Abstract
Tuberculosis remains a major public health hazard worldwide, with neonates and young infants potentially more susceptible to infection than adults. BCG, the only vaccine currently available, provides some protection against tuberculous meningitis in children but variable efficacy in adults, and is not safe to use in immune compromised individuals. A safe and effective vaccine that could be given early in life, and that could also potentiate subsequent booster immunization, would represent a significant advance. To test this proposition, we have generated gene-based vaccine vectors expressing Ag85B from Mycobacterium tuberculosis (Mtb) and designed experiments to test their immunogenicity and protective efficacy particularly when given in heterologous prime-boost combination, with the initial DNA vaccine component given soon after birth. Intradermal delivery of DNA vaccines elicited Th1-based immune responses against Ag85B in neonatal mice but did not protect them from subsequent aerosol challenge with virulent Mtb H37Rv. Recombinant adenovirus vectors encoding Ag85B, given via the intranasal route at six weeks of age, generated moderate immune responses and were poorly protective. However, neonatal DNA priming following by mucosal boosting with recombinant adenovirus generated strong immune responses, as evidenced by strong Ag85Bspecific CD4+ and CD8+ T cell responses, both in the lung-associated lymph nodes and the spleen, by the quality of these responding cells (assessed by their capacity to secrete multiple antimicrobial factors), and by improved protection, as indicated by reduced bacterial burden in the lungs following pulmonary TB challenge. These results suggest that neonatal immunization with gene-based vaccines may create a favorable immunological environment that potentiates the pulmonary mucosal boosting effects of a subsequent heterologous vector vaccine encoding the same antigen. Our data indicate that immunization early in life with mycobacterial antigens in an appropriate vaccine setting can prime for protective immunity against Mtb.
Keywords: Neonatal vaccination, Prime-boosting, Pulmonary T cell immunity, Tuberculosis, Vaccine vectors
1. Introduction
Tuberculosis (TB) remains one of the deadliest infectious diseases globally with an estimated 9.6 million new cases and approximately 1.5 million deaths in 2014 alone (1). The situation has worsened due to human immunodeficiency virus co-infection and the emergence of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB). Infants and young children have the greatest risk of developing tuberculosis following exposure to Mycobacterium tuberculosis (Mtb) (2) and childhood infection is often more severe due to the relative immaturity of the immune system (3). Approximately 1.0 million new cases and 140,000 deaths occur in children annually (1). An effective vaccine given early in life could help to protect children from Mtb infection. Mycobacterium bovis bacille Calmette-Guerin (BCG), the only vaccine currently available, is protective against tuberculous meningitis and disseminated infections in children (4). BCG-induced protection against tuberculosis may last up to 60 years (5), although its effectiveness in preventing TB in adults has been variable, with efficacies in the range of 0% to 80% reported from multiple clinical trials (6). The protective efficacy of BCG immunization may be diminished by prior exposure to mycobacteria (7), while BCG is not suitable for HIV-infected or otherwise immune-compromised individuals (8). Alternative approaches may therefore be required for the development of safe and effective vaccines against TB infection in children, as well as in adults.
Compared with adults, neonates have 10–100-fold fewer T cells (9), even fewer dendritic cells (10), and have still to establish competent T cell-mediated immunity. Thus, neonates and young children are generally more susceptible to microbial infections, including tuberculosis. Early work in mice suggested that exposure to antigen very early in life could induce unresponsiveness referred to as neonatal tolerance (11,12), or generate immune responses biased toward Th2-type activity (13). Other studies indicated that the neonate can develop both Th1 and Th2 CD4+ cells at similar frequencies during primary responses to antigen before skewing towards Th2 responsiveness (14), corresponding to upregulated responsiveness to IL-4 (15,16). Delayed maturity of dendritic cells and insufficient IL-12 production may also contribute to the apparent Th2 bias in neonates (17). Despite Th2-skewed neonatal immunity, Th1 cell-promoting agents, such as DNA vaccines (18), or live, replicating vaccines, such as BCG (19,20), can elicit potent Th1 immunity in neonates. Optimization of vaccination regimens to promote Th1 immunity in neonates in order to protect against infectious diseases such as tuberculosis may therefore be feasible.
Heterologous prime-boost immunization is a promising generalized immunization strategy, both in terms of safety and robust generation of both CD4+ and CD8+ T cells (21–23). The synergistic combination of priming with a vaccine vector encoding the antigen of interest and subsequent boosting with a different (usually viral) vaccine vector encoding common antigens often results in the induction and preferential expansion of high levels of antigen-specific CD4+ and CD8+ T cells. Previous studies in this laboratory have shown that vector-based heterologous prime-boosting can generate sustained immune responses, both in the spleen and in mucosal tissues, which are both quantitatively and qualitatively superior to those induced by conventional approaches (21–27). Although immune correlates of protection are not yet fully defined in TB, strong cell-mediated immune responses against the pathogen may be critical, with several current approaches that have reached clinical trials targeting induction of robust CD4+ and CD8+ T cell responses through heterologous prime-boost vaccine protocols (28).
In the present study, we have used a prime-boost strategy to test the efficacy of neonatal immunization in a mouse model of pulmonary TB infection. We generated DNA vaccines, DNA-85B, and recombinant adenovirus (Ad) vectors, Ad-85B, both of which encode antigen 85B (Ag85B) from Mtb. Ag85B is a mycolyltransferase secreted by actively replicating Mtb within host cells and in culture, where it accounts for more than 20% of the protein in culture filtrates (29). Actively secreted mycobacterial proteins, including Ag85B, are targets of the immune system and have been shown to elicit effective immune responses (30). Multiple CD4+ and CD8+ T cell epitopes have been identified within Ag85B (31), and Ag85B-based constructs can induce strong immune responses and some protection against Mtb challenge in animal models (30, 32). Our recombinant DNA and Ad vaccines were used, either alone or in heterologous prime-boost combination, with the DNA vaccine component first delivered soon after birth. Intradermal delivery of DNA-85B at 4 days of age elicited specific Th1-type immune responses, and ehanced the subsequent effects of Ad-85B given intranasally at 6 weeks of age, both in terms of pulmonary anti-mycobacterial immune responses, and the degree of protection in the lungs against pulmonary Mtb challenge. Our data suggest that immunization early in life with mycobacterial antigens in an appropriate vaccine setting can prime for protective immunity against Mtb infection.
2. Materials and Methods
2.1. Construction of DNA and adenovirus vector vaccines encoding mycobacterial Ag85B
The Ag85B gene was amplified by PCR using Mtb H37Rv genomic DNA (Colorado State University, NIH TBVTRM Contract NO-AI-40091) as template using forward 5’-TTTGGATCCTTCTCCCGGCCGG GGCTGCCGGT-3’ (BamHI) and reverse 5’-AAAGAATTCTCAGCCGGCGCCTAACGAACTCT-3’ (EcoRI) primers (Integrated DNA Technologies, Coralville, IA). The PCR product was digested using BamHI and EcoRI restriction enzymes (Roche Applied Sciences, Branford, CT) and cloned into the DNA vaccine vector-pHis (Coley Pharmaceutical Group, Wellesley, MA). Insert identity and orientation were confirmed by PCR and DNA sequencing. Stocks of DNA vaccines were prepared using the Endo-free plasmid Mega kit (Qiagen, Gaithersburg, MD) according to the manufacturer’s instructions. For intradermal delivery, DNA vaccines were coated onto 1.6 micron gold particles (Bio-Rad) according to the manufacturer’s recommendations. Briefly, 25 mg of gold powder was mixed with 100 µl of spermidine (0.05 M; Sigma) and sonicated. Next, 50 µg of plasmid DNA encoding Ag85B in a 100-µl volume was added. Finally, 100 µl of 1 M CaCl2 was added dropwise to the mixture with gentle vortexing. After a 10-min precipitation step at room temperature, the pellets were washed three times and then resuspended in an ethanol solution containing 0.1 mg/ml polyvinylpyrrolidone (Bio-Rad). The DNA/gold particle suspension was loaded into Gold-Coat tubing using the Tubing Prep Station (Gold-Coat tubing; Bio-Rad). 0.5 inch cartridges containing nitrogen-dried DNA-coated gold particles (1 µg DNA per cartridge) were stored at −20°C until use.
Recombinant adenovirus type 5 (rAd5) vaccine vectors encoding Ag85B were constructed using Gateway® pENTR2B entry pAd/CMV/V5-DEST destination vectors (Invitrogen), purified, concentrated, and titrated as described elsewhere (26). The PCR amplified Ag85B gene insert was generated as above, but using a forward primer containing the “CACC” sequence at the 5’ end that facilitated vector construction using the Gateway® system.
2.2. Mice
All procedures were conducted under the guidelines of the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center (LSUHSC) in accordance with the National Institutes of Health guide for the care and use of Laboratory animals. Mice were housed in the LSUHSC Animal Care Facility. Pregnant Balb/c mice were purchased from Charles River (Wilmington, MA) and housed individually. Neonates were weaned and separated according to sex at 3 weeks of age. All Mtb-challenged mice were housed in a Bio-containment Level 3 Laboratory operated in accordance with the appropriate safety precautions recommended by the Centers for Disease Control and Prevention and monitored by the LSUHSC Institutional Biosafety Committee.
2.3. Immunization
Neonatal BALB/c mice at 4 days of age were immunized with DNA vaccine-coated gold particles using the Helios Gene Gun System (Bio-Rad) at a helium discharge pressure of 350 psi (33). Neonates received two non-overlapping shots on the back, each delivering 0.5 mg of 1.6 micron gold particles coated with 1 µg of plasmid DNA encoding Ag85B or DNA control vaccine. Six weeks later, some groups of mice were given 5×108 plaque-forming units (pfu) of recombinant adenovirus vaccine by pipetting 10 µl of viruses diluted in PBS into each nostril. Control mice were given 1×105 cfu of BCG, or were mock-immunized with saline (naïve group), intradermally via footpad at 4 weeks prior to Mtb challenge.
2.4. Isolation of mononuclear cells
At three or six weeks post-immunization, animals were euthanized, and spleens and lung associated lymph nodes, including deep cervical in addition to anterior and poster mediastinal lymph nodes, were isolated by gross dissection and mononuclear cells were isolated as described elsewhere (27).
2.5. Peptides
Peptides HSWEYWGAQLNAMKGDLQ (peptide 262) and MPVGGQSSF (peptide 8) were synthesized by GenScript Corporation (Piscataway, NJ). These peptides have been shown to represent H2d-restricted CD4+ and CD8+ T cell epitopes within Ag85B, respectively (31) and were used to stimulate antigen-specific responses in IFN-γ ELISpot and ICS assays.
2.6. IFN-γ ELISPOT assay
Assays for Ag85B-specific CD4+ and CD8+ T cell responses to peptides were performed as described elsewhere (26). Data are presented as spot-forming cells (SFCs) per million cells ± SEM.
2.7. Intracellular Cytokine Staining (ICS)
ICS was performed on single cell suspensions as described elsewhere (27). The cells were stained for intracellular cytokines using phycoerythrin-conjugated anti-mouse IL2 (BD Pharmingen, Cat. 554428), phycoerythrin-Cy7-conjugated anti-mouse TNFα (BD Pharmingen, Cat. 557644) and allophycocyanin-conjugated anti-mouse IFNγ (BD Pharmingen, Cat. 554413). Data were analyzed using FlowJo Software (version 8.8.6, TreeStar Inc., Ashland, OR, USA), and assessment of T cell multifunctionality was performed using the Boolean gating combination of the Software. The hierarchical gating strategy is shown in Fig. S1.
2.8. Aerosolized Mtb H37Rv challenge
Mtb strain H37Rv (ATCC No. 27294, Rockville, MD) was grown in Middlebrook 7H11 broth at 37°C for 14 days. This culture was concentrated by centrifugation, gently sonicated at 95 W for 10 seconds in a cup-horn sonicator, and stored in aliquots at −80°C after titration. At the time of inoculation, an aliquot was thawed, gently sonicated, and diluted in endotoxin-free phosphate-buffered saline (PBS) to the desired concentration. Six weeks after adenovirus vaccine booster immunization, mice were infected with Mtb H37Rv by the aerosol route in a Glas-Col inhalation exposure system (Glas-Col). Exposure times were calibrated to deliver 50 to 100 CFU into the lungs of each infected mouse. Mice were sacrificed at six weeks after infection, and bacterial loads in the lungs and spleens were determined by quadruple serial dilution on 7H11 agar plates supplemented with OADC (BD, Sparks, MD). The plates were incubated at 37°C for 14 to 21 days in sealed plastic bags before the colonies were counted.
2.9. Statistical analysis
ANOVA and Student’s t-test were used to analyze statistical differences using GraphPad Prism software. Data are presented as mean ± SD. P values ≤ 0.05 were considered statistically significant.
3. Results
3.1. Intradermal DNA-85B immunization of neonates elicited antigen-specific T cell responses in the lung-associated lymph nodes and spleen
To determine the immunogenicity of DNA-85B vaccine in neonates, Balb/c mice at 4 days of age were immunized intradermally with DNA-85B or control DNA-c by gene gun. Six weeks later, 5 mice from each group were sacrificed and splenocytes were stimulated with Ag85B peptide 262 or peptide 8 in IFN-γ ELISPOT assays. The results showed that neonatal DNA-85B immunization elicited moderate antigen-specific CD4+ (180±35 SFC per million splenocytes) and CD8+ (567±61 SFC per million splenocytes) T cell responses in the spleens. Twelve weeks after immunization, immune responses in lung-associated lymph nodes were examined in mice that had also been boosted intranasally with ‘empty’ control adenovirus vectors at six weeks after DNA priming. Antigen-specific CD4+ (55±5 SFC per million lymphoid cells) and CD8+ (78±5 SFC per million lymphoid cells) T cell responses were both detected in the lung-associated lymph nodes. Control DNA vaccine elicited T cell responses at only background levels (<25 SFC per million lymphoid cells) in both spleen and lung-associated lymph nodes in these experiments. The data are expressed as the mean ± standard deviation for five mice per group and are representative of two independent experiments, Together, they indicate that neonatal DNA-85B immunization via the intradermal route was able to elicit antigen-specific CD4+ and CD8+ T cell responses in both spleen and in the lung-associated lymph nodes.
3.2. Neonatal DNA-85B priming significantly increased antigen-specific IFN-γ-secreting mucosal CD4+ and CD8+ T cell responses in the lung-associated lymph nodes and spleens of mice boosted with Ad-85B
Next, we used IFN-γ ELISPOT assays to examine the impact of neonatal DNA-85B priming on subsequent immunization with Ad-85B given via the intranasal route six weeks later. Strong IFN-γ+ CD4+ and CD8+ T cell responses were generated in both lung-associated lymph nodes (Fig. 1A and B) and spleens (Fig. 1C and D) following neonatal priming with DNA-85B and intranasal boosting withAd-85B. These responses were also greater than in mice primed with control DNA vaccine prior to Ad-85B boosting, indicative of a priming effect of neonatal DNA-85B. These results suggest that intradermal neonatal DNA-85B priming created an environment favorable for enhancement of the effects of subsequent mucosal Ad-85B boosting.
Fig. 1.
TB-specific CD4+ and CD8+ T cell responses generated following neonatal prime /intranasal boost vaccination. Neonates at 4 days of age were immunized intradermally with DNA-c or DNA-85B, and boosted intranasally 6 weeks later with Ad-85B or Ad-c as described in Materials and Methods. Ag85B-specific CD4+ (A and C) and CD8+ (B and D) T cells were quantified in lung-associated lymph nodes (A and B) and spleens (C and D) by IFN-γ ELISPOT assay at six weeks after boosting. Data are expressed as mean numbers of spot-forming cells (SFC) per million cells ± standard deviation for five mice per group and are representative of two independent experiments. * Denotes statistical significance (P < 0.05) when compared with the DNA-c/Ad-85B group.
3.3. Neonatal DNA-85B/Ad-85B prime-boosting generated quantitatively and qualitatively superior mucosal CD4+ and CD8+ T cell responses in lung-associated lymph nodes
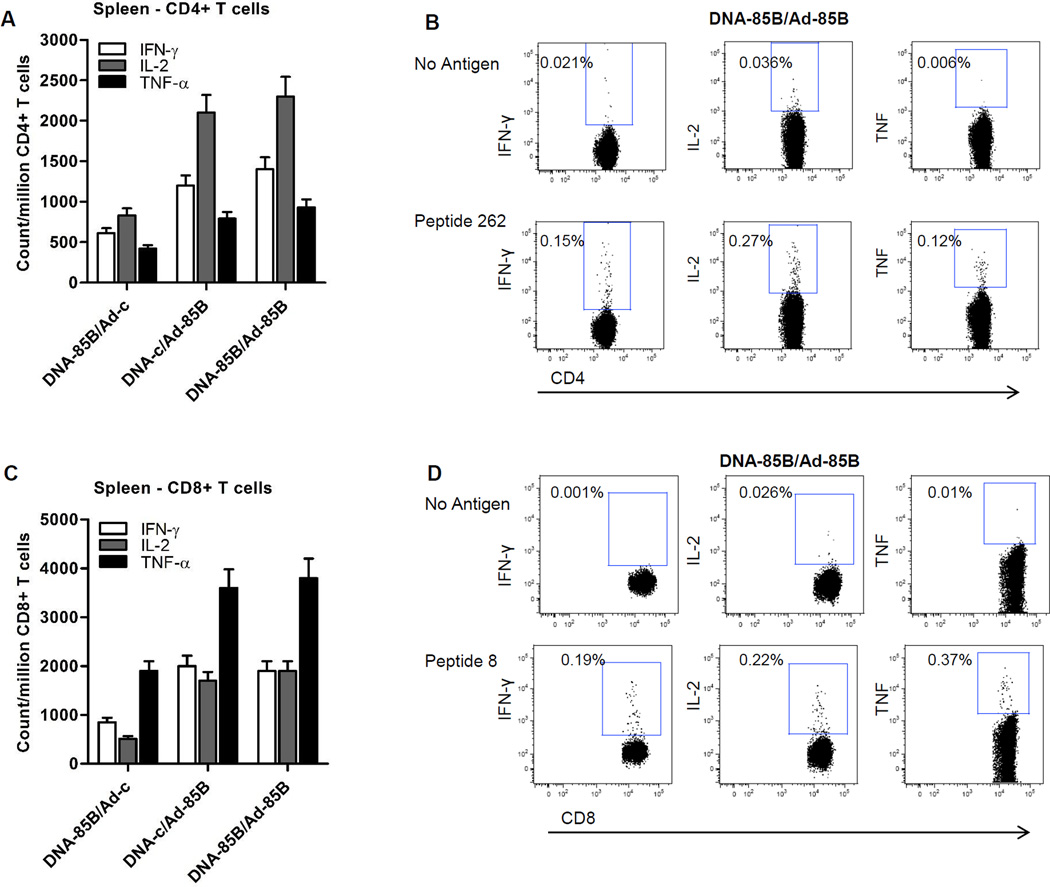
To further characterize the impact of neonatal DNA-85B priming on mucosal T cell responses following DNA-85B/Ad-85B prime-boosting, lung-associated lymph node cells were stimulated with peptide 262 or peptide 8 and cytokine secretion patterns were analyzed by ICS. The results show that frequencies of IFN-γ, IL-2 and TNF-generating CD4+ T cells (Fig. 2A and B) and CD8+ T cells (Fig. 2C and D) in lung-associated lymph nodes were significantly higher in mice primed intradermally with DNA-85B before intranasal Ad boosting than in those primed with DNA-c.
Fig. 2.
TB-specific CD4+ and CD8+ T cell cytokine responses in lung-associated lymph nodes following neonatal prime/intranasal boost vaccination. Cells from lung-associated lymph nodes of mice immunized as described in Fig. 1 were stimulated with peptide 262 or peptide 8 for 6 hours and analyzed for cytokine secretion by ICS as described in Materials and Methods. Data are expressed as cell counts of IFN-γ-, IL-2-, or TNF-generating CD4+ (A) or CD8+ T cells (C) per million CD4+ or CD8+ T cells in each group of five mice and are representative of two independent experiments. **Denotes statistical significance (P < 0.01) where IFN-γ-, IL-2-, or TNF-generating CD4+ or CD8+ T cells in the DNA-85B/Ad-85B group were compared with those in DNA-c/Ad-85B group. Representative ICS data in the DNA85B/Ad-85B group are shown, where lung-associated lymph node cells were stimulated with peptide 262 (B) or peptide 8 (D).
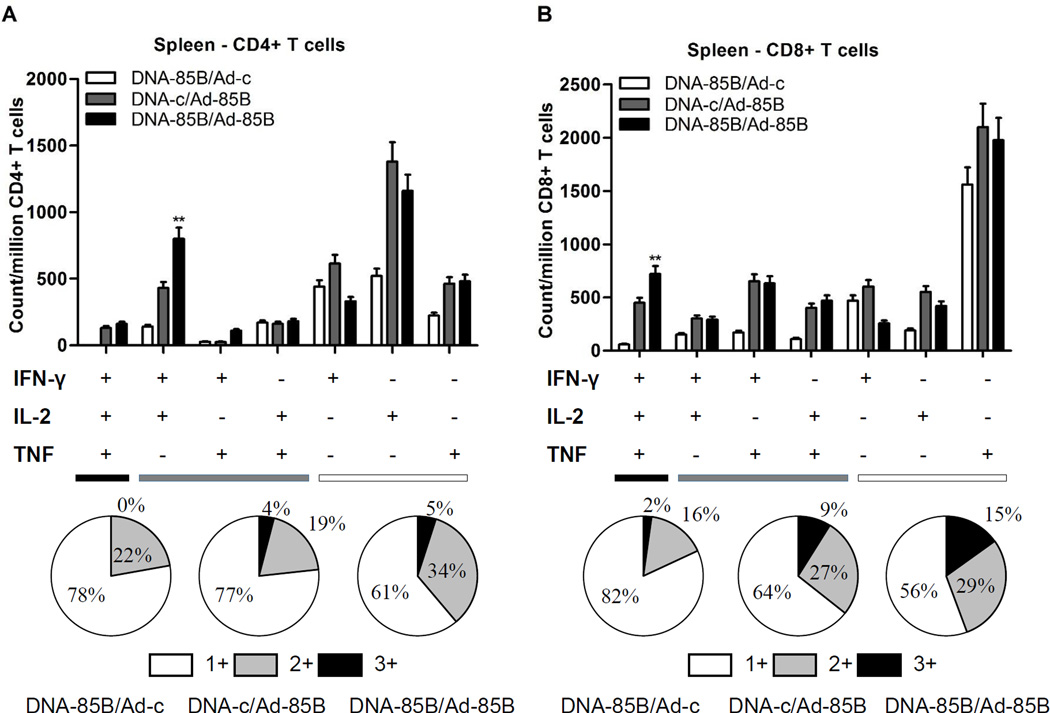
The polyfunctionality of these cell populations was also analyzed as an indication of the quality of vaccine-induced responses in the lung-associated lymph nodes. As discussed below, the generation of T cells simultaneously secreting multiple antimicrobial factors such as IFN-γ, TNF, and also IL-2 has been associated with protective efficacy. As shown in Fig. 3, neonatal priming with DNA-85B clearly enhanced the percentages of responding polyfunctional CD4+ (Fig. 3A) and CD8+ (Fig. 3B) T cell subsets found in the lung-associated lymph nodes. This is reflected in the relative numbers of triple and dual cytokine-secreting cells as a proportion of the responding T cell populations in lung-associated lymph nodes in the different vaccine groups as shown in the pie-charts, ie. DNA-Ag85B/Ad-Ag85B (52% for CD4+, 52% for CD8+) compared to the DNA-c/Ad-Ag85B group (36% for CD4+, 39% for CD8+).
Fig. 3.
Polyfunctional TB-specific CD4+ and CD8+ T cell responses in lung-associated lymph nodes following neonatal prime /intranasal boost vaccination. Cells from lung-associated lymph nodes of mice immunized as described in Fig. 1 were stimulated with peptide 262 or peptide 8 for 6 hours and were analyzed for cytokine secretion by ICS as described in Materials and Methods. Polyfunctional CD4+ T cell (A) and CD8+ T cell (B) cytokine secretion profiles for triple- (3+), dual- (2+) and single-cytokine (1+) secretion, and comparisons between vaccination groups (top), and percentages of triple-, dual- or single-cytokine secreting T cells in each group (pie-charts at bottom), are shown. Data were generated from five mice per group and are representative of two independent experiments. **Denotes statistical significance (P < 0.01) in the DNA-85B/Ad-85B vaccine group compared to the DNA-c/Ad-85B vaccine group for all polyfunctional CD4+ and CD8+ T cell subsets, except for the IFN-γ+ IL-2−TNF+ CD4+ subset.
3.4. Intranasal Ad-85B boosting did not generate heterogeneic polyfunctional CD4+ and CD8+ T cell responses in the spleen following neonatal priming
Analyses of splenic T cell responses by ICS indicated that the frequencies of IFN-γ, TNF and IL-2-secreting CD4+ T cells (Fig. 4A and B) and CD8+ T cells (Fig. 4C and D) did not differ significantly whether mice had been primed with DNA-Ad-85B or control DNA vaccine prior to intranasal boosting with Ad-Ag85B. The polyfunctionality of the Ag85B-specific splenic T cell response was less marked than in the lungs, although neonatal priming with DNA-85B appeared to enhance the levels of responding IFN-γ+IL-2+TNF− CD4+ T cell (Fig. 5A) and IFN-γ+IL-2+TNF+ CD8+ T cell (Fig. 5B) subsets.
Fig. 4.
TB-specific splenic CD4+ and CD8+ T cell cytokine responses following neonatal prime /intranasal boost vaccination. Splenocytes were stimulated with peptide 262 or peptide 8 for 6 hours as described in Materials and Methods and were analyzed for cytokine secretion by ICS. Data are expressed as cell counts of IFN-γ-, IL-2-, or TNF-secreting CD4+ (A) or CD8+ T cells (C) per million CD4+ or CD8+ T cells in each group of five mice, and are representative of two independent experiments. Representative ICS data in the DNA85B/Ad-85B group are shown, where splenocytes were stimulated with peptide 262 (B) or peptide 8 (D).
Fig. 5.
Polyfunctional TB-specific splenic CD4+ and CD8+ T cell responses following neonatal prime /intranasal boost vaccination. Splenocytes from mice immunized as described in Fig. 1 were stimulated with peptide 262 or peptide 8 for 6 hours and analyzed for cytokine secretion by ICS, as described in Materials and Methods. Polyfunctional CD4+ T cell (A) and CD8+ T cell (B) profiles for triple- (3+), dual- (2+) and single-cytokine (1+) secretion and comparisons between vaccination groups (top), and percentages of triple-, dual- or single-cytokine secreting T cells in each group (bottom), are shown. Data were generated from five mice per group and are representative of two independent experiments. **Denotes statistical significance (P < 0.01) in the DNA-85B/Ad-85B vaccine group compared to the DNA-c/Ad-85B vaccine group for the polyfunctional IFN-γ+IL-2+TNF− CD4+ (Fig. 5A) and IFN-γ+IL-2+TNF+ CD8+ (Fig. 5B) T cell subsets.
3.5. Neonatal DNA-85B priming enhanced the protective efficacy of Ad-85B immunization against Mtb H37Rv aerosol challenge
Given the CD4+ and CD8+ T cell responses generated by the neonatal DNA prime/intranasal Ad boost approach, we next looked at the protective efficacy of this strategy in a murine model of pulmonary TB infection. Vaccinated mice were challenged with pathogenic Mtb H37Rv by aerosol at six weeks after Ad-85B boosting and bacillary loads were determined in lungs and spleens six weeks later (Fig.6). Neonatal priming with DNA-85B alone was not protective against Mtb challenge. However, bacillary loads in the lungs at six weeks after aerosolized TB challenge were 5-fold lower in mice that had been primed neonatally with DNA-85B and boosted intranasally with Ad-85B, than in naïve control mice (p<0.01). Less pronounced reductions in bacterial growth were found in the spleens of this vaccine group compared to naïve controls (p<0.05). No significant protection was found in lungs or spleens in the DNA-c/Ad-85B or DNA-85B/Ad-c vaccine groups. It should be noted that bacterial loads in DNA-85B/Ad-85B-vaccinated mice were greater than those seen in ‘positive control’ mice given BCG via footpad at 4 weeks prior to aerosolized Mtb challenge, which were generally 10-fold lower than in the naïve controls, as expected in the murine TB model.
Fig. 6.
Bacillary loads in prime-boost vaccinated mice following pulmonary challenge with Mtb. Neonates were immunized intradermally with DNA-85B or DNA-c at 4 days of age, boosted intranasally with Ad-85B or Ad-c as described in Fig. 1, and challenged with virulent Mtb H37Rv by aerosol 6 weeks later. Control mice were given either saline (naïve) or BCG via the intradermal route at 4 weeks prior to the Mtb challenge, as described in Materials and Methods. All groups of mice were sacrificed at 6 weeks after Mtb challenge and lungs and spleens were examined for bacillary loads. Data represent the mean colony-forming units ± standard deviation for five mice per group and are representative of several experiments. Statistical significance of differences across comparisons between each group are denoted as *** (P < 0.001), ** (P < 0.01), or * (P < 0.05) on the figure.
In conclusion, our data show that neonatal priming with DNA-85B via the intradermal route potentiated both CD4+ and CD8+ T cell responses induced by subsequent intranasal immunization with Ad-85B vaccine, particularly in lung-associated lymph nodes, and that this correlated with enhanced clearance of Mtb from the lungs following aerosolized TB challenge.
4. Discussion
Control of TB infection requires potent T cell-mediated immunity. Th1 CD4+ T cells are pivotal, with activated Th1 CD4+ cells generating a variety of cytokines including IL-2, IFN-γ and TNF-α, which synergistically expand immune responses and activate local antimicrobial activity. CD8+ T cells can also mediate protection against TB infection in the absence of CD4 T cells (34). In this study, we have demonstrated that intradermal vaccination with DNA vaccines encoding Ag85B of Mtb (DNA-85B) as early as 4 days of age can generate antigen-specific CD4+ and CD8+ T cell responses in mice. Furthermore, the neonatal DNA-85B vaccine primed for protective immune responses in the lungs following a heterologous Ad booster vaccine given intranasally. This finding appears contradictory to earlier reports (11–13), but is actually in accordance with our current understanding of neonatal immunology. While neonates have significantly smaller populations of T cells and antigen-presenting cells displaying immature functionality (10, 35–37), and a tendency to mount Th2-biased responses (13), there is now clear evidence that Th1-type responses may be generated in neonates using genetic vaccines or live replicating vectors, such as BCG (18–20,38,39). Our current data serve to underline the capacity of the newborn immune system to prime for strong Th1 cell-mediated antimicrobial immunity despite its relative immaturity. The DNA vaccine delivery technique used in our study may also impact the outcome of immunization. Biolistic techniques propel DNA directly into the intracellular compartments of keratinocytes, Langerhans cells and dermal DC (40). Transfected keratinocytes may serve as a source of antigen (41), while Langerhans cells and dermal DCs can carry antigen to draining lymph nodes to initiate immune responses (42), potentially favoring induction of Th1 immunity and CTL activity (33, 43).
The prime-boost strategy has been widely used in experimental immunology and for vaccine development, including for TB (23,28,44). The booster vaccine, most commonly replication-defective recombinant virus vectors, preferentially expand both antigen-specific CD4+ and CD8+ T cells, typically generating high-frequency, high-avidity T cells with enhanced durability and polyfunctionality (22,45,46). In our model system, we used an adenovirus vector for boosting, and clearly demonstrated that neonatal DNA priming can potentiate the boosting efficacy of intranasally-administered Ad vaccine, particularly in lung-associated lymph nodes, despite the relatively low-level of Th1-type responses induced by the neonatal DNA-85B vaccine when given alone. Indeed, neonatal DNA-85B priming significantly increased numbers of antigen-specific CD4+ and CD8+ T cell responses in both lung-associated lymph nodes and spleens following Ad-85B boosting. This may be due, at least partly, to immune memory established following migration of antigen-loaded Langerhans cells and/or dermal DCs into the lymph nodes after intradermal DNA vaccination in neonates. Polyfunctional T cells simultaneously secrete multiple anti-microbial factors and have been correlated with enhanced protection in different models of infection, including TB (47). In our study, enhanced CD4+ and CD8+ T cell responses generated in neonatally DNA-primed/Ad-boosted mice were associated with reduced bacillary burdens in the lungs following pulmonary TB challenge with aerosolized Mtb. We are currently testing the impact of reducing or lengthening the interval between neonatal priming and vector-directed boosting on the immunogenicity and protective efficacy of this strategy.
Recombinant adenoviruses appear to be particularly suitable as mucosal boosting vaccines in this setting. The Th1-stimulating adjuvant properties of the adenovirus vector backbone may help to enhance low-level DNA-primed antigen-specific responses, while Ad-based vectors have tropism for mucosal surfaces (48). However, neutralizing antibody responses against Ad are present in a large proportion of the population (49), while the STEP and HVTN 505 trials raised concerns that the Ad5 serotype could potentially enhance the risk of HIV acquisition in Ad5-seropositive individuals (50,51). Alternative adenovirus serotypes may ultimately be more suitable as vector vaccine candidates, while other vaccine vectors, including modified vaccinia Ankara strain and fowlpoxvirus, can elicit pulmonary responses following intranasal delivery (21,24,25,52) and might also be used to boost circulating and/or mucosal immune responses following neonatal priming.
In summary, DNA-based neonatal vaccination via the intradermal route elicited only low-level Th1 CD4+ and CD8+ T cell responses, but clearly potentiated the boosting efficacy of Ad-85B vaccine. The combination of neonatal DNA-85B priming via the intradermal route with intranasal Ad-85B boosting generated strong polyfunctional T cell responses, particularly in lung lymphoid tissues, that correlated with reduced bacillary loads in the lungs following pulmonary TB challenge. Our study indicates that neonatal immunization can prime for enhanced local immunity upon subsequent boosting via the respiratory route, and suggests that gene-based prime-boost strategies, either alone or in combination with TB immunogens such as BCG, are worthy of further investigation for vaccination against Mtb and, potentially, other pulmonary infections, in neonatal life.
Supplementary Material
Highlights.
Gene-based vaccination of neonates primes for immune responses in lungs and spleen.
Respiratory boost of primed mice with Ad vector enhances pulmonary T cell responses.
Neonatal prime-boosting enhances protection against pulmonary TB challenge.
Acknowledgments
We thank Elizabeth Porretta for assistance in the BSL3 facility and Olga Nichols for help with flow cytometry. Mtb H37Rv genomic DNA was obtained from Colorado State University under NIH TBVTRM Contract NO-AI-40091. This study was supported by NIH grants R01 AI058810 (AJR) and 5PO1 HL076100 (JES, CM, AJR), and by the Louisiana Vaccine Center funded by the Louisiana Board of Regents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Conflicts of interest: none.
References
- 1.WHO. Geneva: World Health Organization; 2015. Global Tuberculosis Report 2015. [Google Scholar]
- 2.Moyo S, Verver S, Mahomed H, Hawkridge A, Kibel M, Hatherill M, Tameris M, Geldenhuys H, Hanekom W, Hussey G. Age-related tuberculosis incidence and severity in children under 5 years of age in Cape Town, South Africa. Int J Tuberc Lung Dis. 2010;14:149–154. [PubMed] [Google Scholar]
- 3.Mandalakas AM, Starke JR. Current concepts of childhood tuberculosis. Semin Pediatr Infect Dis. 2005;16:93–104. doi: 10.1053/j.spid.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, Snell L, Mangtani P, Adetifa I, Lalvani A, Abubakar I. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. doi: 10.1136/bmj.g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronson NE, Santosham M, Comstock GW, Howard RS, Moulton LH, Rhoades ER, Harrison LH. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: A 60-year follow-up study. JAMA. 2004;291:2086–2091. doi: 10.1001/jama.291.17.2086. [DOI] [PubMed] [Google Scholar]
- 6.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis: Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 7.Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, Sterne JA. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 8.Rezai MS, Khotaei G, Mamishi S, Kheirkhah M, Parvaneh N. Disseminated Bacillus Calmette-Guerin infection after BCG vaccination. J Trop Pediatr. 2008;54:413–416. doi: 10.1093/tropej/fmn053. [DOI] [PubMed] [Google Scholar]
- 9.Garcia AM, Fadel SA, Cao S, Sarzotti M. T cell immunity in neonates. Immunol Res. 2000;22:177–190. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- 10.Wu L, Dakic A. Development of dendritic cell system. Cell Mol Immunol. 2004;1:112–118. [PubMed] [Google Scholar]
- 11.Gammon G, Dunn K, Shastri N, Oki A, Wilbur S, Sercarz EE. Neonatal T-cell tolerance to minimal immunogenic peptides is caused by clonal inactivation. Nature. 1986;319:413–415. doi: 10.1038/319413a0. [DOI] [PubMed] [Google Scholar]
- 12.Clayton JP, Gammon GM, Ando DG, Kono DH, Hood L, Sercarz EE. Peptide-specific prevention of experimental allergic encephalomyelitis. Neonatal tolerance induced to the dominant T cell determinant of myelin basic protein. J Exp Med. 1989;169:1681–1691. doi: 10.1084/jem.169.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N, Field EH. Enhanced type 2 and diminished type 1 cytokines in neonatal tolerance. Transplantation. 1995;59:933–941. doi: 10.1097/00007890-199504150-00002. [DOI] [PubMed] [Google Scholar]
- 14.Adkins B, Du RQ. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J Immunol. 1998;160:4217–4224. [PubMed] [Google Scholar]
- 15.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189:1565–1572. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 17.Lee HH, Hoeman CM, Hardaway JC, Guloglu FB, Ellis JS, Jain R, Divekar R, Tartar DM, Haymaker CL, Zaghouani H. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med. 2008;205:2269–2280. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassett DE, Zhang J, Slifka M, Whitton JL. Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization. J Virol. 2000;74:2620–2627. doi: 10.1128/jvi.74.6.2620-2627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vekemans J, Amedei A, Ota MO, D'Elios MM, Goetghebuer T, Ismaili J, Newport MJ, Del Prete G, Goldman M, McAdam KP, Marchant A. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol. 2001;31:1531–1535. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Hussey GD, Watkins ML, Goddard EA, Gottschalk S, Hughes EJ, Iloni K, Kibel MA, Ress SR. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology. 2002;105:314–324. doi: 10.1046/j.1365-2567.2002.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsay AJ, Kent SJ, Strugnell RA, Suhrbier A, Thomson SA, Ramshaw IA. Genetic vaccination strategies for enhanced cellular, humoral and mucosal immunity. Immunol Rev. 1999;171:27–44. doi: 10.1111/j.1600-065x.1999.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramshaw IA, Ramsay AJ. The prime-boost strategy: exciting prospects for improved vaccination. Immunol Today. 2000;21:163–165. doi: 10.1016/s0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- 23.Dalmia N, Ramsay AJ. Prime-boost approaches to tuberculosis vaccine development. Expert Rev Vaccines. 2012;11:1221–1233. doi: 10.1586/erv.12.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kent SJ, Dale CJ, Ranasinghe C, Stratov I, De Rose R, Chea S, Montefiori DC, Thomson S, Ramshaw IA, Coupar BE, Boyle DB, Law M, Wilson KM, Ramsay AJ. Mucosally-administered human-simian immunodeficiency virus DNA and fowlpoxvirus-based recombinant vaccines reduce acute phase viral replication in macaques following vaginal challenge with CCR5-tropic SHIVSF162P3. Vaccine. 2005;23:5009–5021. doi: 10.1016/j.vaccine.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Ranasinghe C, Eyers F, Stambas J, Boyle DB, Ramshaw IA, Ramsay AJ. A comparative analysis of HIV-specific mucosal/systemic T cell immunity and avidity following rDNA/rFPV and poxvirus-poxvirus prime boost immunisations. Vaccine. 2011;29(16):3008–3020. doi: 10.1016/j.vaccine.2011.01.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auten MW, Huang W, Dai G, Ramsay AJ. CD40 ligand enhances immunogenicity of vector-based vaccines in immunocompetent and CD4+ T cell deficient individuals. Vaccine. 2012;30:2768–2777. doi: 10.1016/j.vaccine.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rady HF, Dai G, Huang W, Shellito JE, Ramsay AJ. Flagellin encoded in gene-based vector vaccines is a route-dependent immune adjuvant. PLoS One. 2016;11(2):e0148701. doi: 10.1371/journal.pone.0148701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines. 2011;10:645–658. doi: 10.1586/erv.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Souza S, Rosseels V, Romano M, Tanghe A, Denis O, Jurion F, Castiglione N, Vanonckelen A, Palfliet K, Huygen K. Mapping of murine Th1 helper T-Cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect Immun. 2003;71:483–493. doi: 10.1128/IAI.71.1.483-493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang-hong S, Xiao-wu W, Hai Z, Ting-fen Z, Li-Mei W, Zhi-kai X. Immune responses and protective efficacy of the gene vaccine expressing Ag85B and ESAT6 fusion protein from Mycobacterium tuberculosis. DNA Cell Biol. 2008;27:199–207. doi: 10.1089/dna.2007.0648. [DOI] [PubMed] [Google Scholar]
- 33.Sudowe S, Ludwig-Portugall I, Montermann E, Ross R, Reske-Kunz AB. Transcriptional targeting of dendritic cells in gene gun-mediated DNA immunization favors the induction of type 1 immune responses. Mol Ther. 2003;8:567–575. doi: 10.1016/s1525-0016(03)00242-9. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Santosuosso M, Ngai P, Zganiacz A, Xing Z. Activation of CD8 T cells by mycobacterial vaccination protects against pulmonary tuberculosis in the absence of CD4 T cells. J Immunol. 2004;173:4590–4597. doi: 10.4049/jimmunol.173.7.4590. [DOI] [PubMed] [Google Scholar]
- 35.Dadaglio G, Sun CM, Lo-Man R, Siegrist CA, Leclerc C. Efficient in vivo priming of specific cytotoxic T cell responses by neonatal dendritic cells. J Immunol. 2002;168:2219–2224. doi: 10.4049/jimmunol.168.5.2219. [DOI] [PubMed] [Google Scholar]
- 36.Dakic A, Shao QX, D'Amico A, O'Keeffe M, Chen WF, Shortman K, Wu L. Development of the dendritic cell system during mouse ontogeny. J Immunol. 2004;172:1018–1027. doi: 10.4049/jimmunol.172.2.1018. [DOI] [PubMed] [Google Scholar]
- 37.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 38.Sarzotti M, Dean TA, Remington MP, Ly CD, Furth PA, Robbins DS. Induction of cytotoxic T cell responses in newborn mice by DNA immunization. Vaccine. 1997;15:795–797. doi: 10.1016/s0264-410x(96)00250-2. [DOI] [PubMed] [Google Scholar]
- 39.Martinez X, Brandt C, Saddallah F, Tougne C, Barrios C, Wild F, Dougan G, Lambert PH, Siegrist CA. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc Natl Acad Sci U S A. 1997;94:8726–8731. doi: 10.1073/pnas.94.16.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raz E, Carson DA, Parker SE, Parr TB, Abai AM, Aichinger G, Gromkowski SH, Singh M, Lew D, Yankauckas MA, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci U S A. 1994;91:9519–9523. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klinman DM, Sechler JM, Conover J, Gu M, Rosenberg AS. Contribution of cells at the site of DNA vaccination to the generation of antigen-specific immunity and memory. J Immunol. 1998;160:2388–2392. [PubMed] [Google Scholar]
- 42.Garg S, Oran A, Wajchman J, Sasaki S, Maris CH, Kapp JA, Jacob J. Genetic tagging shows increased frequency and longevity of antigen-presenting, skin-derived dendritic cells in vivo. Nat Immunol. 2003;4:907–912. doi: 10.1038/ni962. [DOI] [PubMed] [Google Scholar]
- 43.Lauterbach H, Gruber A, Ried C, Cheminay C, Brocker T. Insufficient APC capacities of dendritic cells in gene gun-mediated DNA vaccination. J Immunol. 2006;176:4600–4607. doi: 10.4049/jimmunol.176.8.4600. [DOI] [PubMed] [Google Scholar]
- 44.McShane H, Hill A. Prime-boost immunisation strategies for tuberculosis. Microbes Infect. 2005;7:962–967. doi: 10.1016/j.micinf.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Estcourt MJ, Ramsay AJ, Brooks A, Thomson SA, Medveckzy CJ, Ramshaw IA. Prime-boost immunization generates a high frequency, high-avidity CD8(+) cytotoxic T lymphocyte population. Int Immunol. 2002;14:31–37. doi: 10.1093/intimm/14.1.31. [DOI] [PubMed] [Google Scholar]
- 46.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, Colloca S, Cortese R, Hill AV. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect Immun. 2010;78:145–153. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Derrick SC, Yabe IM, Yang A, Morris SL. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine. 2011;29:2902–2909. doi: 10.1016/j.vaccine.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Santosuosso M, McCormick S, Xing Z. Adenoviral vectors for mucosal vaccination against infectious diseases. Viral Immunol. 2005;18:283–291. doi: 10.1089/vim.2005.18.283. [DOI] [PubMed] [Google Scholar]
- 49.Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen J. More woes for struggling HIV vaccine field. Science. 2013;340:667. doi: 10.1126/science.340.6133.667. [DOI] [PubMed] [Google Scholar]
- 52.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guérin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171(3):1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.