Abstract
Background
Anti-glycyl-tRNA synthetase (anti-EJ) antibody is occasionally positive in patients with interstitial lung disease (ILD). We aimed to define the clinical, radiological and pathological features of patients with anti-EJ antibody-positive ILD (EJ-ILD).
Methods
We retrospectively analyzed the medical records of 12 consecutive patients with EJ-ILD who underwent surgical lung biopsy.
Results
The median follow-up time was 74 months (range, 17–115 months). The median age was 62 years (range, 47–75 years). Seven of 12 patients were female. Eight patients presented with acute onset. Six patients eventually developed polymyositis/dermatomyositis. On high-resolution computed tomography, consolidation and volume loss were predominantly observed in the middle or lower lung zone. Nine patients presented pathologically nonspecific interstitial pneumonia with organizing pneumonia, alveolar epithelial injury and prominent interstitial cellular infiltrations whereas the other three patients were diagnosed with unclassifiable interstitial pneumonia. Although all patients but one improved with the initial immunosuppressive therapy, five patients relapsed. When ILD relapsed, four of the five patients were treated with corticosteroid monotherapy. Four of the six patients without relapse have been continuously treated with combination therapy of corticosteroid and immunosuppressant.
Conclusions
Patients with EJ-ILD often had acute onset of ILD with lower lung-predominant shadows and pathologically nonspecific interstitial pneumonia or unclassifiable interstitial pneumonia with acute inflammatory findings. Although the disease responded well to the initial treatment, relapse was frequent. Because of the diversity of the clinical courses, combination therapy of corticosteroid and immunosuppressant should be on the list of options to prevent relapse of EJ-ILD.
Keywords: Interstitial pneumonia with autoimmune featured (IPAF), Anti-synthetase syndrome, Dermatomyositis, Polymyositis, Idiopathic interstitial pneumonia
Background
Interstitial lung disease (ILD) is caused by various etiologies including collagen vascular disease, drug induced and inhalation exposure. Patients with ILD often have collagen vascular disease-related autoantibodies even when they do not fulfill the diagnostic criteria for any collagen vascular diseases [1]. In 2015, the “European Respiratory Society (ERS)/American Thoracic Society (ATS) Task Force on Undifferentiated Forms of Connective Tissue Disease-associated Interstitial Lung Disease” proposes the term “interstitial pneumonia with autoimmune features” (IPAF) and offers classification criteria organized around the presence of a combination of features from three domains: a clinical domain, a serologic domain and a morphologic domain [2]. Recent report showed autoantibodies against aminoacyl-tRNA synthetase (ARS) have been found to be highly specific for myositis and to associate with complicating ILD, arthritis, Raynaud’s phenomenon, mechanic’s hand and fever. Ten anti-ARS antibodies have been identified: anti-Jo-1, EJ, PL-7, PL-12, OJ, KS, Zo, SC, JS and YRS antibodies [3, 4].
Anti-ARS antibodies are positive in 20%–35% of patients with polymyositis/dermatomyositis (PM/DM) [3, 5]. Among patients with PM/DM, anti-Jo-1 antibody is the most common (15%–30%) while others are seen in less than 10% [6]. Although, anti-EJ antibody, which is against glycyl-tRNA synthetase [7], is generally less common than anti-Jo-1 antibody, it has a higher prevalence than anti-Jo-1 antibody in some case series of patients with ILD positive of anti-ARS antibodies (ARS-ILD) [8, 9]. Meanwhile, over 90% of patients with positive-anti-ARS antibodies show ILD complications [10].
On computed tomography (CT), patients with ARS-ILD often have radiologically traction bronchiectasis, consolidation and volume loss predominantly in the lower lung zones [9, 11, 12]. Histopathological patterns are reported as nonspecific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), acute lung injury (ALI) or diffuse alveolar damage (DAD) [9, 11–13]. Although clinical course of treatment response to ARS-ILD is reportedly good, relapses are frequent [14].
However, there are only a few reports describing the clinical features of patients with ILD with positive-anti-EJ antibody (EJ-ILD) [15, 16]. The aim of this retrospective study was to define the clinical, radiological and pathological features of patients with EJ-ILD with long-term follow-up.
Methods
Study population
We retrospectively analyzed the medical records of 12 consecutive patients with EJ-ILD who underwent surgical lung biopsy at Kanagawa Cardiovascular and Respiratory Center between March 2005 and April 2013 and were subsequently followed up for over one year. Anti-ARS antibodies were detected with an immunoprecipitation assay at Tokai University School of Medicine as previously described method [17].
Clinical information
We obtained the clinical presentations, physical findings, laboratory findings and pulmonary function tests at the initial visit from patients’ medical records. The diagnosis of PM/DM was based on Bohan and Peter’s criteria [18]. Patients satisfying at least two of the five of the criteria were diagnosed with PM/DM. IPAF and anti-synthetase syndrome were diagnosed according to each proposed criteria [2, 19]. The onset of EJ-ILD was divided into two types according to the time from the initial symptoms to the initial hospital visit: 1) within three months (acute onset) and 2) over three months (chronic onset). The pattern of ILD onset in PM/DM patients was divided into three types: 1) PM/DM was diagnosed at least three months earlier than ILD (PM/DM-preceding type), 2) ILD was diagnosed at least three months earlier than PM/DM (ILD-preceding type), and 3) both occurred within three months (simultaneous type) [14].
Radiological analysis
CT images before biopsy were obtained from all the 12 patients. All images were reviewed independently by two experienced radiologists without any prior knowledge of the clinical and pathological information.
To determine the distribution and extent of parenchymal abnormalities, each lung was divided into three zones of upper, middle and lower at the level of the carina and the left inferior pulmonary vein. The following high resolution CT (HRCT) findings were coded as present or absent in each zone: reticulation, ground-glass opacity, consolidation, bronchovascular thickening, traction bronchiectasis, honeycombing, volume loss and emphysema. Radiological assessment was based on those from previous studies [9, 20]. Disagreements between the two radiologists were resolved by consensus.
Pathological analysis
Biopsy was performed in all patients. Multiple specimens mainly from the upper and lower lobes were obtained from 11 of 12 patients. All the specimens were stained with hematoxylin-eosin and Elastica van Gieson and were reviewed independently by two experienced lung pathologists who were not aware of the clinical and radiological findings. Pathological patterns were diagnosed according to the 2002 and 2013 ATS/ERS consensus classification of the idiopathic interstitial pneumonias and 2008 ATS project for idiopathic NSIP [21, 22].
The intensity and extent of the following pathological features were semi-quantitatively graded as absent, mild, moderate or severe: pleural fibrosis, pleuritis, lymphoid follicles with germinal center, organizing pneumonia, alveolar epithelial injury, interstitial cellular infiltration, alveolar wall fibrosis, microscopic honeycombing, fibroblastic focus and collapse. Pathological assessment was based on those from previous studies [13, 23, 24].
Disagreements between the two pathologists were discussed until consensus was reached.
Clinical course and treatment response
The clinical course and treatment response of EJ-ILD was evaluated on the basis of the degree of changes in both chest images and pulmonary functions. Two radiologists evaluated the chest radiograph and/or HRCT. We graded the changes in pulmonary functions according to the 2000 ATS/ERS consensus statement for idiopathic pulmonary fibrosis (Table 1) [25]. In addition, the pattern of disease behavior was divided into the following five types: 1) reversible and selflimited, 2) reversible disease with risk of progression, 3) stable with residual disease, 4) progressive, irreversible disease with potential for stabilization, and 5) progressive, irreversible disease despite therapy [22].
Table 1.
Definition of the initial response to treatment
| Evaluation of pulmonary function testa | ||||
|---|---|---|---|---|
| Improved | Stable | Deteriorated | ||
| Evaluation of images | Improved | Improved | Improved | Deteriorated |
| Stable | Improved | Stable | Deteriorated | |
| Deteriorated | Improved | Deteriorated | Deteriorated | |
a“Improved” meant at least 10% increase in forced vital capacity % predicted (%FVC) or at least 15% increase in diffusing capacity of the lung for carbon monoxide % predicted (%DLCO). Stable was defined as within 10% change of %FVC and within 15% change of %DLCO. “Deteriorated” meant over 10% decrease in %FVC or over 15% decrease in %DLCO.
The initial response was assessed within six months after the initiation of treatment. After the initial evaluation, we assessed all chest radiographs and pulmonary functions until the end of the follow-up period. The relapse of ILD meant that the patients with deterioration needed to reinforced therapy for ILD.
Results
Clinical and laboratory findings of EJ-ILD
The summary of the clinical characteristics of the 12 patients is listed in Table 2. The patients included five males and seven females with median age of 62 years (range, 47–75 years) at the initial visit. Eight patients presented acute onset.
Table 2.
Clinical characteristics and diagnosis of 12 patients
| Clinical characteristics | n = 12 |
| Male/female | 5/7 |
| Age at initial visit [years] | 62 (47–75) |
| Acute onset/chronic onset | 8/4 |
| Smoking status (current or former/never smoker) | 6/6 |
| Serological findings (reference value) | n = 12 |
| CK [U/L] (62–287) | 140 (17–672) |
| LDH [U/L] (70–139) | 252 (212–337) |
| ESR [mm/h] (2–10) | 38 (17–116) |
| CRP [mg/dl] (0–0.1) | 0.63 (0.03–1.79) |
| KL-6 [U/ml] (0–500) | 991 (514–3208) |
| SP-D [ng/ml] (0–110) | 232.3 (56.1–723.1) |
| PaO2 [Torr] (74–108) | 71.5 (53.0–84.5) |
| Pulmonary functions | n = 11 |
| FVC % predicted [%] | 71.5 (42.4–86.1) |
| DLCO % predicted [%] | 66.1 (56.0–112.2) |
Data are presented as n, or median (range)
CK: Creatine kinase, LDH: Lactate dehydrogenase, ESR: Erythrocyte sedimentation rate, CRP: C-reactive protein, KL-6: Klebs von den lungen-6, SP-D: Surfactant protein D, PaO 2: Arterial oxygen pressure, FVC: Forced vital capacity, DL CO: Diffusion capacity for carbon monoxide
At the disease onset, extra-pulmonary symptoms were observed in only five patients, whereas all patients presented pulmonary symptoms. Only one patient was suffering from muscle weakness. Among six patients diagnosed with IPAF and anti-synthetase syndrome, no patient had skin involvement, and only one patient presented articular symptoms. Two patients have not had extra-pulmonary symptoms during entire follow-up period (Table 3). Palmar telangiectasia was not assessed in all patients.
Table 3.
Clinical symptoms and physical findings related to IPAF and anti-synthetase syndrome
| Patient number | Diagnosis | Cough | Dyspnea | Mechanic’s hand, Gottron’s sign | Heliotrope rash | Raynaud’s phenomenon | Articular involvementa | Unexplained digital oedema | Unexplained fever | Muscular involvementb | Distal digital tip ulceration | CK level |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IPAF/ASS | + | - | - | - | - | - | - | - | - | - | 218 |
| 2 | IPAF/ASS | + | + | - | - | - | + | - | - | - | - | 136 |
| 3 | IPAF/ASS | + | - | - | - | - | - | - | - | - | - | 50 |
| 4 | PMI | + | - | - | - | - | +c | - | - | +c | - | 346E |
| 5 | IPAF/ASS | + | + | - | - | - | - | - | +c | +c | - | 51 |
| 6 | DMI | + | - | + | - | +c | - | - | +c | - | - | 143 |
| 7 | IPAF/ASS | + | + | - | - | - | - | - | +c | +c | - | 17 |
| 8 | PMI | + | - | +c | - | +c | +c | +c | - | - | - | 513E |
| 9 | DMI | - | + | +c | - | +c | +c | - | - | +c | - | 137 |
| 10 | IPAF/ASS | + | + | - | - | - | - | - | + | +c | - | 22 |
| 11 | PMI | + | + | - | - | - | + | + | - | +c | - | 230 |
| 12 | PMS | + | + | - | - | - | - | - | + | + | - | 672E |
ainflammatory arthritis, or polyarticular morning joint stiffness > 60mins, bproximal muscular weakness, or myalgia, CK: creatine kinase, IPAF: interstitial pneumonia with autoimmune features, ASS: anti-synthetase syndrome, PM: polymyositis, DM: dermatomyositis, I: interstitial lung disease preceded PM/DM, S: simultaneous onset of PM and interstitial lung disease, csymptoms occurred during follow up, E: The CK level is greater than reference value
Creatine kinase levels were elevated in three patients and lactate dehydrogenase levels were elevated in eight, while all patients presented with the elevation of erythrocyte sedimentation rate, C-reactive protein, Klebs von den Lungen-6 and surfactant protein-D levels. Electromyography, muscle biopsy and magnetic resonance imagings of muscle were performed in only two DM patients.
Specific autoantibodies other than anti-EJ antibody were found in only one patient who had anti-SSA antibody at the initial visit and was diagnosed with Sjögren’s syndrome 20 months after the diagnosis of EJ-ILD. Other patients had neither other autoantibodies nor the diagnosis of any other collagen vascular diseases except for PM/DM.
Six patients were diagnosed with PM/DM during the disease course and five of them were of the ILD-preceding type. Others were all diagnosed with idiopathic interstitial pneumonia and satisfied with the proposed criteria of both IPAF and anti-synthetase syndrome.
Radiological findings
The HRCT findings are described in Table 4. All findings were observed in both lobes. All but one patient presented with reticulation and ground-glass opacity in all lobes. On the other hand, volume loss, bronchovascular thickening, traction bronchiectasis and consolidation were not common in the upper zone, but were predominant in the middle or lower zones in almost all patients. Consolidation was observed along the bronchus from the middle to the subpleural area. Bronchiectasis was noted inside the consolidation, which encompassed the lung architecture. Reticulation and ground-glass opacity were distributed around the consolidation. Typical HRCT images are shown in Fig. 1.
Table 4.
HRCT findings of 12 patients
| Findings | Zones | ||
|---|---|---|---|
| Upper | Middle | Lower | |
| Reticulation | 11 | 12 | 12 |
| Ground-glass opacity | 11 | 12 | 12 |
| Volume loss | 2 | 9 | 12 |
| Bronchovascular thickening | 1 | 10 | 12 |
| Traction bronchiectasis | 5 | 11 | 11 |
| Consolidation | 4 | 11 | 11 |
| Honeycombing | 1 | 1 | 4 |
| Emphysema | 4 | 1 | 1 |
Upper: between apex and carina, Middle: between carina and left inferior pulmonary vein, Lower: between inferior pulmonary vein and diaphragm
Fig. 1.
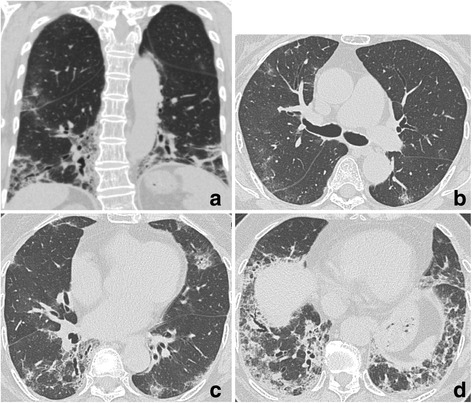
HRCT images of patient #9 (76-years-old female, pathologically cellular NSIP). a The coronal image of the high-resolution computed tomography (HRCT) showed bilateral volume loss and infiltration of lower lung field. b-d Transverse images of the HRCT. Consolidation was observed from middle area to the subpleural area. Traction bronchiectasis was noted inside of consolidation. Reticulation and ground-glass opacity were distributed around consolidation
Pathological findings
The median interval between the date of HRCT and biopsy was 28 days (range, 1–85 days). Four patients were pathologically diagnosed with cellular NSIP (c-NSIP), five were with fibrosing NSIP (f-NSIP), and three were with unclassifiable interstitial pneumonia. Organizing pneumonia, alveolar epithelial injury, interstitial cellular infiltration, pleural fibrosis and collapse with moderate or severe grade were observed in more than half of the patients (Table 5). Especially organizing pneumonia and alveolar epithelial injury with moderate or severe grade were observed in two of four patients with c-NSIP and in four of five f-NSIP patients. Collapse was observed with moderate grade in one of four patients with c-NSIP and with moderate or severe grade in four of five with f-NSIP. Acute inflammatory findings such as organizing pneumonia, alveolar epithelial injury and interstitial cellular infiltration surrounded by collapse with few hyaline membrane were more prominent in unclassifiable interstitial pneumonia than in c-NSIP or f-NSIP. Organizing pneumonia, alveolar epithelial injury and collapse did not show diffuse but rather focal distribution. On the other hand, interstitial cellular infiltration and pleural fibrosis were diffuse. Typical pathological images are shown in Figs. 2, 3, 4.
Table 5.
Pathological diagnosis and findings
| Diagnosis | n = 12 | |||
| cellular-NSIP | 4 | |||
| fibrosing-NSIP | 5 | |||
| unclassifiable interstitial pneumonia | 3 | |||
| Findings | absent | mild | moderate | severe |
| Organizing pneumonia | 1 | 0 | 7 | 4 |
| Alveolar epithelial injury | 1 | 1 | 8 | 2 |
| Interstitial cellular infiltration | 0 | 4 | 5 | 3 |
| Pleural fibrosis | 0 | 4 | 8 | 0 |
| Collapse | 1 | 4 | 6 | 1 |
| Alveolar wall fibrosis | 3 | 4 | 4 | 1 |
| Pleuritis | 2 | 9 | 1 | 0 |
| Lymphoid follicles with germinal centers | 6 | 6 | 0 | 0 |
| Fibroblastic focus | 8 | 2 | 2 | 0 |
| Microscopic honeycombing | 10 | 2 | 0 | 0 |
Organizing pneumonia involved less than 20% of the overall biopsy specimen when the diagnosis was NSIP [21]. Alveolar epithelial injury was defined as obscured border between alveolar septum and alveolar lumina with alveolar epithelial shedding, intra-alveolar cellular infiltration and membranous organization of the alveolar ducts and alveolar sac which meant focal acute lung injury. Collapse meant atelectatic alveoli with intra-alveolar fibrosis and agglutination of alveoli [35].
NSIP: nonspecific interstitial pneumonia
Fig. 2.
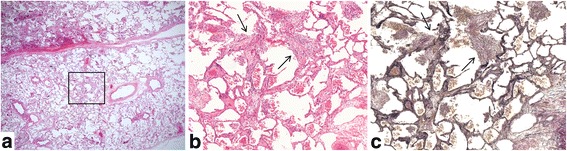
Histopathology of surgical lung biopsies of patient #10 (cellular NSIP, 62-years-old female, right S9). a Low power view of the specimen showed panlobular and homogeneous pattern including interstitial cellular infiltration and organization located in airspace or alveolar septa. b, c High power view of an area of a square of (a) revealed focal membranous organization (arrows) observed in the alveolar duct. Ill-defined border between the alveolar septa and alveolar lumina was seen. Staining: (a, b) Hematoxylin and eosin stain, (c) Elastica van Gieson stein, Magnification: (a) 1×, (b, c) 10×
Fig. 3.
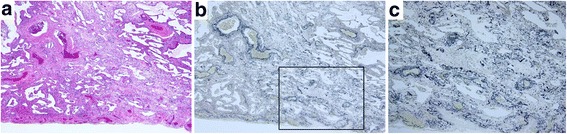
Histopathology of surgical lung biopsies of patient #6 (fibrosing NSIP, 72-years-old female, left S9). a, b Uniform fibrosis of the same age was observed diffusely in alveolar septa. Collapse was observed partially (square). c A higher magnification of an area of a square of (b) showing intraluminal and mural incorporation fibrosis and condensed elastic fiber of the alveolar walls. Staining: (a) Hematoxylin and eosin stain, (b, c) Elastica van Gieson stein, Magnification: (a, b) 4×, (c) 10×
Fig. 4.
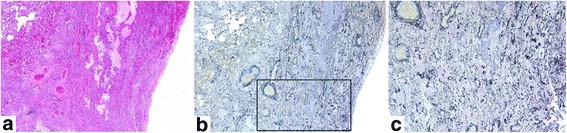
Histopathology of surgical lung biopsies of patient #3 (unclassifiable interstitial pneumonia, 60-years-old male, right S2). The specimen showed mixed fibrosing NSIP pattern and marked collapse with slight inflammatory cell infiltration. a, b Collapse with obliterative intraalveolar fibrosis and disrupted alveolar elastic fibers were observed in the square. c Fragmented elastic fibers of the alveolar walls were seen in a higher magnification of an area of a square of (b). Staining: (a) Hematoxylin and eosin stain, (b, c) Elastica van Gieson stein, Magnification: (a, b) 4×, (c) 10×
Organizing pneumonia and alveolar epithelial injury with moderate or severe grade were observed not only in patients with acute onset but also in those with chronic onset (Table 6).
Table 6.
Number of patients of each pathological finding with moderate or severe grade
| Findings | acute onset (n = 8) | chronic onset (n = 4) |
|---|---|---|
| Organizing pneumonia | 8 | 3 |
| Alveolar epithelial injury | 6 | 4 |
| Interstitial cellular infiltration | 6 | 2 |
| Pleural fibrosis | 5 | 3 |
| Collapse | 3 | 4 |
| Alveolar wall fibrosis | 2 | 3 |
| Pleuritis | 0 | 1 |
| Lymphoid follicles with germinal centers | 0 | 0 |
| Fibroblastic focus | 0 | 2 |
| Microscopic honeycombing | 0 | 0 |
Clinical course and treatment response
Details on the clinical courses and therapy of the 12 patients are shown in Table 7. Seven patients were treated with combination therapy of corticosteroid and immunosuppressant as the initial treatment while five were given corticosteroid monotherapy. The initial dose of corticosteroid was 0.5 mg/kg/day of prednisone in 11 patients. The median time of prednisone dose tapering to 10 mg/day was 91 days (range, 60–132 days).
Table 7.
The clinical courses and therapy of the 12 patients
| Patient number | Response to initial therapy | Disease onset | Diagnosis | Pathological diagnosis | Initial therapy | Observation period (months) | Period from initial therapy to relapse (months) | Therapy at relapsea | Prognosis | Disease behavior |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | improved | acute | IIP | c-NSIP | PDN | 29 | - | - | alive | A |
| 2 | improved | acute | IIP | unclassifiable IP | PDN + CyA | 27 | - | - | alive | A |
| 3 | improved | acute | IIP | unclassifiable IP | PDN + TAC | 90 | - | - | alive | A |
| 4 | improved | chronic | PM | f-NSIP | Low-PDN | 72 | - | - | alive | A |
| 5 | improved | chronic | IIP | c-NSIP | PDN + TAC | 76 | - | - | alive | A |
| 6SS | improved | chronic | DM | f-NSIP | PDN + TAC | 19 | - | - | alive | A |
| 7 | relapsed | acute | IIP | f-NSIP | PDN | 94 | 29 | PDN | alive | B |
| 8 | relapsed | acute | PM | f-NSIP | PDNc | 82 | 61 | PDN | alive | B |
| 9 | relapsed | acute | DM | c-NSIP | PDNc | 70 | 8 | PDN | alive | B |
| 10 | relapsed | acute | IIP | c-NSIP | PDNc + CyA | 115 | 46 | PDN + CyA | alive | B |
| 11 | relapsed | chronic | PM | f-NSIP | PDN + AZA | 104 | 78 | PDN | alive | B |
| 12 | deteriorated | acute | PMb | unclassifiable IP | PDN + CyA | 17 | - | - | dead | C |
aPatients have received these therapies until ILD relapsed., SS: Sjögren’s syndrome, IIP: idiopathic interstitial pneumonia, PM: polymyositis, DM: dermatomyositis, bsimultaneous onset of PM and interstitial lung disease, c-NSIP: cellular nonspecific interstitial pneumonia, f-NSIP: fibrosing nonspecific interstitial pneumonia, IP: interstitial pneumonia, PDN: 0.5 mg/kg/day of prednisone, CyA: cyclosporine A, TAC: tacrolimus, Low-PDN: 0.2 mg/kg/day of prednisone, cstarted with methylprednisone pulse therapy (500 mg/body), AZA: azathioprine, A: progressive, irreversible disease with potential for stabilization, B: reversible disease with risk of progression, C: progressive, irreversible disease despite therapy
The initial response was evaluated as improved in 11 of 12 patients. Radiological findings improved in 11 of 12 patients. Representative images of chest radiograph are shown in Fig. 5, indicating the apparent lung volume recovery. Pulmonary function, evaluated in 10 patients, improved or stabilized in nine patients. Forced vital capacity % predicted (%FVC) and/or diffusing capacity of the lung for carbon monoxide % predicted (%DLCO) increased immediately after the initial treatment and remained almost stable between the initial evaluation period (Fig. 6). On disease behavior, the type of reversible disease with risk of progression were six patients, progressive and irreversible disease with potential for stabilization were five patients, and progressive and irreversible disease despite therapy were one patient. Patient #12 was the only patient who did not respond to the treatment and died during the follow-up period.
Fig. 5.
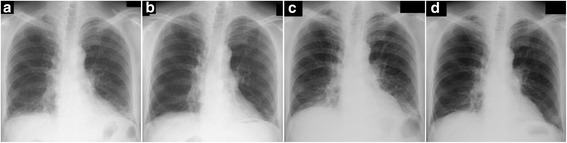
Changes of chest radiographs of patient #11. a The initial chest radiograph before treatment showed infiltration and volume loss of lower lung field. b After four months from the initiation of treatment, consolidation and volume loss improved. c However they arose again at the time of relapse. d At the end of follow-up, treatment improved infiltration and volume
Fig. 6.
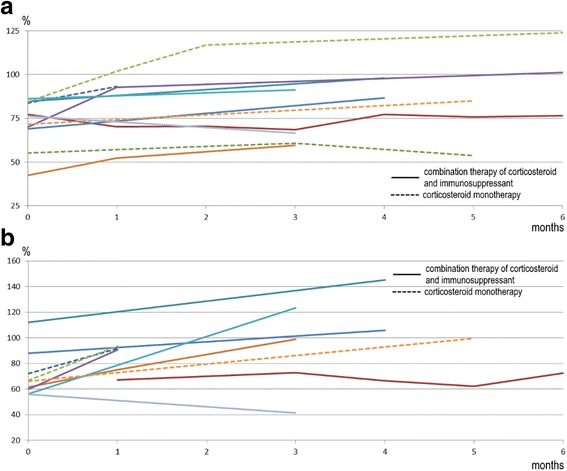
Changes of pulmonary functions of 10 patients during initial evaluation period. Pulmonary function improved or was stable in nine patients. a Line graph of forced vital capacity % predicted. b Line graph of diffusing capacity of the lung for carbon monoxide % predicted
The median follow-up time was 74 months (range, 17–115 months). ILD relapsed in five of the 11 improved patients after a median of 46 months (range, 8–78 months) from treatment initiation. Among the five relapsed patients, four were on prednisone monotherapy and four presented with acute onset of the disease. There were few differences in the changes on chest radiograph and pulmonary function test between the relapsed patients and non-relapsed patients until relapse. However, upon relapse, the loss of lung volume was clearly recognized in chest radiograph, and both %FVC and %DLCO significantly decreased. The addition of immunosuppressant and/or the increase of the dose of corticosteroid reduced the abnormal shadow and prevented deterioration of %FVC and %DLCO (Fig. 7). On the other hand, four of the six patients without relapse had continuously been on combination therapy of corticosteroid and immunosuppressant.
Fig. 7.
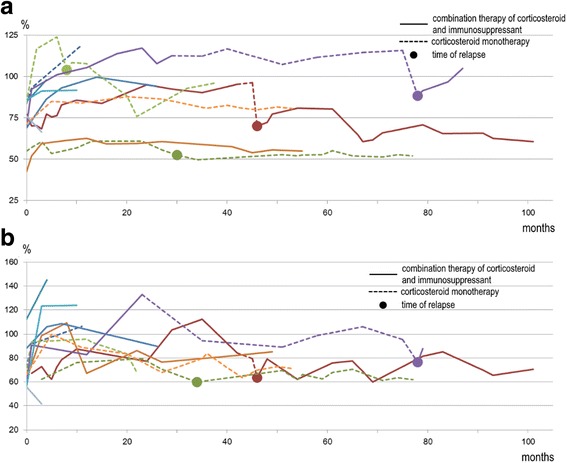
Changes of pulmonary functions of 10 patients during whole follow up period. Four patients deteriorated with corticosteroid monotherapy. a Line graph of forced vital capacity % predicted. b Line graph of diffusing capacity of the lung for carbon monoxide % predicted
Discussion
We retrospectively evaluated the clinical, radiological, pathological features and long-term clinical courses of 12 patients with EJ-ILD who underwent surgical lung biopsy. We have shown that patients with EJ-ILD had often acute onset of ILD with lower-lung predominant shadows with volume loss on HRCT, pathologically NSIP or unclassifiable interstitial pneumonia with acute inflammatory findings, and good response for initial steroid therapy, although relapse was frequent among the patients with corticosteroid monotherapy. In most patients with EJ-ILD clinical disease behavior was reversible or progressive and irreversible disease. Although anti-EJ antibodies correlated well with ILD, there are a few reports which describe the clinical, radiological and pathological findings of EJ-ILD and no reports describe any detailed clinical courses [16, 26]. To our knowledge, this is the first study to describe the detailed clinical features of biopsy-proven EJ-ILD with long-term follow-up period.
The onset of EJ-ILD was often acute in the present study. EJ-ILD progressed within three months in previous literature [15, 16]. Fifty to 70% of patients with anti-ARS antibodies other than anti-EJ antibody reportedly presented with chronic onset of ILD [12, 13, 27–29]. This suggests that onset of the disease varies among ARS-ILD, depending on the type of specific antibody.
Fifty percent of patients in this cohort were ultimately diagnosed with PM/DM, which was consistent with the previous report [8, 10]. However, our study showed that five of six PM/DM patients had ILD-preceding type, whereas only 30%–35% of patients with anti-ARS antibodies were reportedly ILD-preceding disease [12, 14, 17, 28].
Regarding to IPAF and anti-synthetase syndrome, almost no patient presented physical symptoms at initial visit. However, a half of them developed muscular symptoms and fever during follow-up period. This result is also compatible with the previous report [10]. These suggest that it is difficult to distinguish anti-synthetase syndrome from IPAF at initial visit and pulmonary symptoms precede in some patients with anti-EJ antibody before developing anti-synthetase syndrome or PM/DM.
The HRCT findings were basically compatible with those of previous reports on ARS-ILD and ILD associated with PM/DM [9, 20, 30]. Our study revealed consolidation and volume loss along the bronchovascular bundle predominantly in the middle and lower lung zones. These findings were similar to the radiological NSIP pattern [21]. In some cases, changes in lung volume on chest radiograph correlated well with lung function and disease condition. Chest radiograph may be an adjunct indicator when chest CT is unavailable.
Pathologically unclassifiable interstitial pneumonia and NSIP were observed in our study although recent reports showed that the histopathological patterns of ARS-ILD were NSIP, UIP, ALI and DAD [11, 13, 15, 16]. Acute inflammatory findings have not been shown in ARS-ILD patients with chronic onset in previous literature [13, 15, 16]. We here showed that acute inflammatory findings focally distributed with few hyaline membrane were observed not only in acute onset but also in chronic onset. In this point, our results support the recent reports. The pathological diagnosis of EJ-ILD may not match but resemble ALI and DAD because these were reported as indicating the diffuse and uniform widespread involvement of the pulmonary parenchyma and the pulmonary lobule with hyaline membrane [15, 16, 31, 32]. Moreover, the high prevalence of acute inflammatory findings and ILD distribution seem to suggest very similar type ILD as in other anti-synthetase antibodies. However, we cannot reliably draw a conclusion because of a small cohort size.
In this study, the initial treatment response of EJ-ILD and the rate of relapse were similar to those previously reported for ARS-ILD [14]. Although the initial treatment for EJ-ILD was effective for most of the patients, ILD relapsed in nearly half of them. These findings suggest that the patients with EJ-ILD may have the potential high risk of disease progression. We found that those with relapse presented with NSIP, often on acute onset and were on corticosteroid monotherapy in this cohort, whereas the rate of relapse of ILD was lower in patients on combination therapy. To date, it has also been shown that in ARS-ILD the corticosteroid dose reduction might relate to the relapse [12, 14], and combination therapy of corticosteroid and tacrolimus was effective [33]. The combination therapy was also recommended to treat ILD related to PM/DM [34]. These suggest that combination therapy of corticosteroid and immunosuppressant may be preferable to corticosteroid monotherapy especially in case of acute-onset ILD.
This study has several limitations. First, it was a retrospective study with a small sample size. Second, since this study was performed in a specialized single-center for the respiratory diseases, there might be a selection bias for the diagnosis of PM/DM at the initial visit. However, in fact, all patients except patient #12 had muscle weakness at the initial visit.
Conclusions
We delineated the long-term clinical characteristics of EJ-ILD. Because of the diversity of the clinical course, combination therapy of corticosteroid and immunosuppressant should be argued on a case-by-case basis to prevent relapse of EJ-ILD. We need to conduct long-term observation to assess clinical behavior and prevent complications of EJ-ILD.
Acknowledgements
We would like to acknowledge all the participants. We would also like to thank Ms Etsuko Iwata and Ms Miyako Nakagawa who were research assistants of Tokai University School of Medicine to contribute to detection of anti-ARS antibodies. The authors would like to thank Enago (www.enago.jp) for the professional English language review.
Funding
This study is partially supported by a grant from the Ministry of Health, Labour and Welfare of Japan awarded to the Study Group on Diffuse Pulmonary Disorders, Scientific Research/Research on intractable diseases [Grant H26-Nanchi-Ippan-023]. This research is partially supported by the Practical Research Project for Rare Intractable Diseases from Japan Agency for Medical Research and development, AMED [Grant 15ek0109064h0002].
Availability of data and materials
The dataset supporting the conclusions of this article is presented within the article. The detailed clinical data is not available because of patients’ confidentiality.
Authors’ contributions
HS had full access to and takes responsibility for the integrity of the data and the accuracy of the data analysis. EH contributed to data organization, analysis and preparation. HK contributed to envisioning the initial concept and design of this project. YE, NM, TB, SI, KO, TI and TT contributed to data collection, organization, analysis and preparation. SS and YS contributed to detection of anti-ARS antibodies and organization. TO contributed to the data conception, design, organization, analysis and preparation. All authors read and approved the final manuscript.
Competing interests
The authors have reported to BMC Pulmonary Medicine that no potential competing interests exist with any companies/organizations whose products or services may be discussed in this article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the institutional review board of Kanagawa Cardiovascular and Respiratory Center (approval number 25–43). Because of the retrospective nature of the study, the review board waived the need for written informed consent from the patients.
Abbreviations
- %DLCO
Diffusing capacity of the lung for carbon monoxide % predicted
- %FVC
Forced vital capacity % predicted
- ALI
Acute lung injury
- ARS
Aminoacyl-tRNA synthetase
- ARS-ILD
Interstitial lung disease with positive-anti-ARS antibodies
- ATS
American Thoracic Society
- c-NSIP
Cellular nonspecific interstitial pneumonia
- CT
Computed tomography
- DAD
Diffuse alveolar damage
- EJ-ILD
Interstitial lung disease with positive-anti-EJ antibodies
- ERS
European Respiratory Society
- f-NSIP
Fibrosing nonspecific interstitial pneumonia
- HRCT
High-resolution computed tomography
- ILD
Interstitial lung disease
- IPAF
Interstitial pneumonia with autoimmune features
- NSIP
Nonspecific interstitial pneumonia
- PM/DM
Polymyositis/dermatomyositis
- UIP
Usual interstitial pneumonia
Contributor Information
Hajime Sasano, Email: hajimesumire@gmail.com.
Eri Hagiwara, Email: hagiwara@kanagawa-junko.jp.
Hideya Kitamura, Email: kitamura@kanagawa-junko.jp.
Yasunori Enomoto, Email: yasuyasuyasu29@yahoo.co.jp.
Norikazu Matsuo, Email: matsuo_norikazu@med.kurume-u.ac.jp.
Tomohisa Baba, Email: baba@kanagawa-junko.jp.
Shinichiro Iso, Email: si999si999@gmail.com.
Koji Okudela, Email: krascv@yahoo.co.jp.
Tae Iwasawa, Email: tae_i_md@wb3.so-net.ne.jp.
Shinji Sato, Email: shinsjam@gmail.com.
Yasuo Suzuki, Email: y3suzuki@is.icc.u-tokai.ac.jp.
Tamiko Takemura, Email: byori@med.jrc.or.jp.
Takashi Ogura, Phone: +81-45-701-9581, Email: takashiogurajunko@yahoo.co.jp.
References
- 1.Kinder BW, Collard HR, Koth L, Daikh DI, Wolters PJ, Elicker B, Jones KD, King TE., Jr Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? Am J Respir Crit Care Med. 2007;176(7):691–697. doi: 10.1164/rccm.200702-220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, Lee JS, Leslie KO, Lynch DA, Matteson EL, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46(4):976–987. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 3.Lega JC, Fabien N, Reynaud Q, Durieu I, Durupt S, Dutertre M, Cordier JF, Cottin V. The clinical phenotype associated with myositis-specific and associated autoantibodies: a meta-analysis revisiting the so-called antisynthetase syndrome. Autoimmun Rev. 2014;13(9):883–891. doi: 10.1016/j.autrev.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Mimori T, Imura Y, Nakashima R, Yoshifuji H. Autoantibodies in idiopathic inflammatory myopathy: an update on clinical and pathophysiological significance. Curr Opin Rheumatol. 2007;19(6):523–529. doi: 10.1097/BOR.0b013e3282f01a8c. [DOI] [PubMed] [Google Scholar]
- 5.Targoff IN. Immune manifestations of inflammatory muscle disease. Rheum Dis Clin North Am. 1994;20(4):857–880. [PubMed] [Google Scholar]
- 6.Hirakata M, Nagai S. Interstitial lung disease in polymyositis and dermatomyositis. Curr Opin Rheumatol. 2000;12(6):501–508. doi: 10.1097/00002281-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Targoff IN, Trieu EP, Plotz PH, Miller FW. Antibodies to glycyl-transfer RNA synthetase in patients with myositis and interstitial lung disease. Arthritis Rheum. 1992;35(7):821–830. doi: 10.1002/art.1780350718. [DOI] [PubMed] [Google Scholar]
- 8.Takato H, Waseda Y, Watanabe S, Inuzuka K, Katayama N, Ichikawa Y, Yasui M, Fujimura M. Pulmonary manifestations of anti-ARS antibody positive interstitial pneumonia--with or without PM/DM. Respir Med. 2014;107(1):128–133. doi: 10.1016/j.rmed.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe K, Handa T, Tanizawa K, Hosono Y, Taguchi Y, Noma S, Kobashi Y, Kubo T, Aihara K, Chin K, et al. Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias. Respir Med. 2011;105(8):1238–1247. doi: 10.1016/j.rmed.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Hamaguchi Y, Fujimoto M, Matsushita T, Kaji K, Komura K, Hasegawa M, Kodera M, Muroi E, Fujikawa K, Seishima M, et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One. 2013;8(4):e60442. doi: 10.1371/journal.pone.0060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer A, Swigris JJ, du Bois RM, Lynch DA, Downey GP, Cosgrove GP, Frankel SK, Fernandez-Perez ER, Gillis JZ, Brown KK. Anti-synthetase syndrome in ANA and anti-Jo-1 negative patients presenting with idiopathic interstitial pneumonia. Respir Med. 2009;103(11):1719–1724. doi: 10.1016/j.rmed.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koreeda Y, Higashimoto I, Yamamoto M, Takahashi M, Kaji K, Fujimoto M, Kuwana M, Fukuda Y. Clinical and pathological findings of interstitial lung disease patients with anti-aminoacyl-tRNA synthetase autoantibodies. Intern Med. 2010;49(5):361–369. doi: 10.2169/internalmedicine.49.2889. [DOI] [PubMed] [Google Scholar]
- 13.Yousem SA, Gibson K, Kaminski N, Oddis CV, Ascherman DP. The pulmonary histopathologic manifestations of the anti-Jo-1 tRNA synthetase syndrome. Mod Pathol. 2010;23(6):874–880. doi: 10.1038/modpathol.2010.65. [DOI] [PubMed] [Google Scholar]
- 14.Yoshifuji H, Fujii T, Kobayashi S, Imura Y, Fujita Y, Kawabata D, Usui T, Tanaka M, Nagai S, Umehara H, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity. 2006;39(3):233–241. doi: 10.1080/08916930600622884. [DOI] [PubMed] [Google Scholar]
- 15.Hara Y, Tanaka T, Tabata K, Shiraki A, Hayashi K, Kashima Y, Hayashi T, Fukuoka J. Anti-glycyl tRNA synthetase antibody associated interstitial lung disease without symptoms of polymyositis/dermatomyositis. Pathol Int. 2014;64(3):148–150. doi: 10.1111/pin.12140. [DOI] [PubMed] [Google Scholar]
- 16.Schneider F, Yousem SA, Bi D, Gibson KF, Oddis CV, Aggarwal R. Pulmonary pathologic manifestations of anti-glycyl-tRNA synthetase (anti-EJ)-related inflammatory myopathy. J Clin Pathol. 2014;67(8):678–683. doi: 10.1136/jclinpath-2014-202367. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Kuwana M, Hirakata M. Clinical characteristics of Japanese patients with anti-OJ (anti-isoleucyl-tRNA synthetase) autoantibodies. Rheumatology (Oxford) 2007;46(5):842–845. doi: 10.1093/rheumatology/kel435. [DOI] [PubMed] [Google Scholar]
- 18.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 19.Lega JC, Reynaud Q, Belot A, Fabien N, Durieu I, Cottin V. Idiopathic inflammatory myopathies and the lung. Eur Respir Rev. 2015;24(136):216–238. doi: 10.1183/16000617.00002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mino M, Noma S, Taguchi Y, Tomii K, Kohri Y, Oida K. Pulmonary involvement in polymyositis and dermatomyositis: sequential evaluation with CT. AJR Am J Roentgenol. 1997;169(1):83–87. doi: 10.2214/ajr.169.1.9207505. [DOI] [PubMed] [Google Scholar]
- 21.Travis WD, Hunninghake G, King TE, Jr, Lynch DA, Colby TV, Galvin JR, Brown KK, Chung MP, Cordier JF, du Bois RM, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med. 2008;177(12):1338–1347. doi: 10.1164/rccm.200611-1685OC. [DOI] [PubMed] [Google Scholar]
- 22.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer A, West SG, Swigris JJ, Brown KK, du Bois RM. Connective tissue disease-associated interstitial lung disease: a call for clarification. Chest. 2010;138(2):251–256. doi: 10.1378/chest.10-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vij R, Noth I, Strek ME. Autoimmune-featured interstitial lung disease: a distinct entity. Chest. 2011;140(5):1292–1299. doi: 10.1378/chest.10-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 26.Hara H, Inoue Y, Sato T. Clinical and pathological findings of patients with interstitial lung disease associated with antisynthetase. Nihon Kokyuki Gakkai Zasshi. 2005;43(11):652–663. [PubMed] [Google Scholar]
- 27.Hervier B, Wallaert B, Hachulla E, Adoue D, Lauque D, Audrain M, Camara B, Fournie B, Couret B, Hatron PY, et al. Clinical manifestations of anti-synthetase syndrome positive for anti-alanyl-tRNA synthetase (anti-PL12) antibodies: a retrospective study of 17 cases. Rheumatology (Oxford) 2010;49(5):972–976. doi: 10.1093/rheumatology/kep455. [DOI] [PubMed] [Google Scholar]
- 28.Marie I, Hachulla E, Cherin P, Dominique S, Hatron PY, Hellot MF, Devulder B, Herson S, Levesque H, Courtois H. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum. 2002;47(6):614–622. doi: 10.1002/art.10794. [DOI] [PubMed] [Google Scholar]
- 29.Tillie-Leblond I, Wislez M, Valeyre D, Crestani B, Rabbat A, Israel-Biet D, Humbert M, Couderc LJ, Wallaert B, Cadranel J. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax. 2008;63(1):53–59. doi: 10.1136/thx.2006.069237. [DOI] [PubMed] [Google Scholar]
- 30.Debray MP, Borie R, Revel MP, Naccache JM, Khalil A, Toper C, Israel-Biet D, Estellat C, Brillet PY. Interstitial lung disease in anti-synthetase syndrome: initial and follow-up CT findings. Eur J Radiol. 2015;84(3):516–523. doi: 10.1016/j.ejrad.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 31.Katzenstein AL, Bloor CM, Leibow AA. Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review Am J Pathol. 1976;85(1):209–228. [PMC free article] [PubMed] [Google Scholar]
- 32.Katzenstein AL, Myers JL, Mazur MT. Acute interstitial pneumonia. A clinicopathologic, ultrastructural, and cell kinetic study. Am J Surg Pathol. 1986;10(4):256–267. doi: 10.1097/00000478-198604000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV. Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis Rheum. 2005;52(8):2439–2446. doi: 10.1002/art.21240. [DOI] [PubMed] [Google Scholar]
- 34.Takada K, Kishi J, Miyasaka N. Step-up versus primary intensive approach to the treatment of interstitial pneumonia associated with dermatomyositis/polymyositis: a retrospective study. Mod Rheumatol. 2007;17(2):123–130. doi: 10.3109/s10165-007-0553-3. [DOI] [PubMed] [Google Scholar]
- 35.Burkhardt A. Alveolitis and collapse in the pathogenesis of pulmonary fibrosis. Am Rev Respir Dis. 1989;140(2):513–524. doi: 10.1164/ajrccm/140.2.513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is presented within the article. The detailed clinical data is not available because of patients’ confidentiality.


