Abstract
Glutathione-S-transferases mu 2 (GSTM2), a kind of important Phase II antioxidant enzyme of eukaryotes, is degraded by nonsense mediated mRNA decay due to a C27T substitution in the fifth exon of pigs. As a reproductive performance-related gene, GSTM2 is involved in embryo implantation, whereas, functional deficiency of GSTM2 induces pre- or post-natal death in piglets potentially. To have some insight into the role of GSTM2 in embryo development, high throughput RNA sequencing is performed using the swine testis cells (ST) with the deletion of GSTM2. Some embryo development-related genes are observed from a total of 242 differentially expressed genes, including STAT1, SRC, IL-8, DUSP family, CCL family and integrin family. GSTM2 affects expression of SRC, OPN, and SLCs. GSTM2 suppresses phosphorylation of STAT1 by binding to STAT1. In addition, as an important transcription factor, STAT1 regulates expression of uterus receptive-related genes including CCLs, IRF9, IFITs, MXs, and OAS. The present study provides evidence to molecular mechanism of GSTM2 modulating embryo development.
Glutathione S-transferases (GSTs), as members of a supergene family of multifunctional enzymes, play an important role in the defense to a wide array of toxic and carcinogenic substances1,2 by catalyzing the conjugation of glutathione (GSH) with a broad range of electrophilic compounds. Up to now, eight distinct classes of GSTs have been identified1, whereas, different class of GST enzymes have different functions. Mu class (GSTMs) are mainly involved in eliminations of free radicals, peroxides, electrophilic reagents, heavy metals, also, they mediates and regulates cells protection and growth. Among the members of GSTMs, GSTM2 is a potential candidate involved in reproductive regulation due to high expression level in spermaduct, epididymis, testis, ovary, and oviduct, which was mentioned by a study for mammalian reproduction2. It is reported that ova resists the endogenous and exogenous toxic substances by GSTM2 in ovary3, which characterizes GSTM2 as a protector for germ cells. GSTM2 participates in the generation of prostaglandin E2 (PGE2)4 that is essential for testis maturation and embryo implantation5,6,7,8. GSTM2 is up-regulated forcefully in luminal epithelium of uterine at the day 3 and day 4 after pregnancy9, and additionally, progesterone is probably involved in up-regulation of GSTM2, which shows the necessity of GSTM2 in the preparation of uterine in blastocyst implantation process9. Interestingly, the high expression of GSTM2 in progression of embryonic reactivation10 suggests the potential effect on embryo development.
In a previous study of our lab, it has been identified that a premature translation termination codon (PTC) caused by a nonsense mutation (CGA→TGA) resulting from a C27T substitution in the fifth exon of GSTM2. Nonsense-mediated mRNA decay (NMD) could degrade the mutated porcine GSTM2 mRNA11 because of the specifical identification and degradation of aberrant transcripts harboring a premature termination codon (PTC)12,13. Interestingly, the homozygous genotype TT was not found in 164 individuals from Large White, Landrace, Meishan and Qingping pigs11. The embryo with a GSTM2 TT genotype would be most likely to die or abort. To give insight into the role of GSTM2 in embryo development, RNA-seq was performed from ST cells treated with siRNAs targeting GSTM2.
Results
Small interfering RNA treatment repressed GSTM2 in swine testis cells
Three pairs of siRNAs named si1, si2, and si3, were designed to suppress expression of GSTM2 in ST cells. The mRNA and protein level of GSTM2 was decreased significantly (P < 0.01) at 24 h after transfection (Supplementary Fig. S1a), and furthermore, si2 worked best for the suppression (Supplementary Fig. S1b).
Sequence quality and saturation analysis
RNA integrity was assessed by BioAnalyzer in the present study. The RIN value of all samples were over 7. The raw data which contained adaptor sequences were transformed into clean tags. Preparation and experiments of sample RNA were thought to be convincing if the tags contains N were less than 10% of total raw data, and copy number less than 2 tags was no more than 20%. The data showed that all the samples conformed to sequencing requirement and the experiments were successful (Supplementary Fig. S2a). In addition, the repeatability of RNA-seq was tested (Supplementary Fig. S2b). R values both from Spearman and Pearson analyses over 8 showed a high repeatability between two samples. The saturation analysis could be performed to check whether the number of detected genes keep increasing when sequencing amount (total tag number) increases. The data showed that when sequencing amount reaches 2 M or higher, the number of detected genes almost ceases to increase (Supplementary Fig. S2c). Thereby, the result indicated that the high-throughput Illumina sequencing data were exhaustive.
Data analysis of RNA-seq
In the present study, six cDNA libraries (TR1, TR2, TR3, CK1, CK2 and CK3) were established by reverse transcription of total RNA from three wells of treated ST cells (with siRNA2) and three wells of un-treated ST cells respectively. The standard analyses for quality control14 was conducted to ensure the quality of RNA met the requirement for sequencing (Supplementary Fig. S3). A total of 11,927,452 (98.78%), 11,865,576 (99.01%), 11,870,090 (98.74%) clean reads were obtained from three treatment groups respectively. 11,790,005 (98.79%), 12,129,113 (98.75%), and 12,192,917 (98.85%) clean reads were obtained from three control groups respectively (Supplementary Table S2a). A total of 22,966,999 reads of treatment groups and 24,559,030 reads of control groups were mapped to reference gene. A total of 26,394,293 reads of treatment groups and 27,449,663 reads of control groups were mapped to reference genome respectively (Supplementary Table S2b). The raw data was obtained from transformed sequence data by Base Calling, and then the raw reads that contained adaptor sequence data and N > 10% were removed. Furthermore sequence data were filtered for low-quality reads at high level of stringency. After these steps, approximately 99% reads of the raw data were clean reads, whereas only 1% of the data came from adaptor sequences attached to empty vectors. The clean reads were mapped to the reference gene and the reference genome of pig (Supplementary Table S2b and Supplementary Table S3). The evaluation of gene expression levels were displayed in reads per kilo base of gene per million reads (RPKM)15. We used a standard to classify genes into three expression levels, including low expression (0 < RPKM < 5), moderate expression (10 < RPKM < 50), and high expression (RPKM > 500).
Differentially expressed genes (DEGs) between treatment groups and control groups
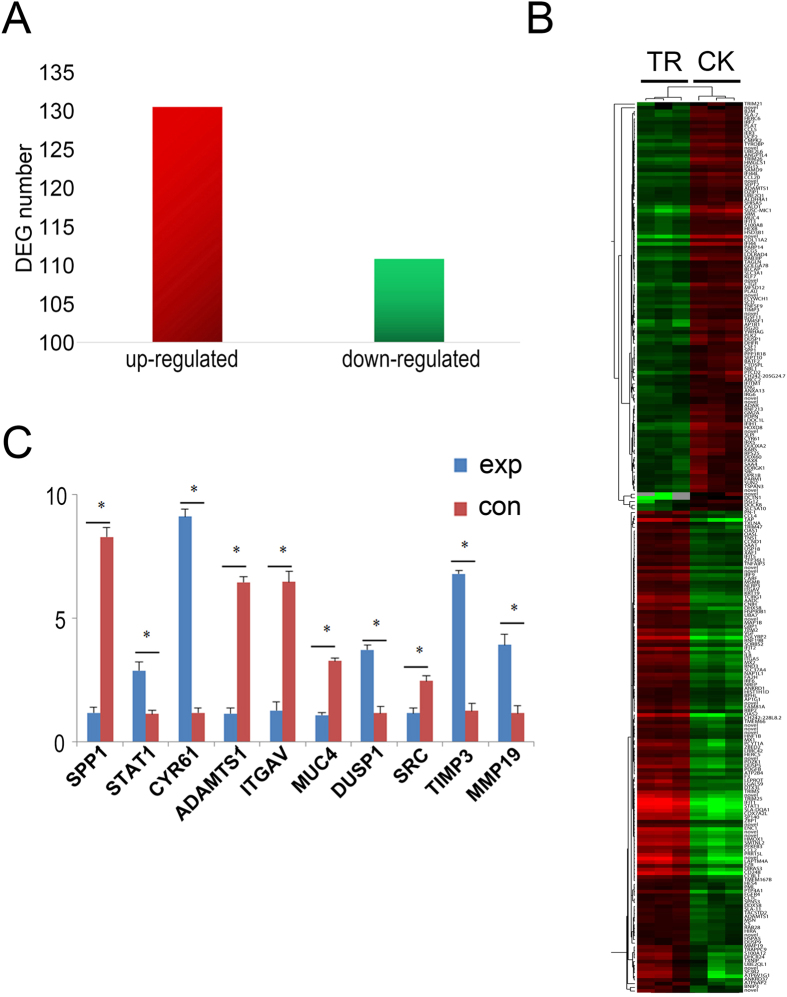
Total of 242 different transcripts within 131 up-regulated DEGs and 111 down-regulated DEGs were identified through the EdgeR analysis (|log2Raito| ≥ 1, FDR ≤ 0.001) in treatment groups compared to control group (Fig. 1 and Supplementary Table S4).
Figure 1. Analysis of differentially expressed genes.
(A) DEGs (FDR ≤ 0.001 and |log2 Ratio| ≥ 1) identified between TR and CK. (B) The heatmap of the subset DEGs in different samples (TR1, TR2, TR3, CK1, CK2, CK3). (C) Several DEGs related to embryo development from RNA-seq data were screened for validation by qRT-PCR.
Verification of DEGs with quantitative real-Time PCR (qRT-PCR)
Several DEGs related to embryo development from RNA-seq data were screened for validation by qRT-PCR (Fig. 1C). The results showed that the expression of SPP1, ADAMTS1, ITGAV, MUC4, and SRC was down-regulated, whereas STAT1, CYR61, DUSP1, TIMP3 and MMP19 was up-regulated, which were consistent with the expression profile of RNA-seq.
Gene ontology and KEGG pathway enrichment analysis for DEGs
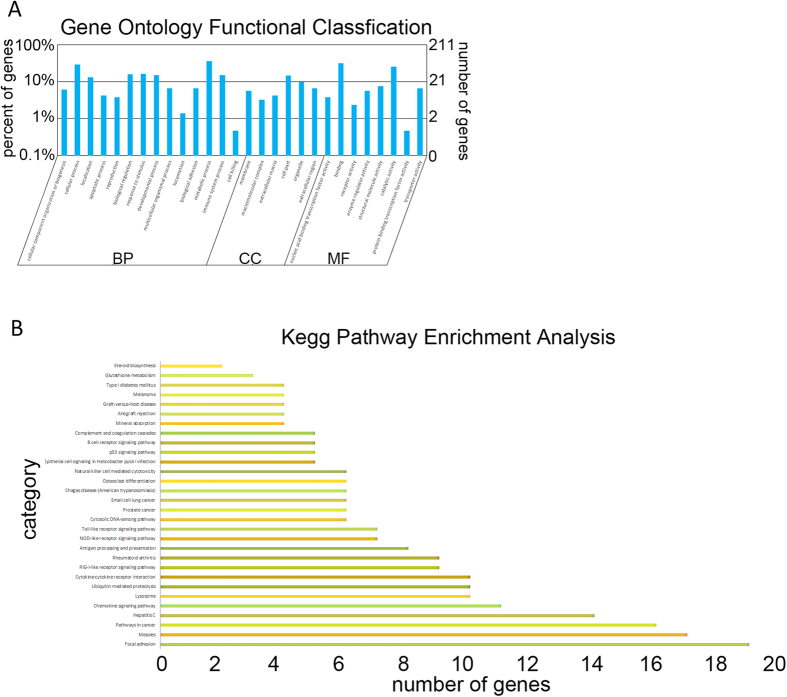
GO classification of the DEGs revealed that 110 genes are involved in metabolic processes, 37 in stimuli, 38 in immune system processes, and 13 in biological adhesion, respectively (Fig. 2A). These 242 DEGs mainly participated in over 30 pathways according to PANTER, including inflammation mediated by chemokine and cytokine signaling pathways, integrin signaling pathways, Parkinson’s disease, angiogenesis, gonadotropin releasing hormone receptor pathway, interleukin signaling pathway, EGF receptor signaling pathway, FGF signaling pathway etc. (Fig. 2B). Detail information of GO and pathway analysis was showed in Supplementary Table S5 and Supplementary Table S6, respectively.
Figure 2. Histogram of Gene Ontology (GO) enrichment and pathway analysis of DEGs.
(A) All the GO terms are summarized in three main categories which are biological process, cellular component and molecular function. (B) The differentially expressed genes were clustered and enriched in several pathways including focal adhesion, measies, pathways in cancer, hepatitis C, chemokine signaling pathway, and etc.
Genes involved in the Maternal-Placental Interface and embryonic development
The knockdown of GSTM2 in ST cells alerts the expression of some genes involved in maternal-placental interface and embryonic development. Three SLC family genes (SLC5A10, SLC3A1, and SLC37A4) were down-regulated in GSTM2 knock-down ST cells. Another down-regulated DEGs is FGFR4, which is a member of the fibroblast growth factor receptor (FGFR) family (Supplementary Fig. S4A). Furthermore, Six genes related to the process of cell adhesion were down-regulated in the GSTM2 knock-down ST cells, including tetraspanin 3 (TSPAN3), a disintegrin and metalloproteinase with thrombospondin motifs 1 (ADAMTS1), integrin, alpha V (ITGAV), GTP-binding RAS-like 3 (DIRAS3), immunoglobulin superfamily 11 (IGSF11) and secreted phosphoprotein 1 (SPP1, also known as osteopontin (OPN)) (Supplementary Fig. S4B). More than 30 IFN-stimulated genes were up-regulated, including ISG family, IFIT, IRF, MX etc. (Supplementary Fig. S4C). In addition, IFN-stimulated genes, some cytokines and chemokines in the endometrium related to implantation, including CCL2, CCL4, CCL20, CCL5, and CXCL816,17,18,19, were also up-regulated. Another up-regulated gene, CSF1, is a specific hemopoietic growth factor regulating survival, proliferation and differentiation of mononuclear phagocytes.
Suppression of STAT1 phosphorylation by overexpression of GSTM2
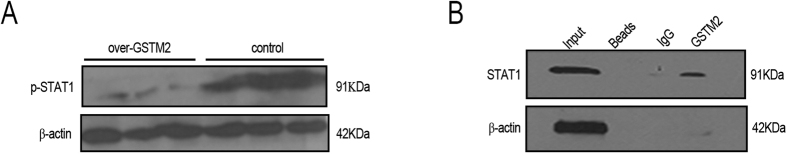
In order to provide more insight into relationship between GSTM2 and STAT1, we overexpressed GSTM2 in ST cells. Protein level of STAT1 was decreased (Supplementary Fig. S5A,C) when GSTM2 was overexpressed. In addition, the mRNA expression level of downstream targets of STAT1 including ITGAV, SRC, and OPN were up-regulated (Supplementary Fig. S5B). Overexpression of GSTM2 suppressed the phosphorylation of STAT1 (Fig. 3A). Furthermore, co-immunoprecipitation assay indicated that GSTM2 could bind to STAT1 (Fig. 3B).
Figure 3. GSTM2 suppressed STAT1 phosphorylation by binding STAT1.
(A) Detection of phosphorylation of STAT1 after the overexpression of GSTM2. (B) Co-immunoprecipitation experiment detected the combination between GSTM2 and STAT1. β-actin was used as internal control. *P < 0.05, **P < 0.01.
Overexpression and interference assay of STAT1
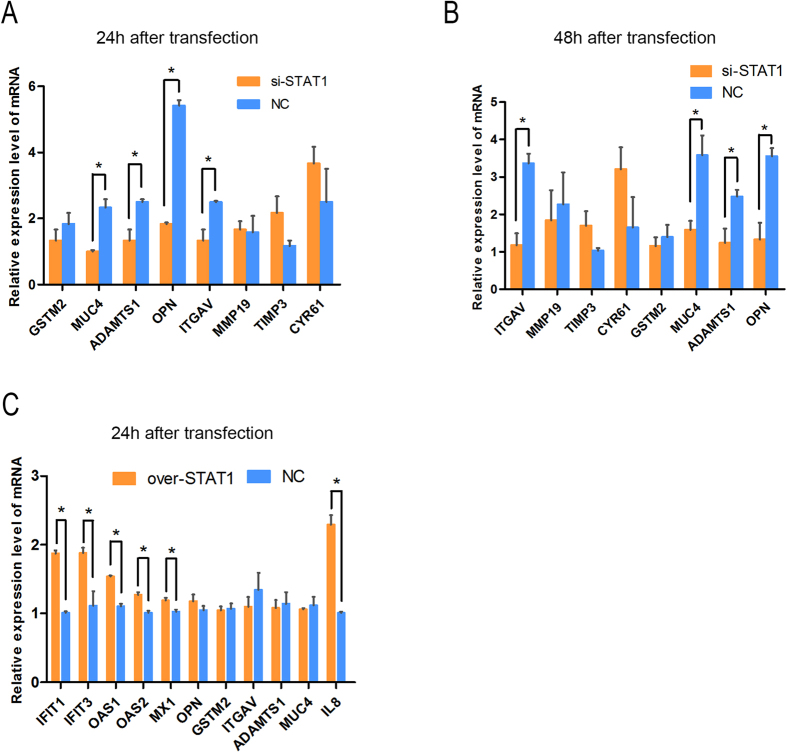
STAT1 was knocked down in ST cells by RNAi (Supplementary Figure S6 A–D). The mRNA expression of MUC4, ADAMTS1, OPN, and ITGAV, which belong to downstream targets of STAT1, were decreased (P < 0.05, Fig. 4A,B), but did not have a significant change (P > 0.05) when STAT1 was overexpressed (Supplementary Figure S6 E,F). IFIT1, IFIT3, MX1, OAS1, OAS2, and immune-related genes were up-regulated (P < 0.05, Fig. 4C) in the presence of overexpression of STAT1.
Figure 4. Knockdown and overexpression experiment of STAT1 in ST cells.
(A) Knockdown STAT1 in ST cells using siRNA and detect downstream genes at 24 h using q-PCR. (B) Knockdown STAT1 in ST cells using siRNA and detect downstream genes at 48 h using q-PCR. (C) Overexpress STAT1 in ST cells and detect downstream genes at 24h using q-PCR. *P < 0.05.
Expression profile of GSTM2 and STAT1 in porcine endometrium
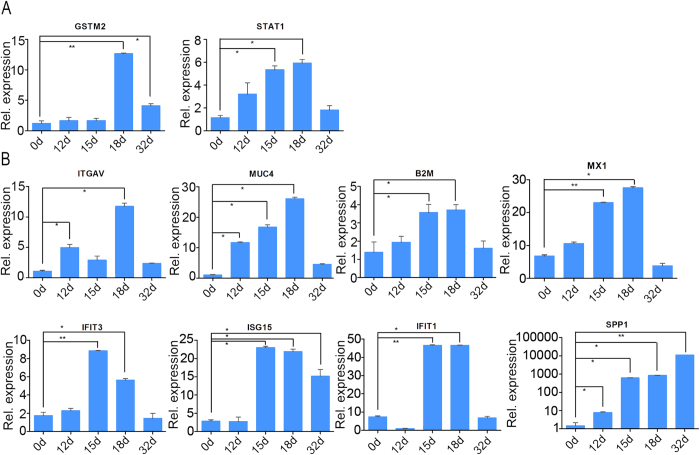
The expression level of GSTM2, STAT1 and some other DEGs was detected in porcine endometrium with different developmental stages, including day 0, day 12, day 15, day 18 and day 32 of gestation stages. The expression level of GSTM2 was increased at day 18 and day 32 compared to day 0 and day 12, whereas the STAT1 was increased at day 12, day 15, and day 18 gradually compared to 0d. Both GSTM2 and STAT1 had a highest expression level at day 18, whereas, the expression sharply decreased at day 32 (Fig. 5A). The expression level of downstream targets of STAT1 including immune-related genes IFIT1, IFIT3, ISG15, B2M, and adhesion process-related genes SPP1, MUC4, ITGAV and MX1, was also detected. Interestingly, all these genes expressed abundantly at day 15 and day 18 compared to day 0 and day 12 except for ITGAV, whereas, expression of SPP1, MUC4 and ITGAV began to increase at day 12. Furthermore, the expression of ISG15 and SPP1 was still high at day 32, whereas the others were resumed to the expression level of day 0. The expression pattern of MUC4, B2M and MX1 was similar to that of STAT1 (Fig. 5B).
Figure 5. Temporal expression profiles of interesting genes.
Expression level of GSTM2, STAT1, SPP1, ITGAV, MUC4, IFIT3, ISG15, B2M, IFIT1, and MX1 in porcine endometrium. Day 0, 12, 15, 18, and 32 were selected for the detection. *P < 0.05, **P < 0.01.
Discussion
As one kind of important phase II antioxidant enzymes in vivo, GSTs were a wide array of toxic and carcinogenic substances. In this superfamily, mu class of GST could eliminate free radicals, peroxides, electrophilic reagents, heavy metals, and mediate cell protection and the regulation of cell growth. GSTM2, a member of GST mu class, was widely and highly expressed in various tissues including embryos, and testis11. In gestation stage of mice, GSTM2 expressed at a low level in luminal epithelium at day 3, but expressed highly at day 4 during early pregnancy9. In a previous study from our lab, the NMD-induced degradation of GSTM2 was most likely to cause embryonic death or abortion. Furthermore, this genotype that could be degraded by NMD was not found in 164 adults from Large White, Landrace, Meishan and Qingping pigs11. Here we knocked down GSTM2 by small interference RNAs in vitro to simulate the degradation of GSTM2 in vivo. High-throughput sequencing with the GSTM2-knockdown ST cells would be helpful to deeply understand the function of GSTM2.
The transcriptome data obtained from the present study were useful for the elucidation of function of GSTM2. Usually more than 18M reads from RNA-seq analyses are required for each sample to attain a saturated state for novel gene discovery and expressional analysis14,20. The quality control of our data revealed that the RNA-seq data were well qualified (Supplementary Table S2).
Bioinformatics analyses of DEGs discovered DEGs between the GSTM2-knockdown group and control group. After filtering with the standard, 242 DEGs were obtained from there six samples (Fig. 1B). Some down-regulated genes, including TSPAN3, ADAMTS1, ITGAV, DIRAS3, IGSF11 and SPP1, were involved in embryo implantation. Among them, TSPAN3 as a member of transmembrane 4 superfamily (TM4SF) involved in the adhesion, migration, perliferation, differentiation and signal transduction of cells21 has a high expression at the blastocyst of Xenopus Laevis, and furthermore played an important role in the communication between blastocyst and endometrium in mammals22,23,24 especially in cow25.
According to our data, three SLC family genes (SLC5A10, SLC3A1, and SLC37A4), one FGFR family gene (FGFR4), and six cell adhesion-related genes (TSPAN3, ADAMTS1, ITGAV, DIRAS3, IGSF11, and OPN) were down-regulated in GSTM2-knockdown ST cells. Whereas, a lot of IFN-stimulated genes including ISG family, IFIT, IRF, MX, and some cytokines/chemokines including CCL2, CCL4, CCL20, CCL5, and CXCL816,17,18,19 were up-regulated. Among these genes, OPN was a powerful gene involved in embryo implantation. The expression of OPN was modulated by estrogen in pregnancy26. OPN is also one component of the extracellular matrix (ECM) serving as an integrin ligand. Additionally, it was expressed in a small number of stroma cells at day 9 of pregnancy, the uterine LE adjacent to conceptus tissue beginning at day 12 and throughout the LE surface by day 2027. Although OPN was clustered to estrogen-responsible gene, the concrete regulation of estrogen on expression of OPN was indirect through its interactions with ERα28. The SRC family members enhanced the transcriptional activity of a variety of nuclear receptors, including ERα, ERβ and PR29,30,31,32. The expression of GSTM2, SRC and OPN was down-regulated significantly at the interface. Therefore, we suspected that GSTM2 regulated the expression of SRC that affected the activity of ERα and resulted in embryo implantation by suppressing OPN expression. OPN expressed both in endometrial epithelial cells and in trophoblast cells due to the interaction between OPN and trophoblast expression of integrin. This interaction achieved embryo adhesion and early communication to mother directly. OPN binds to the receptors including CD44 and integrins on the cell surface, and then initiated a variety of kinase cascades, including focal adhesion kinase (FAK) and phosphatidylinositol 3-kinase (PI3K)/apoptosis signal-regulating kinase 1 (AKT) signaling33,34,35. The increase of adhesion complex assembly in OPN treated blastocysts was mediated through FAK- and PI3K-dependent signaling pathways26. GSTM2 was a downstream gene of PI3K36. Thus, knockdown GSTM2 may affect embryo adhesion by modulating interactions between OPN and integrins. OPN as a kind of ECM protein was degraded by MMPs. According to the data of RNA-seq, expression of MMP19 was raised in the GSTM2 knockdown group, which was likely to lower expression of OPN. However, the protease activity of MMPs was correlated with TIMPs, whereas, both of these proteins maintained the stability of the extracellular matrix. The expression of TIMP3 gene was increased in the GSTM2 knockdown group, whereas expression of MMP19 was elevated as well. Only if the expression of TIMPs/MMPs was balanced, can embryo implantation be carried out. The knock-down of GSTM2 may break the balance. Therefore we assumed that GSTM2 repressed the activity of ER via the regulation of SRC, then the expression level of OPN changed.
IFN-stimulated genes and some cytokines/chemokines were identified from DEGs, but there was no evidence to prove that GSTM2 could regulate these genes. STAT1 is involved in JAK-STAT pathway, which could be stimulated by some IFNs and cytokines, and regulates its downstream targets including some IFN-induced genes (IFNGs) and immune-related genes. Interestingly, previous study indicated that the interaction between GSTP1 and STAT3 regulated the phosphorylation of STAT337. For this reason, we assumed that GSTM2 would regulate phosphorylation of STAT1. The result of co-IP experiment indicated that GSTM2 could bind to STAT1 (Fig. 3B), the phosphorylation level, furthermore, was reduced by the overexpression of GSTM2 (Fig. 3A). It provided a better explanation for expression changes of downstream targets of STAT1.
We performed the overexpression and interference assay of STAT1 to confirm whether these IFN-stimulated genes and some cytokines/chemokines were the downstream targets of STAT1. When STAT1 was knocked down, the expression of MUC4, ADAMTS1, OPN, and ITGAV were decreased (P < 0.05, Fig. 4A,B), whereas, IFIT1, IFIT3, MX1, OAS1 and OAS2, immune-related genes were up-regulated (P < 0.05, Fig. 4C) in the presence of overexpression of STAT1. Hence the activation of STAT1 would induce the up-regulation of IFNGs. IFNGs could enhance the uterus acceptance, however, the high expression of IFNGs would induce the blastocyst delay38. Maternal immune recognition of embryos occurs through two major pathways. One is that the immune system can detect the presence of alloantigens or receptor ligands on the conceptus. The other is that the immune system could be activated by chemokines and cytokines produced by the conceptus39. There were two classes of major histocompatibility complex (MHC) antigens, whereas, only MHC class I antigens were expressed on the surface of the conceptus. MHC class I molecules or transcripts could be detected throughout development to the blastocyst stage in cow40,41,42. However, at the period of placental attachment, little MHC antigens were expressed. Available evidence has indicated the down-regulation of MHC antigen class I in porcine trophoblast from days 14 to 2543. Some chemokines and cytokines, including interferons and CCL, activated the immune system, started the pregnancy recognition and protected against some bacteria during the peri-attachment period. Some of MHC genes were up-regulated with the knock-down of GSTM2, including MHC class I antigens SLA7, SLA11 and SUSC-MIC1, and MHC class Π antigens SLA-DQA1. Porcine conceptus trophectoderm cells induced the expression of SLA class I and β2m genes through secretion of IFN-δ or IFN- γ in uterine stromal, but this expression was silenced in LE in order to prevent immune rejection at the uterine-placental interface44. SLA-DQ was expressed responding to IFN- γ from the conceptus. Additionally, it likely regulated immune responses at the maternal-fetal interface in order to support the maintenance of pregnancy in pigs45. Many IFN-stimulated genes were up-regulated, including ISG family, IFIT, IRF, MX etc. (Supplementary Fig. S4C), whereas the effects of expression of Mx were different in pregnancy recognition mechanism in the early pregnancy uterus of different domestic farm species46,47. Changes of related genes suggested that GSTM2 was associated to immunological recognition of the conceptus. STAT1 participated in type I and type II interferon signaling pathways48,49. In the type I interferon activate signaling pathways, GSTM2 may be involved in IFNs signaling pathways by regulating STAT1.
We also detected the expression of related genes in vivo. The expression level of GSTM2 was increased at day 18 and day 32 compared to day 0 and day 12, whereas the STAT1 was increased at day 12, 15, and 18 gradually compared to day 0. The high level of STAT1 at day 12 and day 15 did not induce the increase of expression of GSTM2. Both GSTM2 and STAT1 expressed highest at 18d, whereas, the expression sharply decreased at day 32 (Fig. 5A). Day 18 is essential time point for pig embryo implantation. Maybe more GSTM2 was needed to suppress the effect of STAT1. The expression level of downstream targets of STAT1 including immune-related genes IFIT1, IFIT3, ISG15, B2M, and adhesion process-related genes SPP1, MUC4, MX1 expressed highly at day 15 and day 18 compared to day 0 and day 12, whereas, expression of SPP1, MUC4 and ITGAV began to increase at day 12. Furthermore, the expression of ISG15 and SPP1 was still high at day 32, whereas the others were resumed to the expression level of day 0. The expression pattern of MUC4, B2M and MX1 was similar to that of STAT1 (Fig. 5B). A previous study about implantation showed that GSTM2 was highly expressed during the early stage of implantation, and this condition just occurred in a very short period9. In addition, IFN was able to increase the capacity of the endometrium to adapt new conceptus, whereas overexpression of IFNG may induce delay of conceptus sometimes by 15–23 days38. In order to protect conceptus from the delay induced by IFNG, expression of GSTM2 was up-regulated in the early stage of implantation. Porcine embryo implantation started at day 13 and finished at day 24 after pregnancy50. In general, we assumed that GSTM2-STAT1 promoted embryo implantation. Blastocyst attachment to the LE was only achieved by the transitional labilization and the remodeling of uterine epithelium polarity after the synchronous exchange of signals between the conceptus and endometrial cells51. Vascular permeability increases within 13 days of pig pregnancy52, whereas, by day 15 of pregnancy, IFNs up-regulated a large array of IFN induced genes in the underlying stroma and glandular epithelium, including ISG15, IRF1, STAT1, SLAs and B2M, which were likely to play roles in uterine remodeling to support placentation53. Another gene is F3, a tissue factor (TF) that existed in endometrial stromal cells and may play an important role during the period of embryo54. Matrix metalloproteinases (MMPs) are a family of zinc-dependent neutral endopeptidases that regulated tissue remodeling during embryonic development, angiogenesis and wound healing55,56,57. Among these, MMP19 was expressed throughout the menstrual cycle, thereafter, affected cell proliferation and angiogenesis, which were crucial for endometrial receptivity58. Tissue inhibitor of matrix metalloproteinases (TIMPs) is an MMP inhibitor, and an abnormal balance between MMPs and TIMPs has been related to tumor invasion and metastasis in various human cancers, including endometrial cancers59,60. In general, we constructed a potential network of GSTM2-STAT1 at the maternal-fetal interface in pigs (Fig. 6).
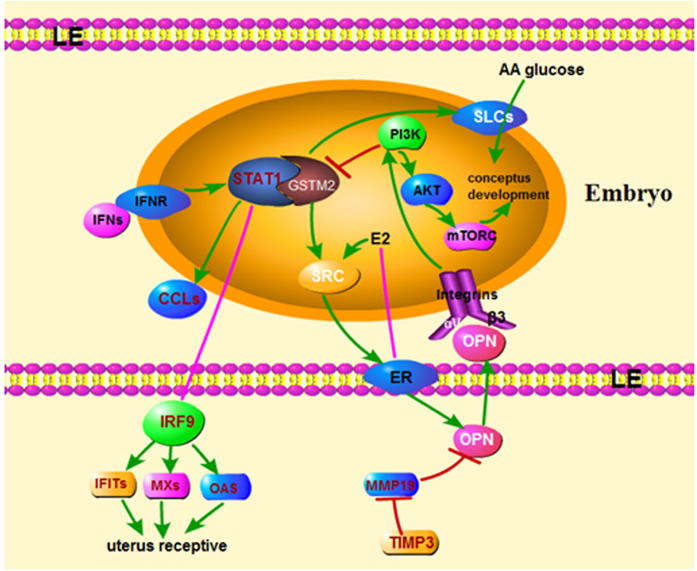
Figure 6. Potential network of GSTM2-STAT1 at the maternal-fetal interface in pigs.
On the one hand, IFNs binds to IFNR and stimulates STAT1, whereas up-regulated STAT1 promotes the expression of CCLs and regulates uterus receptive through IRF9 and the downstream targets IFITs, MXs, OAS. However, GSTM2 could bind to STAT1 and suppress the phosphorylation of STAT1. Hence, the high expression of these IFNGs which was regulated by STAT1 would be balanced by GSTM2, so that the uterus receptive was promoted. On the other hand, GSTM2 regulates OPN through SRC and ER, furthermore, OPN binds to integrin to regulate conceptus development through PI3K-AKT-mTORC signaling. GSTM2 could also promote conceptus development by up-regulating expression of SLCs and increasing the AA and glucose into embryo.
Methods
Samples Collection and Animal Care Protocol
All animals were raised under the same conditions. We obtained endometrium of day 0, 12, 15, 18, and 32 clinically healthy gestation sow from the Fine Farm of HuaZhong Agricultural University. Tissue samples from endometrium were frozen in liquid nitrogen immediately after collection and stored at −80 °C prior to RNA extraction. The methods were performed in accordance with the approved guidelines from Huazhong Agricultural University, and scientific, ethical and legal principles of the Hubei Regulations for the Administration of Affairs Concerning Experimental Animals. All experimental protocols were approved by the Ethics Committee of Huazhong Agricultural University.
Cell culture
Swine testis (ST) cells were purchased from CCTCC (China Center for Type Culture Collection, Wuhan, China) were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% (v/v) bovine calf serum (Gibico, USA) in a culture flask at 37 °C under a humidified atmosphere of 5% CO2. At 60–70% confluence, cells were trypsin-digested for further sub-culturing or seeded into 6-well plates (2 ml/well) at a concentration of 1–2 × 105 cells per ml for siRNA transfection.
RNA interference
GSTM2 was widely expressed in various tissues including embryos, and testis with relative high expression11. Hence, GSTM2 could be expressed by normal ST cells. Three pairs of GSTM2-specific siRNAs were designed targeting corresponding regions of porcine GSTM2 mRNA (Ambion). In addition, scrambled siRNA (Ambion) was used as a negative control. Briefly, the RNA interference transfection was performed as follows. Cell suspensions were reverse transfected in triplicate with siRNA via LipofectamineTM 2000 (Invitrogen, Carlsbad, CA, USA) and Optimem-I reduced serum media (OPTI-MEM I, Invitrogen) with the siRNA pooled at equal amounts to a final concentration of 50 nM. Six hours after transfection (Supplementary Fig. S1), the medium was refreshed, and the cells were further incubated for another 24 h. Triplicate wells of non-transfected cells were also included. To evaluate the effects of GSTM2 knock-down on cells and transfection efficiency, we assessed morphology and cell numbers using fluorescence microscopy. The other two siRNAs, negative control and mock control followed the same treatments. Three independent experiments were performed.
RNA isolation and real-time PCR
Total RNA isolated from transfected and control cells were assessed for integrity using a BioAnalyzer 2100 (Agilent, Santa Clara, USA) and for concentration and purity by the NanoDropTM 1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). After washes with Phosphate Buffered Saline (PBS), cells of each well were homogenized with 1 ml TRIzol reagent (Invitrogen) according to the manufacturer’s protocol followed by modifications after re-dissolving the RNA. The RNA was dissolved in 100 μL RNase-free water by mixing up and down with a pipette and stored at −80 °C freezer.
Real-time PCR was performed using a Roche LightCycler 480 detection system (Roche, Switzerland) and the following PCR program: denaturation at 95 °C for 30 sec, amplification for 40 cycles at 95 °C for 5 s, annealing and extension at 58 °C for 20 s and 72 °C for 10 s. Primer sequences and expected product sizes are shown in Supplementary Table S1. Specific amplification for certain PCRs was assessed by melting curve. One negative control reaction in which cDNA template was replaced by water was performed to avoid potential contamination. The sample from each well was repeated three times, and the comparative Ct (△△Ct) value method was used for relative quantification. Four genes, eukaryotic translation elongation factor 1 alpha 1(EEF1A1), heat shock protein 90 kDa alpha (cytosolic), class B member 1 (HSPCB), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and beta actin (ACTB), were chosen as potential housekeeping genes based on their uniformly high expression levels across groups from the sequencing data. Expression levels of these genes were assessed and used to normalize target genes via geNorm software61. Expression levels were considered undetectable when the Ct value of the targeted gene exceeded 35.
Western blotting
Western blotting was used to further validate the effect of RNAi on GSTM2 before RNA-seq. Transfected cells were homogenized in 1 ml of 25 mM Tris/1 mM ethylenediaminetetraacetic acid pH buffer, pH = 7.5. Homogenates were separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (SDS-PAGE) and transferred to a PVDF membrane (Millipore, Bedford, MA) using a semidry electrophoretic apparatus. The blocked membranes (5% BSA in TBS buffer containing 0.1% Tween 20) were incubated with anti-GSTM2 (1:1,000; AbCam, Cambridge, MA) and anti-beta-actin antibodies (1:1,000; AbCam, Cambridge, MA) overnight at 4 °C. The blots were extensively washed three times with TBST buffer for 10 min and incubated under gentle agitation with the secondary antibodies for immunodetection. The antigen-antibody reaction was incubated for 1 h, and the cross-reacting proteins were detected. Prestained molecular weight markers 10–170 kD in weight (Fermentas, Canada) were used as standards.
RNA-seq
Deep sequencing was carried out at BGI (Shenzhen, China). Briefly, the total RNA samples were first treated with DNase I to degrade any possible contaminating DNA. Then, the mRNA was enriched using oligo (dT) magnetic beads (for eukaryotes) or by removing rRNAs from the total RNA (for prokaryotes). Mixed with the fragmentation buffer, the mRNA was fragmented into short fragments (approximately 200 bp). Then, the first strand of cDNA was synthesized using random hexamer primers. Buffer, dNTPs, RNase H and DNA polymerase I were added to synthesize the second strand. The double-stranded cDNA was purified with magnetic beads. End reparation and 3′-end single nucleotide A (adenine) addition was then performed. Finally, sequencing adaptors were ligated to the fragments, which were then enriched by PCR amplification. During the QC step, the Agilent 2100 Bioanaylzer and ABI StepOnePlus Real-Time PCR System were used to qualify and quantify the sample library. Total six cDNA libraries were constructed including three GSTM2-knock down groups and three negative control groups. The library products were sequenced using an Illumina HiSeqTM 2000. The RNA used in sequencing was the same with the samples for q-PCR analysis.
Differential gene expression analysis
Values of RPKM were used to evaluate the total number of genes expression in each well of ST cells sample and the DEGs among each comparison62. The DEGs were analyzed based on an algorithm as previously described62. The P-value corresponds to a differential gene expression test in which False Discovery Rate (FDR) was used to determine the threshold of the P-value in multiple tests. The Cluster 3.063 was used to the clustering analysis. The R heatmap package64 was applied to the analysis of Pearson and Spearman clustering. The functional classification of genes was performed using KEGG65 pathway analysis.
Bioinformatics and data analysis
Identification of both pathway network and gene ontology (GO) categories was performed using IPA and the online tool PANTHER. All of the probe sequences from differential and positively expressed probes were first re-annotated with pig RefSeq RNA database from the porcine genome (Sscrofa10.2) from NCBI (Index of ftp://ftp.ncbi.nih.gov/genomes/Sus_scrofa/RNA/, last updated on October 2011). The unique gene symbol list from differential and positively detected probes was then uploaded into PANTHER (http://www.pantherdb.org/). The genes, transcripts, and proteins related to the Gene Ontology (GO) terms were identified. Then biological processes and pathways was obtained by these GO terms. The Sus scrofa genome was used as the reference gene list, which allowed for the identification of statistically significant biological processes and pathways from GO terms, which are represented in the over- and under-expressed between gene lists. More details related to the expected value and P-value calculation can be obtained online under Binomial Statistic Help from PANTHER.
Co-immunoprecipitation assay
ST cells were plated in 100 mm dishes the day before transfection. Then cells were transfected with GFP-pcDNA3.1 containing the cDNA encoding GSTM2 and incubated at 37 °C in a humidified atmosphere of 5% CO2 for 24 h for transient expression. The GFP was used to confirm the equal amount of plasmid transfected each dish. The cells were washed with cold PBS three times and lysed in 1000 mL cell lysis buffer (Sangon Biotech, Shanghai). The lysate was centrifuged at 12,000 rpm for 5 min at 4 °C. The supernatant was divided into two equal parts, one added with 2 μg anti-GSTM2, the other added with 2 μL IgG as control, whereafter incubated at 4 °C for 5 h. Each aliquot was added with 50 μL Protein A/G beads (Millipore, USA) and incubated for 30 min. The beads were collected and rinsed with 1 ml PBS (containing 0.05% Tween 20) for 5 times (5 min one time). The beads were added with 20 μL water and 10 μL 5 × SDS PAGE buffer, boiled at 98 °C for 10 min and laid on ice for 5 min. The supernatant was saved for western blotting analysis.
Overexpression and interference experiments
Complete CDS sequences of GSTM2 and STAT1 were obtained from NCBI. The Primers with restriction sites for full-length amplification were designed by Primer 5 software. Primer pairs were as follows: forward primer for GSTM2 (5′-GGGGTACCCCGCGAGCAGGTCAGGGGAGAA-3′, underlined sequences recognized by Kpn1), reverse primer for GSTM2 (5′-GCTCTAGAGCCATCTCCTGCTTCCAGGGCA-3′, underlined sequences recognized by Xba1), forward primer for STAT1 (5′-CGGGATCCCGATGTCCCAGTGGTATGAGCTTC-3′, underlined sequences recognized by BamH1), reverse primer for STAT1 (5′-GCTCTAGAGCTTAGTCAAGGTTCATAGTTCCAGAG-3′, underlined sequences recognized by Xba1). The amplification products were obtained by PCR with reaction mixture including 2 × Taq Master Mix 25 μL, cDNA library of ST cells 1 μL, forward primer 1 μL, reverse primer 1 μL, and dd water added to 50 μL. Then the PCR product was recovered by E.Z.N.A Gel Extraction Kit according to instructions (OMEGA), and additionally, double enzyme digestion reaction was performed with both PCR product and pcDNA3.1. The expression vectors of GSTM2 and STAT1 were acquired after connectivity, transformation, identification and DNA sequencing analysis. The expression vector of GSTM2 or STAT1 was transfected into ST cells followed by the same method as RNA interference experiment which was described previous, expect for the dose of expression vector for transfection was 4 μg (Supplementary Figs S5,S6).
Statistical analyses
All experiments were repeated three times. Data were given as mean ± SD. Student’s t-test was used for statistical comparisons. P value < 0.05 was considered to be statistically significant.
Additional Information
How to cite this article: Lv, Y. et al. Deep sequencing of transcriptome profiling of GSTM2 knock-down in swine testis cells. Sci. Rep. 6, 38254; doi: 10.1038/srep38254 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by Science and Technology Program of Wuhan, China (No. 2016020101010091) and the National Key Technology Support Program of China (No. 2015BAI09B06, No. 2014BAD20B01). The porcine endometrium tissues were given by Prof. Minggang Lei and Prof. Dequan Xu from Huazhong Agricultural University, Wuhan, P.R. China.
Footnotes
Author Contributions Conceived and designed the experiments: Z.R., Y.L. Performed the experiments: Y.L., Y.Z. Analyzed the data: Y.L., Y.J., Y.Z., J.J. Contributed reagents/materials/analysis tools: Y.L., Z.M., Y.J. Wrote the paper: Y.J., Y.L., Z.R.
References
- Fanucchi M. V. et al. Development of phase II xenobiotic metabolizing enzymes in differentiating murine clara cells. Toxicology and applied pharmacology 168, 253–267, doi: 10.1006/taap.2000.9020 (2000). [DOI] [PubMed] [Google Scholar]
- Hayes J. D. & Pulford D. J. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Critical reviews in biochemistry and molecular biology 30, 445–600, doi: 10.3109/10409239509083491 (1995). [DOI] [PubMed] [Google Scholar]
- Lim J. & Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biology of reproduction 84, 775–782, doi: 10.1095/biolreprod.110.088583 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckmann C. T., Fujimori K., Urade Y. & Hayaishi O. Identification of mu-class glutathione transferases M2-2 and M3-3 as cytosolic prostaglandin E synthases in the human brain. Neurochemical research 25, 733–738 (2000). [DOI] [PubMed] [Google Scholar]
- Baskar J. F. et al. Inhibition of hatching of mouse blastocysts in vitro by various prostaglandin antagonists. Journal of reproduction and fertility 63, 359–363 (1981). [DOI] [PubMed] [Google Scholar]
- Didolkar A. K. & Roychowdhury D. Effect of prostaglandins A-1, E-2 and F-2 alpha on spermatogenesis in rats. Journal of reproduction and fertility 58, 275–278 (1980). [DOI] [PubMed] [Google Scholar]
- Holmes P. V. & Gordashko B. J. Evidence of prostaglandin involvement in blastocyst implantation. Journal of embryology and experimental morphology 55, 109–122 (1980). [PubMed] [Google Scholar]
- Kennedy T. G. Prostaglandins and increased endometrial vascular permeabiltiy resulting from the application of artificial stimulus to the uterus of the rat sensitized for the decidual cell reaction. Biology of reproduction 20, 560–566 (1979). [DOI] [PubMed] [Google Scholar]
- Ni H. et al. Progesterone regulation of glutathione S-transferase Mu2 expression in mouse uterine luminal epithelium during preimplantation period. Fertility and sterility 91, 2123–2130, doi: 10.1016/j.fertnstert.2008.04.053 (2009). [DOI] [PubMed] [Google Scholar]
- Fu Z. et al. Integral proteomic analysis of blastocysts reveals key molecular machinery governing embryonic diapause and reactivation for implantation in mice. Biology of reproduction 90, 52, doi: 10.1095/biolreprod.113.115337 (2014). [DOI] [PubMed] [Google Scholar]
- Huang J. et al. Cloning, sequence analysis and identification of a nonsense mutation-mediated mRNA decay of porcine GSTM2 gene. Acta biochimica et biophysica Sinica 39, 560–566 (2007). [DOI] [PubMed] [Google Scholar]
- Bhuvanagiri M., Schlitter A. M., Hentze M. W. & Kulozik A. E. NMD: RNA biology meets human genetic medicine. The Biochemical journal 430, 365–377, doi: 10.1042/BJ20100699 (2010). [DOI] [PubMed] [Google Scholar]
- Pulak R. & Anderson P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes & development 7, 1885–1897 (1993). [DOI] [PubMed] [Google Scholar]
- Garber M., Grabherr M. G., Guttman M. & Trapnell C. Computational methods for transcriptome annotation and quantification using RNA-seq. Nat Methods 8, 469–477, doi: 10.1038/nmeth.1613 (2011). [DOI] [PubMed] [Google Scholar]
- Feng C. et al. Transcriptomic analysis of Chinese bayberry (Myrica rubra) fruit development and ripening using RNA-Seq. BMC Genomics 13, 19, doi: 10.1186/1471-2164-13-19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsalem H. et al. Identification of a novel neutrophil population: proangiogenic granulocytes in second-trimester human decidua. Journal of immunology 193, 3070–3079, doi: 10.4049/jimmunol.1303117 (2014). [DOI] [PubMed] [Google Scholar]
- Di Pietro C. et al. Altered transcriptional regulation of cytokines, growth factors, and apoptotic proteins in the endometrium of infertile women with chronic endometritis. American journal of reproductive immunology 69, 509–517, doi: 10.1111/aji.12076 (2013). [DOI] [PubMed] [Google Scholar]
- Dimitriadis E., White C. A., Jones R. L. & Salamonsen L. A. Cytokines, chemokines and growth factors in endometrium related to implantation. Human reproduction update 11, 613–630, doi: 10.1093/humupd/dmi023 (2005). [DOI] [PubMed] [Google Scholar]
- Hannan N. J., Jones R. L., White C. A. & Salamonsen L. A. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biology of reproduction 74, 896–904, doi: 10.1095/biolreprod.105.045518 (2006). [DOI] [PubMed] [Google Scholar]
- Sang Y., Brichalli W., Rowland R. R. & Blecha F. Genome-wide analysis of antiviral signature genes in porcine macrophages at different activation statuses. PloS one 9, e87613, doi: 10.1371/journal.pone.0087613 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik F. A., Sanders A. J., Kayani M. A. & Jiang W. G. Effect of expressional alteration of KAI1 on breast cancer cell growth, adhesion, migration and invasion. Cancer Genomics Proteomics 6, 205–213 (2009). [PubMed] [Google Scholar]
- Kashef J., Diana T., Oelgeschlager M. & Nazarenko I. Expression of the tetraspanin family members Tspan3, Tspan4, Tspan5 and Tspan7 during Xenopus laevis embryonic development. Gene expression patterns: GEP 13, 1–11, doi: 10.1016/j.gep.2012.08.001 (2013). [DOI] [PubMed] [Google Scholar]
- Spell A. R., Beal W. E., Corah L. R. & Lamb G. C. Evaluating recipient and embryo factors that affect pregnancy rates of embryo transfer in beef cattle. Theriogenology 56, 287–297 (2001). [DOI] [PubMed] [Google Scholar]
- Wolf E. et al. Embryo-maternal communication in bovine - strategies for deciphering a complex cross-talk. Reproduction in domestic animals = Zuchthygiene 38, 276–289 (2003). [DOI] [PubMed] [Google Scholar]
- Ponsuksili S. et al. Gene expression and DNA-methylation of bovine pretransfer endometrium depending on its receptivity after in vitro-produced embryo transfer. PloS one 7, e42402, doi: 10.1371/journal.pone.0042402 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaen T. et al. Estrogen-dependent uterine secretion of osteopontin activates blastocyst adhesion competence. PloS one 7, e48933, doi: 10.1371/journal.pone.0048933 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlow J. E. et al. Analysis of osteopontin at the maternal-placental interface in pigs. Biology of reproduction 66, 718–725 (2002). [DOI] [PubMed] [Google Scholar]
- Xie Q. Z. et al. Uterine micro-environment and estrogen-dependent regulation of osteopontin expression in mouse blastocyst. International journal of molecular sciences 14, 14504–14517, doi: 10.3390/ijms140714504 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H., Kohli K., Garabedian M. J. & Stallcup M. R. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Molecular and cellular biology 17, 2735–2744 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Gomes P. J. & Chen J. D. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proceedings of the National Academy of Sciences of the United States of America 94, 8479–8484 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onate S. A., Tsai S. Y., Tsai M. J. & O’Malley B. W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270, 1354–1357 (1995). [DOI] [PubMed] [Google Scholar]
- Wong C. W., Komm B. & Cheskis B. J. Structure-function evaluation of ER alpha and beta interplay with SRC family coactivators. ER selective ligands. Biochemistry 40, 6756–6765 (2001). [DOI] [PubMed] [Google Scholar]
- Fong Y. C. et al. Osteopontin increases lung cancer cells migration via activation of the alphavbeta3 integrin/FAK/Akt and NF-kappaB-dependent pathway. Lung cancer 64, 263–270, doi: 10.1016/j.lungcan.2008.09.003 (2009). [DOI] [PubMed] [Google Scholar]
- Fujita Y. et al. CD44 signaling through focal adhesion kinase and its anti-apoptotic effect. FEBS letters 528, 101–108 (2002). [DOI] [PubMed] [Google Scholar]
- Rangaswami H., Bulbule A. & Kundu G. C. Osteopontin: role in cell signaling and cancer progression. Trends in cell biology 16, 79–87, doi: 10.1016/j.tcb.2005.12.005 (2006). [DOI] [PubMed] [Google Scholar]
- Bhattacharya P., Madden J. A., Sen N., Hoyer P. B. & Keating A. F. Glutathione S-transferase class mu regulation of apoptosis signal-regulating kinase 1 protein during VCD-induced ovotoxicity in neonatal rat ovaries. Toxicology and applied pharmacology 267, 49–56, doi: 10.1016/j.taap.2012.12.013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou X., Chen N., Feng Z., Luo L. & Yin Z. GSTP1 negatively regulates Stat3 activation in epidermal growth factor signaling. Oncology letters 5, 1053–1057, doi: 10.3892/ol.2012.1098 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels J. M., Linton N. F., Croy B. A. & Tayade C. A review of molecular contrasts between arresting and viable porcine attachment sites. American journal of reproductive immunology 58, 470–480, doi: 10.1111/j.1600-0897.2007.00534.x (2007). [DOI] [PubMed] [Google Scholar]
- Hansen P. J. The immunology of early pregnancy in farm animals. Reproduction in domestic animals = Zuchthygiene 46 Suppl 3, 18–30, doi: 10.1111/j.1439-0531.2011.01850.x (2011). [DOI] [PubMed] [Google Scholar]
- Doyle J. et al. Classical and non-classical Major Histocompatibility Complex class I gene expression in in vitro derived bovine embryos. Journal of reproductive immunology 82, 48–56, doi: 10.1016/j.jri.2009.06.125 (2009). [DOI] [PubMed] [Google Scholar]
- Low B. G., Hansen P. J., Drost M. & Gogolin-Ewens K. J. Expression of major histocompatibility complex antigens on the bovine placenta. Journal of reproduction and fertility 90, 235–243 (1990). [DOI] [PubMed] [Google Scholar]
- Templeton J. W., Tipton R. C., Garber T., Bondioli K. & Kraemer D. C. Expression and genetic segregation of parental BoLA serotypes in bovine embryos. Animal genetics 18, 317–322 (1987). [DOI] [PubMed] [Google Scholar]
- Ramsoondar J. J. et al. Lack of class I major histocompatibility antigens on trophoblast of periimplantation blastocysts and term placenta in the pig. Biology of reproduction 60, 387–397 (1999). [DOI] [PubMed] [Google Scholar]
- Joyce M. M. et al. Uterine MHC class I molecules and beta 2-microglobulin are regulated by progesterone and conceptus interferons during pig pregnancy. Journal of immunology 181, 2494–2505 (2008). [DOI] [PubMed] [Google Scholar]
- Kim M. et al. Swine leukocyte antigen-DQ expression and its regulation by interferon-gamma at the maternal-fetal interface in pigs. Biology of reproduction 86, 43, doi: 10.1095/biolreprod.111.094011 (2012). [DOI] [PubMed] [Google Scholar]
- Hicks B. A. et al. Expression of the uterine Mx protein in cyclic and pregnant cows, gilts, and mares. Journal of animal science 81, 1552–1561 (2003). [DOI] [PubMed] [Google Scholar]
- Kim M. S., Min K. S. & Imakawa K. Regulation of Interferon-stimulated Gene (ISG)12, ISG15, and MX1 and MX2 by Conceptus Interferons (IFNTs) in Bovine Uterine Epithelial Cells. Asian-Australasian journal of animal sciences 26, 795–803, doi: 10.5713/ajas.2012.12529 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Type I. IFN Activity Predicts Response to MEK Inhibition in Melanoma. Cancer discovery 5, 343, doi: 10.1158/2159-8290.CD-RW2015-035 (2015). [DOI] [Google Scholar]
- Wang Z., Zhang D. X. & Zhao Q. Infection-stimulated Anemia Results Primarily from Interferon Gamma-dependent, Signal Transducer and Activator of Transcription 1-independent Red Cell Loss. Chinese medical journal 128, 948–955, doi: 10.4103/0366-6999.154303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q., Guan S., Fu J. & Wang A. Temporal and spatial expression of Muc1 during implantation in sows. International journal of molecular sciences 11, 2322–2335, doi: 10.3390/ijms11062322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker H. W. Implantation: a cell biological paradox. The Journal of experimental zoology 266, 541–558, doi: 10.1002/jez.1402660606 (1993). [DOI] [PubMed] [Google Scholar]
- Keys J. L., King G. J. & Kennedy T. G. Increased uterine vascular permeability at the time of embryonic attachment in the pig. Biology of reproduction 34, 405–411 (1986). [DOI] [PubMed] [Google Scholar]
- Johnson G. A., Burghardt R. C., Bazer F. W. & Spencer T. E. Osteopontin: roles in implantation and placentation. Biology of reproduction 69, 1458–1471, doi: 10.1095/biolreprod.103.020651 (2003). [DOI] [PubMed] [Google Scholar]
- Altmae S. et al. Tissue factor and tissue factor pathway inhibitors TFPI and TFPI2 in human secretory endometrium--possible link to female infertility. Reproductive sciences 18, 666–678, doi: 10.1177/1933719111400633 (2011). [DOI] [PubMed] [Google Scholar]
- Christiane Y., Emonard V. & Emonard H. [Involvement of matrix metalloproteinases in obstetrics: from implantation to delivery]. Revue medicale de Liege 69, 46–50 (2014). [PubMed] [Google Scholar]
- Nagase H. & Woessner J. F. Jr. Matrix metalloproteinases. The Journal of biological chemistry 274, 21491–21494 (1999). [DOI] [PubMed] [Google Scholar]
- Vu T. H. & Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes & development 14, 2123–2133 (2000). [DOI] [PubMed] [Google Scholar]
- Othman R. et al. Microarray profiling of secretory-phase endometrium from patients with recurrent miscarriage. Reproductive biology 12, 183–199 (2012). [DOI] [PubMed] [Google Scholar]
- Bourboulia D. & Stetler-Stevenson W. G. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Seminars in cancer biology 20, 161–168, doi: 10.1016/j.semcancer.2010.05.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graesslin O. et al. Metalloproteinase-2, -7 and -9 and tissue inhibitor of metalloproteinase-1 and -2 expression in normal, hyperplastic and neoplastic endometrium: a clinical-pathological correlation study. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 17, 637–645, doi: 10.1093/annonc/mdj129 (2006). [DOI] [PubMed] [Google Scholar]
- Vandesompele J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3, RESEARCH0034 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audic S. & Claverie J. M. The significance of digital gene expression profiles. Genome Res 7, 986–995 (1997). [DOI] [PubMed] [Google Scholar]
- Eisen M. B., Spellman P. T., Brown P. O. & Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95, 14863–14868 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S. & Huber W. Differential expression analysis for sequence count data. Genome Biol 11, R106, doi: 10.1186/gb-2010-11-10-r106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M. et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res 36, D480–484, doi: 10.1093/nar/gkm882 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.