Abstract
We report a case of a man with traumatic brain injury. He was started on to prophylactic topiramate which led to a mixed acid-base disorder. He had severe metabolic acidosis secondary to renal tubular acidification defect and respiratory alkalosis secondary to hyperventilation. Withdrawal of the offending drug led to the prompt resolution of the acid-base disturbance.
Keywords: Metabolic acidosis, respiratory alkalosis, topiramate, traumatic brain injury
Introduction
Topiramate is an antiepileptic with carbonic anhydrase inhibitor activity which can lead to acidosis in patients. Because of its ability to cross the blood–brain barrier, it can stimulate hyperventilation secondary to cerebrospinal fluid (CSF) acidosis.
Case Report
A 24-year-old man was admitted in an unconscious state after a head injury. He was put on mechanical ventilation elsewhere in view of poor neurological status. Baseline investigations revealed normal creatinine and electrolytes [Table 1]. The blood picture was within normal limits. Urine examination showed specific gravity of 1.015 and pH of 6.5 with no proteinuria and 1–2 pus cells and 5–6 red blood cell/high power field. Computed tomography of the brain revealed an irregular hyperdense lesion (acute interparenchymal hematoma in the left posterior capsuloganglionic region extending to thalamus with perilesional edema and adjacent pressure effect). The ultrasound abdomen showed normal kidneys (right kidney 9.1 cm × 4.3 cm, left kidney 9.2 cm × 4.8 cm) with normal echotexture.
Table 1.
Initial investigations before administration of topiramate

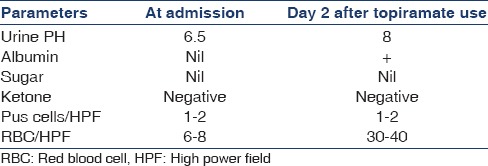
He was initiated on levetiracetam at a dose of 500 mg twice a day for prevention of seizures. He was extubated on the day 3 and showed improvement in his neurological state. On the day 5 of hospital admission, he developed thrombocytopenia (platelet count 29,000/cu mm) thought to be related to levetiracetam, and he was initiated on topiramate 50 mg twice a day. After a day of topiramate he had one spike of fever for which a complete urine examination, and urine culture was sent as part of fever evaluation along with a blood culture. Urine was cloudy and the examination revealed an alkaline pH [Table 2].
Table 2.
Salient features of complete urine examination during the hospital stay
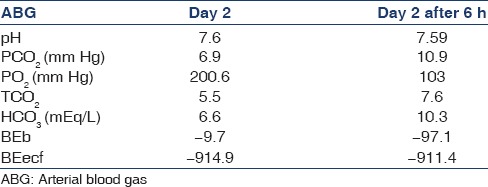
Meanwhile, respiratory rate increased to 35/min and sensorium worsened on the day 8 of hospital stay. Arterial blood gas (ABG) showed a pH of 7.6 with PCO2 of 7.0 mm of Hg with PO2 of 163.00 with bicarbonate of 6.70, SPO2 of 99.80 on 2 L of oxygen. A repeat ABG was done 6 h later [Table 3].
Table 3.
Serial arterial blood gas after initiation of topiramate
The blood picture revealed a neutrophilic leukocytosis. Nephrology consultation was sought, and a thorough history and clinical examination was not suggestive of any previous renal disorders. The drug chart included the above-mentioned antibiotics along with topiramate at a dose of 50 mg twice daily. The blood gas analysis was suggestive of acute respiratory alkalosis with metabolic acidosis. The anion gap was high (32). He was febrile, and there was leukocytosis with toxic granules and hypersegmented neutrophils. The serum lactate levels were 23.9 mg/dl (4–20).
In view of severe hyperventilation with incumbent respiratory fatigue, he was intubated and kept on mechanical ventilation mechanically ventilated. The urine output was normal throughout the above episode of acid-base disorder.
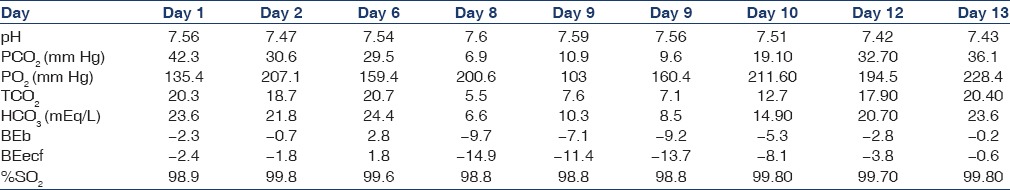
The most striking feature was that, despite significant metabolic acidosis, urine pH was alkaline at 9. The urinary electrolytes showed sodium of 150.00 mmol/L, chloride of 111.20 mmol/L, and potassium of 35 mmol/L (urinary anionic gap >10). The above two indicators were pointing toward renal tubular acidification defect. Topiramate was stopped. As there was severe metabolic acidosis and in view of effectiveness of hemodialysis in removing topiramate, one session of hemodialysis was done. The subsequent course in the hospital was that of progressive improvement in level of consciousness over the next few days. He was taken off ventilator after 2 days and was eventually discharged. At discharge, serum creatinine (0.6 mg/dl) and electrolytes were normal. Serial ABG reports during the hospital course have been shown in Table 4.
Table 4.
Serial arterial blood gas analyses reports
Discussion
The advent of newer and more potent antiepileptics has helped neurologists to better control seizure disorders. However, their varied mechanisms of actions in control of seizure have led to recognition of new array of adverse events in view of interference in normal metabolism. Topiramate is a sulfamate-substituted monosaccharide derived from D-fructose. Multiple mechanisms are thought to be related to its antiepileptic activity, including the voltage-dependent blocking of sodium channels, potentiation of gamma-aminobutyrate receptors, and glutamate receptor subtype kainate antagonism.[1] The major side effects include confusion, somnolence, dizziness, ataxia, weight loss, and nephrolithiasis.[2] With widespread use of topiramte (TPM), there have been case reports of metabolic acidosis secondary to renal acidification defects.[3,4,5,6] The mechanism involves the inhibition of the enzyme carbonic anhydrase (CA) impairing both the normal proximal reabsorption of filtered HCO3 − and distal excretion of hydrogen ion.[2] Topiramate-induced renal tubular acidosis can make patients acutely ill and chronically and lead to nephrolithiasis and osteoporosis.[7] Central hyperventilation secondary to topiramate use has been reported in pediatric age group with resolution of hyperpnea within 24 h of stopping of TPM.[8] The hyperventilation induced by topiramate was thought to be due to blockade of CA at the central level, resulting in impaired exchange of carbon dioxide across the blood–brain barrier. The increase in carbon dioxide and acidosis in CSF continued to stimulate the central chemoreceptors, resulting in hyperventilation.[9] In our case, we have seen the simultaneous presentation of renal tubular acidification defect and hyperventilation in the same individual with 48 h of initiation of topiramate, albeit other concomitant causes for metabolic acidosis as evident by increase in lactate and sepsis were present. The occurrence of respiratory alkalosis secondary to hyperventilation superimposed on a renal acidification defect resulted in altered sensorium and severe tachycardia and tachypnea. The other corroborative evidence is the immediate resolution of hyperventilation after extracorporeal removal of the offending drug was achieved with the help of hemodialysis. Hemodialysis was shown to be effective in removing topiramate with a mean clearance of 123 ml/min versus 10.8 ml/min in patients with moderate to severe renal impairment.[10] The subsequent period of the hospital stay confirmed that symptoms were temporally associated with drug administration. To our knowledge, simultaneous presentation of metabolic acidosis and hyperventilation in adults at this low dose has not been reported.
Conclusion
Use of topiramate can be associated with rapid onset of renal acidification defects and central hyperventilation. The drug should be used with caution even in patients with normal renal function. Serum bicarbonate level needs to be regularly monitored during administration for early recognition of metabolic complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE. An overview of the preclinical aspects of topiramate: Pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(Suppl 1):S3–9. [PubMed] [Google Scholar]
- 2.Mirza N, Marson AG, Pirmohamed M. Effect of topiramate on acid-base balance: Extent, mechanism and effects. Br J Clin Pharmacol. 2009;68:655–61. doi: 10.1111/j.1365-2125.2009.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montenegro MA, Guerreiro MM, Scotoni AE, Guerreiro CA. Predisposition to metabolic acidosis induced by topiramate. Arq Neuropsiquiatr. 2000;58:1021–4. doi: 10.1590/s0004-282x2000000600007. [DOI] [PubMed] [Google Scholar]
- 4.Garris SS, Oles KS. Impact of topiramate on serum bicarbonate concentrations in adults. Ann Pharmacother. 2005;39:424–6. doi: 10.1345/aph.1E437. [DOI] [PubMed] [Google Scholar]
- 5.Groeper K, McCann ME. Topiramate and metabolic acidosis: A case series and review of the literature. Paediatr Anaesth. 2005;15:167–70. doi: 10.1111/j.1460-9592.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 6.Burmeister JE, Pereira RR, Hartke EM, Kreuz M. Topiramate and severe metabolic acidosis: Case report. Arq Neuropsiquiatr. 2005;63:532–4. doi: 10.1590/s0004-282x2005000300032. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-de Orueta L, Esteban-Fernández J, Aichner HF, Casillas-Villamor A, Rodríguez-Álvarez S. Topiramate-induced metabolic acidosis: A case study. Nefrologia. 2012;32:403–4. doi: 10.3265/Nefrologia.pre2011.Dec.11308. [DOI] [PubMed] [Google Scholar]
- 8.Laskey AL, Korn DE, Moorjani BI, Patel NC, Tobias JD. Central hyperventilation related to administration of topiramate. Pediatr Neurol. 2000;22:305–8. doi: 10.1016/s0887-8994(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 9.Ko CH, Kong CK. Topiramate-induced metabolic acidosis: Report of two cases. Dev Med Child Neurol. 2001;43:701–4. doi: 10.1017/s0012162201001268. [DOI] [PubMed] [Google Scholar]
- 10.Manitpisitkul P, Curtin CR, Shalayda K, Wang SS, Ford L, Heald DL. Pharmacokinetics of topiramate in patients with renal impairment, end-stage renal disease undergoing hemodialysis, or hepatic impairment. Epilepsy Res. 2014;108:891–901. doi: 10.1016/j.eplepsyres.2014.03.011. [DOI] [PubMed] [Google Scholar]


