Abstract
Plasmodium falciparum is a predominant malaria species that infects humans in the African continent. A recent WHO report estimated 95% and 5% of P. falciparum and P. vivax malaria cases, respectively, in Sudan. However many laboratory reports from different areas in Sudan indicated otherwise. In order to verify, we selected four hundred suspected malaria cases from Aljabalain area located in the White Nile state, central Sudan, and diagnosed them with quality insured microscopy and species-specific nested PCR. Our results indicated that the proportion of P. vivax infections among suspected malaria cases was high. We found that on average 20% and 36.5% of malaria infections in both study areas were caused by P. vivax using both microscopy and PCR, respectively. This change in pattern is likely due to the recent demographic changes and high rate of immigration from neighbouring countries in the recent years. This is the first extensive clinical study of its kind that shows rising trend in P. vivax malaria cases in White Nile area, Sudan.
1. Background
Malaria is an infectious disease of humans and other animals caused by Plasmodium parasites [1]. According to the latest WHO estimates, there were about 198 million cases of malaria in 2013 (with an uncertainty range of 124 million to 283 million) and an estimated 584 000 deaths (with an uncertainty range of 367 000 to 755 000) in the world [2]. Malaria mortality rates have fallen by 47% globally since 2000 and by 54% in the WHO African Region [2].
Five species of plasmodia can infect humans. The vast majority of deaths in Sub-Saharan regions are caused by P. falciparum, while P. vivax, P. ovale, P. malariae, and P. knowlesi cause generally milder form of malaria which other than P. knowlesi is rarely fatal [1, 3].
P. vivax is responsible for most malaria cases in Asia and Latin America but it is almost absent from most of central Africa due to the absence of Duffy antigen, the receptor which P. vivax uses to invade human erythrocytes [4]. In eastern and southern Africa, P. vivax represents around 10% to 40% of malaria cases but <1% of cases in western and central Africa [4, 5].
In Sudan until recently the majority of malaria cases were caused by P. falciparum. P. vivax is relatively rare; 95% of cases are caused by P. falciparum and the other 5% are caused by P. vivax [6]. However, in recent years many clinicians observed recurrent relapses of malaria infections in different areas in Sudan suggesting perhaps a higher than expected transmission of non-falciparum malaria parasites (most likely P. vivax since it is the second most important malaria parasite species in Sudan). The objective of this study was to document the suggested rise in the proportion of P. vivax infections among suspected malaria cases in White Nile state in Sudan.
2. Materials and Methods
2.1. Ethical Considerations
The study was approved by the ethical committee of the Institute of endemic diseases, University of Khartoum. Informed consent was obtained from each patient before participation in the study.
2.2. Study Area and Sample Collection
This study was a cross-sectional study carried out in the White Nile area which is one of the central Sudan states, Figure 1. It lies between latitudes 33 and 30-31 north and longitude 13 and 30–12 east, occupying an area of around 675000 km2, with population of 1.675 million. The study was conducted in Aljabalain area, 80 km south of Rabak town, the capital of White Nile state. The area is considered mesoendemic for malaria; transmission follows mainly the rainy season (July to October). Four hundred suspected malaria cases based on patient's symptoms (fever, headache, sweating, nausea, and vomiting) and signs (pyrexia, pallor) were chosen randomly from Aljabalain hospital and Aljabalain military hospital aged >1 year old regardless of gender. Samples were collected during the rainy season (July–September) in 2012. All suspected malaria cases were treated with a standard regimen (artemisinin based combination therapy) by the local malaria control program according to the Sudanese Malaria Treatment Guidelines in Sudan National Malaria Control Program (NMCP), and the National Protocol for Treatment of Malaria (Federal Ministry of Health, Khartoum, Sudan, 2013, unpublished).
Figure 1.
Study site: White Nile area in Sudan.
2.3. Diagnosis of Malaria
Two and a half mL of venous blood was obtained from each patient. Malaria was diagnosed using blood film microscopy and confirmed with PCR. Both thick and thin blood films were used, fields were read at least twice, and the procedure was followed according to quality control guidelines of WHO. PCR was done for both P. falciparum and P. vivax. The PCR was performed at the Institute of Endemic Diseases, University of Khartoum, with quality control in place (both positive and negative control were used). Parasite genomic DNA was extracted from whole blood samples using Chelex method. A fragment of the plasmodial 18S rRNA gene was amplified by PCR and species identification was performed with species-specific oligoprobes using the following primers: for P. falciparum, rPLU5: 5′ CTTGTTGTTGCCTTAAACTTC-3′, rPLU6: 5′-TTAAAATTGTTGCATTAAAACG-3′; for P. vivax, rVIV1: 5′-CGCTTCTAGCTTAACCACATAACTGATAC-3′, rVIV2: 5′-ACTTCCAAGCCGAAG CAAAGA AAG TCC TTA-3′, as described previously [7].
2.4. Statistical Analysis
Data were analyzed using SPSS (statistical package for the social sciences) version twentieth software.
3. Results
In our study, both males and females are affected by malaria; however more females were represented in Aljabalain hospital (64.4%) and more males were represented from Aljabalain military hospital (64%). The average haemoglobin level in all patients from the two study sites was 11.9 g/dL. The prevalence of anaemia among malaria patients was high, more than two-thirds in both study sites. The majority of malaria cases (more than 90%) had low parasite level (≤500 parasite/μL of blood), Table 1.
Table 1.
The mean age, gender and haemoglobin level in Aljabalain hospital and military hospital.
| Aljabalain hospital | Military hospital | |
|---|---|---|
| Number of patients | 200 | 200 |
| Mean age ± SD, range (years) | 30 ± 15 (2–80) | 31 ± 13 (1–70) |
| Less than 15 (%) | 19 (9.5%) | 17 (8.5%) |
| 15–30 (%) | 93 (46.5%) | 92 (46.3%) |
| More than 30 (%) | 88 (44%) | 90 (45.2%) |
| Gender | ||
| Males (%) | 70 (34.7%) | 128 (64%) |
| Females (%) | 130 (64.4%) | 72 (36%) |
| Mean Hb level ± SD, range (g/dL) | 11.8 ± 2.2 (4–18) | 12 ± 2.1 (5–19) |
| Anaemia∗ (%) | 135 (67.5%) | 146 (73%) |
| Parasitaemia∗∗ | ||
| Low (%) | 180 (90%) | 185 (92.5%) |
| High (%) | 20 (10%) | 15 (7.5%) |
∗Anaemia was defined as Hb level less than 13.5 g/dL for males and 12.5 g/dL for females.
∗∗Low parasitaemia was defined as number of asexual parasites ≤500/µL of blood. High parasitaemia: >500 asexual parasites/µL of blood.
In both study sites, the proportion of P. vivax infections among suspected malaria cases was high. Microscopy results showed that 30 (15%) and 50 (25%) of malaria infections in Aljabalain hospital and Aljabalain military hospital were caused by P. vivax. The results were even higher with PCR (Figure 2); 66 (33%) and 80 (40%) of samples from Aljabalain hospital and Aljabalain military hospital were positive for P. vivax, respectively. Mixed infections (P. falciparum + P. vivax) were detected in 2.25% of samples in average from both study areas, Table 2.
Figure 2.
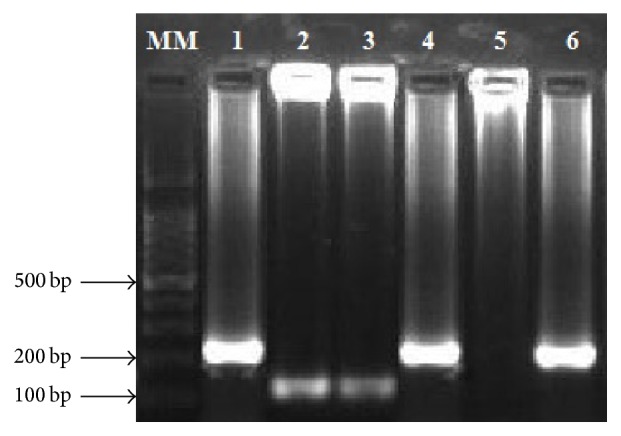
Detection of Plasmodium 18S rRNA gene using nested PCR from Sudanese malaria patients; MM: 100 bp ladder (iNtRON Biotechnology, South Korea), 1, 4, 6: P. falciparum, 2, 3: P. vivax, and 5: negative control.
Table 2.
Positive results of blood films and PCR for P. falciparum and P. vivax in Aljabalain hospital and military hospital.
| Aljabalain hospital (200) | Military hospital (200) | |||
|---|---|---|---|---|
| Microscopy | PCR | Microscopy | PCR | |
| N (%) | N (%) | N (%) | N (%) | |
| P. falciparum | 107 (53.5%) | 115 (57.5%) | 93 (46.5%) | 112 (56%) |
| P. vivax | 30 (15%) | 66 (33%) | 50 (25%) | 80 (40%) |
| Mixed (P. falciparum + P. vivax) | 0 (0%) | 5 (2.5%) | 0 (0%) | 4 (2%) |
| Negative | 63 (31.5%) | 14 (7%) | 57 (28.5%) | 4 (2%) |
4. Discussion
This study was carried out in an area characterized by seasonal and unstable malaria transmission. The most remarkable result in this study was the unexpected high proportion (about 40% by PCR) of P. vivax infections among suspected malaria cases, eight times more than that previously reported in Sudan [4]. This change in pattern is most likely due to the recent varied composition of the community resulting from several migrations of people from several Asian and African countries to work at petroleum and new sugar companies in White Nile area especially from Ethiopia where high prevalence of P. vivax infection (31%) among malaria cases was found [6].
Another suggested explanation for the emergence of P. vivax is parasite's development of alternative mechanisms to invade human erythrocytes other than the Duffy antigen. This is a plausible explanation since P. vivax infection of Duffy negative genotype was reported previously in many African countries [8–16]. This is the first study of its kind to document the significant rise in malaria P. vivax transmission in Sudan. And it has important health policy implications since P. vivax infection requires eradication of liver stages with primaquine due to presence of dormant hypnozoites within hepatocytes [17, 18]. Recent data showed that P. vivax infection is becoming more severe especially in children, and further studies are required to understand the exact causes of this pattern [19].
The sampled population (four hundred cases) of this study was selected based on their highly suspected symptoms of malaria and positive blood film microscopy done in hospitals' laboratories. Blood films were later proved only 50% positive in more quality insured settings. This obvious result of malaria overdiagnosis by a factor of two is probably due to lack of training in general hospitals, low quality control microscopy, and high work load. The same result was previously found in Tanzania where malaria was overdiagnosed by a factor of five [20, 21].
PCR diagnosis for malaria is accurate especially for differentiating between plasmodia species, but it is expensive and needs well-trained personnel. In this study quality control microscopy allowed for 20% of Plasmodium vivax diagnosis, while by PCR it was 38.7%. This may be due to difficulty in differentiation between species based on a single ring form especially for untrained personnel. In this study more than 90% of slides had low parasite density (≤500 parasite/μL); this also makes differentiation between plasmodia species very difficult.
5. Conclusion
Our study confirmed the observed high percentage of P. vivax infections in White Nile area, central Sudan. This result has important implications for the malaria control and necessitates modification of current guidelines for the treatment of malaria in Sudan.
Acknowledgments
The authors send their gratitude to the patients and families who participated in this study. This study was funded by TWAS research Grant agreement no. 13-145 RG/BIO/AF/AC_G.
Abbreviations
- BFFM:
Blood film for malaria
- PCR:
Polymerase chain reaction
- Pf:
Plasmodium falciparum
- Pv:
Plasmodium vivax.
Competing Interests
All authors declare no competing interests.
Authors' Contributions
Muzamil Mahdi Abdel Hamid, Makarim M. Adam Suliman, and Bushra M. Hamad made substantial contributions to the conception and the design of the study. Musab M. Ali Albasheer did the lab work. Analysis and interpretation of data was done by Muzamil Mahdi Abdel Hamid, Makarim M. Adam Suliman, and Bushra M. Hamad. Muzamil Mahdi Abdel Hamid, Maha Elobied, and Mutaz Amin Mustafa revised the manuscript and Mutaz Amin Mustafa wrote it. Muzamil Mahdi Abdel Hamid gave final approval of the version to be published; all authors read and approved the final manuscript.
References
- 1.Centers for Disease Control and Prevention. Malaria Worldwide. September 2015 http://www.cdc.gov/malaria/malaria_worldwide/index.html.
- 2.WHO. Malaria. 2015, http://www.who.int/mediacentre/factsheets/fs094/en/
- 3.Baird J. K. Evidence and implications of mortality associated with acute plasmodium vivax malaria. Clinical Microbiology Reviews. 2013;26(1):36–57. doi: 10.1128/cmr.00074-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendis K., Sina B. J., Marchesini P., Carter R. The neglected burden of Plasmodium vivax malaria. The American Journal of Tropical Medicine and Hygiene. 2001;64(1-2, supplement):97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- 5.Golassa L., Baliraine F. N., Enweji N., Erko B., Swedberg G., Aseffa A. Microscopic and molecular evidence of the presence of asymptomatic Plasmodium falciparum and Plasmodium vivax infections in an area with low, seasonal and unstable malaria transmission in Ethiopia. BMC Infectious Diseases. 2015;15(1, article 310) doi: 10.1186/s12879-015-1070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo E., Yewhalaw D., Zhong D., et al. Molecular epidemiology of Plasmodium vivax and Plasmodium falciparum malaria among duffy-positive and duffy-negative populations in Ethiopia. Malaria Journal. 2015;14, article 84 doi: 10.1186/s12936-015-0596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snounou G., Singh B. Nested PCR analysis of Plasmodium parasites. Methods in molecular medicine. 2002;72:189–203. doi: 10.1385/1-59259-271-6:189. [DOI] [PubMed] [Google Scholar]
- 8.Sondén K., Castro E., Trönnberg L., Stenström C., Tegnell A., Färnert A. High incidence of Plasmodium vivax malaria in newly arrived Eritrean refugees in Sweden since may 2014. Eurosurveillance. 2014;19(35):1–4. doi: 10.2807/1560-7917.es2014.19.35.20890. [DOI] [PubMed] [Google Scholar]
- 9.Mathews H. M., Armstrong J. C. Duffy blood types and vivax malaria in Ethiopia. American Journal of Tropical Medicine and Hygiene. 1981;30(2):299–303. doi: 10.4269/ajtmh.1981.30.299. [DOI] [PubMed] [Google Scholar]
- 10.Ménard D., Barnadas C., Bouchier C., et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(13):5967–5971. doi: 10.1073/pnas.0912496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendes C., Dias F., Figueiredo J., et al. Duffy negative antigen is no longer a barrier to Plasmodium vivax—molecular evidences from the African West Coast (Angola and Equatorial Guinea) PLoS Neglected Tropical Diseases. 2011;5(6, article e1192) doi: 10.1371/journal.pntd.0001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercereau-Puijalon O., Ménard D. Plasmodium vivax and the Duffy antigen: a paradigm revisited. Transfusion Clinique et Biologique. 2010;17(3):176–183. doi: 10.1016/j.tracli.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Ngassa Mbenda H. G., Das A. Molecular evidence of Plasmodium vivax mono and mixed malaria parasite infections in Duffy-negative native Cameroonians. PloS one. 2014;9(8) doi: 10.1371/journal.pone.0103262.e103262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan J. R., Stoute J. A., Amon J., et al. Evidence for transmission of Plasmodium vivax among a Duffy antigen negative population in Western Kenya. American Journal of Tropical Medicine and Hygiene. 2006;75(4):575–581. [PubMed] [Google Scholar]
- 15.Woldearegai T. G., Kremsner P. G., Kun J. R. F. J., Mordmüller B. Plasmodium vivax malaria in duffy-negative individuals from Ethiopia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2013;107(5):328–331. doi: 10.1093/trstmh/trt016. [DOI] [PubMed] [Google Scholar]
- 16.Wurtz N., Mint Lekweiry K., Bogreau H., et al. Vivax malaria in Mauritania includes infection of a Duffy-negative individual. Malaria Journal. 2011;10, article 336 doi: 10.1186/1475-2875-10-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernando D., Rodrigo C., Rajapakse S. Primaquine in vivax malaria: an update and review on management issues. Malaria Journal. 2011;10, article 351 doi: 10.1186/1475-2875-10-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulden L., Hulden L. Activation of the hypnozoite: a part of Plasmodium vivax life cycle and survival. Malaria Journal. 2011;10, article 90 doi: 10.1186/1475-2875-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahgoub H., Gasim G. I., Musa I. R., Adam I. Severe Plasmodium vivax malaria among Sudanese children at New Halfa Hospital, Eastern Sudan. Parasites & Vectors. 2012;5, article 154 doi: 10.1186/1756-3305-5-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harchut K., Standley C., Dobson A., et al. Over-diagnosis of malaria by microscopy in the Kilombero Valley, Southern Tanzania: an evaluation of the utility and cost-effectiveness of rapid diagnostic tests. Malaria Journal. 2013;12(1, article 159) doi: 10.1186/1475-2875-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reyburn H., Mbatia R., Drakeley C., et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: A Prospective Study. British Medical Journal. 2004;329(7476):1212–1215. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]


