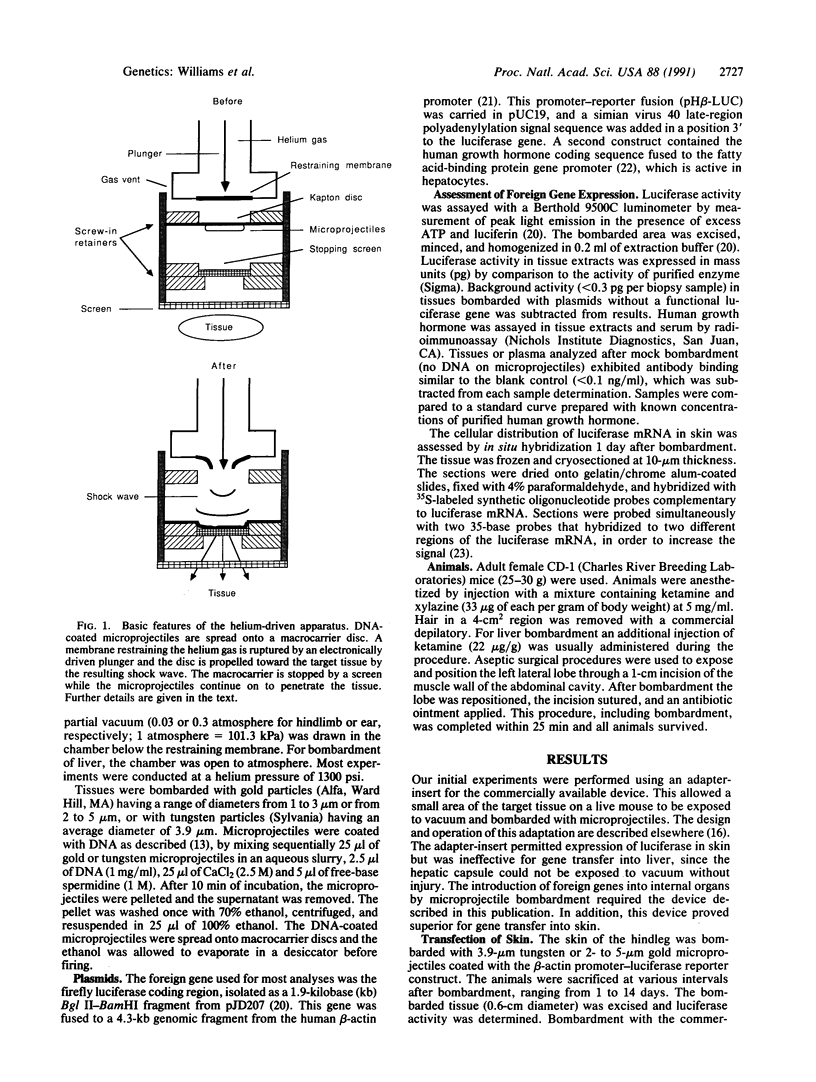
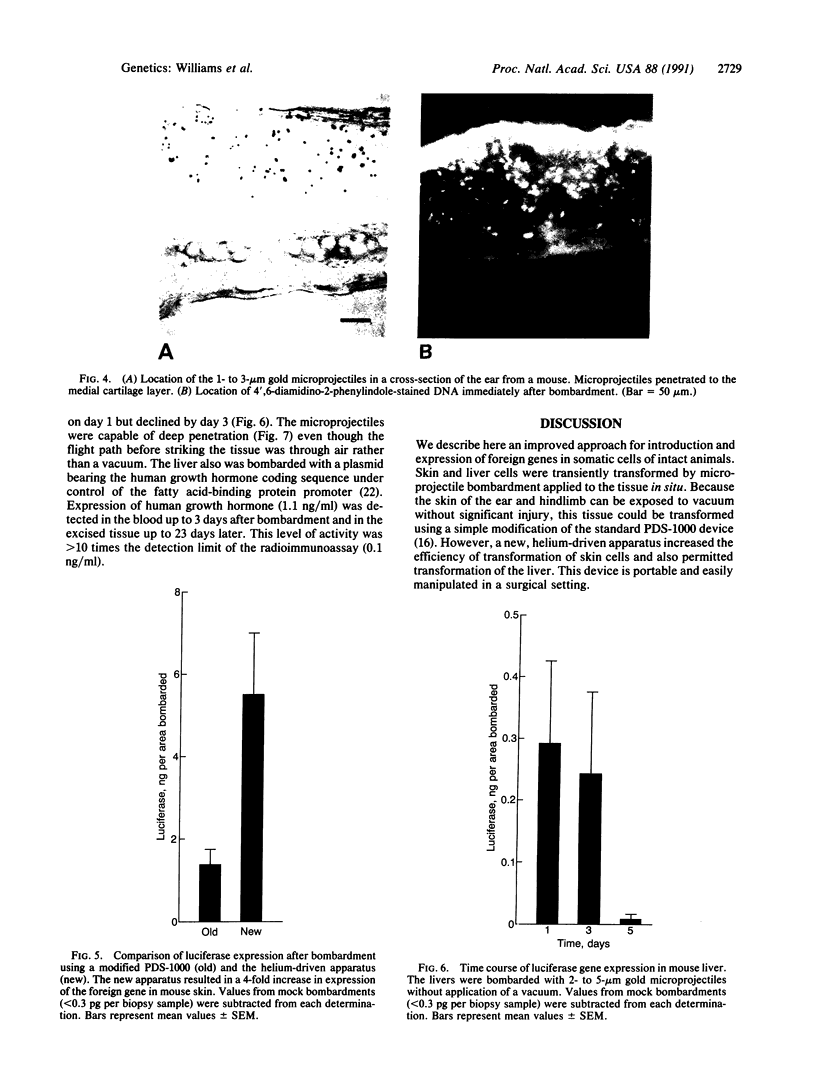
Foreign genes were expressed in liver and skin cells of live mice by using a new apparatus to accelerate DNA-coated microprojectiles into tissues. After introduction of a plasmid in which the firefly luciferase gene was controlled by the human beta-actin promoter, luciferase activity was detectable for up to 14 days in mouse tissues (skin and liver). In situ hybridization histochemistry revealed that microprojectiles penetrated through multiple cell layers without evidence of tissue injury and that 10-20% of the cells in the bombarded area expressed the foreign gene. An advantage of the new design is that internal organs, such as liver, can be transfected without subjecting the tissue to a vacuum. This procedure potentially is applicable to a wide variety of tissues and cell types for studies of transcriptional control elements and for expression of foreign proteins in intact animals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armaleo D., Ye G. N., Klein T. M., Shark K. B., Sanford J. C., Johnston S. A. Biolistic nuclear transformation of Saccharomyces cerevisiae and other fungi. Curr Genet. 1990 Feb;17(2):97–103. doi: 10.1007/BF00312852. [DOI] [PubMed] [Google Scholar]
- Benvenisty N., Reshef L. Direct introduction of genes into rats and expression of the genes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9551–9555. doi: 10.1073/pnas.83.24.9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Christou P., McCabe D. E., Swain W. F. Stable Transformation of Soybean Callus by DNA-Coated Gold Particles. Plant Physiol. 1988 Jul;87(3):671–674. doi: 10.1104/pp.87.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Kamine J., Bakker A., Silva D., Woychik R. P., Sakai D. D., Rottman F. M. Synthesis of bovine growth hormone in primates by using a herpesvirus vector. Mol Cell Biol. 1985 Oct;5(10):2796–2803. doi: 10.1128/mcb.5.10.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubensky T. W., Campbell B. A., Villarreal L. P. Direct transfection of viral and plasmid DNA into the liver or spleen of mice. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7529–7533. doi: 10.1073/pnas.81.23.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick-McElligott S., Card J. P., Lewis M. E., Baldino F., Jr Neuronal localization of prosomatostatin mRNA in the rat brain with in situ hybridization histochemistry. J Comp Neurol. 1988 Jul 22;273(4):558–572. doi: 10.1002/cne.902730410. [DOI] [PubMed] [Google Scholar]
- Holt C. E., Garlick N., Cornel E. Lipofection of cDNAs in the embryonic vertebrate central nervous system. Neuron. 1990 Feb;4(2):203–214. doi: 10.1016/0896-6273(90)90095-w. [DOI] [PubMed] [Google Scholar]
- Johnston S. A., Anziano P. Q., Shark K., Sanford J. C., Butow R. A. Mitochondrial transformation in yeast by bombardment with microprojectiles. Science. 1988 Jun 10;240(4858):1538–1541. doi: 10.1126/science.2836954. [DOI] [PubMed] [Google Scholar]
- Kaneda Y., Iwai K., Uchida T. Increased expression of DNA cointroduced with nuclear protein in adult rat liver. Science. 1989 Jan 20;243(4889):375–378. doi: 10.1126/science.2911748. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Porreca P., Ng S. Y., Lin C. S., Kedes L. Molecular cloning and characterization of mutant and wild-type human beta-actin genes. Mol Cell Biol. 1984 Oct;4(10):1961–1969. doi: 10.1128/mcb.4.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolau C., Le Pape A., Soriano P., Fargette F., Juhel M. F. In vivo expression of rat insulin after intravenous administration of the liposome-entrapped gene for rat insulin I. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1068–1072. doi: 10.1073/pnas.80.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. The cloned genome of ground squirrel hepatitis virus is infectious in the animal. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5849–5852. doi: 10.1073/pnas.81.18.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser D. A., Birkenmeier E. H., Hoppe P. C., McKeel D. W., Gordon J. I. Mechanisms underlying generation of gradients in gene expression within the intestine: an analysis using transgenic mice containing fatty acid binding protein-human growth hormone fusion genes. Genes Dev. 1988 Oct;2(10):1318–1332. doi: 10.1101/gad.2.10.1318. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Huang L. pH-sensitive immunoliposomes mediate target-cell-specific delivery and controlled expression of a foreign gene in mouse. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7851–7855. doi: 10.1073/pnas.84.22.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Receptor-mediated gene delivery and expression in vivo. J Biol Chem. 1988 Oct 15;263(29):14621–14624. [PubMed] [Google Scholar]
- Zelenin A. V., Titomirov A. V., Kolesnikov V. A. Genetic transformation of mouse cultured cells with the help of high-velocity mechanical DNA injection. FEBS Lett. 1989 Feb 13;244(1):65–67. doi: 10.1016/0014-5793(89)81163-9. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]