Abstract
Frailty is a critical aging-related syndrome marked by diminished physiologic reserve and heightened vulnerability to stressors, predisposing to major adverse clinical outcomes, including hospitalization, institutionalization, disability, and death in the general population of older adults. As the proportion of older adults living with HIV increases in the era of antiretroviral therapy, frailty is increasingly recognized to be of significant clinical and public health relevance to the HIV-infected population. This article reviews current knowledge on the epidemiology and biology of frailty and its potential role as a target for reducing disparities in outcomes in HIV; conceptual frameworks and current approaches to frailty measurement; existing data on frailty interventions; and important areas for future research focus necessary to develop and advance effective strategies to prevent or ameliorate frailty and its marked adverse consequences among people living with HIV.
Keywords: Physical frailty phenotype, Deficit accumulation index, Immunosenescence, Physical activity, Clinical outcomes, Disparities
Introduction
Aging and HIV
With the advent of effective combination antiretroviral therapy (cART), HIV-infected persons are living longer and the proportion of older adults living with HIV is increasing. Approximately 10–30 % of the over 36 million people living with HIV worldwide are now over 50 years of age, with this number expected to triple over the next three decades [1, 2]. With increasing age has come an increasing burden of aging-associated conditions and complications, and significant adverse aging-associated syndromes [1–15]. This burden is heightened in the HIV-infected population relative to their HIV-uninfected counterparts. These shifts have necessitated an increased focus on reducing vulnerability to adverse aging-related outcomes among HIV-infected persons.
Frailty Definition
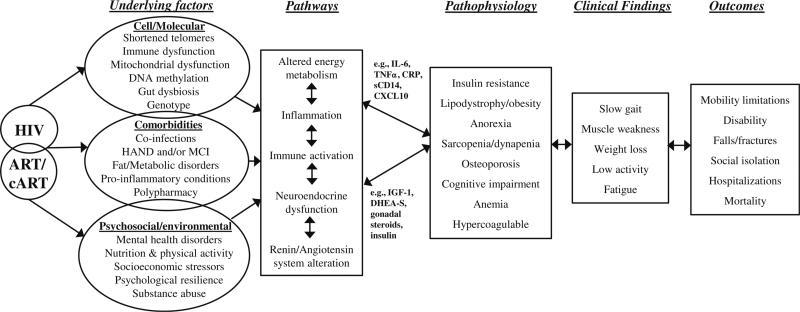
Initially recognized as a clinical entity over four decades ago, frailty has been defined as a key adverse aging-related syndrome of vulnerability, predisposing to adverse clinical outcomes of major public health importance. Initially conceived as a syndrome of older adults (65 years and older), the frailty construct has been conceptualized as a cumulative loss of physiologic reserve with diminished homeostasis, resulting in increased susceptibility and decreased resilience to stress, ultimately precipitating marked adverse outcomes including increased hospitalization, institutionalization, and death (Fig. 1) [16–23]. In early theoretical frameworks and in recent expert consensus, frailty has been considered related but distinct from disability, and in several constructs considered distinct from comorbidity [17–20, 24]. Further, while aging-related, frailty has been characterized as distinct from chronologic age [17, 18, 25].
Fig. 1.
Potential integrative context around which to consider frailty in HIV-infected adults, adapted from Walston et al. [79]. The proposed pathways are based on existing evidence derived from the general population, HIV-specific frailty literature, and emerging evidence and multidisciplinary ideas about psychosocial and physiological inter-relationships among contributors, confounders, pathogenesis, phenotypes, and recognized outcomes or behaviors in frailty. HAND, HIV-associated neurocognitive disorder. MCI, mild cognitive impairment. IL-6, interleukin-6. TNFα, tumor necrosis factor alpha. CRP, C-reactive protein. CXCL10, C-X-C motif chemokine 10 or interferon gamma-induced protein. IGF-1, insulin-like growth factor-1, DHEA-S, dehydroepiandrosterone sulfate
Frailty Measurement and Burden
Frailty Measurement in Older Adults
Multiple instruments have been employed for frailty assessment among older adults. Such instruments have been applied to the study of clinical risk, methodologic assessments, frailty burden, frailty etiology, biomarker evaluation, and to delineate frail individuals for inclusion or exclusion in clinical studies [26]. While no gold standard measure exists, a recent systematic review identified nine highly cited frailty instruments of which two—the physical frailty phenotype and the deficit accumulation index—have been most predominantly applied.
Derived from a theoretically based evolution of frailty conceptual frameworks, the physical frailty phenotype (PFP) has been the most commonly used frailty construct in the research literature to date [26, 27]. First operationalized in the Cardiovascular Health Study of older US adults, the PFP is defined by the presence of three or more of five phenotypic domains: weight loss, weakness, low physical activity, poor endurance, and slow gait. In initial validation, the PFP was found to be related but distinct from comorbidity and disability. The PFP was subsequently externally validated as a syndrome in the Women's Health and Aging Study [28].
The Deficit Accumulation Index (DAI) conceptualizes frailty as vulnerability engendered by the accumulation of nonspecific age-related health deficits without a defined underlying pathophysiology. This index includes measures of physical function, cognition, comorbidity, social, sensory, and demographic factors [29–34]. Though nonspecific, assessment of a minimum number of deficits has been proposed necessary for this construct. Both the PFP and DAI have been associated with significantly increased risk for key adverse aging-related outcomes [27–31, 33], though reflect different conceptual frameworks for the frailty construct and its putative underlying pathophysiology [23, 35, 36].
Frailty Burden in Older Adults
Regardless of instrument, frailty burden in older adults has been found to be substantial. Recent nationally representative estimates based on PFP assessment among US adults 65 years and older demonstrate an overall frailty prevalence of 15 % [37]. An even higher frailty burden (30–40 %) has been observed in low and middle income countries [38]. Among older adults, multiple studies demonstrate increased frailty burden with age, particularly among women and persons with increased chronic comorbid disease [27, 28].
Frailty Measurement and Burden in HIV
Recognition of similarities in the biology and clinical phenotype between older adults and HIV-infected adults led to the study of frailty in HIV. The first evaluation of frailty in HIV was a retrospective analysis in the Multicenter AIDS Cohort Study (MACS) of HIV-infected men who have sex with men (MSM), using self-reported measures to approximate four out of five PFP domain criteria [39]. In this study, HIV infection was strongly associated with a heightened burden of this frailty-related phenotype (FRP). Subsequent studies demonstrated the significant association of the FRP with advanced HIV disease, specifically low CD4 counts [40]. Additional prospective studies incorporating objective measures of gait speed and grip strength as in the original PFP measure and using all five PFP domains have demonstrated a heightened burden of frailty in HIV, particularly with poorly controlled HIV infection or advanced HIV disease. The frailty burden reported in these studies ranged from 5 to 19 % though direct comparative assessment of these estimates is limited by different internal cutoffs for component measures and differences in populations between studies [41–49]. Congruent with findings in older adults, these HIV cohort studies have demonstrated increased frailty burden with age, among women, and with increased chronic comorbid disease [19, 41–44, 46, 47, 49, 50].
A few studies have adopted the DAI approach to the study of frailty in HIV. Guaraldi and colleagues recently constructed a 37-item index that consisted of metabolic, hematologic, and coagulation parameters, hepatitis B and C status, polypharmacy, low physical activity, and unemployment; a 45-item index included comorbidity variables; and a 53-item index added eight HIV-related variables. Frailty index scores in this study were all strongly associated with increased age [51]. A continuous index was also derived in the Veterans Aging Cohort Study (VACS index) [52]. This index was initially developed as a prognostic tool for survival in HIV-infected Veterans. It includes HIV parameters (CD4 count and HIV-1 RNA) and several markers of chronic comorbid disease of greater prevalence in HIV, including renal dysfunction, markers of liver fibrosis, anemia, and hepatitis C. Distinct from other measures, the VACS index incorporates age in its calculation for risk assessment.
Frailty Outcomes in HIV
Multiple studies have now demonstrated a heightened burden of frailty in the HIV-infected population as described above. However, the median ages in cohorts of HIV-infected participants in which frailty has been studied are notably younger than the 65 years and older populations in which the frailty construct was initially conceived, operationalized, and applied. Thus, a critical question has been whether the frailty construct is relevant to aging, but younger HIV-infected populations, particularly in relation to clinical outcomes.
While studies remain limited, there is a growing evidence base that the frailty construct finds applicability to younger HIV-infected persons and their high risk uninfected counterparts. In initial studies in the MACS, FRP status prior to cART initiation was significantly associated with an increased risk of a composite outcome of AIDS and death after cART [53]. In more recent studies by Piggott and colleagues applying the PFP in the AIDS Linked to the IntraVenous Experience (ALIVE) cohort of HIV-infected and uninfected persons with a history of injection drug use, being HIV infected or frail was associated with an approximately 3-fold increased risk of death, while being both HIV infected and frail was associated with an over 7-fold increased risk of death, independent of comorbidity and HIV disease stage [47]. Subsequent studies have demonstrated significant associations of both an adapted self-reported FRP and frailty DAI indices with mortality among people living with HIV [51, 54].
Frailty has also been associated with an increased risk of falls and hospitalizations in HIV (Fig. 1). In a cross-sectional study, Erlandson and colleagues demonstrated that each one point worsening of the PFP increased the odds of falls by 3.1 (CI, 2.3 to 4.2; P < 0.001) [55], and that frailty by the PFP was strongly associated with an increased risk of hospitalizations (unpublished data), polypharmacy, and multimorbidity [50]. Onen and colleagues similarly demonstrated an increased odds of hospitalization with the PFP in the Washington University HIV clinic [44]. Both the adapted FRP and the VACS index have been associated with increased hospitalization risk in the Veterans population [54]. Further, in prospective analysis in ALIVE, the PFP has been significantly associated with increased hospitalization risk (manuscript submitted).
Does Increased Frailty Burden in HIV Represent Accelerated Aging?
Debate continues on whether untreated or cART-treated HIV prematurely accelerates aging-related pathophysiology [56]. The relationship of HIV infection to several biological aging-related pathophysiologic pathways has provided some support for this hypothesis [57–60]. Conversely, several recent epidemiologic studies provide support for an accentuated rather than accelerated aging-related process in the HIV-infected population, with no difference in the mean age at onset of several aging-related disease conditions between HIV-infected and uninfected persons [61, 62]. In studies in the MACS, the increased frailty burden with HIV was most notable over 50 years of age [41]. However, these studies reflect observations of heightened frailty prevalence rather than incidence and thus do not yet provide evidence for “accelerated” frailty in the HIV-infected population. Further, unlike multiple non-AIDS chronic disease conditions, frailty has been considered dynamic in HIV-infected and uninfected populations alike [41, 63]. With this putative reversibility, without a better understanding of underlying frailty biology (Fig. 1), it will be difficult to assess the role of HIV infection or prior and current cART exposure as mediators of an accelerated frailty pathway.
Frailty and HIV: Shared Disparities
Marked disparities in frailty burden exist within the general population of older adults [64–67]. At the individual level, several studies report an increased frailty burden among older adults facing heightened socioeconomic challenges. In recent data, frailty burden was notably greater among minority and low income populations, across US geographic regions [37]. Frailty amelioration may thus be critical to reducing vulnerability and disparity and promoting equitable health outcomes among older adults.
Significant geographic overlap exists in the regions with the most severe frailty and HIV burden [37, 68]. As observed for frailty in older adults, HIV also disproportionately impacts minority and socioeconomically challenged populations [69–76]. These populations not only bear the greatest burden of HIV infection but also suffer the most severe morbidity and mortality with HIV disease. Frailty also has been significantly associated with heightened socioeconomic challenge including low educational attainment, low income, and individual unemployment in HIV-infected cohorts [41, 42, 44, 46, 47, 49, 50]. Frailty may thus also be a critical target to reduce disparities in health outcomes within the HIV-infected population and among their high risk counterparts.
Frailty Biology
Frailty Biology in Older Adults
Developing interventions to prevent or ameliorate frailty and its major adverse clinical consequences requires an understanding of the pathophysiologic pathways that precipitate its onset and progression (Fig. 1). Frailty has been associated with a range of aberrant, dynamic, multisystem physiologic stress responses among older HIV-uninfected adults affecting neuroendocrine, metabolic, musculoskeletal, cognitive, and immune systems [17, 35, 77–79]. Dysregulated pathways proposed to contribute to frailty pathophysiology include disruption of key stress response systems including the sympathetic nervous system; hypothalamic-pituitary axis; hormonal dysregulation with disruption of glucose, amino acid, and lipid metabolism; brain blood flow and anatomy; and innate immune dysregulation—driven by underlying aging-related cellular and molecular pathogeneses (Fig. 1) [79–81]. These aberrant pathways have been proposed to adversely impact muscle mass and quality, strength, endurance, and efficiency of energy expenditure.
Frailty Biology in HIV
Increasing evidence exists for a role of HIV infection in promoting aberrant aging-related biology (Fig. 1) [7]. HIV infection has been associated with telomere attrition, aging-related epigenetic modifications (e.g., methylome), endocrine dysregulation, cardiometabolic complications, and mitochondrial dysfunction [58, 59, 82–90]. The strongest biological evidence for the role of HIV pathophysiology in promoting aging-associated conditions lies in the realm of dysregulated inflammation and immunity. Dysregulated inflammation has been strongly associated with both specific aging-associated diseases (e.g., diabetes, cardiovascular disease, cancer) and adverse aging-associated syndromes, such as frailty in the general population of older adults [91–94].
A chronic, persistent pro-inflammatory state, despite effective cART, also appears central to HIV-related pathogenesis (Fig. 1). HIV infection leads to potent dysregulation and activation of the innate immune system through toll-like receptor activation, microbial translocation through gut CD4+ T cell and lymphoid tissue depletion and alteration in the gut microbiome, facilitation of co-infections, and promotion of cell death [95–97]. Even with cART, residual inflammation and immune cell activation persist and have been associated with increased morbidity and mortality among HIV-infected persons [95, 98, 99]. In the University of Colorado HIV cohort study, heightened inflammation (serum IL-6) and immune activation (CD8+ T cell activation) were significantly associated with low physical function as assessed by a composite measure of the PFP and a Short Physical Performance Battery [84]. In recent reports from ALIVE, the PFP was found to be significantly associated with inflammation as measured by an NFkB related, biologically informed inflammatory index (combined measure of IL-6 and soluble TNF receptor 1), itself a significant independent predictor of mortality [100].
Frailty Interventions
Consensus exists for the frailty construct as amenable to and a key target for intervention [20, 21]. Although many studies have tested clinical interventions on components of the frailty phenotype (i.e., strength, activity), few studies have tested clinical interventions to ameliorate frailty as a syndrome in any population or group. Existing studies in physical frailty have been primarily focused on exercise training or increased physical activity as primary interventions [101–104]. Randomized, controlled trials of progressive exercise training interventions have targeted aerobic, resistance, balance, and flexibility domains in community-dwelling frail older adults and have reported some protection against loss of muscle mass (sarcopenia) and strength (dynapenia) with functional benefits [105–107]. However, sarcopenia and dynapenia primarily adversely affect physical function dimensions of frailty. The potential beneficial effects of increased physical activity, muscle mass and strength, and cardiovascular endurance on cognitive and immune function remain to be determined, especially in people living with HIV. Further, in people living with HIV and taking effective cART, the magnitude and incidence of muscle wasting over time is similar to age-matched seronegative adults [108]. So, among frail, HIV-infected adults, muscle-building interventions alone may not be entirely effective against physical frailty.
One important recent trial (LIFE-P study) reported efficacy of increased physical activity as an intervention for frailty, defined by the PFP [109]. This study included 424 community-dwelling older adults with a mean age of 76.8 years. Participants were randomized to a 12-month physical activity intervention or to a successful aging education group. There was an equivalent frailty prevalence at baseline between the two groups of approximately 23 %. A significant difference in frailty prevalence was observed after 12 months: 10 % in the physical activity intervention group and 19.1 % in the group receiving educational counseling.
Several trials have suggested a potential role for multimodal interventions in frailty. The Australian FIT trial randomized 216 participants to receive an intervention targeting individual components of the PFP as compared to usual health care and support services [110]. Participants were all frail by PFP criteria at study initiation. There was a significant 14.7 % lower prevalence of frailty observed at 12 months in the intervention group compared to usual care. In a trial of 117 older Taiwanese adults, participants were randomized to 3 months of combined exercise and nutrition intervention or to an educational program [111]. The primary outcome was improvement in the PFP by at least one category: a significant improvement was noted in the intervention group (45 %) compared to the control (27 %). Little data exists on the role of solitary nutritional interventions for frailty, with several multimodal interventions for frailty ongoing among the general population of older adults, several of which have incorporated nutrition [21, 112–114]. Few data exist on such interventions for frailty in HIV-infected adults.
Advances in understanding frailty biology will be key to the development of effective pathogenesis-based therapeutics for frailty. Pharmacologic interventions proposed to target frailty or its components have been studied in the general population and include endocrine agents (androgenic steroids, vitamin D, growth hormone, and insulin-like growth factor-1 or their secretagogues), ACE inhibitors, cytokine targeting agents, anti-inflammatory medications, and other immunomodulators [115]. Significant research remains to be done in this sphere, among HIV-infected adults and in the general population.
Conclusion
Future Directions
Much success has been achieved in reducing AIDS-related morbidity and overall mortality in the HIV-infected population. Yet, notable disparities remain in clinical outcomes between HIV-infected and uninfected persons and between subgroups within the aging HIV-infected population. There is now increasing evidence for the relevance of frailty as a key construct in identifying vulnerability among HIV-infected persons, and frailty may be a key target to reduce disparities in HIV. The relatively younger ages at which frailty has been found pertinent in HIV cohorts also suggests the importance of early life course frailty intervention in both HIV-infected and uninfected persons. However, several key challenges and critical research needs remain for the study of frailty in HIV, particularly relative to frailty measurement, clinical application, frailty biology, and the need for effective frailty interventions.
Increasing epidemiologic and biological evidence exists for the similarity of the frailty construct in both older adults and among younger but aging HIV-infected individuals. However, further investigation into the underpinnings of frailty will require careful attention to the instruments used for frailty measurement. While no gold standard exists, agreement exists that any instrument should be multi-faceted, clinically feasible, and subject to change [26]. Some suggest that frailty instrument selection may vary by the setting and need. However, a significant challenge raised in the application of different instruments to frailty assessment is the potential discordance in the underlying theoretical construct being captured across instruments. In this regard, application of instruments to frailty measurement should be attentive to established principles of validation. Validation should extend beyond solely predictive validity or an instrument's association with increased risk for clinical events. For example, many disease conditions and clinical syndromes may predict or be strongly associated with increased risk for death, while qualitatively distinct in their underlying pathophysiology and manifestations. Thus, careful attention should be paid to the underlying theoretical framework of the frailty construct in the application of any instrument to frailty measurement (Fig. 1). Ultimately, achieving consensus on frailty measurement, attentive to the aforementioned principles, will remain an important goal for advancing the frailty research agenda across HIV-infected and general populations alike.
One particular recent theoretical challenge for the field of frailty has been the relationship of physical frailty to cognitive impairment. Cognitive impairment has been associated with physical frailty in some studies and incorporated into frailty instruments in others [116–120]. Whether cognitive impairment belongs to the same latent construct as frailty remains an active area of research inquiry among older adults in general, and similar studies are needed on the relationship of cognition to frailty in HIV.
Despite the significant public health importance of the frailty construct, few studies in older adults have applied frailty instruments to clinical decision-making. The frailty construct has been used for risk stratification and to guide treatment decisions in surgical and oncology populations [121, 122]. However, little data exist on such use in the HIV-infected population. A recent study in an Arizona clinic did report the potential feasibility of incorporating the PFP into HIV clinical practice [48]. Further study on implementation and utility in HIV clinical practice is needed.
One particular challenge to the clinical application of frailty in HIV is the paucity of proven frailty targeted interventions for use once frailty has been identified. Development of frailty interventions thus remains an active area of research need. The efficacy of targeting frailty as a complex, aggregate syndrome or targeting its putative components tailoring interventions to individual vulnerabilities requires future study. Ultimately, elucidation of frailty biology will be key to the development of such interventions. In order to better understand the biology of frailty in HIV, it will be necessary to further untangle the relationships of frailty, comorbidity, and disability and better understand whether frailty has its own underlying biology distinct from the latter two syndromes. Finally, mechanisms by which HIV biology may converge upon and precipitate frailty biology requires further investigation and could significantly inform frailty interventions to reduce vulnerability and disparity in the HIV-infected population and their high risk HIV-uninfected counterparts.
Ultimately, research advances in frailty measurement, biology, and interventions in HIV and among older adults are likely to continue to evolve in tandem.
Acknowledgments
The authors were supported by National Institutes of Health grants K23-AI-108357 (DAP), K23 AG050260 (KME), P41-GM103422, P30-DK056341, and P30-DK020579 (KEY).
Conflict of Interest Damani A. Piggott reports grants from NIH.
Kristine M. Erlandson reports grants from NIH, Gilead, and personal fees from Theratechnologies.
Kevin E. Yarasheski reports grants from NIH, grants and non-financial support from Merck, and consultant fees from EMD Serono.
Footnotes
This article is part of the Topical Collection on Complications of Antiretroviral Therapy
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Mills EJ, Barnighausen T, Negin J. HIV and aging—preparing for the challenges ahead. N Engl J Med. 2012;366(14):1270–3. doi: 10.1056/NEJMp1113643. [DOI] [PubMed] [Google Scholar]
- 2.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15(7):810–8. doi: 10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60(4):351–8. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond MB, Kirk GD. HIV-associated obstructive lung diseases: insights and implications for the clinician. Lancet Respir Med. 2014;2(7):583–92. doi: 10.1016/S2213-2600(14)70017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 6.Guaraldi G, Silva AR, Stentarelli C. Multimorbidity and functional status assessment. Curr Opin HIVAIDS. 2014;9(4):386–97. doi: 10.1097/COH.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 7.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(Suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirk GD, Mehta SH, Astemborski J, Galai N, Washington J, Higgins Y, et al. HIV, age, and the severity of hepatitis C virus-related liver disease: a cohort study. Ann Intern Med. 2013;158(9):658–66. doi: 10.7326/0003-4819-158-9-201305070-00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2014;59(9):e96–138. doi: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monroe AK, Glesby MJ, Brown TT. Diagnosing and managing diabetes in HIV-infected patients: current concepts. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2015;60(3):453–62. doi: 10.1093/cid/ciu779. [DOI] [PubMed] [Google Scholar]
- 11.Nightingale S, Winston A, Letendre S, Michael BD, McArthur JC, Khoo S, et al. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 2014;13(11):1139–51. doi: 10.1016/S1474-4422(14)70137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nou E, Lo J, Hadigan C, Grinspoon SK. Pathophysiology and management of cardiovascular disease in patients with HIV. Lancet Diabetes Endocrinol. 2016 doi: 10.1016/S2213-8587(15)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52(5):611–22. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sico JJ, Chang CC, So-Armah K, Justice AC, Hylek E, Skanderson M, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84(19):1933–40. doi: 10.1212/WNL.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverberg MJ, Lau B, Achenbach CJ, Jing Y, Althoff KN, D'Souza G, et al. Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med. 2015;163(7):507–18. doi: 10.7326/M14-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bortz WM., 2nd A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57(5):M283–8. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 17.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8(1):1–17. [PubMed] [Google Scholar]
- 18.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 19.Hogan DB, MacKnight C, Bergman H. Steering Committee CIoF, aging. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15(3 Suppl):1–29. [PubMed] [Google Scholar]
- 20.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–7. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Manas L, Feart C, Mann G, Vina J, Chatterji S, Chodzko-Zajko W, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62–7. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varadhan R, Seplaki CL, Xue QL, Bandeen-Roche K, Fried LP. Stimulus–response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech Ageing Dev. 2008;129(11):666–70. doi: 10.1016/j.mad.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walston JD, Bandeen-Roche K. Frailty: a tale of two concepts. BMC Med. 2015;13:185. doi: 10.1186/s12916-015-0420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB. Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr HIV/AIDS Rep. 2014;11(3):279–90. doi: 10.1007/s11904-014-0215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bortz W. Understanding frailty. J Gerontol A Biol Sci Med Sci. 2010;65(3):255–6. doi: 10.1093/gerona/glp162. discussion 7. [DOI] [PubMed] [Google Scholar]
- 26.Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2015;26:53–61. doi: 10.1016/j.arr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a pheno-type. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 28.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, et al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 29.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. TheScientificWorldJOURNAL. 2001;1:323–36. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59(6):M627–32. doi: 10.1093/gerona/59.6.m627. [DOI] [PubMed] [Google Scholar]
- 31.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–43. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 32.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–7. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 33.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975–9. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 34.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ: Can Med Assoc J = J Assoc Med Can. 2011;183(8):E487–94. doi: 10.1503/cmaj.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427–34. doi: 10.1093/gerona/glv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarado BE, Zunzunegui MV, Beland F, Bamvita JM. Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci. 2008;63(12):1399–406. doi: 10.1093/gerona/63.12.1399. [DOI] [PubMed] [Google Scholar]
- 39.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62(11):1279–86. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 40.Desquilbet L, Margolick JB, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr. 2009;50(3):299–306. doi: 10.1097/QAI.0b013e3181945eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Althoff KN, Jacobson LP, Cranston RD, Detels R, Phair JP, Li X, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci. 2014;69(2):189–98. doi: 10.1093/gerona/glt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gustafson DR, Shi Q, Thurn M, Holman S, Minkoff H, Cohen M, et al. Frailty and constellations of factors in aging HIV-infected and uninfected women—the Women's Interagency HIV Study. J Frailty Aging. 2016;5(1):43–8. doi: 10.14283/jfa.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kooij KW, Wit FW, Schouten J, van der Valk M, Godfried MH, Stolte IG, et al. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. Aids. 2016;30(2):241–50. doi: 10.1097/QAD.0000000000000910. [DOI] [PubMed] [Google Scholar]
- 44.Onen NF, Agbebi A, Shacham E, Stamm KE, Onen AR, Overton ET. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect. 2009;59(5):346–52. doi: 10.1016/j.jinf.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Onen NF, Patel P, Baker J, Conley L, Brooks JT, Bush T, et al. Frailty and pre-frailty in a contemporary cohort of HIV-infected adults. J Frailty Aging. 2014;3(3):158–65. doi: 10.14283/jfa.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pathai S, Gilbert C, Weiss HA, Cook C, Wood R, Bekker LG, et al. Frailty in HIV-infected adults in South Africa. J Acquir Immune Defic Syndr. 2013;62(1):43–51. doi: 10.1097/QAI.0b013e318273b631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piggott DA, Muzaale AD, Mehta SH, Brown TT, Patel KV, Leng SX, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One. 2013;8(1):e54910. doi: 10.1371/journal.pone.0054910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rees HC, Ianas V, McCracken P, Smith S, Georgescu A, Zangeneh T, et al. Measuring frailty in HIV-infected individuals. Identification of frail patients is the first step to amelioration and reversal of frailty. J Vis Exp: JoVE. 2013;(77) doi: 10.3791/50537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terzian AS, Holman S, Nathwani N, Robison E, Weber K, Young M, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health (Larchmt) 2009;18(12):1965–74. doi: 10.1089/jwh.2008.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erlandson KM, Allshouse AA, Jankowski CM, Duong S, Mawhinney S, Kohrt WM, et al. Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials. 2012;13(6):324–34. doi: 10.1310/hct1306-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guaraldi G, Brothers TD, Zona S, Stentarelli C, Carli F, Malagoli A, et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. Aids. 2015;29(13):1633–41. doi: 10.1097/QAD.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 52.Justice AC, McGinnis KA, Skanderson M, Chang CC, Gibert CL, Goetz MB, et al. Towards a combined prognostic index for survival in HIV infection: the role of ‘non-HIV’ biomarkers. HIV Med. 2010;11(2):143–51. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A, Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akgun KM, Tate JP, Crothers K, Crystal S, Leaf DA, Womack J, et al. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr. 2014;67(4):397–404. doi: 10.1097/QAI.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erlandson KM, Allshouse AA, Jankowski CM, Duong S, MaWhinney S, Kohrt WM, et al. Risk factors for falls in HIV-infected persons. J Acquir Immune Defic Syndr. 2012;61(4):484–9. doi: 10.1097/QAI.0b013e3182716e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69(7):833–42. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angelovich TA, Hearps AC, Maisa A, Martin GE, Lichtfuss GF, Cheng WJ, et al. Viremic and virologically suppressed HIV infection increases age-related changes to monocyte activation equivalent to 12 and 4 years of aging, respectively. J Acquir Immune Defic Syndr. 2015;69(1):11–7. doi: 10.1097/QAI.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 58.Horvath S, Levine AJ. HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis. 2015;212(10):1563–73. doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levine AJ, Quach A, Moore DJ, Achim CL, Soontornniyomkij V, Masliah E, et al. Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. J Neurovirol. 2015 doi: 10.1007/s13365-015-0406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu JC, Leung JM, Ngan DA, Nashta NF, Guillemi S, Harris M, et al. Absolute leukocyte telomere length in HIV-infected and uninfected individuals: evidence of accelerated cell senescence in HIV-associated chronic obstructive pulmonary disease. PLoS One. 2015;10(4):e0124426. doi: 10.1371/journal.pone.0124426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2015;60(4):627–38. doi: 10.1093/cid/ciu869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010;153(7):452–60. doi: 10.1059/0003-4819-153-7-201010050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166(4):418–23. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 64.Harttgen K, Kowal P, Strulik H, Chatterji S, Vollmer S. Patterns of frailty in older adults: comparing results from higher and lower income countries using the Survey of Health, Ageing and Retirement in Europe (SHARE) and the Study on Global AGEing and Adult Health (SAGE). PLoS One. 2013;8(10):e75847. doi: 10.1371/journal.pone.0075847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herr M, Robine JM, Aegerter P, Arvieu JJ, Ankri J. Contribution of socioeconomic position over life to frailty differences in old age: comparison of life-course models in a French sample of 2350 old people. Ann Epidemiol. 2015;25(9):674–80. e1. doi: 10.1016/j.annepidem.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Soler-Vila H, Garcia-Esquinas E, Leon-Munoz LM, Lopez-Garcia E, Banegas JR, Rodriguez-Artalejo F. Contribution of health behaviours and clinical factors to socioeconomic differences in frailty among older adults. J Epidemiol Community Health. 2016;70(4):354–60. doi: 10.1136/jech-2015-206406. [DOI] [PubMed] [Google Scholar]
- 67.Szanton SL, Seplaki CL, Thorpe RJ, Jr, Allen JK, Fried LP. Socioeconomic status is associated with frailty: the women's health and aging studies. J Epidemiol Community Health. 2010;64(1):63–7. doi: 10.1136/jech.2008.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hall HI, An Q, Tang T, Song R, Chen M, Green T, et al. Prevalence of diagnosed and undiagnosed HIV infection—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2015;64(24):657–62. [PMC free article] [PubMed] [Google Scholar]
- 69.Bachhuber MA, Southern WN. Hospitalization rates of people living with HIV in the United States, 2009. Public Health Rep. 2014;129(2):178–86. doi: 10.1177/003335491412900212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lesko CR, Cole SR, Miller WC, Westreich D, Eron JJ, Adimora AA, et al. Ten-year survival by race/ethnicity and sex among treated, HIV-infected adults in the United States. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2015;60(11):1700–7. doi: 10.1093/cid/civ183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linley L, Prejean J, An Q, Chen M, Hall HI. Racial/ethnic disparities in HIV diagnoses among persons aged 50 years and older in 37 US States, 2005–2008. Am J Public Health. 2012;102(8):1527–34. doi: 10.2105/AJPH.2011.300431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Losina E, Schackman BR, Sadownik SN, Gebo KA, Walensky RP, Chiosi JJ, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2009;49(10):1570–8. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Millett GA, Peterson JL, Flores SA, Hart TA, Jeffries WL, Wilson PA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet. 2012;380(9839):341–8. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 74.Rosenberg ES, Millett GA, Sullivan PS, Del Rio C, Curran JW. Understanding the HIV disparities between black and white men who have sex with men in the USA using the HIV care continuum: a modeling study. Lancet HIV. 2014;1(3):e112–e8. doi: 10.1016/S2352-3018(14)00011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siddiqi AE, Hu X, Hall HI. Centers for disease C, prevention. Mortality among blacks or African Americans with HIV infection—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2015;64(4):81–6. [PMC free article] [PubMed] [Google Scholar]
- 76.Simard EP, Fransua M, Naishadham D, Jemal A. The influence of sex, race/ethnicity, and educational attainment on human immunodeficiency virus death rates among adults, 1993–2007. Arch Intern Med. 2012;172(20):1591–8. doi: 10.1001/archinternmed.2012.4508. [DOI] [PubMed] [Google Scholar]
- 77.Fedarko NS. The biology of aging and frailty. Clin Geriatr Med. 2011;27(1):27–37. doi: 10.1016/j.cger.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, et al. From bedside to bench: research agenda for frailty. Sci Aging knowl Environ: SAGE KE. 2005;2005(31):e24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 79.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 80.Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, et al. HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis. 2010;201(3):336–40. doi: 10.1086/649899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walston J. Frailty—the search for underlying causes. Sci Aging Knowl Environ: SAGE KE. 2004;2004(4):e4. doi: 10.1126/sageke.2004.4.pe4. [DOI] [PubMed] [Google Scholar]
- 82.Beaupere C, Garcia M, Larghero J, Feve B, Capeau J, Lagathu C. The HIV proteins Tat and Nef promote human bone marrow mesenchymal stem cell senescence and alter osteoblastic differentiation. Aging Cell. 2015;14(4):534–46. doi: 10.1111/acel.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang LL, et al. Shortened telomeres in the expanded CD28–CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. Aids. 1996;10(8):F17–22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Erlandson KM, Allshouse AA, Jankowski CM, Lee EJ, Rufner KM, Palmer BE, et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Inf Dis. 2013;208(2):249–59. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jain S, Desai N, Bhangoo A. Pathophysiology of GHRH-growth hormone-IGF1 axis in HIV/AIDS. Rev Endocr Metab Disord. 2013;14(2):113–8. doi: 10.1007/s11154-013-9245-9. [DOI] [PubMed] [Google Scholar]
- 86.Maagaard A, Kvale D. Mitochondrial toxicity in HIV-infected patients both off and on antiretroviral treatment: a continuum or distinct underlying mechanisms? J Antimicrob Chemother. 2009;64(5):901–9. doi: 10.1093/jac/dkp316. [DOI] [PubMed] [Google Scholar]
- 87.Payne BA, Wilson IJ, Hateley CA, Horvath R, Santibanez-Koref M, Samuels DC, et al. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genet. 2011;43(8):806–10. doi: 10.1038/ng.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perrin S, Cremer J, Roll P, Faucher O, Menard A, Reynes J, et al. HIV-1 infection and first line ART induced differential responses in mitochondria from blood lymphocytes and monocytes: the ANRS EP45 “Aging” study. PLoS One. 2012;7(7):e41129. doi: 10.1371/journal.pone.0041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith RL, de Boer R, Brul S, Budovskaya Y, van Spek H. Premature and accelerated aging: HIV or HAART? Front Genet. 2012;3:328. doi: 10.3389/fgene.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolthers KC, Wisman GBA, Otto SA, Husman AMD, Schaft N, de Wolf F. T cell telomere length in HIV-1 infection: no evidence for increased CD4(+) T cell turnover. Science. 1996;274(5292):1543–7. doi: 10.1126/science.274.5292.1543. [DOI] [PubMed] [Google Scholar]
- 91.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–71. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 92.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–29. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the cardiovascular health study. Arch Intern Med. 2002;162(20):2333–41. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 94.Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. 2011;27(1):79–87. doi: 10.1016/j.cger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Erlandson KM, Campbell TB. Inflammation in chronic HIV infection: what can we do? J Infect Dis. 2015;212(3):339–42. doi: 10.1093/infdis/jiv007. [DOI] [PubMed] [Google Scholar]
- 97.Zapata HJ, Shaw AC. Aging of the human innate immune system in HIV infection. Curr Opin Immunol. 2014;29C:127–36. doi: 10.1016/j.coi.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210(8):1228–38. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–59. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piggott DA, Varadhan R, Mehta SH, Brown TT, Li H, Walston JD, et al. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci. 2015;70(12):1542–7. doi: 10.1093/gerona/glv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men ≥76 yr old. Am J Phys. 1999;277(1 Pt 1):E118–25. doi: 10.1152/ajpendo.1999.277.1.E118. [DOI] [PubMed] [Google Scholar]
- 102.Liu CK, Fielding RA. Exercise as an intervention for frailty. Clin Geriatr Med. 2011;27(1):101–10. doi: 10.1016/j.cger.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Labra C, Guimaraes-Pinheiro C, Maseda A, Lorenzo T, Millan-Calenti JC. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr. 2015;15:154. doi: 10.1186/s12877-015-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fried LP. Interventions for human frailty: physical activity as a model. Cold Spring Harbor Perspect Med. 2016 doi: 10.1101/cshperspect.a025916. doi: 10.1101/cshperspect.a025916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Binder EF, Schechtman KB, Ehsani AA, Steger-May K, Brown M, Sinacore DR, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50(12):1921–8. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 106.Binder EF, Yarasheski KE, Steger-May K, Sinacore DR, Brown M, Schechtman KB, et al. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2005;60(11):1425–31. doi: 10.1093/gerona/60.11.1425. [DOI] [PubMed] [Google Scholar]
- 107.Tarazona-Santabalbina FJ, Gomez-Cabrera MC, Perez-Ros P, Martinez-Arnau FM, Cabo H, Tsaparas K, et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc. 2016;17(5):426–33. doi: 10.1016/j.jamda.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 108.Yarasheski KE, Scherzer R, Kotler DP, Dobs AS, Tien PC, Lewis CE, et al. Age-related skeletal muscle decline is similar in HIV-infected and uninfected individuals. J Gerontol A Biol Sci Med Sci. 2011;66(3):332–40. doi: 10.1093/gerona/glq228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cesari M, Vellas B, Hsu FC, Newman AB, Doss H, King AC, et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci. 2015;70(2):216–22. doi: 10.1093/gerona/glu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cameron ID, Fairhall N, Langron C, Lockwood K, Monaghan N, Aggar C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chan DC, Tsou HH, Yang RS, Tsauo JY, Chen CY, Hsiung CA, et al. A pilot randomized controlled trial to improve geriatric frailty. BMC Geriatr. 2012;12:58. doi: 10.1186/1471-2318-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cesari M, Demougeot L, Boccalon H, Guyonnet S, Vellas B, Andrieu S. The Multidomain Intervention to preveNt disability in ElDers (MINDED) project: rationale and study design of a pilot study. Contemp Clin Trials. 2014;38(1):145–54. doi: 10.1016/j.cct.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 113.Michel JP, Cruz-Jentoft AJ, Cederholm T. Frailty, exercise and nutrition. Clin Geriatr Med. 2015;31(3):375–87. doi: 10.1016/j.cger.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 114.Romera L, Orfila F, Segura JM, Ramirez A, Moller M, Fabra ML, et al. Effectiveness of a primary care based multifactorial intervention to improve frailty parameters in the elderly: a randomised clinical trial: rationale and study design. BMC Geriatr. 2014;14:125. doi: 10.1186/1471-2318-14-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morley JE. Developing novel therapeutic approaches to frailty. Curr Pharm Des. 2009;15(29):3384–95. doi: 10.2174/138161209789105045. [DOI] [PubMed] [Google Scholar]
- 116.Gross AL, Xue QL, Bandeen-Roche K, Fried LP, Varadhan R, McAdams-DeMarco MA, et al. Declines and impairment in executive function predict onset of physical frailty. J Gerontol A, Biol Sci Med Sci. 2016 doi: 10.1093/gerona/glw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Halil M, Cemal Kizilarslanoglu M, Emin Kuyumcu M, Yesil Y, Cruz Jentoft AJ. Cognitive aspects of frailty: mechanisms behind the link between frailty and cognitive impairment. J Nutr Health Aging. 2015;19(3):276–83. doi: 10.1007/s12603-014-0535-z. [DOI] [PubMed] [Google Scholar]
- 118.Houles M, Canevelli M, van Kan GA, Ousset PJ, Cesari M, Vellas B. Frailty and cognition. J Frailty Aging. 2012;1(2):56–63. doi: 10.14283/jfa.2012.11. [DOI] [PubMed] [Google Scholar]
- 119.McAdams-DeMarco MA, Tan J, Salter ML, Gross A, Meoni LA, Jaar BG, et al. Frailty and cognitive function in incident hemodialysis patients. Clin J Am Soc Nephrol: CJASN. 2015;10(12):2181–9. doi: 10.2215/CJN.01960215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12(4):840–51. doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 121.Korc-Grodzicki B, Downey RJ, Shahrokni A, Kingham TP, Patel SG, Audisio RA. Surgical considerations in older adults with cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2014;32(24):2647–53. doi: 10.1200/JCO.2014.55.0962. [DOI] [PubMed] [Google Scholar]
- 122.Robinson TN, Walston JD, Brummel NE, Deiner S, Brown CH, Kennedy M, et al. Frailty for surgeons: review of a National Institute on Aging Conference on Frailty for Specialists. J Am Coll Surg. 2015;221(6):1083–92. doi: 10.1016/j.jamcollsurg.2015.08.428. [DOI] [PMC free article] [PubMed] [Google Scholar]