Abstract
Background
High-intensity stepping practice may be a critical component to improve gait following motor incomplete spinal cord injury (iSCI). However, such practice is discouraged by traditional theories of rehabilitation that suggest high-intensity locomotor exercise degrades gait performance. Accordingly, such training is thought to reinforce abnormal movement patterns, although evidence to support this notion is limited.
Objective
The purposes of this study were: (1) to evaluate the effects of short-term manipulations in locomotor intensity on gait performance in people with iSCI and (2) to evaluate potential detrimental effects of high-intensity locomotor training on walking performance.
Design
A single-day, repeated-measures, pretraining-posttraining study design was used.
Methods
Nineteen individuals with chronic iSCI performed a graded-intensity locomotor exercise task with simultaneous collection of lower extremity kinematic and electromyographic data. Measures of interest were compared across intensity levels of 33%, 67%, and 100% of peak gait speed. A subset of 9 individuals participated in 12 weeks of high-intensity locomotor training. Similar measurements were collected and compared between pretraining and posttraining evaluations.
Results
The results indicate that short-term increases in intensity led to significant improvements in muscle activity, spatiotemporal metrics, and joint excursions, with selected improvements in measures of locomotor coordination. High-intensity locomotor training led to significant increases in peak gait speed (0.64–0.80 m/s), and spatiotemporal and kinematic metrics indicate a trend for improved coordination.
Limitations
Measures of gait performance were assessed during treadmill ambulation and not compared with a control group. Generalizability of these results to overground ambulation is unknown.
Conclusions
High-intensity locomotor exercise and training does not degrade, but rather improves, locomotor function and quality in individuals with iSCI, which contrasts with traditional theories of motor dysfunction following neurologic injury.
Spinal cord injury (SCI) results in profound deficits in sensorimotor function, which can contribute to the loss of functional mobility. Following motor incomplete spinal cord injury (iSCI), indicating partial sparing of descending motor pathways, recovery of walking is reported as a primary goal of rehabilitation.1,2 Consistent with principles of motor learning3 and experience-dependent neural plasticity,4 the amount and specificity of practice are thought to be important parameters of exercise interventions to optimize locomotor recovery following neurological injury.5–7 Specific data from animal models and humans with SCI indicate that large amounts of stepping practice can elicit significant improvements in gait speed, endurance, and independence.8–13
Additional training parameters have been suggested to influence motor recovery after neurologic injury.6,7 Specifically, emerging evidence suggests that intensity of locomotor practice also may be a critical parameter to improve locomotor function after neurologic injury. Locomotor intensity is defined here as rate of work (ie, workload) or power output, and it is commonly manipulated through alterations in walking speeds and measured indirectly through cardiovascular (heart rate) or metabolic (oxygen consumption) measures. Specific studies in patients poststroke have demonstrated greater improvements in locomotor performance following training at faster versus slower speeds.14,15 In addition, more recent meta-analyses suggest that locomotor training at high cardiovascular intensities is both safe and effective in people poststroke, with significant improvements in walking speed and endurance.16–18 Improvements in locomotor function with high-intensity training may be due to adaptations in cardiopulmonary fitness and peak metabolic capacity19 and related to plasticity of the neuromuscular system.20–22 Particularly, studies in both humans and animal models have demonstrated a positive relationship between locomotor exercise intensity and the expression of neurotrophins, or neural growth factors,23–26 which can promote motor recovery following SCI.27–32 However, data supporting the benefits of exercise intensity in patients with iSCI are lacking.
A potential barrier of performing high-intensity locomotor training with patients with iSCI or other upper motoneuron disorders is the long-standing belief33–35 that such training can exaggerate spastic motor behaviors. For example, a study in individuals poststroke demonstrated an increased magnitude of spastic motor behaviors and altered movement patterns in the affected upper extremity (ie, elbow and finger flexion) with increases in locomotor intensity (ie, speed).36 Such behaviors are traditionally minimized during clinical practice, and high-intensity training is discouraged, in part, due to concerns of reinforcing abnormal movement patterns or inducing maladaptive neuroplastic changes.4 However, the effect of increasing locomotor intensity on gait quality, particularly measures of joint kinematics and muscle timing, is not clear. A single case study has demonstrated increased spastic motor behavior at higher walking speeds in an individual with SCI.37 Whether such behavior leads to abnormal gait kinematics or reinforces poor kinematic patterns and altered muscle activity with high-intensity training is uncertain, as no studies have attempted to quantify these effects. Rather, previous data from animal models of SCI suggest that practice of unconstrained locomotor tasks may lead to improved and more consistent locomotor kinematics.38,39
The goal of this study was to evaluate the effects of short- and long-term exposure to high-intensity walking in people with incomplete SCI on key measures of gait performance, including measures of function (speed) and quality (kinematics). Based on available evidence, our hypothesis was that gait quality in people with incomplete SCI would degrade at higher walking speeds and intensity, yet improve with repeated high-intensity locomotor practice.
Method
The present study details 2 separate experiments designed to delineate the effects of short-term and repeated exposure to high-intensity stepping exercise on lower extremity kinematics and muscle activity patterns during walking. Nineteen individuals with iSCI participated in an evaluation of the effects of changes in locomotor exercise intensity on gait kinematics and muscle activity and timing within a single session. In 9 of these individuals, gait kinematics and muscle timing were evaluated following a 12-week, high-intensity locomotor training paradigm. Inclusion criteria for participation were history of chronic iSCI (>1 year) with anatomical lesions at T10 or above, age between 18 and 75 years, and ability to walk without physical assistance at up to 0.3 m/s during a graded-intensity treadmill test, as described below. Exclusion criteria were concurrent illness that could limit walking performance, including unhealed decubiti; uncontrolled cardiopulmonary disease, including orthostatic hypotension and recurrent autonomic dysreflexia; active heterotopic ossification; and other peripheral or central neurologic injury. For evaluation of changes in locomotor function following training, additional exclusion criteria included concurrent physical therapist services or enrollment in a training study less than 3 months prior to the training initiation. All participants had received medical clearance and given written informed consent to participate.
Data Collection
Prior to initial locomotor testing, clinical measures of strength, spasticity, and overground walking ability were assessed by a licensed physical therapist to capture the participants' clinical characteristics. Lower extremity strength was assessed using the International Standards for Neurological Classification of Spinal Cord Injury lower extremity motor score.40,41 Spastic motor activity was evaluated using the Spinal Cord Assessment Tool for Spastic Reflexes (SCATS)42 and modified Ashworth scale testing of bilateral knee flexors and extensors with participants in a supine position.43 Raw scores for the modified Ashworth scale were converted to an ordinal scale to allow for calculation of a composite score.44 Spinal Cord Assessment Tool for Spastic Reflexes and modified Ashworth scale scores were summed within legs to obtain a composite score for both measures. The Walking Index for Spinal Cord Injury II45,46 was used to quantify overground walking ability through the use of braces, assistive devices, and therapist assistance for locomotion at self-selected speeds. Measurements of overground self-selected walking speed were collected over a 3.85-m instrumented walkway (GaitMat II, Equitest, Chalfont, Pennsylvania), with instructions to participants to walk at their normal, comfortable pace and with the average calculated over 2 trials.
Following clinical assessment, included individuals participated in a graded-intensity locomotor testing paradigm on a motorized treadmill. Participants were fitted with an overhead safety harness without weight support and instructed to walk on the treadmill at 0.1 m/s for 2 minutes, with 0.1-m/s increases in speed every 2 minutes until they required support from the safety harness to maintain an upright posture (determined by tension in the shoulder straps of the harness) or voluntarily terminated the test. To evaluate whether these changes in gait speed were an effective means of manipulating exercise intensity, participants were asked to report perceived intensity at each speed reached during this testing paradigm using the Rating of Perceived Exertion (RPE) scale.47 During testing, lower extremity kinematic data (spatiotemporal and angular joint excursions) were collected with 32 spherical reflective markers affixed to the participants' lower extremities using a previously described modified Cleveland Clinic marker set.48 Data were sampled at 100 Hz with a 6-camera motion capture system (Motion Analysis Corp, Santa Rosa, California). Bilateral lower extremity electromyographic (EMG) activity was assessed with surface recordings from the rectus femoris, vastus lateralis, medial hamstring, tibialis anterior, medial gastrocnemius, and soleus muscles using a 32-channel dynamic EMG system (Noraxon Inc, Scottsdale, Arizona). Electromyographic data were sampled at 1,000 Hz and collected synchronously with kinematic data using Cortex software (Motion Analysis Corp). All data were collected in two 30-second intervals for a maximum collection interval of 1 minute at each speed. To allow accommodation to each speed, data collection was initiated 30 seconds after each speed increase. Data collected within this single testing session were used to assess the short-term effects of locomotor exercise intensity. To evaluate the effects of repeated exposure to high-intensity locomotor activity, the same data collection procedures were utilized posttraining in the relevant subset of participants (n=9) who were willing and able to participate in repeated locomotor training over 12 weeks.
Training Paradigm
For the 9 individuals who participated in high-intensity training, sessions were scheduled for 1 hour, 3 times per week for 12 weeks, with a goal of up to 45 minutes of high-intensity walking per session. Participants walked on a treadmill with the use of a harness and body-weight support only as needed (up to 40% of body weight, depending on level of ability) to ensure continuous stepping at a high intensity. Limb swing assistance was provided by a single therapist and use of elastic bands attached to the front of the treadmill and to the knee, ankle, or both, with assistance of up to 5% body weight (evaluated in standing position) to allow for continued stepping. Locomotor training intensity was manipulated primarily by increases in treadmill speed to ensure that individuals worked at a high intensity. Exercise intensity during training sessions was monitored using the RPE scale,47 with targeted intensities of greater than 14 during training. Measures of heart rate were not routinely used to assess training intensity, as cardiac responses are often blunted in this patient population compared with individuals with an intact central nervous system.49 Blood pressures were monitored intermittently during rest breaks and kept below the American College of Sports Medicine thresholds for continuing exercise.50
Data Analysis
The effects of increases in exercise intensity on gait kinematics and muscle activity were evaluated. Data collected during speeds closest to 33% and 67% of peak speed and at peak speeds were analyzed to evaluate the effects of low, moderate, and high levels of intensity, respectively, on measures of locomotor performance. In individuals who received high-intensity training, data from peak speeds achieved at pretraining and posttraining evaluations were compared and indicated as comparisons at “fastest” speeds to assess the kinematic strategies utilized to achieve higher speeds. In addition, metrics were compared from baseline peak speed with the “matched” speed reached posttraining (termed “fastest matched”). This latter comparison was performed to minimize the confounding effect of gait speed on locomotor performance. Analysis of kinematic and EMG data collected during treadmill testing was performed with data collected from the more impaired limb, as determined by strength scores (or increased spasticity scores if strength was equal bilaterally). All measurements were calculated as an average over all step cycles taken within the relevant collection windows, as previously described.51
For kinematic measures, a bilateral 6-degree of freedom model of each participant's lower limbs (pelvis, thighs, shanks, and feet) was created from the reflective marker data during static standing using Visual3D software (C-Motion Inc, Germantown, Maryland). This was done on each day of testing for participants who were tested before and after training. The generated model was applied to filtered marker data (low-pass, second-order recursive Butterworth filter, cutoff frequency=10 Hz) collected during treadmill testing, and 3-dimensional joint positions and intersegmental angles were calculated from transformations between model segments.
Kinematic metrics were averaged across multiple normalized gait cycles (from heel-strike to heel-strike, defined by the maximum anterior position of the calcaneal marker) during the collection windows. A minimum of 10 full gait cycles were utilized for the majority of analyses. In 6 cases, however, participants had fewer than 10 step cycles (range=3–9) for analysis secondary to the low cadence values at slower walking speeds. In addition, data from 2 participants who wore bilateral ankle-foot orthoses are included in the analysis. Metrics of interest included stride length, cadence, and specific measures of sagittal-plane joint kinematics. Specific kinematic measures included total range of motion (ROM) and peak flexion and extension angles at the hip, knee, and ankle during swing and stance, respectively. Measures of step-to-step kinematic variability also were calculated at different intensities (speeds). For metrics with a true zero (cadence, stride length, and total joint ROM), the percent coefficient of variation (COV=standard deviation/mean) was calculated; for peak angular joint excursion (measures without a true zero), the standard deviations of these measures are presented. Furthermore, the average coefficient of correspondence (ACC) was calculated to quantify the intralimb consistency between hip and knee joint excursions across multiple normalized gait cycles.52 A value of 1.0 represents perfectly consistent coordination of hip and knee joint trajectories between step cycles, whereas a value of .0 indicates no consistency. Individuals without neurologic injury demonstrate ACC values from .94 to .97.52,53
Data for EMG activity collected during graded-intensity treadmill ambulation was post-processed using custom-designed software (Visual3D, Matlab, The MathWorks Inc, Natick, Massachusetts). Raw EMG signals were filtered (fourth-order recursive Butterworth filter, band-pass 30–450 Hz, and band-stop 58–62 Hz), rectified, and smoothed using a low-pass filter (20-Hz fourth-order recursive Butterworth filter) to create linear envelopes. The EMG data were normalized to gait cycle as a percentage from heel-strike to heel-strike and averaged over all step cycles. From these data, the average integrated area throughout the gait cycle was calculated as a measure of overall muscle activity during locomotion for comparison across different intensities during single-day testing.
Evaluation of changes in muscle timing within session and following training was performed using a variation of the Spastic Locomotor Disorder Index (SLDI). The integrated EMG area during predetermined normative periods of quiescence (“off” period) was divided by that during periods of activation (“on” periods) throughout the gait cycle of each tested muscle.54 Values of SLDI closer to 0.0 represent appropriate muscle timing, consistent with normative data from individuals with an intact central nervous system, whereas higher values indicate abnormal muscle activation patterns during gait.55
Statistical Analysis
All statistical analyses were performed in StatView, version 5.0.1 (SAS Institute Inc, Cary, North Carolina). The effects of short-term changes in locomotor intensity on locomotor metrics were assessed using repeated-measures analyses of variance with significance set at α=.05. Changes in ordinal RPE scores were assessed with a Friedman test with significance also set to α=.05. With significant findings, post hoc pair-wise comparisons were performed with a Bonferroni correction for intensity-dependent comparisons (α=.017). For evaluation of training effects, gait measurements were compared between the fastest and fastest-matched treadmill speeds reached before and after training using paired t tests for kinematic metrics and a Wilcoxon signed rank test for muscle timing. Based on a previous report,48 we anticipated that some combination of spatiotemporal and kinematic parameters would contribute to increases in peak gait speed following training. Potential correlations between changes in peak gait speed and spatiotemporal and kinematic parameters from pretraining to posttraining were assessed using Pearson correlation analyses. For pretraining to posttraining comparisons, a P value of <.05 was considered significant.
Role of the Funding Source
This study was funded by the following grants: H133N110014 and RO1-NS079751 to Dr Hornby, 1F31NS084723, and the Foundation for Physical Therapy (PODS I and PODS II) to Dr Leech.
Results
Demographic and clinical characteristics of the individuals who participated in one or both portions of the study are detailed in Table 1. Nineteen individuals (6 female, 13 male) with chronic iSCI participated in the first portion of this study aimed at assessing the short-term effects of locomotor exercise intensity on the magnitude and variability of gait kinematics and muscle activity and timing. A subset of 9 individuals who were willing and available participated in the second portion of the study to evaluate the effects of repeated exposure to high-intensity exercise. The flowchart of participants in the study is shown in eFigure 1.
Table 1.
Participants' Clinical and Demographic Characteristicsa

Measures that capture scores for individual muscle strength or spastic motor activity are provided as the summation of scores from each tested muscle on the more impaired lower extremity. For all measures, larger scores indicate increased strength, spastic motor behavior, or independence with ambulation. LOI=neurologic level of injury, DOI=duration of injury, LEMS=International Standards for Neurological Classification of Spinal Cord Injury lower extremity motor score (range=0–25), mAsh=modified Ashworth scale (range=0–10), SCATS=Spinal Cord Assessment Tool for Spastic Reflexes (range=0–15), WISCI II=Walking Index for Spinal Cord Injury-II (range=0–20), SSS=self-selected gait speed, M=male, F=female, Y=yes, N=no.
Effects of Short-Term Manipulations in Locomotor Exercise Intensity
Graded-intensity treadmill testing indicated that participants achieved significantly higher walking speeds at low-, moderate-, and high-intensity levels (X̅=0.28 [SD=0.15], 0.59 [SD=0.27], and 0.87 [SD=0.43] m/s, respectively; P<.001 for all post hoc comparisons). Rating of Perceived Exertion scale scores were consistently reported at each of these intensity levels by a subset of participants (n=10/19) during testing, with inconsistent reporting from other participants. Significant differences in median (interquartile range) values of RPE at low (11 [7.5–12]), moderate (14 [10–15]), and high (17 [14.5–19]) intensities indicate that gait speed manipulation effectively modulates exercise intensity.
Increases in speed across levels of intensity were accomplished by significant increases in stride length and cadence, with few changes in the COV of these measures across levels of intensity (Tab. 2). Specifically, only COV of stride length decreased significantly at moderate intensity, whereas changes in COV of cadence across intensity levels only approached significance.
Table 2.
Changes in Magnitude and Variability of Spatiotemporal Parameters of Gait and Sagittal-Plane Lower Extremity Joint Kinematics at Different Locomotor Exercise Intensitiesa

Values are presented as mean±standard deviation. COV=percent coefficient of variation of the mean, ROM=range of motion, NS=not significant. Bolded values are statistically significant.
Analysis of lower-extremity joint kinematics revealed significant increases in the total ROM of the ankle and hip joints with increasing exercise intensity, with changes in knee ROM approaching significance (Tab. 2). Single-subject data representative of these changes are presented in eFigure 2. Related changes in the peak angular excursions of the ankle, knee, and hip across gait speeds are presented in eTable 1. No differences were found in the COV of the total ROM of the ankle, knee, or hip joints (Tab. 2) or the standard deviations of peak joint angles (not detailed in eTab. 1). One exception was the standard deviation of peak ankle plantar flexion, which was significantly greater at high intensities compared with moderate intensities (P<.017). Evaluation of the hip-knee ACC (n=18) demonstrated a significant effect of intensity (P=.007), with greater values at moderate intensities compared with low intensities (Fig. 1A; P<.01) and increases at low and high intensities that approached significance (P=.06).
Figure 1.
Assessment of hip-knee average coefficient of correspondence (ACC) values with short-term increases in locomotor exercise intensity (A) and before and after high-intensity training at fastest speeds (B) and fastest-matched speeds (C). Short-term increases in locomotor exercise intensity resulted in increases in hip-knee ACC (A). High-intensity training led to nearly significant and nonsignificant increases in hip-knee ACC at fastest speeds (B) and fastest-matched speeds (C), respectively. *P<.01.
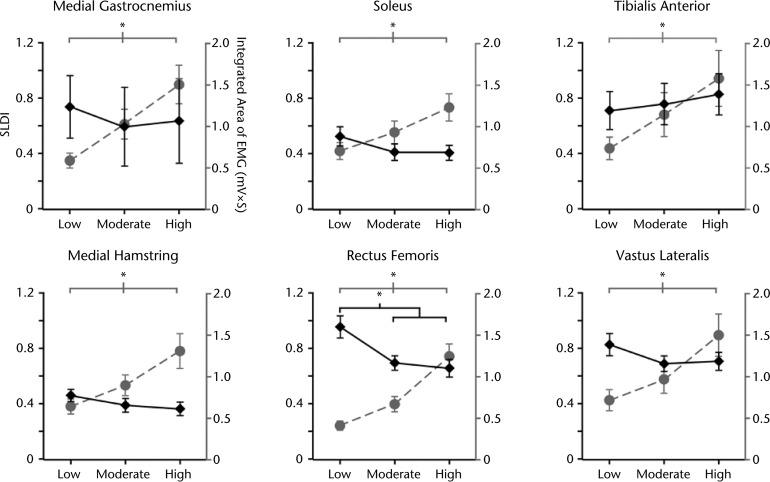
Changes in EMG activity were scaled with increases in exercise intensity, as all 6 tested muscles demonstrated significant increases in activity with increasing intensities (Fig. 2). Evaluation of muscle timing using the SLDI demonstrated minimal changes across intensities, as only rectus femoris muscle activity demonstrated significant decreases (Fig. 2), whereas all other muscles demonstrated nonsignificant changes.
Figure 2.
Muscle activity and Spastic Locomotor Disorder Index (SLDI) in specific lower extremity muscles at different levels of exercise intensity. Increases in locomotor exercise intensity led to significant increases in measures of overall muscle activity (plotted in gray) in each of the tested muscles (n=16). Most tested muscles demonstrated a nonsignificant trend for decreased SLDI (plotted in black) with increases in exercise intensity (n=16). Exceptions include the rectus femoris muscle, in which the SLDI significantly decreased, and the tibialis anterior muscle, which nonsignificantly increased at higher levels of intensity. *P<.01. EMG=electromyography.
Effects of High-Intensity Locomotor Training
Intensity of locomotor training was monitored by RPE during activity with a target intensity of greater than 14. Values of RPE were collected over an average of 85% (SD=13%) of training sessions completed across participants. The average RPE reported during these training sessions was 15 (SD=1), confirming that the participants reached the targeted high-intensity levels. There were no dropouts during the study (all participants finished 12 weeks of high-intensity motor training), although the number of training sessions varied from 24 to 36. Participants ambulated at least 25 minutes per session, with the exception of those with unrelated illness (flu-like symptoms), which limited tolerance during training in 2 participants. Common minor adverse events during training included subjective reports of muscle soreness with concomitant increased stiffness or spasticity (n=3) and low back pain (n=1) early posttraining, which subsided.
Assessment of the effects of high-intensity training on measures of gait performance revealed significant increases in peak speeds (from X̅=0.64 [SD=0.26] m/s to X̅=0.80 [0.25] m/s, P<.01). At fastest speeds, comparisons of spatiotemporal measures demonstrated significant increases in both stride length and cadence, with no changes in COV of either measure (Tab. 3). Correlations between changes in stride length and cadence and changes in peak gait speed following training demonstrated a positive, significant correlation between changes in stride length and changes in peak gait speed (R=.76, P=.02), but not between changes in cadence and changes in peak gait speed (R=.55, P=.12). Pretraining to posttraining comparisons of joint excursion at fastest speeds indicated increased total knee ROM, with more limited changes in total hip and ankle ROM (Tab. 3). Correlations between changes in total joint excursion and peak gait speed following training demonstrated a significant correlation only between changes in total hip excursion and gait speed (R=.73, P=.03). Changes in peak joint angles also revealed increases only in peak knee flexion angle at fastest speeds (eTab. 2). No changes in the COV of total joint ROM (Tab. 3) or standard deviation of the peak joint angles from pretraining to posttraining were observed, although changes in the hip-knee ACC at the fastest speeds approached significance (Fig. 1B; P=.05).
Table 3.
Pretraining and Posttraining Measurements of Magnitude and Variability of Spatiotemporal Parameters of Gait and Sagittal-Plane Lower Extremity Joint Kinematics at Fastest and Fastest-Matched Speedsa
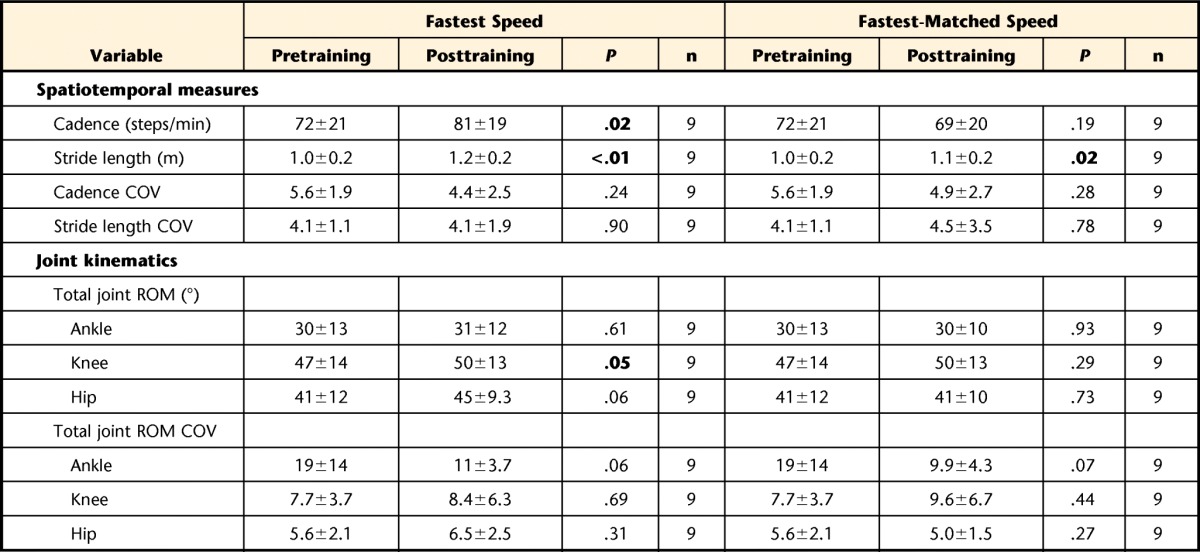
Values are presented as mean±standard deviation. COV=percent coefficient of variation of the mean, ROM=range of motion. Bolded values are statistically significant.
Comparisons of these measures at fastest-matched speeds from pretraining to posttraining demonstrated few changes in the magnitude and variability of locomotor parameters. For example, a significant increase was found only in magnitude of stride length and peak knee flexion angle at matched speeds (Tab. 3). There was a nonsignificant increase in the hip-knee ACC at fastest-matched speeds from pretraining to posttraining (Fig. 1C; P=.18).
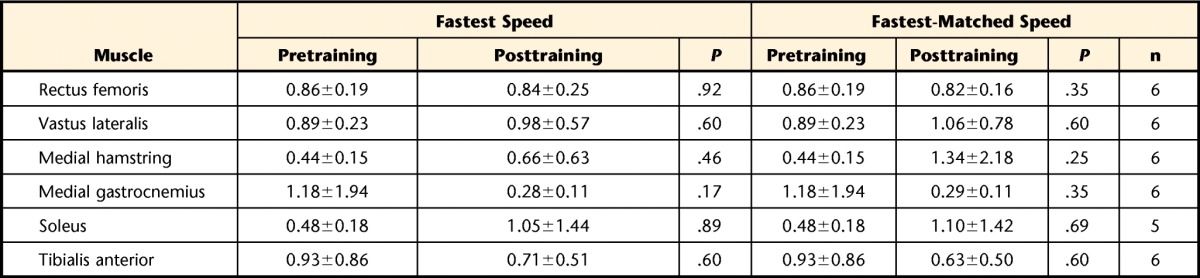
Pretraining to posttraining comparisons of muscle timing were assessed with data from 6 participants for all tested muscles, with the exception of the soleus muscle, which was assessed in 5 participants. Loss of EMG recordings occurred more often during higher walking speeds, and data from all participants could not be included in the analysis. For the timing of muscle activity throughout the gait cycle, no significant changes were found at fastest or fastest match speeds (Tab. 4).
Table 4.
Pretraining and Posttraining Spastic Locomotor Disorder Index Values for Tested Lower Extremity Muscles at Fastest and Fastest-Matched Speeds
Discussion
Contrary to our hypothesis, the primary finding of this investigation was that increases in locomotor exercise intensity did not degrade gait performance. Specifically, short-term increases in locomotor exercise intensity significantly improved spatiotemporal parameters and joint kinematics toward more normal kinematic excursions and consistency. Higher intensities of stepping also elicited significant increases in lower extremity muscle activity without aberrant changes in muscle timing (ie, higher SLDI). Repeated training at high intensities performed in a subset of participants resulted in minimal changes in gait parameters at matched speeds, but led to significant increases in peak gait speed, with associated gains in stride length and cadence, but smaller, inconsistent changes in joint kinematics and muscle timing.
Effects of Short-Term Changes in Locomotor Intensity
Significant improvements in spatiotemporal patterns and joint excursions with increasing locomotor intensity are consistent with previous reports of kinematic changes utilized for accommodation to changes in gait speed in individuals with SCI56 and healthy control participants.57,58 The average peak speeds and total joint excursions achieved by these participants during treadmill walking approximated those observed in healthy older adults during comfortable walking speeds performed overground.59,60 Such changes were likely driven largely by changes observed in overall muscle activity (consistent with previous reports in individuals with SCI56 and control participants61).
The observations of few intensity-dependent changes in the variability of gait parameters or deteriorations of muscle timing were of significant interest. In those measures where significant differences were found, decreases in variability (ie, increases in consistency) or improvements in muscle timing were observed with increasing intensity. These findings are not consistent with our initial hypothesis, based primarily on traditional theories of the relationship between exercise intensity and spastic motor behaviors during training of patients with damage to descending pathways (upper motoneuron syndromes).35 Rather, the present results are consistent with findings in healthy control participants62–64 and individuals poststroke,65 demonstrating increased consistency of movement with increased gait speeds. Importantly, the data suggest that altering locomotor task demands through increasing intensity leads to a consequent increase in motor output (eg, EMG activity, joint ROM) without increasing kinematic variability or muscle timing during gait. As such, these single-session results provide preliminary support for repetitive locomotor practice at high intensities with little concern for the reinforcement of poor kinematic patterns or locomotor control strategies or the induction of maladaptive plasticity.
High-Intensity Locomotor Training
Following locomotor training at high intensities, improvements in gait speed were associated with altered spatiotemporal patterns, with greater contributions from stride length than cadence. Similarly, comparisons at fastest matched speeds at pretraining and posttraining evaluations demonstrated increases in stride length, with nonsignificant decreases in cadence. Although previous studies56,66 suggest that individuals with SCI have a reduced capacity to modify step cadence to accommodate to increases in gait speed, the present findings suggest that high-intensity training may result in changes in both spatial and temporal gait parameters to achieve faster walking speeds.
Our results also suggest that high-intensity training did not result in a worsening of poor kinematic patterns; rather, gait kinematics improved, or became more “normal” at higher intensities during single exercise bouts. This finding is supported by the trends of increasing joint excursions at fastest or fastest matched speeds. We further found that high-intensity training did not increase gait variability or impair muscle timing throughout the gait cycle. Rather, decreases in gait variability or improvements in intralimb kinematic consistency were observed with faster speeds. The combined findings support the hypothesis that high-intensity training may improve both gait quality and function in individuals with SCI.
Our findings highlight the need for this proposed link between increases in exercise intensity and impaired gait quality to be reevaluated. Understanding the interaction between exercise intensity and gait quality is of particular importance given the pervasive influence of these and similar theories on current standard clinical practice.67 Furthermore, given the evidence for the benefit of high-intensity exercise across multiple physiological (cardiovascular19 and neuromuscular20,21) domains, the present work adds to the premise that patients may benefit from the incorporation of higher-intensity interventions into standard clinical care. However, significant research remains to be done through larger, controlled studies to rigorously evaluate the efficacy of high-intensity locomotor training relative to other interventions.
Limitations
In addition to sample size for selected measures, other limitations should be considered. First, in order to assess different levels of locomotor exercise intensity, it was necessary to utilize a treadmill to allow for systematic manipulations of gait speed. Dingwell and Cavanagh68 demonstrated that ambulation on a treadmill can stabilize locomotor output, with greater step-to-step variability demonstrated during overground versus treadmill ambulation. Therefore, a similar assessment of different locomotor intensities overground may reveal differences in gait variability that were not found in the present study.
Next, other reports of gait kinematics and muscle activity in individuals with neurologic injury evaluated changes in different parameters at key events in the gait cycle, such as examination of soleus muscle activity at push-off or ankle angle at heel contact.56,65 The data analyses performed in this study were utilized to capture a broader picture of the kinematic pattern and EMG activity throughout the gait cycle; however, this analysis may limit comparability of these results to previous reports. In addition, the SLDI was calculated using predetermined percentages of the gait cycle identified as “on” and “off” times for each muscle.51,54 Our previous work54 demonstrated little change in muscle activity patterns throughout the gait cycle across different speeds in healthy control participants. However, a study by Pepin et al56 in people with SCI demonstrated that the percentage of the gait cycle spent in stance and swing is altered with increases in gait speed. This finding suggests that the use of predetermined percentages of the gait cycle to calculate the SLDI at different speeds may confound the results. The methods utilized in the present study are consistent with previous reports.51,54
Finally, the lack of a control group limited our ability to evaluate the efficacy of high-intensity locomotor training compared to other interventions. We were not specifically interested in the comparative efficacy of this training protocol in the current paradigm, but were rather interested in whether such training may contribute to altered locomotor control strategies in this patient population.
Clinical Relevance
This study demonstrates that locomotor exercise at high intensities elicits increased muscle activity and kinematics more similar to that of healthy controls at similar speeds. Participants exhibited no significant intensity-dependent changes in variability of movement or aberrant muscle activity during walking, suggesting there is no detriment to gait performance as previously suggested.35 Furthermore, consistent with previous reports of positive effects of high-intensity locomotor exercise in individuals with stroke,14,15 high-intensity locomotor practice led to increases in peak gait speed, with specific improvements in the gait pattern. Importantly, higher-intensity locomotor activity is driven, in large part, by the neural and muscular activity required to accomplish the task.69 Studies performed in basic70 and applied71 research have demonstrated that increased neural activity leads to improved synaptic function. This finding suggests that intensity of practice may be an important factor in potentiating experience-dependent neuroplasticity. In this context, the results of this study strongly support the utility of intensity as a parameter of rehabilitation to promote functional recovery after SCI.
Footnotes
Dr Leech, Ms Kinnaird, Dr Kahn, and Dr Hornby provided concept/idea/research design. Dr Leech, Dr Hollaran, and Dr Hornby provided writing. All authors provided data collection. Dr Leech, Ms Kinnaird, and Dr Kahn provided data analysis. Dr Leech and Dr Hornby provided project management. Dr Hornby provided fund procurement, facilities/equipment, and institutional liaisons. Dr Leech, Dr Holleran, and Dr Kahn provided participants. Ms Kinnaird, Dr Kahn, and Dr Hornby provided consultation (including review of manuscript before submission).
All procedures were conducted in accordance with the Declaration of Helsinki and approved by the Northwestern University Institutional Review Board (Chicago, Illinois).
This project was a secondary analysis of data extracted from 2 larger studies (registered on ClinicalTrials.gov: NCT02560506 and NCT01538693) in which we evaluated the effects of both short-term increases in locomotor exercise intensity and high-intensity locomotor training in individuals with iSCI.
This study was funded by the following grants: H133N110014 and RO1-NS079751 to Dr Hornby, 1F31NS084723, and the Foundation for Physical Therapy (PODSI and PODSII) to Dr Leech.
References
- 1. Ditunno PL, Patrick M, Stineman M, et al. Cross-cultural differences in preference for recovery of mobility among spinal cord injury rehabilitation professionals. Spinal Cord. 2006;44:567–575. [DOI] [PubMed] [Google Scholar]
- 2. Estores IM. The consumer's perspective and the professional literature: what do persons with spinal cord injury want? J Rehabil Res Dev. 2003;40:93–98. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt R, Lee T. Motor Control and Learning: A Behavioral Emphasis. Champaign, IL: Human Kinetics Inc; 1999. [Google Scholar]
- 4. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–S239. [DOI] [PubMed] [Google Scholar]
- 5. Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil Neural Repair. 2003;17:3–11. [DOI] [PubMed] [Google Scholar]
- 6. Hornby TG, Straube DS, Kinnaird CR, et al. Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Top Stroke Rehabil. 2011;18:293–307. [DOI] [PubMed] [Google Scholar]
- 7. Barbeau H, Nadeau S, Garneau C. Physical determinants, emerging concepts, and training approaches in gait of individuals with spinal cord injury. J Neurotrauma. 2006;23:571–585. [DOI] [PubMed] [Google Scholar]
- 8. Dobkin B, Apple D, Barbeau H, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dobkin B, Barbeau H, Deforge D, et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized Spinal Cord Injury Locomotor Trial. Neurorehabil Neural Repair. 2007;21:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barbeau H, Norman K, Fung J, et al. Does neurorehabilitation play a role in the recovery of walking in neurological populations? Ann NY Acad Sci. 1998;860:377–392. [DOI] [PubMed] [Google Scholar]
- 11. Yang JF, Musselman KE, Livingstone D, et al. Repetitive mass practice or focused precise practice for retraining walking after incomplete spinal cord injury? A pilot randomized clinical trial. Neurorehabil Neural Repair. 2014;28:314–324. [DOI] [PubMed] [Google Scholar]
- 12. de Leon R, See PA, Chow CH. Differential effects of low versus high amounts of weight supported treadmill training in spinal rats. J Neurotrauma. 2011;28:1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cha J, Heng C, Reinkensmeyer DJ, et al. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J Neurotrauma. 2007;24:1000–1012. [DOI] [PubMed] [Google Scholar]
- 14. Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke. 2002;33:553–558. [DOI] [PubMed] [Google Scholar]
- 15. Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002;83:683–691. [DOI] [PubMed] [Google Scholar]
- 16. Mehta S, Pereira S, Janzen S, et al. Cardiovascular conditioning for comfortable gait speed and total distance walked during the chronic stage of stroke: a meta-analysis. Top Stroke Rehabil. 2012;19:463–470. [DOI] [PubMed] [Google Scholar]
- 17. Pang MY, Charlesworth SA, Lau RW, Chung RC. Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence-based exercise prescription recommendations. Cerebrovasc Dis. 2013;35:7–22. [DOI] [PubMed] [Google Scholar]
- 18. Tang A, Eng JJ, Krassioukov AV, et al. Exercise-induced changes in cardiovascular function after stroke: a randomized controlled trial. Int J Stroke. 2014;9:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Macko RF, Ivey FM, Forrester LW. Task-oriented aerobic exercise in chronic hemiparetic stroke: training protocols and treatment effects. Top Stroke Rehabil. 2005;12:45–57. [DOI] [PubMed] [Google Scholar]
- 20. Luft AR, Macko RF, Forrester LW, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke. 2008;39:3341–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roy RR, Baldwin KM, Edgerton VR. The plasticity of skeletal muscle: effects of neuromuscular activity. Exerc Sport Sci Rev. 1991;19:269–312. [PubMed] [Google Scholar]
- 22. Hoppeler H, Baum O, Lurman G, Mueller M. Molecular mechanisms of muscle plasticity with exercise. Compr Physiol. 2011;1:1383–1412. [DOI] [PubMed] [Google Scholar]
- 23. Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. [DOI] [PubMed] [Google Scholar]
- 24. Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity—exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 2010;40:765–801. [DOI] [PubMed] [Google Scholar]
- 25. Huang T, Larsen KT, Ried-Larsen M, et al. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: a review. Scand J Med Sci Sports. 2014;24:1–10. [DOI] [PubMed] [Google Scholar]
- 26. Gomez-Pinilla F, Ying Z, Roy RR, et al. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. [DOI] [PubMed] [Google Scholar]
- 27. Jakeman LB, Wei P, Guan Z, Stokes BT. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp Neurol. 1998;154:170–184. [DOI] [PubMed] [Google Scholar]
- 28. Boyce VS, Tumolo M, Fischer I, et al. Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J Neurophysiol. 2007;98:1988–1996. [DOI] [PubMed] [Google Scholar]
- 29. Boyce VS, Park J, Gage FH, Mendell LM. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur J Neurosci. 2012;35:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vavrek R, Girgis J, Tetzlaff W, et al. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129:1534–1545. [DOI] [PubMed] [Google Scholar]
- 31. Ying Z, Roy RR, Zhong H, et al. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cote MP, Azzam GA, Lemay MA, et al. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Sullivan SB, Schmitz TJ, Fulk G. Physical Rehabilitation. 6th ed Philadelphia, PA: FA Davis Co; 2014. [Google Scholar]
- 34. Umphred DA, Lazaro RT, Roller M, Burton G. Umphred's Neurological Rehabilitation. 6th ed St Louis, MO: Mosby; 2012. [Google Scholar]
- 35. Bobath B. Adult Hemiplegia: Evaluation and Treatment. Oxford, United Kingdom: Butterworth-Heinemann; 1990. [Google Scholar]
- 36. Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain. 2007;130:159–169. [DOI] [PubMed] [Google Scholar]
- 37. Gross R, Leboeuf F, Remy-Neris O, Perrouin-Verbe B. Unstable gait due to spasticity of the rectus femoris: gait analysis and motor nerve block. Ann Phys Rehabil Med. 2012;55:609–622. [DOI] [PubMed] [Google Scholar]
- 38. Shah PK, Gerasimenko Y, Shyu A, et al. Variability in step training enhances locomotor recovery after a spinal cord injury. Eur J Neurosci. 2012;36:2054–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ziegler MD, Zhong H, Roy RR, Edgerton VR. Why variability facilitates spinal learning. J Neuroscience. 2010;30:10720–10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. El Masry WS, Tsubo M, Katoh S, et al. Validation of the American Spinal Injury Association (ASIA) motor score and the National Acute Spinal Cord Injury Study (NASCIS) motor score. Spine (Phila Pa 1976). 1996;21:614–619. [DOI] [PubMed] [Google Scholar]
- 41. Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International Standards for Neurological Classification of Spinal Cord Injury (revised 2011). J Spinal Cord Med. 2011;34:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Benz EN, Hornby TG, Bode RK, et al. A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch Phys Med Rehabil. 2005;86:52–59. [DOI] [PubMed] [Google Scholar]
- 43. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. [DOI] [PubMed] [Google Scholar]
- 44. Thompson CK, Jayaraman A, Kinnaird C, Hornby TG. Methods to quantify pharmacologically induced alterations in motor function in human incomplete SCI. J Vis Exp. 2011;50:2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dittuno PL, Ditunno JF., Jr Walking Index For Spinal Cord Injury (WISCI II): scale revision. Spinal Cord. 2001;39:654–656. [DOI] [PubMed] [Google Scholar]
- 46. Gunja N, Collins M, Graudins A. A comparison of the pharmacokinetics of oral and sublingual cyproheptadine. J Toxicol Clin Toxicol. 2004;42:79–83. [DOI] [PubMed] [Google Scholar]
- 47. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 48. Lewek MD, Cruz TH, Moore JL, et al. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Phys Ther. 2009;89:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Machač S, Radvanský J, Kolář P, Kříž J. Cardiovascular response to peak voluntary exercise in males with cervical spinal cord injury. J Spinal Cord Med. 2015;1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. American College of Sports Medicine's Guidelines for Exercise Testing and Prescription. 9th ed Baltimore, MD: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 51. Leech KA, Kinnaird CR, Hornby TG. Effects of serotonergic medications on locomotor performance in humans with incomplete spinal cord injury. J Neurotrauma. 2014;31:1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Field-Fote EC, Tepavac D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys Ther. 2002;82:707–715. [PubMed] [Google Scholar]
- 53. Pang MY, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. J Physiology. 2000;528(pt 2):389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hornby TG, Kinnaird CR, Holleran CL, et al. Kinematic, muscular, and metabolic responses during exoskeletal-, elliptical-, or therapist-assisted stepping in people with incomplete spinal cord injury. Phys Ther. 2012;92:1278–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fung J, Barbeau H. A dynamic EMG profile index to quantify muscular activation disorder in spastic paretic gait. Electroencephalogr Clin Neurophysiol. 1989;73:233–244. [DOI] [PubMed] [Google Scholar]
- 56. Pepin A, Norman KE, Barbeau H. Treadmill walking in incomplete spinal-cord-injured subjects, 1: adaptation to changes in speed. Spinal Cord. 2003;41:257–270. [DOI] [PubMed] [Google Scholar]
- 57. Grieve DW, Gear RJ. The relationships between length of stride, step frequency, time of swing and speed of walking for children and adults. Ergonomics. 1966;9:379–399. [DOI] [PubMed] [Google Scholar]
- 58. Grillner S, Halbertsma J, Nilsson J, Thorstensson A. The adaptation to speed in human locomotion. Brain Res. 1979;165:177–182. [DOI] [PubMed] [Google Scholar]
- 59. Judge JO, Ounpuu S, Davis RB., III Effects of age on the biomechanics and physiology of gait. Clin Geriatr Med. 1996;12:659–678. [PubMed] [Google Scholar]
- 60. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack Inc; 1992. [Google Scholar]
- 61. Franz JR, Kram R. The effects of grade and speed on leg muscle activations during walking. Gait Posture. 2012;35:143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Winter DA. Biomechanical motor patterns in normal walking. J Motor Behav. 1983;15:302–330. [DOI] [PubMed] [Google Scholar]
- 63. Jordan K, Challis JH, Newell KM. Walking speed influences on gait cycle variability. Gait Posture. 2007;26:128–134. [DOI] [PubMed] [Google Scholar]
- 64. Wuehr M, Schniepp R, Pradhan C, et al. Differential effects of absent visual feedback control on gait variability during different locomotion speeds. Exp Brain Res. 2013;224:287–294. [DOI] [PubMed] [Google Scholar]
- 65. Tyrell CM, Roos MA, Rudolph KS, Reisman DS. Influence of systematic increases in treadmill walking speed on gait kinematics after stroke. Phys Ther. 2011;91:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pepin A, Ladouceur M, Barbeau H. Treadmill walking in incomplete spinal-cord-injured subjects: 2. Factors limiting the maximal speed. Spinal Cord. 2003;41:271–279. [DOI] [PubMed] [Google Scholar]
- 67. MacKay-Lyons MJ, Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: is the intensity adequate to induce a training effect? Arch Phys Med Rehabil. 2002;83:1378–1383. [DOI] [PubMed] [Google Scholar]
- 68. Dingwell JB, Cavanagh PR. Increased variability of continuous overground walking in neuropathic patients is only indirectly related to sensory loss. Gait Posture. 2001;14:1–10. [DOI] [PubMed] [Google Scholar]
- 69. Brummer V, Schneider S, Struder HK, Askew CD. Primary motor cortex activity is elevated with incremental exercise intensity. Neuroscience. 2011;181:150–162. [DOI] [PubMed] [Google Scholar]
- 70. Lisman J, Spruston N. Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity. Nat Neurosci. 2005;8:839–841. [DOI] [PubMed] [Google Scholar]
- 71. Peinemann A, Reimer B, Loer C, et al. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiology. 2004;115:1519–1526. [DOI] [PubMed] [Google Scholar]