Abstract
Cancer survivors (CA) tend to demonstrate metabolic, cardiac, and ventilatory alterations due to previous chemotherapy and radiation that may impair adaptability following aerobic exercise training. Exercise training adaptations of CA finished with primary treatment compared to non-cancer participants (NC) have not yet been extensively elucidated. Thus, the present study compared physiologic responses of CA versus NC following a low-to-moderate intensity, 8-wk aerobic training program. Thirty-seven previously sedentary participants (CA: n = 14, 12 females; NC: n = 23, 19 females) with no heart or metabolic disease did not differ in age, height, weight, and body mass index (51 ± 2 y, 1.66 ± 0.02 m, 83.8 ± 3.2 kg, and 30.5 ± 1 kg·m-2). Each participant underwent baseline, 3-, 6-, and 8-wk VO2peak treadmill testing using the USAFSAM protocol and walked on a treadmill three times per week at 80-90% of ventilatory threshold (VT) for approximately 40-min·session-1. Variables obtained on the VO2peak tests included: HR at stage 2 (HR@stage2), rating of perceived exertion at stage 2 (RPE@stage2), lactate threshold (LT), ventilatory threshold (VT), salivary cortisol at 30-min post VO2peak test (SC@30-minPost),VO2peak level, time of fatigue (TOF), and maximal heart rate (HRmax). NC had significantly (p < 0.05) higher VO2peak, TOF, and HRmax at baseline, 3- and 6-wks of training but not at 8-wks. There were no differences between groups on RPE@stage2 except at baseline (p < 0.05). A significant (p < 0.05) interaction was observed only for RPE@stage2 with CA rating their initial RPE significantly greater at baseline versus NC. CA notably improved submaximal and maximal exercise capacity during 8 weeks of aerobic training and did not show altered adaptability compared to NC. We suggest prescribing aerobic exercise training at low/moderate intensity and duration initially, with progressive increases in duration and intensity after approximately 8-weeks. If available and supported, we advise clinicians to utilize submaximal threshold concepts obtained from cardiopulmonary exercise testing to prescribe more precise aerobic exercise training parameters.
Key points.
Cancer survivors will most likely begin an exercise program after cancer therapy with a diminished functional capacity whereby baseline cardiopulmonary testing is recommended.
By about 8-weeks of individualized aerobic exercise training at 80-90% of VT, cancer survivors’ functional capacity improves similar to non-cancer exercisers.
Fine tuning the aerobic exercise prescription in cancer survivors, especially after 8-weeks of training, should be an on-going process and discussion.
Key words: Ventilatory threshold, rating of perceived exertion, VO2peak, cortisol
Introduction
About 40 percent of U.S. citizens will be diagnosed with cancer at some point during their lifetime and nearly 14.5 million people have lived with or been treated for some form of cancer (i.e., all cancer sites included) in 2014 (ACS, 2015). Based on data between 2008 and 2012 of the National Cancer Institute, the incidence of all cancer sites was 455 per 100,000 men and women per year and the number of deaths was 171 per 100,000 men and women per year. With early detection of some of the more common cancers (i.e., prostate, breast, colon, lung) the risk of pre-mature death decreases (ACS, 2015). Between 2005 and 2011, the 5 year surviving rate was 67% for all types of cancer (ACS, 2015). Research that focuses on improving quality of life, affecting cancer recurrence, enhancing functional capacity, attenuating cancer-related fatigue, and heightening survival rate has become essential (Courneya et al. 2003; Schneider et al., 2003; 2007a; 2007b; Schwartz et al., 1999; Winningham et al., 1994).
Cancer (CA) patients undergo many forms of treatment, including surgery, radiation therapy, chemotherapy, and/or biological therapies. Cancer treatments not only affect tumor cells, but also negatively affect normal cells (DeVita et al., 2001). This can lead to cancer- related fatigue, decreased functional capacity, muscle weakness, and depression (Dimeo et al., 2003; Maguire et al., 1978; Schneider et al., 2007c; Winningham et al., 1994; 2001).
Aerobic exercise training has been proven to attenuate side effects by improving metabolic function, functional capacity, and immune system before, during, or after cancer treatment (Fairey et al., 2005; Newton et al., 2008; McNeely et al., 2006; McTiernan et al., 2004; Schmitz and Speck, 2010). However, exercise training at higher intensities (e.g., above the anaerobic threshold) may impair immune function with potentially negative effects on CA patient’s health (Nieman et al., 1997). Therefore, cardiopulmonary exercise testing should be considered as a pre-participatory screening tool to assess physical function and fitness (Scharhag-Rosenberger et al., 2015). An appealing method to determine the appropriate exercise intensity also applicable to CA survivors is to assess their ventilatory threshold (VT) or lactate threshold (LT) during an incremental, symptom-limited peak treadmill test (i.e., VO2peak) prior to the initiation of aerobic training (Meyer et al., 2005; Scharhag-Rosenberger et al., 2015). Training below and at the ventilatory or lactate threshold has been shown to induce beneficial aerobic training and VO2max or VO2peak adaptations (Hagberg et al., 1989; Seals et al. 1984) with a decrease in ventilatory efforts, HR, and RPE at a given exercise intensity (Hagberg et al., 1989; Yerg et al., 1985) when comparing before and after training. However, the use of VT or LT to determine exercise intensity in cancer survivors remains relatively unexplored. An earlier study by Klika et al. (2009) revealed that a 12-week aerobic exercise training program based on a percentage of LT from a VO2peak test elicited significant improvement in power (Watts) at LT, peak power, and VO2peak in CA survivors who were in or post cancer treatment. Also Dimeo and coworkers found that CA accumulates less blood lactate at a certain workload after six weeks of aerobic training (Dimeo et al., 1997; 1998). The line of research on cancer and exercise is, however, limited related to precise exercise prescription guidelines for cancer survivors, even though individualized and supervised exercise training recommendations has been repeatedly claimed to be beneficial (Klika et al., 2009; Schneider et al., 2007b; Spence et al., 2010). Thus, training prescriptions based on cardiopulmonary exercise testing using VT in terms of investigating aerobic adaptability throughout the process of training is highly recommended; however, we concede this should occur per the availability or desire of skilled clinicians to administer appropriate testing. Lastly, we sought to objectively measure the peak exercise stress of participants via cortisol analysis 30-min post exercise test (Buono et al., 1986).
The purpose of this study was to evaluate select, physiological variables over an 8-week aerobic exercise training period where intensity was based on VT determined during cardiopulmonary exercise testing. Primary variables were: heart rate at stage 2 of the exercise testing/VO2peak protocol (HR@stage2), rating of perceived exertion at stage 2 (RPE@stage2), power at the lactate threshold (LT), ventilatory threshold (VT), and salivary cortisol at 30-min post VO2peak (SC@30-minPost). Secondary variables included: VO2peak, HRmax, and time of fatigue (TOF) during cardiopulmonary testing. It was hypothesized that CA survivors would begin the aerobic exercise training program with a lower exercise capacity, including substandard values on all objective physiological measures on maximal and submaximal levels (e.g., submaximally inflated HR@stage2, decremented VT, decremented HRmax, decremented TOF) with higher rates of subjective perceived exertion initially. We further investigated whether CA exercisers would improve at a similar progression rate compared to their healthy counterparts within 8-weeks. Hence, we hypothesized the CA exercisers would show a different rate of improvement (e.g., HR@stage2 or HRmax would remain consistently elevated over 8-weeks of training vs. controls) referring to altered adaptability to aerobic training stimuli.
Methods
Participants
This study was conducted at the Rocky Mountain Cancer Rehabilitation Institute (RMCRI) in Greeley, CO at the University of Northern Colorado (UNC). A sample of local and sedentary CA survivors (n = 14) and NC participants (n = 23) were recruited. CA and NC groups mostly consisted of women (CA: 12 females/2 males; NC: 19 females/4 males) and did not notably differ (p > 0.05) in terms of age (54 ± 2.2 vs 48 ± 2.1 years), height (1.65 ± 2.80 vs 1.66 ± 1.95 m), weight (85.1 ± 2.9 vs 81.2 ± 3.2 kg) and body mass index (BMI, 31.5 ± 1.2 vs 29.5 ± 1.3 kg·m-2) at baseline level. None of the participants suffered from cardiovascular and/or metabolic disease and were sedentary based on less than two days/week of aerobic activity for < 20-min/day over the past year (Bellman et al., 1986; Cassaburi et al., 1995). CA survivors were on average 4 ± 1 months removed from having finished cancer therapies (e.g., surgery, radiation, chemotherapy) and included a multitude of cancer types (i.e., breast, ovarian, lung, uterine, renal, colorectal, cervical; see Table 1). This study was approved by the UNC Institutional Review Board and RMCRI Medical Director. All participants gave written informed consent prior to participation and therefore voluntarily entered into the 8-week aerobic exercise study.
Table 1.
Cancer history.
| Cancer type | Canser stage | Treatment type | Month following treatment | Sex |
|---|---|---|---|---|
| R. Breast | cna | Surgery | 3 months | F |
| Ovarian | IA | Surgery, chemo | 2 months | F |
| R. Breast | I | Radiation, surgery | 3 months | F |
| L. Breast | II | Surgery, | 4 months | F |
| R. Breast | cna | Radiation, surgery | 1 month | F |
| Lung | cna | Chemo, radiation | 1 month | F |
| R. Breast | I | Surgery | 2 months | F |
| Uterine | I | Surgery | 2 months | F |
| Renal cell | III | Surgery | 5 months | M |
| L. Breast | II | Surgery, chemo | 5 months | F |
| R. Breast | cna | Surgery | 8 months | F |
| R. Breast | I | Surgery | 9 months | F |
| Colorectal | II | Surgery | 10 months | M |
| Cervical | IB | Radiation | 1.5 months | F |
cna = could not assess; R = right; L = left; F = female; M = male.
Study design
The study was outlined as a controlled study with parallel group design. Participants were recruited for an 8-week, individualized aerobic exercise intervention three times per week with 40-minutes of treadmill walking at 80-90% of VT with a cancer exercise specialist supervising each session. VO2peak testing (breath by breath analysis using an automatic gas analyzer, the Medgraphic CPX Ultima; Medical Graphic Corporation, St. Paul, MN, USA) using the United States Air Force School of Aerospace Medicine (USAFSAM) protocol was conducted on all participants at baseline, 3-weeks, 6-weeks, and 8- weeks of aerobic exercise training to assess physiological outcome variables. To confirm, the primary variables assessed during VO2peak testing included: HR@stage2, RPE@stage2, concentration of blood lactate at lactate threshold (LT), VT, and SC@30-minPost. Notably, 30-min post VO2peak test is reportedly when cortisol reaches its greatest concentration after a peak exercise bout (Buono et al., 1986; Jacks et al., 2002). Eighty to 90% of VT was used to prescribe a HR-range (within ± 5 bpm) for each treadmill walking session (3×/week for 8-weeks) and was updated to reflect cardiorespiratory conditioning after each VO2peak test. The aforementioned VT training range was considered to be low to moderate exertion and thought to be easily attainable by previously sedentary participants (i.e., CA and NC groups). LT was also calculated after each peak test and quantified to assess improvements in participant fitness over time. Recall, secondary variables were also reviewed and included: VO2peak, HRmax, and TOF during peak testing over 8-weeks.
Assessments
VO2peak was determined as the maximal value achieved on each peak test and was assessed via the Medgraphic CPX Ultima, as mentioned prior. HR was observed using a Polar heart rate monitor (transmitter/chest strap and receiver/watch; Polar Inc., Lake Success, NY) and recorded every minute during VO2peak testing and each training session. RPE was assessed utilizing a Categorical-Ratio Borg scale, a 0-10 assessment scale where 0 indicates no exertion and 10 equals maximal effort (Noble et al. 1983; Borg, 1998). LT was determined from plotting blood lactate values recorded during each incremental treadmill test with the use of a handheld, portable lactate analyzer (Accutrend® Portable Lactate Analzyer, Sport Resource Group Inc., www.lactate.com). Capillary blood was sampled at rest, the last minute of each stage, immediate post exercise, 5-min of recovery, and 10-min of recovery with approximately 20-25 µL of blood drawn into a capillary tube and dispensed onto a test strip positioned in the handheld analyzer via a reflotron applicator (Sport Resource Group Inc., www.lactate.com) (Beaver et al., 1986). A log-log transformation was employed to accurately assess the LT from the intersection of two lines using linear regression for each, where one line represented a slow increase in lactate concentration [La-] and the other depicted a phase of rapid increase. The intersection of the two lines was used as the LT. Overall; the plot of lactate during the VO2peak test was defined by log [La-] vs log VO2 (where VO2 is oxygen uptake). This LT determination technique is thoroughly described elsewhere (Beaver et al., 1986). VT was assessed via the V-slope method as described by Hughson et al. (1995) and Beaver et al. (1986). In brief, the V-slope is based on computerized regression analysis of the slope of CO2 concentration (VCO2) vs O2 uptake (VO2) plots and relies on the fact that excess CO2 is produced and detected when [H+] are buffered from lactic acid by a bicarbonate ion (HCO3 ).
Salivary cortisol (SC) was collected in volumes of about 0.05 to 2 mL in 15 mL plastic tubes, then immediately frozen at -20 ºC for later analysis (Chatard et al., 2002; Jacks et al., 2002; Kirschbaum et al., 1994; McDowell et al., 1992). Participants were instructed to abstain from ingesting food (with water ad libitum) three hours prior to all testing and we attempted to test each person at the same time of day each session. During each VO2peak assessment, samples, via “spitting” into the 15 mL tubes, were taken at rest, immediate post exercise, 5-min of recovery, 15- min of recovery, and 30-min of recovery (Buono et al., 1986; Jacks et al., 2002). Regular flavor Trident gums, routinely used in salivary cortisol collection, helped participants develop a greater concentration of saliva. Samples were sent to the University of Colorado Denver where an immunoassay (EIA) technique/kit (Salimetrics, LLC, State College, PA) was utilized to detect cortisol concentrations. All assays were conducted in triplicate and completed by trained personnel.
Exercise training
During the exercise intervention all participants were enrolled in personalized treadmill walking three times per week for 40-min·session-1, which included a 5- min warm-up and 5-min cool down of gentle/easy walking (ACSM, 2003; Schneider et al., 2003). During 30 minutes of each session, participants exercised within a HR-range (80-90% VT) and were continuously monitored by a cancer exercise specialist (Schneider et al., 2003) employed to specifically record minute by minute HR and RPE values. According to Casaburi et al. (1995), the aforementioned intensity relates to approximately 66 ± 11% HRmax and 43 ± 8% VO2peak. Overall, the ACSM (2003) and Schneider et al. (2007; 2007b; 2007c) recommend low to moderate exercise intensity for cancer survivors.
Statistical analysis
Data are presented as mean ± standard error of measurement (SEM) and the level of significance for all statistical tests was set at P < 0.05. SAS version 9.1 was used for analysis and all data were examined by a biostatistician. A repeated measures analyses of variance (rANOVA) was employed with post-hoc, pair wise comparisons when a significant main effect or interaction was observed for each, primary dependent variable at each time point (e.g., baseline, 3-weeks, 6-weeks, and 8-weeks of training during the VO2peak test). Trends were denoted by percent changes in selected variables without statistical significance calculated. Secondary variables were observed to denote trends and included VO2peak, TOF, and HRmax (assessed during VO2peak tests).
Results
Baseline physiological characteristics
HR@stage 2 did not differ between both groups at all time periods observed (p > 0.05, Figure 1A). Notably, both groups changed their HR at the same rate over the 8-week aerobic exercise period (time effect: p<0.05, np² = 0.08). No significant group differences were found for HR@stage2, LT, VT, and SC@30-minPost at baseline level. However, the effect sizes of group differences ranged from moderate to very large (0.8<d<2.1). Conversely, CA presented significantly higher values for RPE@stage2 (at 3.3 mph and 0% grade) versus NC prior to the initiation of an 8-week exercise program (4.1 ± 0.45 vs. 2.9 ± 0.23, respectively, p = 0.01, d = 3.2, Figure 1B).
Figure 1.
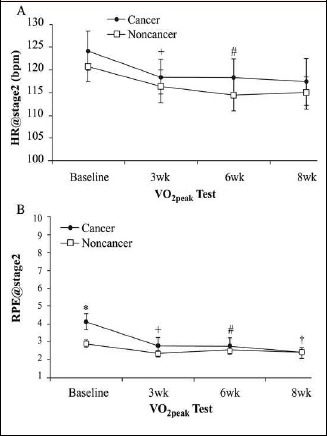
Comparison between cancer survivors and non-cancer participants for heart rate response at stage 2 (HR@stage2, A) and perceived exertion at stage 2 (RPE@stage2; B) at baseline, 3-wks, 6wks, and 8-wks of aerobic training. * Significant difference between CA and NC (p < 0.05). + Significant difference from baseline to 3wk (main effect, p < 0.05). # Significant difference from 3wk to 6wk (main effect, p < 0.05). † Significant difference from 6wk to 8wk (main effect, p < 0.05). Values are means + SEM.
Submaximal perceived exertion
A significant moderate interaction effect (p < 0.05, np² = 0.09) occurred for the time course of perceived exertion level. Post hoc testing revealed a significant decline for CA between baseline to 3-weeks (p < 0.01, d = 1.9) and 6-weeks to 8-weeks (p < 0.05, d = 0.5) (Figure 1B). Moderate to large group differences were found at baseline (p < 0.01, d = 2.0), 3-weeks (p < 0.05, d = 1.2), and 6-weeks (p = 0.05, d = 0.63), indicating that CA began to rate their perceived effort progressively less stressful.
Submaximal lactate and ventilatory thresholds
No main or interaction effects with small effect sizes for the time course of lactate concentration at stage 2 (p > 0.05, np² = 0.03) were observed (Figure 2A). Irrespective of groups, a significant but small main time effect was observed (p < 0.05, np² = 0.04). Post hoc testing revealed significant differences with moderate effect sizes from baseline to 3-weeks (p = 0.05, d=0.6), 3-weeks to 6-weeks (p = 0.05, d = 0.5), and 6-weeks to 8-weeks (p = 0.05, d = 0.7), indicating that the VT (L·min-1) slowly increased across all time periods throughout the 8-week training program (Figure 2B). However, statistical significance level disappeared after multiple corrections as the initial interaction effect was not significant.
Figure 2.
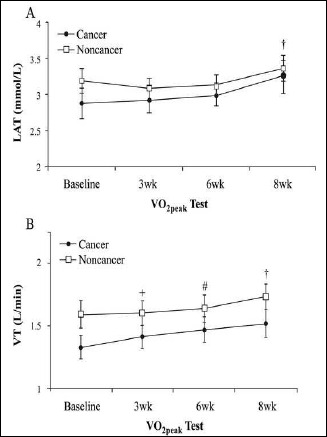
Comparison between cancer survivors and non-cancer participants for lactate concentration at the lactate threshold (LT, A) and ventilatory threshold (VT, B) responses at baseline, 3-wks, 6-wks, and 8-wks of aerobic training. + Significant difference from baseline to 3wk (main effect, p < 0.05). # Significant difference from 3wk to 6wk (main effect, p < 0.05). † Significant difference from 6wk to 8wk (main effect, p < 0.05). Values are means + SEM.
Cortisol concentration 30-min post VO2peak testing
No significant interaction effects occurred (p > 0.05). A significant main time effect was found (p < 0.05). Irrespective of groups, cortisol levels differed from baseline to 3-weeks, 3-weeks to 6-weeks (p < 0.01), and 6-weeks (p < 0.05) to 8-weeks (p < 0.05), indicating that cortisol changed over time progressively increased due to the higher aerobic capacity at stage 2 in both groups. The highest average values were found after 8 weeks (see Table 2 for an overview of cortisol data).
Table 2.
Salivary cortisol at 30-min post-exercise. Values are reported as means (±SEM).
| Group | SC@30-minPost (μg/dL) | ||||
|---|---|---|---|---|---|
| Baseline | 3-wks | 6-wks | 8-wks | %Δ (B to 8-wk) | |
| CA | .21 (.05) | .390 (.09) | .37 (.17) | .56 (.13) | 165% |
| NC | .36 (.07) | .44 (.08) | .68 (.12) | .60 (.09) | 66% |
CA = cancer group; NC = non-cancer group; %Δ = percent change; B = baseline. No significant differences between groups at each time point (p > 0.05).
Maximal exercise capacity
With very large effect sizes (1.6<d<2.1), significant differences between groups were observed at baseline (p < 0.05), 3- and 6-weeks (p < 0.05), but not after 8-weeks (p > 0.05) of aerobic exercise training on all secondary variables (Figure 3). Related to each variable, the CA achieved lower VO2peak, HRmax, and TOF values at all time-points (i.e., baseline, 3-weeks, 6-weeks, and 8-weeks) (Table 3).
Figure 3.
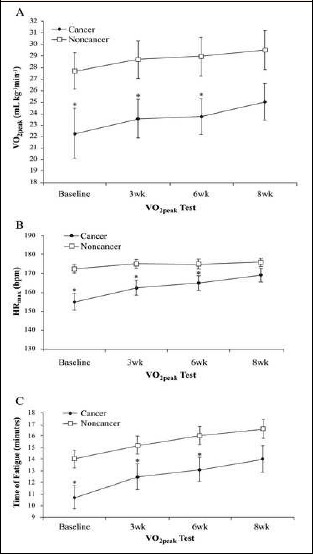
Comparison between cancer survivors (black circles) and non-cancer participants (white squares) for VO2peak (A), HRmax (B), and TOF (C) responses at baseline, 3-wks, 6-wks, and 8-wks of aerobic training. *Significant differences between cancer survivors and non-cancer participants (p < 0.05). Values are means (±SEM).
Table 3.
Baseline physiological characteristics for selected secondary dependent variables.
| Variable | Group | Mean (±SD) | P-value/d |
|---|---|---|---|
|
VO2peak (mL·kg-1· min-1) |
CA | 22.3 (6.3)* | .03/0.7 |
| NC | 27.7 (7.6) | ||
|
HRmax (bpm) |
CA | 155(16) * | .001/1.3 |
| NC | 172(10) | ||
|
TOF (minutes) |
CA | 10.7 (3.7) * | .01/0.9 |
| NC | 14.0 (3.5) | ||
|
BLa@stage2 (mmol/L) |
CA | 2.70 (.79) | .2/0.25 |
| NC | 2.90 (.81) | ||
|
SC@rest (μg/dL) |
CA | .13 (.05) | .59/0.2 |
| NC | .14 (.07) |
CA = cancer group; NC = non-cancer group; VO2peak = peak volume of oxygen uptake; HR = heart rate; TOF = time of fatigue; BLa = blood lactate accumulation; SC = salivary cortisol concentration. *Significantly different (p < 0.05) versus the NC group. “d” indicates Cohen’s d as a measure of effect size.
Discussion
The present study demonstrated that an 8-week low-to-moderate intensity, supervised aerobic exercise training program, 3 times a week, is effective. Hence, a similar rate of change on physiological variables related to aerobic capacity or fitness in inactive cancer survivors compared to healthy counterparts was observed. While CA might show a greater baseline physiological dysfunction (i.e., HR, LT, VT, VO2peak, HRmax, and TOF) and increased perceived and objectively measured stress levels (i.e., increased RPE and [SC]) after cancer treatment, our participants revealed a similar and, in part, improved adaptability to 2 months of aerobic exercise training. Notably, it was the submaximal perceived exertion that improved in the CA group.
After only 3-weeks of training, CA decreased their submaximal rate of perceived exertion by 33% versus 19% for NC and continued to change at a different rate throughout 8-weeks of training. A possible reason for a greater initial drop of perceived exertion in CA compared to healthy controls might be that CA entered the training program with greater fatigue and loss of physical function due to the sequel of their cancer and subsequent treatments (Porth, 2007, Schwartz, 2003). However, CA and NC trained for 8-weeks at a similar RPE of 2.2 and 2.5, respectively, and at the same relative intensity (80-90% VT). As CA were more deconditioned versus their matched NC counterparts at baseline, a greater improvement in reported, subjective measures of exertion may naturally occur. Notably, clinicians should be aware of how rapidly cancer survivors might adjust to the perceived effort of training and therefore may possibly have an augmented ability to perform activities of daily living and/or return to work. Also regarding training HR, CA exercised at an average HR of 111 bpm or about 67% of age predicted HRmax. The NC group exercised at an average HR of 113 bpm or about 66% of age predicted HRmax. This intensity is similar to a study by Dimeo et al. (2003) in which they implemented a “moderate” intensity training HR of up to 70% of HRmax at an average walking speed of 3.4-3.6 mph (5.5-5.8 km·h-1) in cancer survivors (age = 48 ± 15 years, mean ± SD) immediately after chemotherapy. However, they did not report HR responses during maximal incremental exercise testing. In the current study, the average heart rate at stage 2 (3.3 mph or 5.3 km/h) during the VO2peak test across all time points for CA and NC were 119 and 117 bpm, respectively. This indicated that stage 2 of the VO2peak test at 3.3 mph and 0% grade was a moderate intensity, overall HR response (Dimeo et al., 2003). Interestingly, a consistent definition for the classification of exercise intensity as low, moderate, or high does not seem to exist between study protocols in the literature; therefore, we report a low-to-moderate exercise training protocol and attempt to relate it to similar protocols.
Researchers have stressed the need to advance strict, specific or evidence-based exercise guidelines for cancer survivors (Humple and Iverson, 2005; Lucia et al., 2003; Oldervoll et al., 2004), seemingly different to that required or prescribed for the general, non-diseased population. This seems to indicate that cancer survivors will respond differently to the same exercise program versus another population, such as a sedentary, non-diseased group. Despite this, we observed that exercising cancer survivors improved on physiological measures similar to NC exercisers. Thus, ACSM guidelines (ACSM Position Stand (1998), which recommend appropriate frequency, intensity, and duration of exercise to the general, unfit public, could aptly be applied to cancer survivors; especially individuals recently finished with cancer treatment. However, we caution to initially utilize lower intensity and duration, per the fluctuating health of CA. Notably, cardiopulmonary exercise testing is an important means to exclude cardiac dysfunction and recommend valid training prescription in cancer survivors (Scharhag-Rosenberger et al., 2015). Clinicians should always keep in mind and recognize the fluctuating nature of cancer toxicities, which can persist for many months up to years post treatment or tumor burden (Schneider et al., 2007a; 2007b). Therefore, cancer survivors also require extensive clinical examination prior to exercise training. There may be a need to exercise test each cancer survivor (or seemingly healthy person) prior to the initiation of an exercise program so that an individualized heart rate or percentage of VT (or LT) training recommendation can be developed based upon the client’s current level of fitness (Klika et al., 2009; 2011).
The ACSM (1998) recognizes that middle-aged sedentary and older exercisers, including the very frail, may have to exercise for several weeks to adapt to the stresses of initial exercise training. The current authors’ HR and RPE data support this in that CA and NC decreased HR- and RPE-responses at stage 2 throughout the entire training program, with positive gains already evident after only 3-weeks of training.
On another note, CA changed at a similar rate (main effect, no interaction) versus their healthy control counterparts and had similar gains in submaximal and maximal response (LT, VT, VO2peak, HRmax, and TOF) at baseline to 3-, 6-, and 8-weeks of training. Interestingly, significant differences between CA and NC persisted (with CA showing a decrement) through 6-weeks of training for VO2peak, HRmax, and TOF but no differences were observed between groups after 8-weeks of aerobic exercise training on the previously mentioned variables. Despite lower functional capacity at the beginning of the training, CA increased functional capacity closely related to their healthy counterparts.
A benefit of improved physiological response to exercise training, such as a decreased HR response to the same workload, may be related to a decrease in cancer-related fatigue. For instance, Lucia et al. (2003) - in their review about cancer-related fatigue - found that improvements in functional capacity were related to a decrease in HR (and blood lactate) at submaximal workloads and as a result most patients carried out normal activities of daily living with no fatigue (Lucia et al., 2003). With the above in mind, Knols et al. (2005) reviewed the literature and summarized randomized and controlled clinical studies regarding physical exercise in cancer survivors during and after medical treatment (Knols et al., 2005). The authors concluded that exercise trained cancer survivors, at varying intensities and durations, accrue many physiological and psychological benefits. However, contrary to the current results, the authors underscored the finding that exercise benefits may differ depending on the type of cancer; cancer stage; medical treatment; nature, intensity, and duration of exercise training; and patient lifestyle.
Our findings are partly in contrast to Knols et al. (2005). In particular, post cancer treatment exercise training may be implemented based on established ACSM aerobic exercise guidelines for sedentary healthy persons, beginning with low/moderate intensity (e.g., 80-90% VT or ~ 67% HRmax or 2-3 RPE on 1-10 scale), duration (e.g., 30-40 min of walking), and frequency (e.g., 3·wk-1). Furthermore, the ACSM’s recent roundtable discussion on exercise guidelines for cancer survivors by Schmitz et al. (2010) also supports the use of generalized exercise prescription for cancer survivors with the recommendation that clinicians follow the 2008 Physical Activity Guidelines for Americans (Schmitz et al., 2010). A key finding of our study is that although CA started less fit versus their healthy counterparts, they systematically improved with a greater magnitude related to percent change from baseline to 8-weeks across all variables measured (when comparing CA versus NC: HR@stage2 (-5.5% vs. -4.7%), RPE@stage2 (-43% vs. -16%), LT (13% vs. 5%), VT (14% vs. 9%), SC@30-minPost (165% vs. 66%), VO2peak (12% vs. 7%), HRmax (9% vs. 2%), and TOF (31% vs. 19%). This may underscore the point that CA was most likely very deconditioned at the inception of the 8-week training period and possibly improved their fitness to a greater level vs NC; even though both groups exercised at the same relative intensity.
Lastly, the current authors are aware of the limited sample size, gender bias toward females, and age disparity between groups, whereby cancer survivors tended to be older (although not found to be significant vs controls). In light of this, we view our conclusions as suggestions or observations to be applied with caution and within the context of our limited study group. Foremost, we hope our findings add to the conversation about fine tuning exercise training parameters in cancer survivors.
Conclusion
The current researchers showed that cancer survivors’ physiological variables improved throughout 8-weeks of aerobic exercise training. This was illustrated by a lower HR and RPE at the same absolute intensity, improved VT, and greater production of cortisol after a peak test, indicating the potential ability to “push harder” after 8-weeks of exercise. An under-reported finding was that cancer survivors responded in a similar manner to comparable healthy controls undergoing the same, periodic peak exercise testing protocol and exercise program. Because cancer survivors were significantly lower on secondary variables (i.e., VO2peak, HRmax, and TOF) through 6-weeks but not at 8-weeks of training versus NC participants, there appears to be a threshold (i.e., at or after 8-weeks) beyond which CA is able to increase their training volume and possibly intensity similar to a non-cancer exerciser. This information may help future clinicians and exercise physiologists understand the importance of implementing an 8-week exercise period that is low/moderate intensity (i.e., ~ 80-90% VT or ~ 66% HRmax) and duration (i.e., ≤ 40-min, including warm-up and cool-down) prior to increasing volume or intensity in a cancer survivor’s exercise plan.
Acknowledgments
This study was supported by funding provided by the University of Northern Colorado (Greeley, CO) in the Sport and Exercise Science Department for exercise testing supplies and analysis of salivary cortisol. The authors thank Dr. Mark Laudenslager of the University of Colorado Denver (Denver, CO) for analyzing salivary cortisol in his lab, Behavior Immunology and Endocrinology Laboratory. The authors also thank Dr. Michelle Roozeboom, Senior Analytics Manager at Buccaneer, A Vangent Company (West Des Moines, IA) for her statistical help. There are no conflicts of interest.
Biographies

Scott N. DRUM
Employment
Northern Michigan University
Degree
PhD, FACSM
Research interests
Exercise testing and training in clinical and non-traditional sport populations; rock climbing and ultra-running performance physiology.
E-mail: sdrum@nmu.edu

Riggs J. KLIKA
Employment
Pepperdine University; Aspen Cancer Survivor Center
Degree
PhD, FACSM
Research interests
Cancer and exercise; sport performance; wellness coaching
E-mail: riggs.klika@pepperdine.edu

Susan D. CARTER
Employment
Northern Colorado Medical Center
Degree
MD, FACSM
Research interests
Cancer and exercise; exercise is medicine

Lisa K. SPROD
Employment
University of North Carolina at Wilmington
Degree
PhD, MPH
Research interests
Benefits of physical activity for cancer survivorship.
E-mail: sprodl@uncw.edu

Lars DONATH
Employment
University of Basel
Degree
PhD
Research interests
Improving seniors' fall risk factors, occupational and general physical activity promotion, exercise training programs in clinical settings and balance, endurance and strength training in general.
E-mail: lars.donath@unibas.ch
References
- ACS-American Cancer Society. (2015) Cancer Treatment & Survivorship Facts & Figures 2014-2015. Available from URL: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf.
- American College of Sports Medicine. (2003) ACSM’s Exercise management for persons with chronic diseases and disabilities. 2nd edition Champaign, IL: Human Kinetics. [Google Scholar]
- American College of Sports Medicine. (2009) Resource Manual for Guidelines for Exercise Testing and Prescription. 6th edition Philadelphia, PA: Lippincott Williams & Wilkins; 448-462. [Google Scholar]
- American College of Sports Medicine. Position Stand. (1998) The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Medicine and Science in Sports and Exercise 31, 916-920. [DOI] [PubMed] [Google Scholar]
- Beaver W.L., Wasserman K., Whipp B.J. (1986) A new method for detecting anaerobic threshold by gas exchange. Journal of Applied Physiology 60, 2020-2027. [DOI] [PubMed] [Google Scholar]
- Borg G.A.V. (1998) Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics. [Google Scholar]
- Buono M.J., Yeager O.E., Hodgdon J.A. (1986) Plasma adrenocortitropin and corisol responses to brief high-intensity exercise in humans. Journal of Applied Physiology 61,1337-39. [DOI] [PubMed] [Google Scholar]
- Casaburi R., Storer T.W., Sullivan C.S., Wasserman K. (1995) Evaluation of blood lactate elevation as an intensity criterion for exercise training. Medicine and Science in Sports and Exercise 27, 852-862. [PubMed] [Google Scholar]
- Chatard J.C., Atlaoui D., Lac G., et al. (2002) Cortisol, DHEA, performance and training in elite swimmers. International Journal of Sports Medicine 23, 510-515. [DOI] [PubMed] [Google Scholar]
- Courneya K.S. (2003) Exercise in cancer survivors: an overview of research. Medicine & Science in Sports & Exercise 35, 1846-1852. [DOI] [PubMed] [Google Scholar]
- Davis J.A., Frank M.H., Whipp B.J., Wasserman K. (1979) Anaerobic threshold alterations caused by endurance training in middle-aged men. Journal of Applied Physiology 46, 1039-46. [DOI] [PubMed] [Google Scholar]
- DeVita V.T., Hellman S., Rosenberg S.A. (2001) Cancer principles and practice of oncology. 6th edition Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Dimeo F.C., Tilmann M.H., Bertz H., Kanz L., Mertelsmann R., Keul J. (1997) Aerobic exercise in the rehabilitation of cancer patients after high dose chemotherapy and autologous peripheral stem cell transplantation. Cancer 79, 1712-1722. [PubMed] [Google Scholar]
- Dimeo F..C, Rumberger, B.G. and Keul J. (1998) Aerobic exercise as therapy for cancer fatigue. Medicine & Science in Sports & Exercise 30, 475-478. [DOI] [PubMed] [Google Scholar]
- Dimeo F.C., Schwartz S., Fietz T., Wanjura T., Boning D., Thiel E. (2003) Effects of endurance training on the physical performance of patients with hematological malignancies during chemotherapy. Support Care Cancer 11, 623-628. [DOI] [PubMed] [Google Scholar]
- Fairey A.S., Courneya K.S., Field C.J., Bell G.J., Jones L.W., Martin B.S., Mackey J.R. (2005) Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain Behavior and Immunology 19, 381-388. [DOI] [PubMed] [Google Scholar]
- Gabriel H., Kindermann W. (1997) The acute immune response to exercise: what does it mean? International Journal of Sports Medicine 18(Suppl 1), S28-45. [DOI] [PubMed] [Google Scholar]
- Hagberg J.M., Graves J.E., Limacher M., Woods D.R., Leggett S.H., Cononie C., Gruber J.J., Pollock M.L. (1989) Cardiovascular responses of 70- to 79-yr-old men and women to exercise training. Journal of Applied Physiology 66, 2589-2594. [DOI] [PubMed] [Google Scholar]
- Hughson R.L., Green H.J., Sharratt M.T. (1996) Gas exchange, blood lactate, and plasma catecholamines during incremental exercise in hypoxia and normoxia. Journal of Applied Physiology 79, 1134-1141. [DOI] [PubMed] [Google Scholar]
- Humpel N., Iverson D.C. (2005) Review and critique of the quality of exercise recommendations for cancer patients and survivors. Support Care Cancer 13, 493-502. [DOI] [PubMed] [Google Scholar]
- Jacks D.E., Sowash J., Anning J., McGloughlin T., Andres F. (2002) Effect of exercise at three exercise intensities on salivary cortisol. Journal of Strength and Conditioning Research 16, 286-289. [PubMed] [Google Scholar]
- Kirschbaum C., Hellhammer D.H. (1994) Salivary cortisol in psychoneuroendocrine research: Recent developments and application. Psychoneuroendocrinology 19, 313-333. [DOI] [PubMed] [Google Scholar]
- Klika R.J., Callahan K.E., Drum S.N. (2009) Individualized 12-week exercise training programs enhance aerobic capacity of cancer survivors. Physician and Sports Medicine 3, 68-77. [DOI] [PubMed] [Google Scholar]
- Klika R.J., Golik K.S., Drum S.N., Callahan K.E., Thorland W.G. (2011) Comparison of physiological response to cardiopulmonary exercise testing among cancer survivors and healthy controls. European Journal of Applied Physiology 111, 1167-1176. [DOI] [PubMed] [Google Scholar]
- Knols R., Aaronson N.K., Uebelhart D., Fransen J., Aufdemkampe G. (2005) Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. Journal of Clinical Oncology 23, 3830-3842. [DOI] [PubMed] [Google Scholar]
- Lucia A., Earnest C., Perez M. (2003) Cancer-related fatigue: can exercise physiology assist oncologists? Lancet Oncology 4, 616-625. [DOI] [PubMed] [Google Scholar]
- Maguire G.P., Lee E.G., Bevington D.J., Kuchemann C.S., Crabtree R.J., Cornell C.E. (1978) Psychiatric problems in the first year after mastectomy. British Medical Journal 1, 963-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell S.L., Hughes R.A., Hughes R.J., Housh T.J., Johnson G.O. (1992) The effect of exercise training on salivary immunoglobulin A and cortisol responses to maximal exercise. International Journal of Sports Medicine 13, 577-580. [DOI] [PubMed] [Google Scholar]
- McNeely M.L., Campbell K.L., Rowe B.H., Klassen T.P., Mackey J.R., Courneya K.S. (2006) Effects of exercise on breast cancer patients and survivors: a systematic review and metaanalysis. Canadian Medical Association Journal 175, 34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTiernan A. (2004) Physical activity after cancer: physiologic outcomes. Cancer Investigation 22, 68-81. [DOI] [PubMed] [Google Scholar]
- Meyer T., Lucia A., Earnest C.P., Kindermann W. (2005) A conceptual framework for performance diagnosis and training prescription from submaximal gas exchange parameters--theory and application. International Journal of Sports Medicine 26(Suppl 1), S38-48. [DOI] [PubMed] [Google Scholar]
- Newton R.U., Galvão D.A. (2008) Exercise in prevention and management of cancer. Current Treatment Options in Oncology 9, 135-146. [DOI] [PubMed] [Google Scholar]
- Nieman D.C. (1997) Exercise immunology: practical applications. International Journal of Sports Medicine 18(Suppl 1), S91-100. [DOI] [PubMed] [Google Scholar]
- Noble B.J., Borg G.A.V., Jacobs I., Ceci R., Kaiser P. (1983) A category-ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Medicine and Science in Sports and Exercise 15, 523-528. [PubMed] [Google Scholar]
- Oldervoll L.M., Kaasa S., Hjermstad M.J., Lund J.A., Loge J.H. (2004) Physical exercise results in the improved subjective well-being of a few or is effective rehabilitation for all cancer patients? European Journal of Cancer 40, 951-962. [DOI] [PubMed] [Google Scholar]
- Porth C.M. (2007) Essentials of pathophysiology – concepts of altered health states. 2nd edition. Philadelphia, PA:Lippincott Williams & Wilkins; 79-104. [Google Scholar]
- Scharhag-Rosenberger F., Wiskemann J., Scharhag J. (2015) Cardiopulmonary exercise testing in cancer patients: should we really refrain from considering it for preparticipation screening? Oncologist 20, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz K.H., Courneya K.S., Matthews C., Demark-Wahnefried W., Galvão D.A., Pinto B.M., Irwin M.L., Wolin K.Y., Segal R.J., Lucia A., Schneider C.M., von Gruenigen V.E., Schwartz A.L. (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine & Science in Sports & Exercise 42, 1409-1426. [DOI] [PubMed] [Google Scholar]
- Schmitz K.H., Speck R.M. (2010) Risks and benefits of physical activity among breast cancer survivors who have completed treatment. Womens Health (Lond Engl) 6, 221-238. [DOI] [PubMed] [Google Scholar]
- Schneider C.M., Dennehy C.A., Carter S.D. (2003) Exercise and cancer recovery. Champaign, Il: Human Kinetics Publisher, Inc. [Google Scholar]
- Schneider C.M., Hsieh C.C., Sprod L.K., Carter S.D., Hayward R. (2007a) Cancer treatment-induced alterations in muscular fitness and quality of life: the role of exercise training. Annals of Oncology 18, 1957-1962. [DOI] [PubMed] [Google Scholar]
- Schneider C.M., Hsieh C.C., Sprod L.K., Carter S.D., Hayward R. (2007b) Effects of supervised exercise training on cardiopulmonary function and fatigue in breast cancer survivors during and after treatment. Cancer 110, 918-925. [DOI] [PubMed] [Google Scholar]
- Schneider C.M., Hsieh C.C., Sprod L.K., Carter S.D., Hayward R. (2007c) Exercise training manages cardiopulmonary function and fatigue during and following cancer treatment in male cancer survivors. Integrative Cancer Therapy 6, 235-241. [DOI] [PubMed] [Google Scholar]
- Schwartz A.L. (1999) Fatigue mediates the effects of exercise on quality of life. Quality of Life Research 8, 529-538. [DOI] [PubMed] [Google Scholar]
- Schwartz A.L. (2003) Cancer. In: ACSM’s Exercise Management for Persons with Chronic Diseases and Disabilities. Eds: Durstine J.L., Moore G.E. Champaign, IL: Human Kinetics; 166-172. [Google Scholar]
- Seals D.R., Hagburg JM., Hurley B.F., Ehsani A.A., Holloszy J.O. (1984) Endurance training in older men and women I. Cardiovascular responses to exercise. Journal of Applied Physiology 57, 1024-1029. [DOI] [PubMed] [Google Scholar]
- Spence R.R., Heesch K.C., Brown W.J. (2010) Exercise and cancer rehabilitation: a systematic review. Cancer Treatment Reviews 36(2), 185-194. [DOI] [PubMed] [Google Scholar]
- Winningham M.L. Nail L.M. Burke M.B. Brophy L. Limprich B. Jones L.S. et al. (1994) Fatigue and cancer experience: the state of the knowledge. Oncology Nursing Forum 21, 23-26. [PubMed] [Google Scholar]
- Winningham M.L. (2001) Strategies for managing cancer-related fatigue syndrome. Cancer 92(Suppl. 4), 988-997. [DOI] [PubMed] [Google Scholar]
- Yerg J.E., 2nd., Seals D.R., Hagberg J.M., Holloszy J.O. (1985) Effect of endurance exercise training on ventilatory function in older individuals. Journal of Applied Physiololgy 58(3), 791-794. [DOI] [PubMed] [Google Scholar]


