Abstract
To investigate the association between long-term changes of serum total bile acid and hepatocellular carcinoma in chronic hepatitis B patients, we did a retrospective cohort study of 2262 chronic hepatitis B patients with regular antiviral treatment using data from the Hepatitis Biobank at Southwest Hospital Program from 2004 to 2014. Patients in the study were classified into 3 groups according to persistence of elevated serum total bile acid during follow-up: none-low, medium, and high persistence of elevated serum total bile acid. The association between persistence of elevated serum total bile acid and hepatocellular carcinoma was estimated using Cox proportional hazard models and Kaplan-Meier analysis including information about patients’ demographic and clinical characteristics. There were 62 hepatocellular carcinoma cases during a total follow-up of 14756.5 person-years in the retrospective study. Compared to patients with none-low persistence of elevated total bile acid, the multivariate adjusted hazard ratios (95% confidence interval) were 2.37 (1.16–4.84), and 2.57 (1.28–5.16) for patients with medium, and high persistence of elevated total bile acid. Our findings identified persistence of elevated serum total bile acid as an independent risk factor of hepatocellular carcinoma in chronic hepatitis B patients.
Chronic hepatitis B virus (HBV) infection accounts for about 50% of the global hepatocellular carcinoma (HCC) cases1. Antiviral treatment has been reported to reduce but not eliminate the risk of HCC in chronic hepatitis B (CHB) patients2,3,4,5,6. Some risk factors unamenable to antiviral treatment might contribute to HCC development in CHB patients7.
Bile acid has been associated with hepatocellular carcinoma (HCC) in human and mice with bile acid receptor deficiency8,9,10, and in mice with altered bile acid metabolism11. Elevated serum bile acid has been observed in CHB patients12,13, while there is currently no epidemiological study to evaluate the independent contribution of elevated serum bile acid to HCC in CHB patient population. A small-sample prospective study shows that HBV-related HCC patients usually had long-term elevated serum bile acid before HCC development14. This study provides important information but does not provided enough evidences to support the persistence of elevated serum bile acid as an independent driving factor of HCC in CHB patients, as the authors simply adjusted for demographic factors (age and sex) in the analyses14.
Using data from the Hepatitis Biobank at Southwest Hospital Program by the Department of Infectious Diseases at Southwest Hospital, we conducted a retrospective study to evaluate the association between persistence of elevated serum total bile acid (TBA) and HCC in CHB patients. Commonly recognized HCC risk factors, both demographic characteristics (age and sex) and clinical characteristics (liver damage, HBV virus replication, and liver cirrhosis), were included in the analyses as covariates15,16,17,18.
Methods
Study design and participants
The Hepatitis Biobank at Southwest Hospital Program by the Department of Infectious Diseases at Southwest Hospital covers all the records of patients visiting the Department of Infectious Diseases at Southwest Hospital since 2001. The records include information on the patients’ demographic characteristics, blood test, imaging test, histology test, and treatments. As serum HBV DNA records before 2004 are unavailable in the database, we checked records of the program from 2004 onwards. The study cohort included patients who were positive for serum hepatitis B surface antigen (HBsAg), had a follow-up for at least 4 years, and had serum TBA records in most (more than 90%) of the calendar years of their follow-up. Patients were excluded if they were positive for hepatitis C virus (HCV)/hepatitis D virus (HDV)/human immunodeficiency virus (HIV) infection, if they were diagnosed as autoimmune hepatitis, if they were diagnosed as cancers other than HCC, if they had received liver transplantation/resection surgery before HCC development, if they did not have HBV DNA or hepatitis B e antigen (HBeAg) records during the first year of follow-up, or if they did not have imaging or histology test records. Patients diagnosed as HCC during their first year of follow-up were also excluded to improve the temporal causal relationship evaluation.
Serum HBV DNA and HBeAg as indicators of HBV virus replication, serum alanine aminotransferase (ALT) as indicator of liver damage, liver cirrhosis, age and sex were commonly recognized as HCC risk factors in CHB patients and were chosen as covariates along with serum TBA in this study.
Verification of HCC
Of the total 62 HCC cases diagnosed during follow-up: 26 cases were verified with a biopsy examination and at least 1 imaging technique (abdominal ultrasonography, computed tomography, or magnetic resonance imaging), 29 cases were verified with at least 2 imaging techniques, and the remaining 7 cases were verified with 1 imaging technique (6 by computed tomography and 1 by ultrasonography) and a serum α–fetoprotein level ≧400 ng/mL.
Procedures
The scheduled end of follow-up was set at December 31, 2014. The follow-up of each participant began at his first visit to the hospital and ended at HCC diagnosis or censoring. Patients were censored at loss to follow-up (>365 days since the last visit), or at December 31, 2014, whichever came first.
Liver cirrhosis was verified by patients’ image or biopsy test records. We categorized exposure to cirrhosis based on diagnosis of cirrhosis during follow-up, as image or biopsy test data were not available for a large part (1593, 70.4%) of our patients in their first year of follow-up. Of the total 1082 cirrhosis cases diagnosed during follow-up: 901 cases were verified with 1 imaging technique alone, 173 cases were verified with at least two imaging techniques, and 8 cases were verified with biopsy and at least 1 imaging technique. Development of ascites was used as an indicator of the severity of cirrhosis. A total of 145 cirrhotic patients developed ascites during follow-up as revealed by abdominal imaging. Patients’ last aspartate aminotransferase to platelet ratio index (APRI) score in the last year of follow-up was also used to evaluate the liver fibrosis at the end of follow-up19. A total of 245 patients without APRI score in the last year of follow-up were excluded from analyses concerning this covariate.
To precisely characterize the long-term changes of serum TBA during follow-up, we divided participants’ follow-up time into separate intervals by 180 days starting from their first visit, as patients in our study cohort usually paid a visit to the hospital at an interval of 3–6 months. The exposure threshold of serum TBA was set at ≧10 μmol/L. The values of serum TBA test results in each 180-day interval were used to evaluate the exposure levels in that 180-day interval. For the 180-day interval without test results, we replaced the missing value with the value from the closest previous visit. The persistence of elevated serum TBA during follow-up was stratified into 3 levels: “none-low persistence of elevated serum TBA” for patients who had elevated serum TBA (≧10 μmol/L) in <1/3 of their total 180-day intervals, “medium persistence of elevated serum TBA” for patients who had elevated serum TBA in 1/3–2/3 of their total 180-day intervals, and “high persistence of elevated serum TBA” for patients who had elevated serum TBA in ≧2/3 of their total 180-day intervals. The persistence of elevated serum ALT and HBV DNA during follow-up was stratified similarly. For serum HBV DNA, the exposure threshold was set at ≧10000 copies/mL according to a previous report17. For serum ALT. the exposure threshold was set at ≧45 IU/L. Of the total 31103 180-day intervals created, 25446 (81.8%) 180-day intervals contain serum ALT test results, 25441 (81.8%) 180-day intervals contain serum TBA test results, and 25679 (82.6%) 180-day intervals contain serum HBV DNA test results.
Statistical analyses
We assessed the characteristics of retrospective cohort by patient groups with different long-term TBA change patterns using κ2 test (for categorical data) and Mann-Whitney U test (for continuous data). Cumulative incidence of HCC by patient groups with different long-term TBA change patterns was assessed with Kaplan-Meier analyses, and we tested for crude differences among the groups using a log-rank test. Cox proportional hazard models were used to calculate unadjusted and adjusted hazard ratios and 95% confidence intervals of HCC. STATA software version 13.0 was used for statistical analyses. Statistical significance was set at P < 0.05 and all tests were 2-tailed.
Human subjects review
The study was approved by the ethics committee of Southwest Hospital (Chongqing, China) and conducted in accordance with The Declaration of Helsinki Principles. Informed consent was obtained from all subjects.
Results
Characteristic of retrospective cohort and crude hazard ratios of HCC
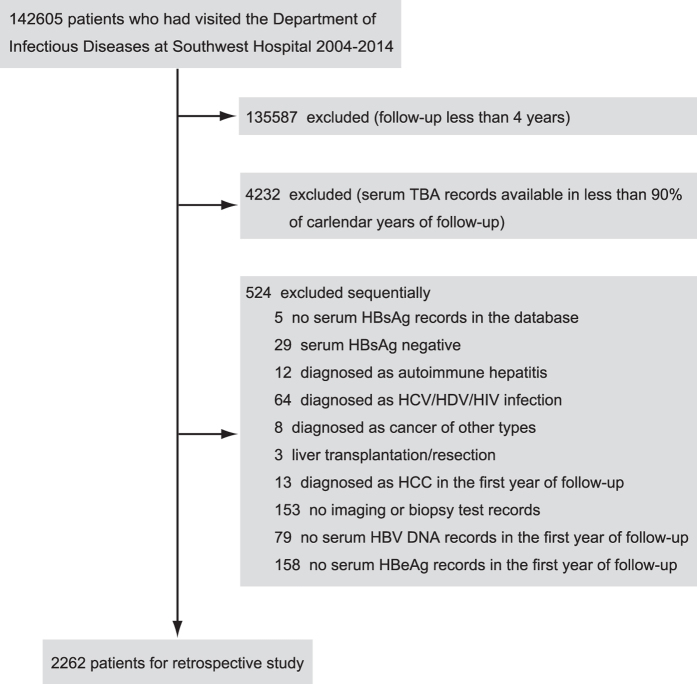
There were 142605 patients who have paid at least 1 visit to the Department of Infectious Diseases at Southwest Hospital from 2004 to 2014. A total of 2262 patients met the inclusion criteria and constituted the final retrospective cohort (Fig. 1).
Figure 1. Retrospective study cohort.
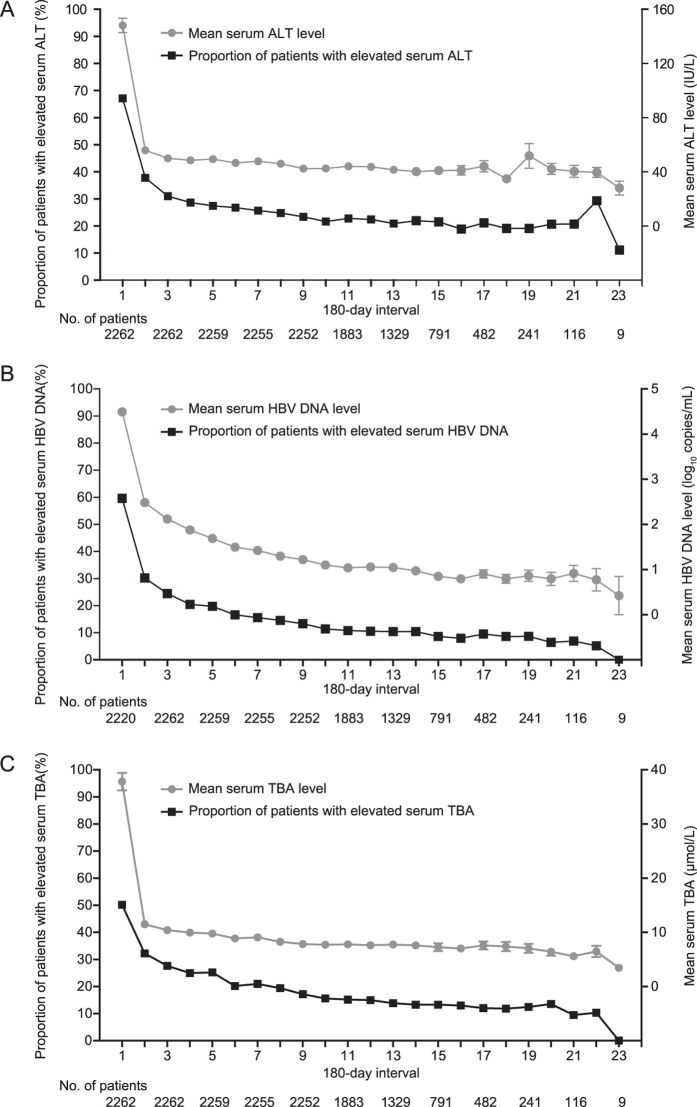
According to the records of drug prescription in the database, most of patients in the cohort had regular antiviral treatment with interferon and/or nucleoside analogue during follow-up. Of the total 2262 patients: only 1 patient had not received any antiviral treatment, 1 patient had received antiviral treatment with interferon alone, and the rest 2260 patients had received antiviral treatment with both interferon and nucleoside analogue. For the total 2261 patients with antiviral treatment, 2259 patients had antiviral treatment in more than 50% of the calendar years of their follow-up. Figure 2 shows the changes in the serum ALT, HBV DNA and TBA levels during follow-up. The serum ALT, HBV DNA, and TBA level decreased progressively with the most marked decline during the initial follow-up possibly due to antiviral treatment (Fig. 2).
Figure 2. Long-term change of serum ALT, HBV DNA, and TBA.
(A) Long-term change of serum ALT. Filled rectangles indicate the proportion of patients with elevated serum ALT in each 180-day interval. Filled circles indicate the mean of serum ALT in each 180-day interval and horizontal bars depict standard error. (B) Long-term change of serum HBV DNA. Filled rectangles indicate the proportion of patients with elevated serum HBV DNA in each 180-day interval. Filled circles indicate the mean of serum HBV DNA (log transformed) in each 180-day interval and horizontal bars depict standard error. For HBV DNA below the detection limit of 50 copies/mL, HBV DNA level was set to 1 copies/mL before log transforming. A total of 42 patients have no HBV DNA records in the first 180-day interval. (C) Long-term change of serum TBA. Filled rectangles indicate the proportion of patients with elevated serum TBA in each 180-day interval. Filled circles indicate the mean of serum TBA in each 180-day interval and horizontal bars depict standard error.
The characteristics of our retrospective cohort and their association with HCC by univariate analysis are shown in Table 1. A total of 62 HCC cases were diagnosed during a total follow-up of 14756.5 person-years. Medium, and high persistence of elevated serum TBA was observed in 267 (11.8%), and 222 (9.8%) patients. Compared with patients with none-low persistence of elevated serum TBA, the univariate hazard ratios were 3.70 (95% confidence interval, 1.89–7.22; P < 0.001), and 8.60 (95% confidence interval, 4.91–15.06; P < 0.001) for patients with medium, and high persistence of elevated serum TBA. Medium, and high persistence of elevated serum ALT was observed in 589 (26.0%), and 238 (10.5%) patients. Compared with patients with none-low persistence of elevated serum ALT, the univariate hazard ratios were 1.78 (95% confidence interval, 1.01–3.14; P = 0.04), and 2.93 (95% confidence interval, 1.52–5.66; P = 0.001) for patients with medium, and high persistence of elevated serum ALT. Medium, and high persistence of elevated serum HBV DNA was observed in 316 (14.0%), and 139 (6.2%) patients. Compared with patients with none-low persistence of elevated serum HBV DNA, the univariate hazard ratios were 1.25 (95% confidence interval, 0.63–2.47; P = 0.52), and 1.16 (95% confidence interval, 0.42–3.23; P = 0.77) for patients with medium, and high persistence of elevated serum HBV DNA. A total of 1082 (47.8%) patients were diagnosed as liver cirrhosis, and 145 of them had developed ascites during the follow-up. Compared with non-cirrhotic patients, the univariate hazard ratios were 31.26 (95% confidence interval, 7.59–128.82; P < 0.001), and 62.38 (95% confidence interval, 14.17–274.51; P < 0.001) for cirrhotic patients without ascites, and cirrhotic patients with ascites. For 2017 patients who had APRI records in their last year of follow-up, 732 (36.3%) patients had the last APRI score 0.5–1.5, and 174 (8.6%) patients had the last APRI score >1.5. Compared with patients with the last APRI score <0.5, the univariate hazard ratios were 3.47 (95% confidence interval, 1.76–6.85; P < 0.001), and 12.10 (95% confidence interval, 5.91–24.77; P < 0.001) for patients with the last APRI score 0.5–1.5, and patients with the last APRI score >1.5. About half of patients (1107, 48.9%) were HBeAg serum positive at baseline. Compared with patients with negative serum HBeAg at baseline, the univariate hazard ratio was 0.44 (95% confidence interval, 0.25–0.76; P = 0.003) for patients with positive serum HBeAg at baseline.
Table 1. Demographical and clinical characteristics of retrospective cohort and their association with HCC.
| Characteristics | Participants, number (%) | Follow-up, person-years | HCC, number | Hazard ratio (95% confidence interval) | P value |
|---|---|---|---|---|---|
| Age at entry, y | |||||
| <30 | 692 (30.6) | 4581.8 | 8 | 1.00 [Reference] | |
| 30–39 | 941 (41.6) | 6204.1 | 20 | 1.84 (0.81–4.18) | 0.14 |
| 40–49 | 433 (19.1) | 2762.6 | 18 | 3.81 (1.66–8.77) | 0.002 |
| ≧50 | 196 (8.7) | 1208.0 | 16 | 7.95 (3.40–18.60) | <0.001 |
| Sex | |||||
| Female | 552 (24.4) | 3541.5 | 11 | 1.00 [Reference] | |
| Male | 1710 (75.6) | 11215.0 | 51 | 1.44 (0.75–2.76) | 0.28 |
| Cirrhosis | |||||
| Non-cirrhosis | 1180 (52.2) | 7863.8 | 2 | 1.00 [Reference] | |
| Cirrhosis without ascites | 937 (41.4) | 5975.3 | 46 | 31.26 (7.59–128.82) | <0.001 |
| Cirrhosis with ascites | 145 (6.4) | 917.4 | 14 | 62.38 (14.17–274.51) | <0.001 |
| APRI at the end | |||||
| <0.5 | 1111 (55.1) | 7335.9 | 12 | 1.00 [Reference] | |
| 0.5–1.5 | 732 (36.3) | 4776.9 | 27 | 3.47 (1.76–6.85) | <0.001 |
| >1.5 | 174 (8.6) | 1064.2 | 20 | 12.10 (5.91–24.77) | <0.001 |
| Persistence of elevated TBA | |||||
| None-low | 1773 (78.4) | 11690.3 | 25 | 1.00 [Reference] | |
| Medium | 267 (11.8) | 1686.7 | 13 | 3.70 (1.89–7.22) | <0.001 |
| High | 222 (9.8) | 1379.5 | 24 | 8.60 (4.91–15.06) | <0.001 |
| Persistence of elevated ALT | |||||
| None-low | 1435 (63.4) | 9357.4 | 28 | 1.00 [Reference] | |
| Medium | 589 (26.0) | 3892.3 | 21 | 1.78 (1.01–3.14) | 0.04 |
| High | 238 (10.5) | 1506.8 | 13 | 2.93 (1.52–5.66) | 0.001 |
| Persistence of elevated HBV DNA | |||||
| None-low | 1807 (79.9) | 11856.6 | 48 | 1.00 [Reference] | |
| Medium | 316 (14.0) | 2020.5 | 10 | 1.25 (0.63–2.47) | 0.52 |
| High | 139 (6.2) | 879.4 | 4 | 1.16 (0.42–3.23) | 0.77 |
| Baseline HBeAg | |||||
| Negative | 1155 (51.1) | 7592.4 | 44 | 1.00 [Reference] | |
| Positive | 1107 (48.9) | 7164.1 | 18 | 0.44 (0.25–0.76) | 0.003 |
Cumulative incidence of HCC by persistence of elevated serum TBA
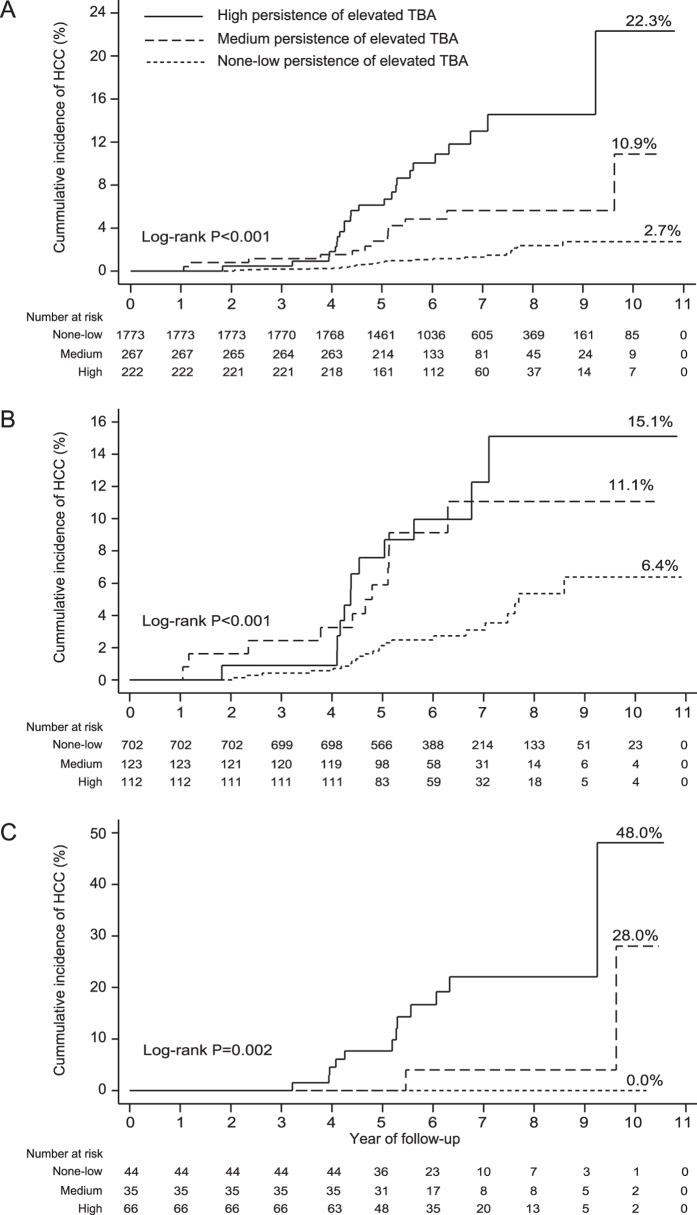
For the whole cohort, the cumulative probability of HCC at the end of follow-up was 2.7%, 10.9%, and 22.3% for patient with none-low, medium, and high persistence of elevated serum TBA, respectively (Log-rank P < 0.001) (Fig. 3A). For the sub-cohort of 937 cirrhotic patients without ascites, the cumulative probability of HCC at the end of follow-up was 6.4%, 11.1%, and 15.1% for patient with none-low, medium, and high persistence of elevated serum TBA, respectively (Log-rank P < 0.001) (Fig. 3B). For the sub-cohort of 145 cirrhotic patients with ascites, the cumulative probability of HCC at the end of follow-up was 0.0%, 28.0%, and 48.0% for patient with none-low, medium, and high persistence of elevated serum TBA, respectively (Log-rank P = 0.002) (Fig. 3C).
Figure 3. Cumulative incidence of HCC, by persistence of elevated serum TBA.
(A) The whole cohort. (B) The sub-cohort of 937 cirrhotic patients without ascites during follow-up. (C) The sub-cohort of 145 cirrhotic patients with ascites during follow-up.
Characteristics of retrospective cohort by persistence of elevated serum TBA
The characteristics of patients groups with different persistence of elevated serum TBA are shown in Table 2. Compared with patients with none-low persistence of elevated serum TBA, patients with medium or high persistence of elevated serum TBA were more likely to have shorter length of follow-up, to have older age at entry, to be diagnosed with cirrhosis and ascites during follow-up, to have higher APRI scores at the end of follow-up, and to have medium or high persistence of elevated serum ALT (P < 0.05) (Table 2).
Table 2. Characteristics of retrospective cohort, by persistence of elevated serum TBA.
| Characteristics | None-low persistence of elevated TBA (n = 1773), number (%) or median (interquartile range) | Medium persistence of elevated TBA (n = 267), number (%) or median (interquartile range) | P value, medium versus none-low | High persistence of elevated TBA (n = 222), number (%) or median (interquartile range) | P value, high versus none-low |
|---|---|---|---|---|---|
| Loss to follow-up | 462 (26.1) | 83 (31.1) | 0.08 | 68 (30.6) | 0.15 |
| Follow-up, y | 6.4 (5.3–7.6) | 6.0 (5.1–7.3) | 0.001 | 6.0 (4.9–7.1) | 0.001 |
| Age at entry, y | |||||
| <30 | 586 (33.0) | 78 (29.2) | 28 (12.6) | ||
| 30–39 | 759 (42.8) | 94 (35.2) | 88 (39.6) | ||
| 40–49 | 294 (16.6) | 73 (27.3) | 0.001 | 66 (29.7) | <0.001 |
| ≧50 | 134 (7.6) | 22 (8.2) | 40 (18.0) | ||
| Sex | |||||
| Female | 452 (25.5) | 55 (20.6) | 45 (20.3) | ||
| Male | 1321 (74.5) | 212 (79.4) | 0.08 | 177 (79.7) | 0.09 |
| Cirrhosis | |||||
| Non-cirrhosis | 1027 (57.9) | 109 (40.8) | 44 (19.8) | ||
| Cirrhosis without ascites | 702 (39.6) | 123 (46.1) | <0.001 | 112 (50.4) | <0.001 |
| Cirrhosis with ascites | 44 (2.5) | 35 (13.1) | 66 (29.7) | ||
| APRI at the end | |||||
| <0.5 | 965 (61.5) | 99 (40.9) | 47 (22.7) | ||
| 0.5–1.5 | 539 (34.4) | 115 (47.5) | <0.001 | 78 (37.7) | <0.001 |
| >1.5 | 64 (4.1) | 28 (11.6) | 82 (39.6) | ||
| Persistence of elevated ALT | |||||
| None-low | 1166 (65.8) | 145 (54.3) | 124 (55.9) | ||
| Medium | 440 (24.8) | 86 (32.2) | 0.001 | 63 (28.4) | 0.003 |
| High | 167 (9.4) | 36 (13.5) | 35 (15.8) | ||
| Persistence of elevated HBV DNA | |||||
| None-low | 1419 (80.0) | 208 (77.9) | 180 (81.1) | ||
| Medium | 242 (13.6) | 45 (16.8) | 0.32 | 29 (13.1) | 0.93 |
| High | 112 (6.3) | 14 (5.2) | 13 (5.9) | ||
| Baseline HBeAg | |||||
| Serum negative | 882 (49.8) | 140 (52.4) | 133 (59.9) | ||
| Serum positive | 891 (50.2) | 127 (47.6) | 0.41 | 89 (40.1) | 0.004 |
Multivariate-adjusted hazard ratios of HCC
Multivariate analyses adjusting for all the covariates listed in Table 1 are shown in Table 3. A total of 245 patients without APRI score in the last year of follow-up were excluded from multivariate analyses. Compared with patients with none-low persistence of elevated serum TBA, the multivariate adjusted hazard ratios were 2.37 (95% confidence interval, 1.16–4.84; P = 0.018), and 2.57 (95% confidence interval, 1.28–5.16; P = 0.008) for patients with medium, and high persistence of elevated serum TBA (Table 3). Compared with non-cirrhotic patients, the multivariate hazard ratios were 17.01 (95% confidence interval, 3.96–73.15; P < 0.001), and 15.42 (95% confidence interval, 3.20–74.32; P = 0.001) for cirrhotic patients without ascites, and cirrhotic patients with ascites (Table 3). Compared with patients with the last APRI score <0.5, the multivariate hazard ratios were 1.60 (95% confidence interval, 0.78–3.27; P = 0.20), and 2.67 (95% confidence interval, 1.12–6.38; P = 0.027) for patients with the last APRI score 0.5–1.5, and patients with the last APRI score >1.5 (Table 3). Compared with patients with none-low persistence of elevated serum HBV DNA, the multivariate hazard ratios were 1.62 (95% confidence interval, 0.78–3.35; P = 0.19), and 3.14 (95% confidence interval, 1.02–9.64; P = 0.045) for patients with medium, and high persistence of elevated serum HBV DNA (Table 3). Age at entry was significantly associated with HCC (P = 0.027) (Table 3). Sex, persistence of elevated serum ALT, and baseline HBeAg were not significantly associated with HCC in the multivariate analyses (P > 0.05) (Table 3).
Table 3. Multivariate analyses of risk for HCC.
| Characteristics | HCC, number (%) | Hazard ratio (95% confidence interval) | P value |
|---|---|---|---|
| Age at entry in 1-year increment | 1.03 (1.00–1.06) | 0.027 | |
| Sex | |||
| Female | 11 (18.6) | 1.00 [Reference] | |
| Male | 48 (81.4) | 1.17 (0.58–2.39) | 0.66 |
| Cirrhosis | |||
| Non-cirrhosis | 2 (3.4) | 1.00 [Reference] | |
| Cirrhosis without ascites | 43 (72.9) | 17.01 (3.96–73.15) | <0.001 |
| Cirrhosis with ascites | 14 (23.7) | 15.42 (3.20–74.32) | 0.001 |
| APRI at the end | |||
| <0.5 | 12 (20.3) | 1.00 [Reference] | |
| 0.5–1.5 | 27 (45.8) | 1.60 (0.78–3.27) | 0.20 |
| >1.5 | 20 (33.9) | 2.67 (1.12–6.38) | 0.027 |
| Persistence of elevated TBA | |||
| None-low | 23 (39.0) | 1.00 [Reference] | |
| Medium | 13 (22.0) | 2.37 (1.16–4.84) | 0.018 |
| High | 23 (39.0) | 2.57 (1.28–5.16) | 0.008 |
| Persistence of elevated ALT | |||
| None-low | 28 (47.4) | 1.00 [Reference] | |
| Medium | 18 (30.5) | 1.26 (0.67–2.39) | 0.47 |
| High | 13 (22.0) | 1.82 (0.86–3.90) | 0.12 |
| Persistence of elevated HBV DNA | |||
| None-low | 45 (76.3) | 1.00 [Reference] | |
| Medium | 10 (16.9) | 1.62 (0.78–3.35) | 0.19 |
| High | 4 (6.8) | 3.14 (1.02–9.64) | 0.045 |
| Baseline HBeAg | |||
| Negative | 43 (72.9) | 1.00 [Reference] | |
| Positive | 16 (27.1) | 0.61 (0.33–1.13) | 0.12 |
Discussion
Our retrospective study identified persistently elevated serum TBA as a major independent risk factor for HCC development in CHB patients receiving regular antiviral treatment. A total of 37 (59.7%) HCC cases in our cohort were contributed by the patient populations with medium or high persistence of elevated serum TBA.
Previous epidemiological studies identified the persistence of elevated serum ALT/HBV DNA as major risk factors of HCC in CHB patient population without antiviral treatment18. Our multivariate analyses also found significant association between persistence of elevated serum HBV DNA and HCC. However, in our multivariate analyses, only patients with high persistence of elevated serum HBV DNA showed significantly increased HCC risk compared with patients with none-low persistence of elevated serum HBV DNA, and only 4 (6.9%) HCC cases were contributed by this patient population (Table 3). The decreased HCC risk burden by persistence of elevated serum HBV DNA in our cohort might be explained by the regular antiviral treatment in our patients. Unlike previous study18, although our univariate analyses showed significant association between persistence of elevated serum ALT and HCC (Table 1), our multivariate analyses did not show significant association between persistence of elevated serum ALT and HCC (Table 3). This discrepancy might result from the different ways of adjusting for cirrhosis in the analyses. Previous study adjusts for cirrhosis by excluding patients with cirrhosis at enrollment18, which would inevitably lead to an underestimation of the effect of cirrhosis on HCC development as most of HBV-related HCC cases (70–90%) occur in patients with cirrhosis1. Our study not only adjusts for diagnosis of cirrhosis during the follow-up but also adjusts for the severity of cirrhosis through ascites and APRI score at the end of follow-up. There is significant association between persistence of elevated serum ALT and APRI score at the end of follow-up in our cohort (P < 0.001). The loss of significant association between persistence of elevated serum ALT and HCC in the multivariate analyses indicates that persistence of elevated serum ALT is not an independent risk factor of HCC when the severity of cirrhosis is taken into consideration.
Bile acid, as product of cholesterol metabolism, is traditionally recognized as detergents for dietary lipid digestion and absorption, while accumulative evidences have identified its role as hormone with distinct receptors in multiple tissues20. Previous studies from mice model reveals a pro-inflammatory role of bile acid21,22, which is thought to cause HCC development by promoting genomic modification of hepatocytes11,23. Except for hepatocyte damage, elevated serum bile acid in CHB patients could also be caused by disrupted bile acid metabolism: Binding of HBV virus to bile acid receptor Na+-taurocholate cotransporting polypeptide (NTCP) could promote hepatic bile acid synthesis24; Bile acid metabolism could also be modulated by gut microbiota in cirrhotic patients25.
Our results should be interpreted within the study’s limitations. Firstly, for lack of image test results for a large part of our patients in the initial follow-up, our study adjusts for cirrhosis based on diagnosis of cirrhosis during follow-up. This shortcoming might overestimate the effect of cirrhosis on HCC because some cirrhotic patients might be free of cirrhosis at entry. Of the 60 HCC cases with cirrhosis in our cohort, 37 (61.7%) cases were diagnosed as cirrhosis at least 1 year after the start of follow-up. Secondly, as persistence of elevated serum TBA was closely associated with diagnosis of cirrhosis during follow-up in our cohort, it is reasonable to suspect that the increased HCC risk in patients with medium or high persistence of elevated serum TBA might simply be a result of advanced cirrhosis in those patients. To address this issue, we included ascites and APRI score into our analyses. Development of ascites is a clinical feature of advanced cirrhosis. The APRI scoring system is less applicable in CHB patients without antiviral treatment due to severe hepatic inflammation26. As the hepatic inflammation decreased progressively in our cohort due to regular antiviral treatment, we used patients’ last APRI score to evaluate liver fibrosis at the end of follow-up. Inclusion of those two factors in the analyses supported the role of persistence of elevated serum TBA as an independent driving factor for HCC. Also worthy of notice, the close association between persistence of elevated serum TBA and severity of cirrhosis in our data do not imply a causal relationship between those two factors, as there is no reliable information about the severity of cirrhosis during initial follow-up of our cohort. Thirdly, due to lack of data, our analyses did not adjust for HBV genotype and HBV mutations. Both HBV genotype and HBV mutation in the precore/enhancer II region and the preS region have been reported to be independent HCC risk factors in CHB patients16,27,28,29,30,31. It is not clear whether persistence of elevated serum TBA is associated with specific HBV genotypes or specific HBV variants in our cohort.
Our findings identified persistence of elevated serum TBA as an independent risk factor of HCC in CHB patients with regular antiviral treatments, which could help to improve routine surveillance for HCC in current clinical practice. Accumulative metabolomics data has found elevated serum bile acid as a core metabolomics phenotype in almost all the types of liver diseases32. It is worthy to apply our findings on those liver diseases.
Additional Information
How to cite this article: Wang, H. et al. Increased hepatocellular carcinoma risk in chronic hepatitis B patients with persistently elevated serum total bile acid: a retrospective cohort study. Sci. Rep. 6, 38180; doi: 10.1038/srep38180 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was funded by National Natural Science Foundation of China (81330038, 91442203, and 81220108024), China 973 Project (2013CB531501), and Third Military Medical University Key Project for Clinical Research (2012XLC05). The funding agencies had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions H.W., G.D., and Y.W. conceived the study questions and designed the study. Q.M. advised on the concept and design of the study. G.D. provided the raw clinical data for retrospective study. X.S., X.X., and X.W. verified and cleaned the raw clinical data for retrospective study. H.W. conducted data analysis, and interpreted results. H.W. and G.D. drafted manuscript. Y.W. critically reviewed manuscript. Q.M., G.D., and Y.W. obtained the fundings. Y.W. supervised the study.
References
- El-Serag H. B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142, 1264–1273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw Y. F. et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 351, 1521–1531 (2004). [DOI] [PubMed] [Google Scholar]
- Hosaka T. et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 58, 98–107 (2013). [DOI] [PubMed] [Google Scholar]
- Cho J. Y. et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut 63, 1943–1950 (2014). [DOI] [PubMed] [Google Scholar]
- Wu C. Y. et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology 147, 143–151 (2014). [DOI] [PubMed] [Google Scholar]
- Chiang C. J. et al. Significant reduction in end-stage liver diseases burden through the national viral hepatitis therapy program in Taiwan. Hepatology 61, 1154–1162 (2015). [DOI] [PubMed] [Google Scholar]
- Papatheodoridis G. V., Chan H. L., Hansen B. E., Janssen H. L. & Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J. Hepatol. 62, 956–967 (2015). [DOI] [PubMed] [Google Scholar]
- Knisely A. S. et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44, 478–486 (2006). [DOI] [PubMed] [Google Scholar]
- Kim I. et al. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28, 940–946 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F. et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 67, 863–867 (2007). [DOI] [PubMed] [Google Scholar]
- Yoshimoto S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013). [DOI] [PubMed] [Google Scholar]
- Yang J. et al. High performance liquid chromatography-mass spectrometry for metabonomics: potential biomarkers for acute deterioration of liver function in chronic hepatitis B. J. Proteome Res. 5, 554–561 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Development and validation of a liquid chromatography-mass spectrometry metabonomic platform in human plasma of liver failure caused by hepatitis B virus. Acta Biochim. Biophys. Sin. 42, 688–698 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. A weighted relative difference accumulation algorithm for dynamic metabolomics data: long-term elevated bile acids are risk factors for hepatocellular carcinoma. Sci. Rep. 5, 8984, doi: 10.1038/srep08984 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. I. et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N. Engl. J. Med. 347, 168–174 (2002). [DOI] [PubMed] [Google Scholar]
- Yu M. W. et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J. Natl. Cancer Inst. 97, 265–272 (2005). [DOI] [PubMed] [Google Scholar]
- Chen C. et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295, 65–73 (2006). [DOI] [PubMed] [Google Scholar]
- Chen C. F. et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 141, 1240–1248 (2011). [DOI] [PubMed] [Google Scholar]
- Wai C.-T. et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38, 518–526 (2003). [DOI] [PubMed] [Google Scholar]
- de Aguiar Vallim T. Q., Tarling E. J. & Edwards P. A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 17, 657–669 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P. et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 127, 261–274 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 32, 58–69 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannelli F. et al. Massive gene amplification drives paediatric hepatocellular carcinoma caused by bile salt export pump deficiency. Nat. Commun. 5, 3850, doi: 10.1038/ncomms4850 (2014). [DOI] [PubMed] [Google Scholar]
- Oehler N. et al. Binding of hepatitis B virus to its cellular receptor alters the expression profile of genes of bile acid metabolism. Hepatology 60, 1483–93 (2014). [DOI] [PubMed] [Google Scholar]
- Kakiyama G. et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 58, 949–955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray Kim W. et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J. Hepatol. 64, 773–780 (2016). [DOI] [PubMed] [Google Scholar]
- Chan H. L. et al. High viral load and hepatitis B virus subgenotype ce are associated with increased risk of hepatocellular carcinoma. J. Clin. Oncol. 26, 177–182 (2008). [DOI] [PubMed] [Google Scholar]
- Yang H. I. et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J. Natl. Cancer Inst. 100, 1134–1143 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen M. F. et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J. Hepatol. 50, 80–88 (2009). [DOI] [PubMed] [Google Scholar]
- Pollicino T., Cacciola I., Saffioti F. & Raimondo G. Hepatitis B virus PreS/S gene variants: pathobiology and clinical implications. J. Hepatol. 61, 408–417 (2014). [DOI] [PubMed] [Google Scholar]
- Yin J. et al. Hepatitis B virus combo mutations improve the prediction and active prophylaxis of hepatocellular carcinoma: a clinic-based cohort study. Cancer Prev. Res. 8, 978–988 (2015). [DOI] [PubMed] [Google Scholar]
- Beyoglu D. & Idle J. R. The metabolomic window into hepatobiliary disease. J. Hepatol. 59, 842–858 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]