Abstract
Snake bite is an occupational hazard in India and important preventable cause of acute kidney injury (AKI). This study was done to estimate the magnitude of snakebite-induced AKI (SAKI) who required renal replacement therapy, prognostic predictors, and final outcome, and to measure the oxidative and carbonyl stress (CS) level in SAKI patient who underwent hemodialysis (HD). All SAKI patients dialyzed between April 2010 and July 2011 in NRS Medical College were included. Demographical, clinical, and biochemical data were analyzed, and patients are followed to discharge or death. Oxidative and CS markers (advanced oxidation protein product [AOPP], advanced glycation end product, pentosidine, dityrosine, thioberbituric acid reactive substance, and methylglyoxal [MG]) were measured in 48 SAKI patient requiring HD. About 155 SAKI patients (M: F 2.2:1) received HD. Of them. The age was 36.2 (range 4–74) years. The most common site of the bite was lower limb (88.7%). Oliguria and bleeding manifestation were the common presentation. Hypotension was found in 52 (33.5%) cases, cellulitis and inflammation were found in about 63%. Mean creatinine was 4.56 ± 0.24 mg/dl. About 42 (27.1%) had disseminated intravascular coagulation (DIC). 36 (78.2%) had cellulites, 24 (52.2%) had hypotension or shock at initial presentation (P < 0.05), bleeding manifestation was found in 37 (80.4%), and 22 (47.8%) had DIC (P < 0.05). Forty-six (29.7%) patient died. DIC and hypotension/shock at initial presentation came out as an independent predictor of death. Among all markers measured for oxidative and CS (n = 48) AOPP and MG came out as an independent predictor (P < 0.05) of adverse outcome. Hypotension, DIC, AOPP, and MG were a poor prognostic marker in SAKI patients requiring dialysis.
Keywords: Carbonyl stress, hemodialysis, oxidative stress, predictor, snakebite-induced acute kidney injury
Introduction
Venomous snakebites are an important medical problem and occupational hazard in South-East Asia. Russell's viper is the major cause of snakebite leading to increased morbidity and mortality following acute kidney injury (AKI).[1] The annual mortality is around 30,000 most of them from South-East Asia and West Africa. Approximately, 10,000 deaths occur in India.[2] An incidence of renal involvement with snakebite envenomation of 1.4–28% has been reported in various series,[3] and incidence of ARF is 10–32%.[4,5,6] Among multifactorial cause for the pathogenesis of snakebite-induced AKI (SAKI), elevated oxidative, and carbonyl stress (CS) leading cause of kidney injury.[7,8] Oxidative stress (OS) results in the modification of protein either directly through the oxidation of amino acid residues by reactive oxygen species (ROS) or indirectly by an increased generation of reactive carbonyl species [Figure 1].[8] Although increased CS and protein modification has been extensively studied in both hyperglycemic and corticotropin-releasing factor and many cases of AKI, proteins damage due to OS and CS in SAKI has not been described well in literature.
Figure 1.
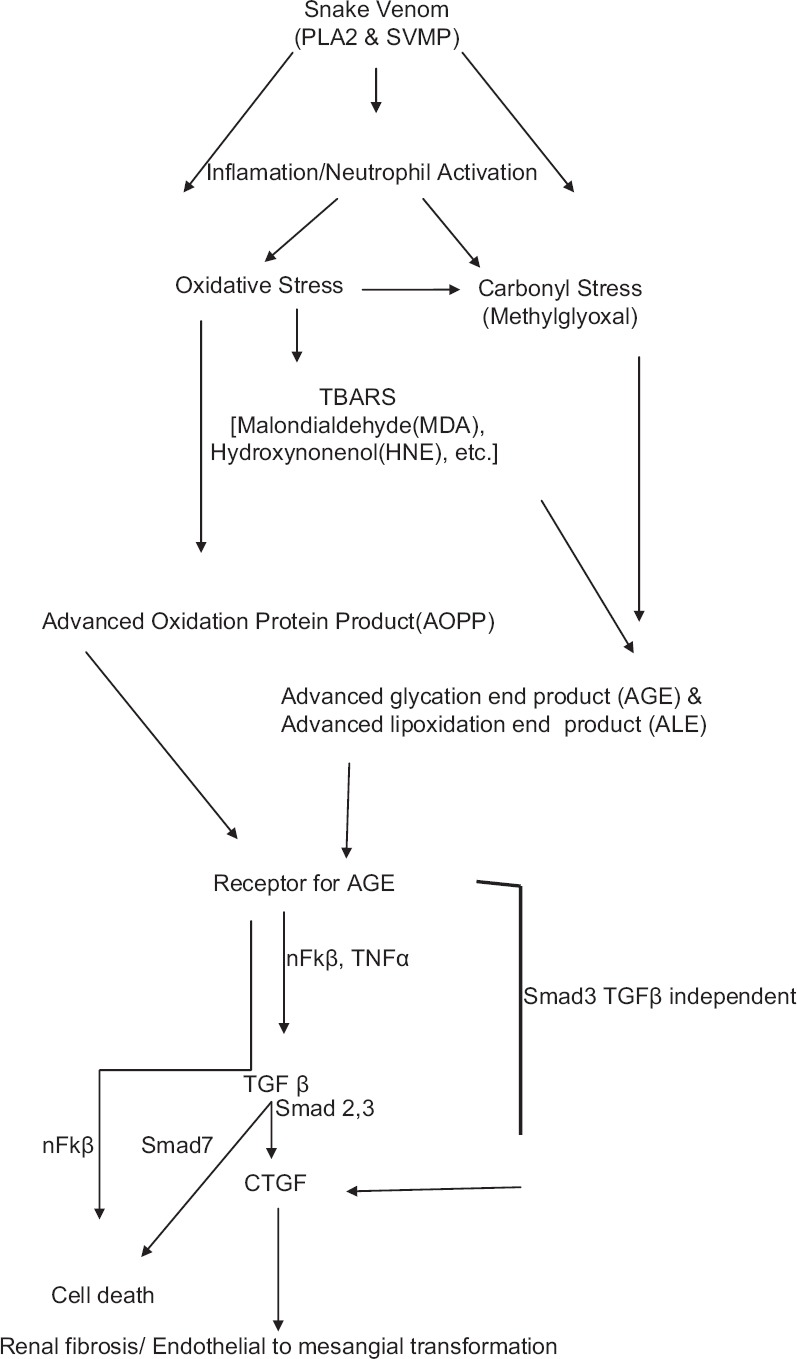
Proposed pathway of snakebite-induced acute kidney injury. PLA2: Phospholipase A2; SVMP: Snake venom metalloproteinase; TBARS: Thiobarbituric acid reacting substances; nFκβ: nuclear factor κβ; TGFβ: Transforming growth factor β; CTGF: Connective tissue growth factor
We estimated the prognostic predictors, and final outcome amongst SAKI requiring renal replacement therapy, and measured the oxidative and CS level in patients who underwent hemodialysis (HD).
Materials and Methods
All snakebite patient who received HD from April 2010 to July 2011 in NRS Medical College were included. The study was approved by the Institutional Ethical Committee of NRS Medical College and Hospital, Kolkata (NMC/Ethi/Gen-25/7671 Dated 02.12.2008). The snake was identified either by the victim or the attendants at the scene or brought a dead snake to the Emergency Department. Snakebite was confirmed by the presence of fang marks. Symptoms and signs were recorded. The snakebite patients admitted during the above mentioned period at NRS Medical College (n = 460) include nonvenomous snakebite - 25%, identified snake - Russell's viper bite - 29%, cobra bite - 4%, and krait bite - 1%. SIAKI was diagnosed according to RIFLE criteria[9] and patients belonging to a failure (F) class requiring HD were enrolled in the study.
Coagulation profile was evaluated along with other biochemical and hematological tests and polyvalent anti-snake venom (ASV) was provided where needed (each vial of serum contains 10 mL, and each 1 mL neutralizes 0.6 mg cobra, 0.6 mg Russell's viper, 0.45 mg common krait, and 0.45mg saw-scaled viper venoms) according to the standard protocol.
SAKI patient with existing kidney disease was excluded. Demographical, clinical, and biochemical data were analyzed, and they are followed from hospitalization to discharge or death.
Biochemical parameters
OS markers like advanced oxidation protein product (AOPP), advanced glycation end product (AGE), pentosidine, dityrosine, thioberbituric acid reactive substance (TBARS), and CS marker methylglyoxal (MG) were measured consecutively in 48 SAKI patient requiring HD according to the standard protocol and compared with 25 normal subject. All markers were measured before initiation of HD and after three consecutive HD.
Total antioxidant status (TAS) was estimated according to the method of Re et al.[10] based on the inhibition of radical cation ABTS+ and expressed as mM Trolox equivalent. Total oxidant status (TOS) was estimated according to the method of Erel,[11] based on the generation of a colored complex of ferric ion, produced by oxidative components, and expressed as μM H2O2 equivalent. OS index (OSI) was calculated from the ratio of TOS and TAS, according to Harma et al.[12] and expressed as arbitrary units. MG was estimated spectrophotometrically, based on 1, 2 diamino benzene reaction following Ghosh et al.[13] method. TBARS was estimated according to the method of Buege and Aust,[14] AOPP was determined according to the method of Witko-Sarsat et al.[15] which is based on the reaction of oxidatively modified protein with potassium iodide in acidic medium to give an absorbance at 340 nm; the result was expressed as μM chloramin T equivalent. AGE (excitation wavelength (Ex) =370 nm and emission wavelength (Em) ≈440 nm),[16] pentosidine (Ex = 335 nm and Em ≈ 385 nm),[17] and dityrosine (Ex = 315 nm and Em ≈ 410 nm)[18] were measured from serum by fluoremetric technique and expressed as arbitrary unit/g protein.
Statistical analysis
Patients were classified into two groups according to the outcome, i.e., survival or death. Differences between groups were compared using Chi-square test or Student's t-test wherever required. Risk factor for both development and outcome of SAKI were determined by univariate and multivariate analysis with logistic regression and receiver operated characteristic curve wherever required, P < 0.05 was considered statistically significant. All calculations were carried out by the statistical program packages SPSS (SPSS v. 16 for Windows, SPSS Inc., Chicago, IL, USA).
Results
Two hundred and three snakebite patients (44.13%) developed AKI and 155 (33.70%) received HD during the period of April 2010 to July 2011. HD could not be initiated in 10 patients due to death, treatment refusal, and unstable health condition. The demographic and clinical characteristics are given in Table 1. Most of the cases were from viper bite. There were 107 males and 48 females. The mean age was 36.2 ± 1.27 (range 4–74) years. Pediatric (<18 years) population were 29 (18.7%) and elderly (>60) were 12 (7.7%). Most patients came from Midnapur district of West Bengal. The most common site of the bite was lower limb (88.7%). About 74.2% had received primary treatment in the form of tetanus toxoid and wound management. Oliguria and bleeding manifestation were the common presentation. Hypotension was found in 52 (33.5%) cases, cellulites and severe inflammation were found in 63.2% patients. Two patients had panhypopituiterism, 8.38% had regional lymphadenopathy. Mean creatinine was 4.56 ± 0.24 mg/dl. About 42 (27.1%) had acute disseminated intravascular coagulation (DIC) as evidenced by thrombocytopenia and low plasma fibrinogen level. Isolated thrombocytopenia was found in 15 (9.68%) patients. Average 21.5 ± 1.94 vial (1 vial = 10 ml) ASV was used during treatment. Median number of HD was required 3 sessions. Mean hospital stay was 11 (range 2–34) days. Bite to HD initiation time was 3.2 days. The most common indication of HD was oliguria and rising creatinine. Forty-six (29.7%) patient died during the hospital stay.
Table 1.
Demographic and clinical characteristics of SAKI patients underwent HD
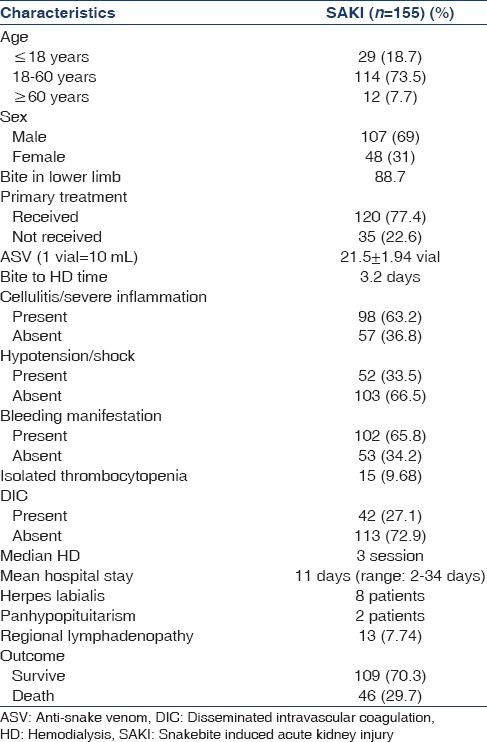
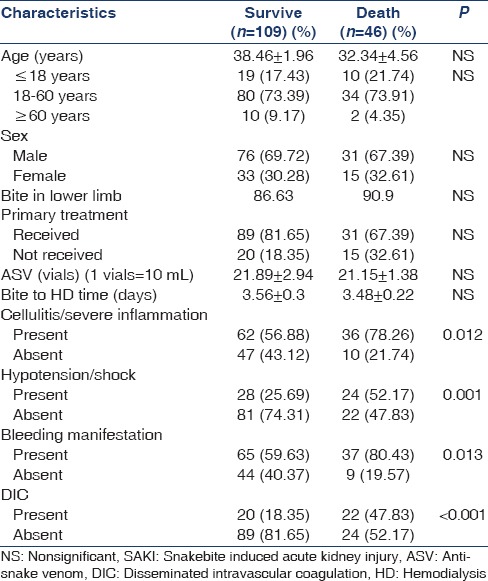
Table 2 represents the comparison of the clinical characteristics of the survived and expired SAKI patients. Among expired patients 10 (34.48%) were <18 years. About 36 (78.2%) had cellulites and/or severe inflammation, 24 (52.2%) had hypotension or shock at initial presentation, bleeding manifestation was found in 37 (80.4%), and 22 (47.8%) had DIC (P < 0.05). Among variables subjected to univariate analysis [Table 2] hypotension, DIC, bleeding manifestation, and cellulitis/severe inflammation were found to predict the adverse outcome (i.e. death) of the SAKI patients underwent HD. Logistic regression analysis yields that cellulitis and severe inflammation (odds ratio [OR] =2.729, confidence interval [CI] =1.23–6.053, P = 0.012) and bleeding manifestation (OR = 2.78, CI = 1.22–6.053, P = 0.012) were confounding risk factor (Wald statistics, P > 0.05) for adverse outcome of SAKI patients whereas DIC (OR = 4.08, CI = 1.917–8.678, P < 0.001) and hypotension/shock (OR = 3.156, CI = 1.535–6.487, P = 0.001) at initial presentation came out as independent predictor of death (Wald statistics, P < 0.05).
Table 2.
Univariate analysis of predictors of SAKI patients
All biochemical parameters studied for oxidative and CS were significantly altered in SAKI patients (n = 48) when compared with normal healthy subjects (n = 25) indicating a state of oxidative and CS [Table 3]. Significantly elevated TOS (P = 0.002) with decreased TAS (P = 0.048) leads to net OS in the patients of SAKI, which was depicted by increased OSI values (P < 0.001). MG, the marker of CS was found to be increased by 3.48 times when compared to the normal subject (P < 0.001). Elevated TBARS levels (P < 0.001) depicts increased oxidative cellular damage in the SAKI patients. Both oxidative and CS leads to increased protein modification as indicated by AOPP, AGE, pentosidine, and dityrosine levels (P < 0.001). When the level of oxidative and CS markers and protein modifications were compared between the survived (n = 31) and expired (n = 17) only AOPP (248.64 ± 17.4 vs. 168.75 ± 12.56, P = 0.001) and MG (39.93 ± 2.11 vs. 28.89 ± 2.41, P = 0.004) were found to be significantly elevated in expired patients than the survived. Area under the curve (AUC) for AOPP (AUC = 0.822, CI = 0.699–0.945, P < 0.001) and MG (AUC = 0.83, CI = 0.714–0.946, P < 0.001) were higher than the reference line [Figure 2].
Table 3.
Comparative status of biochemical parameters of the normal control and SAKI patients
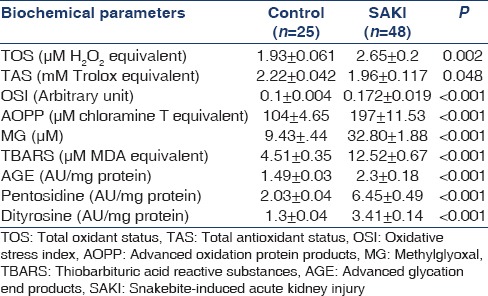
Figure 2.
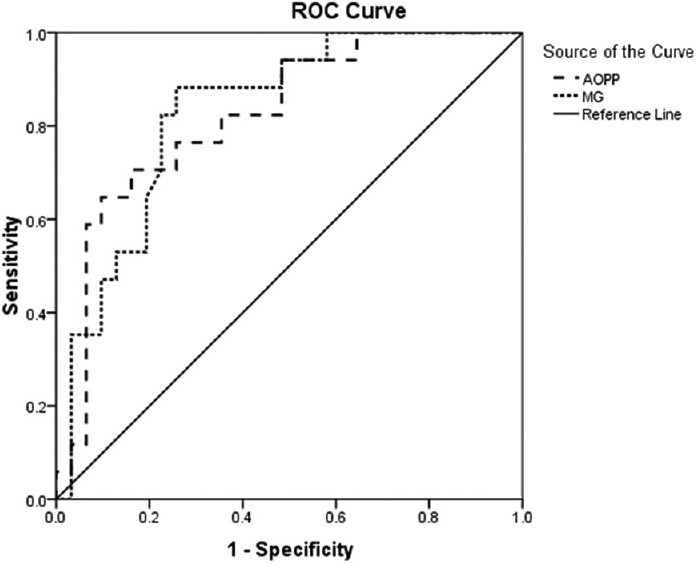
Receiver operating characteristic curve for advanced oxidation protein product and methylglyoxal in snakebite-induced acute kidney injury patients
Discussion
Snake bite is a common and frequently devastating environmental and occupational disease, especially in rural areas of tropical developing countries. In 2009, snakebite was recognized for the first time by the World Health Organization (WHO) as a neglected tropical disease[19] and considered more attention. WHO estimates that India has the highest number of deaths because of snakebites in the world with 80,000 envenomation per year and a case fatality rate of 1.7% to 20%.[20,21]
Data regarding the actual number of SAKI and requiring HD and their complications are inadequate in our country.[22] Most patients in the rural areas do not report to hospitals, and a majority of the victims are treated by unqualified healers. Data from private hospitals are not always available.[23] Among SAKI one study[22] showed 43.5% required HD. In our tertiary center, 155 SAKI patients were received HD from April 2010 to July 2011. The peak incidence of the bite was from July to September around rainy season. About 77.4% patients received some kind of primary treatment before admission here. An average ASV was needed 21.5 ± 1.94 vial and bite to HD time was 3.2 days. ASV requirement was close to national standard (max 25 vial)[24] and also similar study from Northern India.[25]
Oligoanuria and bleeding manifestation were the most common clinical manifestation. Besides this hypotensive shock, cellulitis or feature of severe inflammation and regional lymphadenopathy also observed. Among unusual presentation about eight patients had herpes labialis and two patients had panhypopituiterism. Herpes labialis in SAKI patients was attributed to a possible immunologically altered state in those patients.[26] Between the two cases of hypopituitarism one case presented during the hospital stay and another case was diagnosed on a subsequent visit with deficiencies of gonadal, steroid, and thyroid axes. They showed marked improvement after replacement of anterior pituitary hormones. The probable mechanism was thought to deposition of fibrin microthrombi and hemorrhage in the pituitary gland resulting from the action of venom procoagulant enzymes and hemorrhagin.[27,28]
Forty-six patients (29.7%) died during follow-up. Of them 21.7% below 18 years of age. Athappan et al.[22] showed mortality of 31.9% in their series of the SAKI patient. About 16% death rates when acute tubular necrosis but increased to as high as 80% when cortical necrosis occurs as a result of the toxin.[29]
We have evaluated the predictors of death [Table 2]. Though children were more vulnerable to death in some studies,[30] in our series age and sex were not an independent predictors.[31] Patients who were received primary treatment in the form of the wound dressing, administration of injection of tetanus toxoid, initial fluid management, and the early arrival at the primary care hospital had a better outcome (P = 0.05).[30] There was no correlation between the highest creatinine values and survival. The presence of cellulitis and bleeding manifestation though came out as significant adverse predictor[32] in univariate analysis [Table 2] but become confounding at multivariate level. Hypotensive shock (OR = 5.6, CI = 1.18–5.99, P = 0.018) and DIC (OR = 5.9, CI = 1.23–7.38, P = 0.015) were came out as independent significant predictor of death. Renal biopsies were carried out in three cases, 2 showed feature of acute tubular necrosis and one had patchy cortical necrosis.
The pathogenesis of renal lesions is multifactorial and has been attributed to the nephrotoxicity of venom, hypotension, circulatory collapse, and intravascular hemolysis with hemoglobinuria, myoglobulinuria, DIC, hemolytic uremic syndrome, sepsis, and other factors such as hypersensitivity to venomous or antivenomous protein. Pathological investigations of human fatal cases revealed renal cortical necrosis, distal nephron nephrosis, thrombotic microangiopathy, and acute tubular necrosis.[33]
Whatever the predisposing factor or ultimate renal lesion, it is the interplay of chemical, and immune mediators produce in different pathway responsible for kidney injury. Oxidative and carbonyl stress marker are well known in the pathogenesis of kidney injury.[7] We have measured some oxidative and CS marker in SAKI patient received HD and tried to correlate with an adverse outcome like death. Though all markers were increased, AOPP and MG level were increased sharply before initiating HD and gradually decreased after dialysis or subsequent recovery. Elevated AOPP and MG came out as a strong independent predictor of death. AUC of receiver operated curve for AOPP (AUC = 0.822, CI = 0.699-0.945, P < 0.001) and MG (AUC = 0.83, CI = 0.714-0.946, P < 0.001) were significantly higher than the reference line (AUC = 0.5) indicate their predictive power for the adverse outcome [Figure 2].
The most important finding in our study was that oxidative product, AOPP and carbonyl compounds, and MG were increased rapidly in SAKI patients with a low TAS and glutathione (GSH) concentration along with very high TBARS their serum just within 2-3 days after snakebite in comparison to normal people.[7] It may be suggested that snake venom induces inflammation, shock, which may trigger the immune response leading to a various reaction of neutrophils and macrophages producing ROS that increase the OS. It is known that snake venom of Vipera sp. contains many proteases and lipases,[34] which help in the breakdown of red blood cell (RBC) membranes and cause hemolysis. As a result of protein and lipid breakdown, the threonine, fatty acids, malondialdehyde like compounds (by the breakdown of arachidonic acid of RBC membrane), and carbonyl derivatives are produced rapidly which may be the precursor of MG synthesis[35,36] and the another source of MG accumulation in SAKI. Though the AGE study has not been done in snakebite cases, still the increase of MG may indicate the insufficient renal clearance and increased pathogenesis. Further studies are required to better understand whether the decrease of thiol concentration in SAKI derives only from OS or from other nonoxidative pathway, and the rapid accumulation of MG occurs due to depleted GSH mediated detoxification only or snake venom-induced altered metabolic pathway is linked to it.
Limitations of the study
Our study had some limitations. First, it was a single center study with a limited population. An adequate renal biopsy was not done to confirm the pathogenesis. Follow-up was also of short duration. Details coagulation study was not done in all patients. We were included only severe form of renal failure received dialysis. The pattern of elevation of different stress marker in AKI not requiring HD and snakebite without AKI not studied.
Second, in this study, we have compared dialysis-requiring AKI with controls only, which is not sufficient to generate a clear picture of its effect on the study outcome parameters. Further adequately powered and controlled research studying AKI not requiring dialysis and snakebite without AKI will provide more data on the link of OS with increased mortality in this clinical scenario.
Conclusion
Snake bite is a life-threatening condition with high mortality. Early intervention and aggressive treatment is needed for a favorable outcome. Oxidative and CS may play a crucial role in the pathogenesis of SAKI and MG, and AOPP may be used as a functional biomarker of renal clearance. Glutathione precursor, carbonyl compound quencher, and antioxidant drugs may be used in future to reduce the renal risk.
Financial support and sponsorship
West Bengal State Department of Science and Technology, Kolkata, West Bengal, India (Grant numbers: 647[Sanc.]/ST/P/S and T/9G-7/2009 Dated 29.01.2010 and 368[Sanc.]/ST/P/S and T/9G-7/2009 Dated 16.09.2011).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chugh KS. Snake-bite-induced acute renal failure in India. Kidney Int. 1989;35:891–907. doi: 10.1038/ki.1989.70. [DOI] [PubMed] [Google Scholar]
- 2.Chugh KS, Pal Y, Chakravarty RN, Datta BN, Mehta R, Sakhuja V, et al. Acute renal failure following poisonous snakebite. Am J Kidney Dis. 1984;4:30–8. doi: 10.1016/s0272-6386(84)80023-2. [DOI] [PubMed] [Google Scholar]
- 3.Jha V, Chugh KS. Community-acquired acute kidney injury in Asia. Semin Nephrol. 2008;28:330–47. doi: 10.1016/j.semnephrol.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Pinho FM, Yu L, Burdmann EA. Snakebite-induced acute kidney injury in Latin America. Semin Nephrol. 2008;28:354–62. doi: 10.1016/j.semnephrol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Kanjanabuch T, Sitprija V. Snakebite nephrotoxicity in Asia. Semin Nephrol. 2008;28:363–72. doi: 10.1016/j.semnephrol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Burdmann EA, Jha V, Sitprija V. Acute kidney injury in the tropics. Comprehensive Clinical Nephrology. 4th ed. 67. St. Louis, Missouri: Saunders Elsevier; 2011. pp. 813–20. [Google Scholar]
- 7.Mukhopadhyay S, Ghosh A, Kar M. Methylglyoxal increase in uremia with special reference to snakebite-mediated acute renal failure. Clin Chim Acta. 2008;391:13–7. doi: 10.1016/j.cca.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Miyata T, van Ypersele de Strihou C, Kurokawa K, Baynes JW. Alterations in nonenzymatic biochemistry in uremia: Origin and significance of “carbonyl stress” in long-term uremic complications. Kidney Int. 1999;55:389–99. doi: 10.1046/j.1523-1755.1999.00302.x. [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative Workgroup. Acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 11.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Harma M, Harma M, Erel O. Oxidative stress in women with preeclampsia. Am J Obstet Gynecol. 2005;192:656–7. doi: 10.1016/j.ajog.2004.07.094. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh M, Talukdar D, Ghosh S, Bhattacharyya N, Ray M, Ray S. In vivo assessment of toxicity and pharmacokinetics of methylglyoxal. Augmentation of the curative effect of methylglyoxal on cancer-bearing mice by ascorbic acid and creatine. Toxicol Appl Pharmacol. 2006;212:45–58. doi: 10.1016/j.taap.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 15.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–13. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 16.Münch G, Keis R, Wessels A, Riederer P, Bahner U, Heidland A, et al. Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur J Clin Chem Clin Biochem. 1997;35:669–77. doi: 10.1515/cclm.1997.35.9.669. [DOI] [PubMed] [Google Scholar]
- 17.Meng J, Sakata N, Takebayashi S. Increased glycoxidation and lipoperoxidation in the collagen of the myocardium in hemodialysis patients. Cardiovasc Res. 2000;47:306–13. doi: 10.1016/s0008-6363(00)00105-x. [DOI] [PubMed] [Google Scholar]
- 18.Giulivi C, Davies KJ. Dityrosine and tyrosine oxidation products are endogenous markers for the selective proteolysis of oxidatively modified red blood cell hemoglobin by (the 19 S) proteasome. J Biol Chem. 1993;268:8752–9. [PubMed] [Google Scholar]
- 19.WHO WHO. Snakebite. [Last accessed on 2015 Feb 10]. Available from: http://www.who.int/neglected_diseases/diseases/snakebites/en/index.html .
- 20.Chippaux JP. Snake-bites: Appraisal of the global situation. Bull World Health Organ. 1998;76:515–24. [PMC free article] [PubMed] [Google Scholar]
- 21.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Athappan G, Balaji MV, Navaneethan U, Thirumalikolundusubramanian P. Acute renal failure in snake envenomation: A large prospective study. Saudi J Kidney Dis Transpl. 2008;19:404–10. [PubMed] [Google Scholar]
- 23.Visweswaran RK, George J. Snake bite induced acute renal failure. Indian J Nephrol. 1999;9:156–9. [Google Scholar]
- 24.Indian National Snakebite Protocol. Proceedings of the Indian National Snakebite Protocol Consultation Meeting, New Delhi, India. 2007 Aug 2; [Google Scholar]
- 25.Sharma N, Chauhan S, Faruqi S, Bhat P, Varma S. Snake envenomation in a north Indian hospital. Emerg Med J. 2005;22:118–20. doi: 10.1136/emj.2003.008458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waikhom R, Sapam R, Patil K, Jadhav JP, Sircar D, Roychowdhury A, et al. Herpes labialis in patients with Russell's viper bite and acute kidney injury: A single center experience. Am J Trop Med Hyg. 2011;84:1016–20. doi: 10.4269/ajtmh.2011.10-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonypillai CN, Wass JA, Warrell DA, Rajaratnam HN. Hypopituitarism following envenoming by Russell's vipers (Daboia siamensis and D. russelii) resembling Sheehan's syndrome: First case report from Sri Lanka, a review of the literature and recommendations for endocrine management. QJM. 2011;104:97–108. doi: 10.1093/qjmed/hcq214. [DOI] [PubMed] [Google Scholar]
- 28.Uberoi HS, Achuthan AC, Kasthuri AS, Kolhe VS, Rao KR, Dugal JS. Hypopituitarism following snake bite. J Assoc Physicians India. 1991;39:579–80. [PubMed] [Google Scholar]
- 29.Kohli HS, Sakhuja V. Snake bites and acute renal failure. Saudi J Kidney Dis Transpl. 2003;14:165–76. [PubMed] [Google Scholar]
- 30.Russell FE. Snake Venom Poisoning. Philadelphia: JB Lippincott Company; 1980. pp. 235–85. [Google Scholar]
- 31.Reid HA. In: Animal poisons. Manson's Tropical Diseases. 18th ed. Manson-Bahr PE, Apted FI, editors. London: Bailliere-Tindall; 1982. pp. 544–6. [Google Scholar]
- 32.Russell FE. Snake Venom Poisoning. Philadelphia: JB Lippincott Company; 1980. pp. 291–350. [Google Scholar]
- 33.Vargas-Baldares M. In: Renal lesions in snake bite in Costa Rica. Toxins: Animal, Plant and Microbial. Rosenberg P, editor. Oxford: Pergamon Press; 1978. p. 497. [Google Scholar]
- 34.Pough FH, Janis CN, Heiser JB. Vertebrate Life. Singapore: Pearson Education Pvt. Ltd; 2002. p. 315. [Google Scholar]
- 35.Suzuki D, Miyata T, Saotome N, Horie K, Inagi R, Yasuda Y, et al. Immunohistochemical evidence for an increased oxidative stress and carbonyl modification of proteins in diabetic glomerular lesions. J Am Soc Nephrol. 1999;10:822–32. doi: 10.1681/ASN.V104822. [DOI] [PubMed] [Google Scholar]
- 36.Reichard GA, Jr, Skutches CL, Hoeldtke RD, Owen OE. Acetone metabolism in humans during diabetic ketoacidosis. Diabetes. 1986;35:668–74. doi: 10.2337/diab.35.6.668. [DOI] [PubMed] [Google Scholar]


