Abstract
Ligation of membrane IgM on B lymphocytes causes activation of a protein-tyrosine kinase(s) (PTK) and of phospholipase C (PLC). To determine whether these are elements of a common signal-transduction pathway, the effect of three PTK inhibitors on the rise in intracellular free Ca2+ concentration [( Ca2+]i) in human B-lymphoblastoid cell lines was assessed. Tyrphostin completely suppressed the increase in [Ca2+]i and the generation of inositol phosphates induced by ligation of membrane immunoglobulin (mIg) M. Herbimycin and genistein reduced by 30% and 50%, respectively, the rise in [Ca2+]i caused by optimal ligation of mIgM, and they abolished it in cells activated by suboptimal ligation of mIgM. Tyrphostin had no effect on the capacity of aluminum fluoride to increase [Ca2+]i. To determine whether a function of PTK is the phosphorylation of PLC, immunoprecipitates obtained with anti-phosphotyrosine from detergent lysates of B-lymphoblastoid cells were assayed for PLC activity. Ligation of mIgM increased immunoprecipitable PLC activity 2-fold by 90 sec and 4-fold by 30 min. Specific immunoprecipitation and Western blot analysis identified tyrosine phosphorylation of the gamma 1 isoform of PLC after 60 sec of stimulation. Activation of PLC in B cells by mIgM requires PTK function and is associated with tyrosine phosphorylation of PLC-gamma 1, suggesting a mechanism of PLC activation similar to that described for certain receptor PTKs.
Full text
PDF
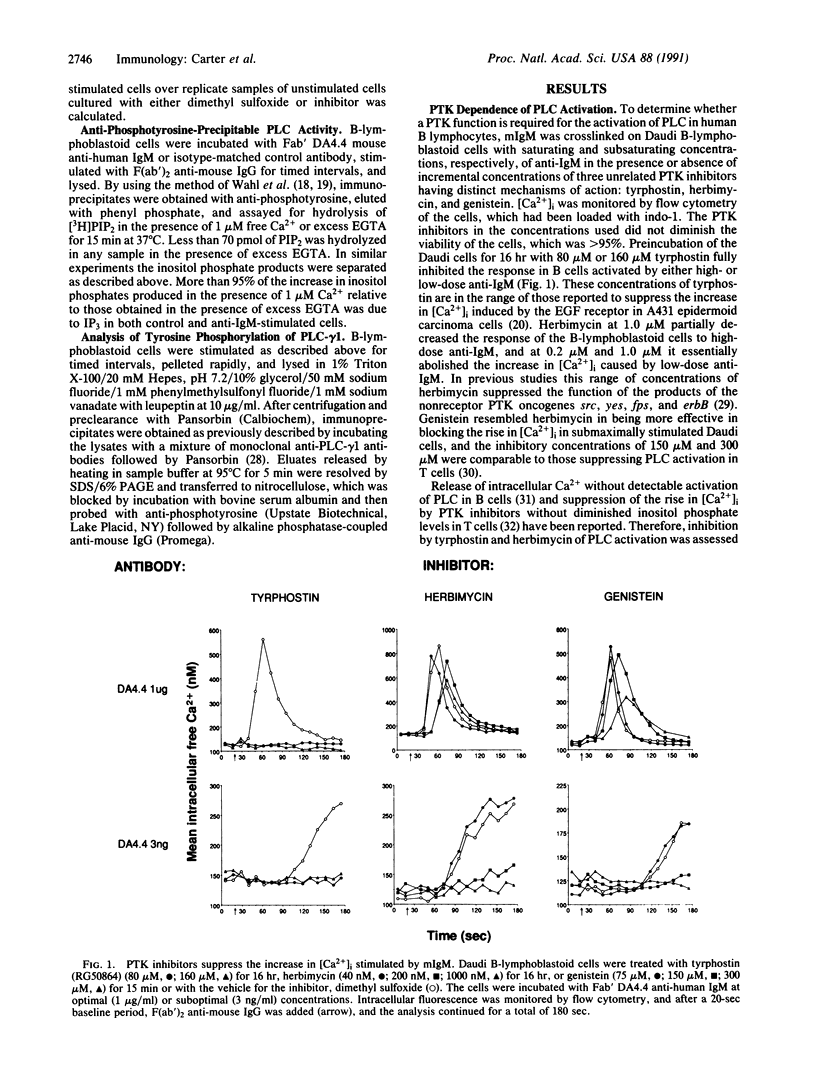
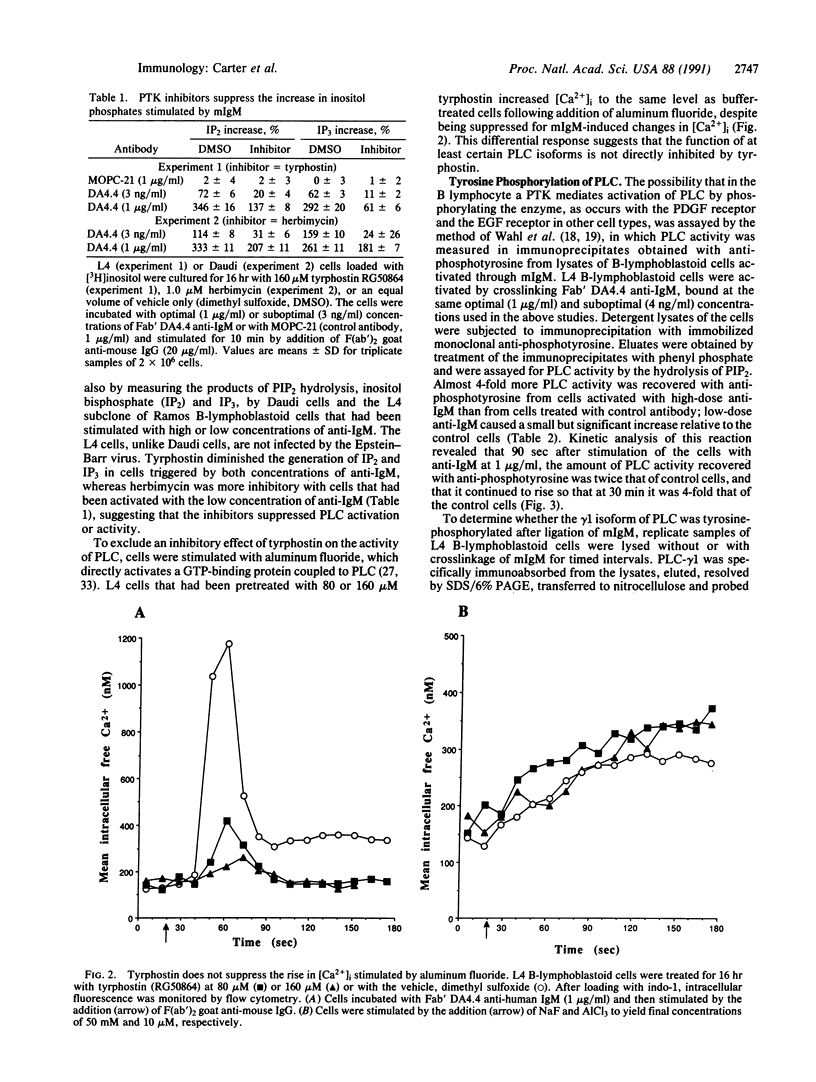
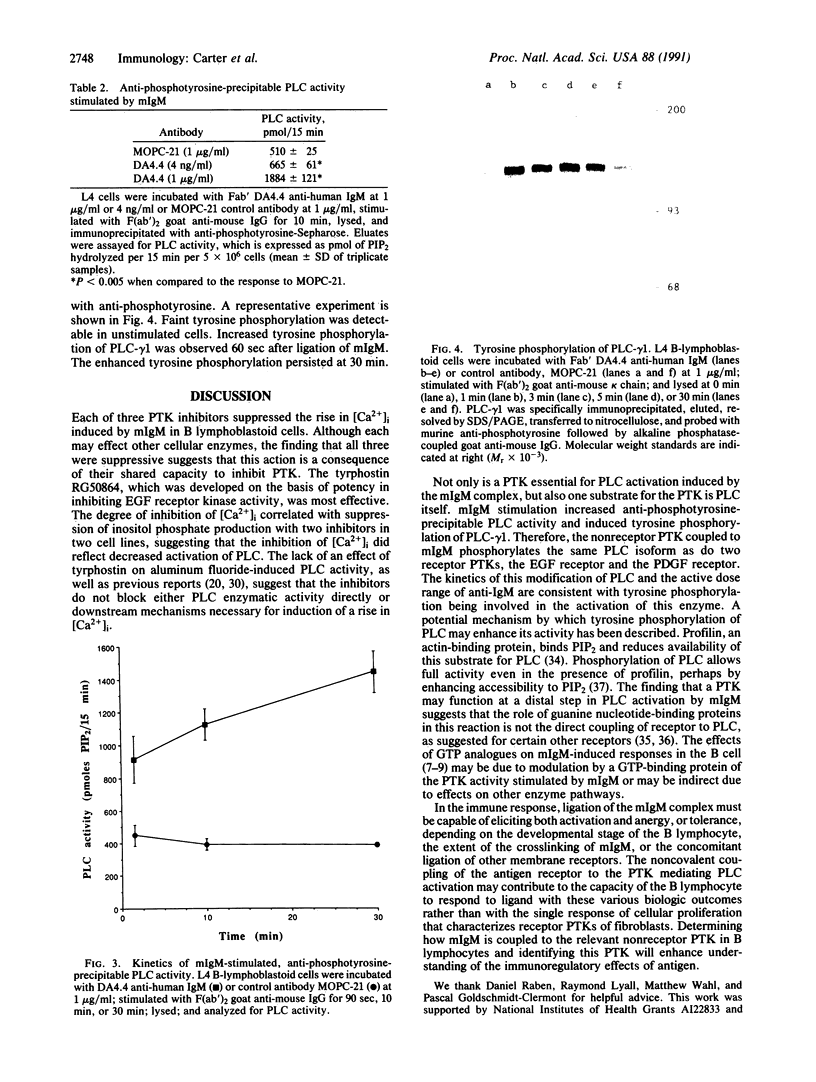

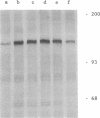
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Meade C. J., Turner G. A., Klaus G. G. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985 Jul;41(3):999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J. 1990 Jul;9(7):2125–2131. doi: 10.1002/j.1460-2075.1990.tb07381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. H., Spycher M. O., Ng Y. C., Hoffman R., Fearon D. T. Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes. J Immunol. 1988 Jul 15;141(2):457–463. [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Chen Z. Z., Coggeshall K. M., Cambier J. C. Translocation of protein kinase C during membrane immunoglobulin-mediated transmembrane signaling in B lymphocytes. J Immunol. 1986 Mar 15;136(6):2300–2304. [PubMed] [Google Scholar]
- Coggeshall K. M., Cambier J. C. B cell activation. VIII. Membrane immunoglobulins transduce signals via activation of phosphatidylinositol hydrolysis. J Immunol. 1984 Dec;133(6):3382–3386. [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Dymecki S. M., Niederhuber J. E., Desiderio S. V. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990 Jan 19;247(4940):332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Jakway J. P., DeFranco A. L. Involvement of a guanine-nucleotide-binding component in membrane IgM-stimulated phosphoinositide breakdown. J Immunol. 1987 Dec 1;139(11):3604–3613. [PubMed] [Google Scholar]
- Gold M. R., Law D. A., DeFranco A. L. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature. 1990 Jun 28;345(6278):810–813. doi: 10.1038/345810a0. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Baldassare J. J., Pollard T. D. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990 Mar 30;247(4950):1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- Graziadei L., Riabowol K., Bar-Sagi D. Co-capping of ras proteins with surface immunoglobulins in B lymphocytes. Nature. 1990 Sep 27;347(6291):396–400. doi: 10.1038/347396a0. [DOI] [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein coupling of antigen receptor-stimulated polyphosphoinositide hydrolysis in B cells. J Immunol. 1988 May 1;140(9):3135–3139. [PubMed] [Google Scholar]
- Harris L. K., Cambier J. C. B lymphocyte activation. Transmembrane signal transduction by membrane immunoglobulin in isolated cell membranes. J Immunol. 1987 Aug 1;139(3):963–970. [PubMed] [Google Scholar]
- Hasegawa-Sasaki H., Lutz F., Sasaki T. Pathway of phospholipase C activation initiated with platelet-derived growth factor is different from that initiated with vasopressin and bombesin. J Biol Chem. 1988 Sep 15;263(26):12970–12976. [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Kim U. H., Kim J. W., Rhee S. G. Phosphorylation of phospholipase C-gamma by cAMP-dependent protein kinase. J Biol Chem. 1989 Dec 5;264(34):20167–20170. [PubMed] [Google Scholar]
- Kumjian D. A., Wahl M. I., Rhee S. G., Daniel T. O. Platelet-derived growth factor (PDGF) binding promotes physical association of PDGF receptor with phospholipase C. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8232–8236. doi: 10.1073/pnas.86.21.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Maruyama S., Kubagawa H., Cooper M. D. Activation of human B cells and inhibition of their terminal differentiation by monoclonal anti-mu antibodies. J Immunol. 1985 Jul;135(1):192–199. [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Mizuguchi J., Ji Y. Y., Nakabayaschi H., Huang K. P., Beaven M. A., Chused T., Paul W. E. Protein kinase C activation blocks anti-IgM-mediated signaling BAL17 B lymphoma cells. J Immunol. 1987 Aug 15;139(4):1054–1059. [PubMed] [Google Scholar]
- Monroe J. G., Haldar S. Involvement of a specific guanine nucleotide binding protein in receptor immunoglobulin stimulated inositol phospholipid hydrolysis. Biochim Biophys Acta. 1989 Oct 9;1013(3):273–278. doi: 10.1016/0167-4889(89)90146-8. [DOI] [PubMed] [Google Scholar]
- Monroe J. G., Seyfert V. L., Owen C. S., Sykes N. Isolation and characterization of a B lymphocyte mutant with altered signal transduction through its antigen receptor. J Exp Med. 1989 Mar 1;169(3):1059–1070. doi: 10.1084/jem.169.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Quintrell N., Lebo R., Varmus H., Bishop J. M., Pettenati M. J., Le Beau M. M., Diaz M. O., Rowley J. D. Identification of a human gene (HCK) that encodes a protein-tyrosine kinase and is expressed in hemopoietic cells. Mol Cell Biol. 1987 Jun;7(6):2267–2275. doi: 10.1128/mcb.7.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevillyan J. M., Lu Y. L., Atluru D., Phillips C. A., Bjorndahl J. M. Differential inhibition of T cell receptor signal transduction and early activation events by a selective inhibitor of protein-tyrosine kinase. J Immunol. 1990 Nov 15;145(10):3223–3230. [PubMed] [Google Scholar]
- Uehara Y., Murakami Y., Mizuno S., Kawai S. Inhibition of transforming activity of tyrosine kinase oncogenes by herbimycin A. Virology. 1988 May;164(1):294–298. doi: 10.1016/0042-6822(88)90649-6. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Daniel T. O., Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science. 1988 Aug 19;241(4868):968–970. doi: 10.1126/science.2457254. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y., Mori S., Yoshida M., Kishimoto T., Inoue K., Yamamoto T., Toyoshima K. Selective expression of a protein-tyrosine kinase, p56lyn, in hematopoietic cells and association with production of human T-cell lymphotropic virus type I. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6538–6542. doi: 10.1073/pnas.86.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Marth J. D., Lewis D. B., Perlmutter R. M. Novel protein-tyrosine kinase gene (hck) preferentially expressed in cells of hematopoietic origin. Mol Cell Biol. 1987 Jun;7(6):2276–2285. doi: 10.1128/mcb.7.6.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]