Abstract
Background
Subclinical cognitive impairment is prevalent in heart failure (HF); however, its role in important clinical outcomes, such as HF treatment adherence, is unclear. Given the complex polypharmacy in HF treatment, cognitive deficits may be important in predicting medication management. Thus, the objective of the current study was to examine the impact of cognitive function on medication adherence among community-dwelling patients with HF using objective assessments.
Methods and Results
A prospective observational cohort design of 309 community-dwelling patients with HF (59.7% male, 68.7±9.7 years) and no history of dementia or neurological disease. Cognition was assessed using a neuropsychological battery at baseline. Medication adherence was objectively measured for 21 days using an electronic pillbox. Regression analyses tested whether attention, executive function, or memory predicted 21-day medication adherence. In unadjusted analyses, lower scores on all three cognitive domains predicted poorer medication adherence (β = .52–85, p = .001–.009). After adjusting for demographic, clinical, and psychosocial variables, memory continued to predict medication adherence (β = 0.51, p = .008), whereas executive function (β = 0.24 p = .075), and attention were no longer a predictor (β = 0.34, p = .131).
Conclusions
Poorer cognitive function, especially in regard to memory, predicted reduced medication adherence among patients with HF and no history of dementia. This effect remained after adjustment for factors known to predict adherence, such as depressed mood, social support, and disease severity level. Future studies should examine the link from cognitive impairment and medication non-adherence to clinical outcomes (e.g., hospitalization and mortality).
Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01461629.
Keywords: heart failure, cognitive function, medication adherence
Nearly 6 million adults in the U.S. have heart failure (HF) 1 and its associated risks of poorer biopsychosocial outcomes,2–4 including multi-domain cognitive impairment.5–7 Although cognitive deficits are most common and severe in inpatient samples (up to 80%), cognitive impairment is also widespread even among community-dwelling HF populations (15–25%).5–7
Though cognitive deficits are pervasive, cognitive screening is not yet a practice guideline for HF management.8 One reason is that these deficits are rarely examined as predictors of objectively-measured patient behaviors or hard clinical outcomes; thus, their clinical impact is not yet known. The lack of evidence on clinical effects is problematic as initial data suggest that cognitive function may impact patients’ abilities to adhere to the complex HF treatment regimen.9,10 Medication adherence may be especially difficult for those with cognitive impairment given the increasingly complex nature of pharmacotherapy for HF.11,12 To our knowledge, only two studies have examined cognitive function in relation to objectively-measured medication adherence in patients with HF.9,10 Both suggest that cognitive deficits contribute to poorer adherence but were limited by lack of sample diversity (i.e., veterans)9 or failure to examine multiple cognitive domains.10
Difficulties with medication adherence might be expected even among cognitively intact patients13 as the typical patient with HF is prescribed 11 different medications, including but not limited to angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB), beta-blockers, aldosterone antagonist, and vasodilators.11,12 Comorbid diseases add complexity to this already extensive regimen with some patients taking more than 20 medications.12 This polypharmacy results in challenging dosing patterns in which patients take more than 10 doses/day.11 As an example, a patient may be taking oral Lisinopril (20 mg) and Spironolactone (50 mg) once/day, and Carvedilol (25 mg) twice/day, and Hydralazine (75 mg) three times/day for heart failure symptoms alone – not to mention medications for hyperglycemia and depression. Unfortunately, poorer adherence to HF medication has serious consequences, as partially adherent and non-adherent HF patients demonstrate more adverse coronary events, hospitalizations, and all-cause mortality compared to their adherent counterparts.14–16
Thus, the role of cognitive function in medication adherence should be further explored using objectively-measured medication adherence as well as multiple cognitive domains. Examining the cognitive function-adherence relationship among community-dwelling HF patients is particularly important as these individuals will likely not benefit from the adherence-promoting mechanisms that are in place for the most cognitively impaired patients (e.g., 24-hour care in nursing facilities). Accordingly, the primary objective of the current study was to examine the impact of cognitive function on medication adherence among community-dwelling patients with HF using objective assessments. To test our hypothesis that poorer cognitive function would predict poorer adherence to medications, we employed a neuropsychological battery and objectively-monitored medication adherence using an electronic pillbox. If cognitive deficits predict patient adherence behaviors, they should be assessed and used to identify patients at risk for non-adherence and poorer clinical outcomes. Such information may also be used to guide interventions to improve adherence efforts among this population.
Methods
Participants
The Heart Adherence Behavior and Cognition (Heart ABC) study (Trial Identifier: NCT01461629 http://clinicaltrials.gov/ct2/show/NCT01461629?term=self+management+heart+failure&rank=5) is an NIH-funded prospective cohort study of patients with HF. Heart ABC enrolled 372 patients recruited from a variety of cardiology practices in two major hospital systems in Northeast Ohio. Eligibility requirements were chosen to maximize the number of HF patients presenting with cognitive impairment but minimize the possibility that the impairment was a result of non-HF-related disease processes (e.g., head injury, psychoticism, Alzheimer’s, substance use disorder, etc.). The criteria were as follows: (1) Aged 50–85 years at enrollment, (2) Systolic HF diagnosis for at least 3 months and verifiable in the medical record (within 3 years of study enrollment), (2) New York Heart Association (NYHA)17 class II or III ≥ 3 months duration (patients with NYHA class IV at baseline were excluded, however, some participants advanced to NYHA class IV during the study), (3) No cardiac surgery within last 3 months, (4) No history of neurological disorder or injury (e.g., Alzheimer’s disease, stroke), (5) No history of moderate or severe head injury, (6) No past or current history of psychotic disorders, bipolar disorder, learning disorder, developmental disability, renal failure requiring dialysis, or untreated sleep apnea, (7) No substance use disorder currently or within the past 5 years, and (8) No current use of home tele-health monitoring program for HF. The primary rationale the majority of the exclusion criteria is that they represent potential adverse impact to cognitive function that may not be related to HF (e.g., recent invasive surgery, head injury, untreated sleep apnea, etc.). Telemonitoring was used as an exclusion due to its possible invention effects in our purported observational study as well as the potential liability and safety concerns associated with connecting the electronic medication pillbox to a patient’s landline that was also connected to his/her telehealth device such as an implanted device or scale. Of note, the analyses in the current study are based on the 309 patients with complete medication adherence data.
Measures
Medication Adherence
Medication adherence was measured objectively using MedSignals® Pillbox (VitalSignals, LLC, Lexington, KY). MedSignals® pillbox was selected because of ease of use for the participants, the ability to monitor multiple medications simultaneously, and the capacity to transmit daily adherence data via Bluetooth and home phone to a secure electronic server. Research assistants were trained on the installation, problem solving, and instructing participants on the use of the pillbox and provided technical support to patients during the trial. The pillbox tracked adherence data for up to four of the more common heart failure medications (chosen according to a predetermined priority list that included beta blockers, see Figure 1) for 21 days. Adherence, which was used as the primary outcome variable in analyses, was defined as the percent of days that the patient was compliant with their personally prescribed medication regimen divided by the number of total days monitored (possible range of scores: 0–100%). The calculations included one time or multiple doses per day. Medication adherence was calculated separately for each of the 4 medication bins according to the instructions for the medication in that bin. Given that the correlations between adherence scores for each bin were high (r ≥ .85), we averaged the scores for each bin to yield one adherence score for analyses. All reminder alerts and alarms were deactivated during the study. We excluded days the participant knowingly did not use the box (e.g., out of town or hospitalized). For descriptive purposes, non-adherence was defined using the standard of < 80% of days compliant with medication regimen to determine the number of non-adherent patients.18
Figure 1.
Pillbox Medication choices
Cognitive Function
Multiple domains of cognitive functioning were measured using neuropsychological tests that have strong validity and reliability and have been previously used among patients with HF.18 The four cognitive domains were as follows:
Global cognitive function: General cognitive ability across a variety of domains. Global cognitive function was examined with the Modified Mini-Mental Status Examination (3MS).19 The 3MS has better validity, reliability, and sensitivity than the MMSE.19,20 Higher scores indicate better global cognitive function (possible range: 0–100) with scores ≤ 90 indicative of some degree of cognitive impairment.21 Importantly, we include the 3MS screener for descriptive and comparative purposes. The 3MS was not used in the primary analyses given that we assessed each cognitive domain separately using gold-standard neuropsychological tests (below).
Attention: The capacity to attend to and process information. Attention was measured by the Stroop Word and Color subtests,22 Trail Making Test A,23 and Letter-Number Sequencing.24
Executive function: The capacity to problem-solve, plan, inhibit, and reason. Executive function was assessed using the Stroop Color Word subtest,22 Trail Making Test B,23 and the Frontal Assessment Battery.25
Memory: The capacity to retain and recall verbal information. Memory was measured using the Rey Auditory Verbal Learning Test Learning Over Time, True Hits, Short Delay, and Long Delay scores.26
Using published age- and education-adjusted normative data,24,27–29 raw scores on each neuropsychological test were converted to scaled scores (M = 10, SD = 3). Scaled scores were converted to T-scores to facilitate interpretation (M = 50, SD = 10), and the T-scores of the relevant tests were averaged to create a composite score for each domain. T-scores ≤ 35 are associated with scores ≥ 1.5 standard deviations (SD) below the test mean for the normative sample and are indicative of cognitive impairment. For the primary analyses, we examined the test scores as continuous variables. A dichotomized variable of cognitively impaired (T-scores ≤ 35) or not was calculated for descriptive purposes.
Demographic, Medical, and Psychosocial Variables
The following demographic and clinical variables were also assessed: age (years), gender (0 = female, 1 = male), race-ethnicity (0 = white, 1 = non-white), education level (1 = no schooling, 2 = 8th grade or less, 3= 9–11th grade, 4 = high school, 5 = technical or trade school, 6 = some college, 7 = bachelor’s degree, 8 = master’s degree), socioeconomic status (SES), self-reported current heart failure severity, medical comorbidity, medication regimen complexity, depressed mood, anxiety, social support, and health literacy. The SES score was calculated as a z-score using indicators of income and education for each zip code30 with zero as the sample mean and higher scores indicating higher socioeconomic status. Heart failure severity was determined by asking patients about their current symptoms and functional limitations (e.g., “Do you markedly reduce physical activity due to tiredness, heart fluttering, shortness of breath, anginal pain?”). Based on their responses, we categorized patients’ HF severity as class I, II, III, or IV based on their current self-reported symptoms and limitations. Medical comorbidity was assessed using the Charlson Comorbidity Index (CCI),31 which yields a summary score of comorbid medical conditions (e.g., diabetes, peripheral vascular disease, myocardial infarction, etcetera). Medication complexity was assessed using the Medication Regimen Complexity Index32 (MRCI); higher scores reflect greater complexity. Depressive symptoms were assessed using the Patient Health Questionnaire-9 (PHQ-9).33 Anxiety was assessed using the 7-item Patient Reported Outcomes Measurement Information System Anxiety Subscale (PROMIS-Anxiety).34 Social support was assessed using the Multidimensional Scale of Perceived Social Support (MSPSS).34 Health literacy was assessed using the Medical Term Recognition Test (METER)35 with higher scores indicative of greater health literacy.
Procedure
All patients were recruited from a variety of cardiology practices in northeast Ohio and gave their written, informed consent to participate. All procedures were approved by the Institutional Review Boards of Kent State University, Summa Health Systems, Inc., and University Hospitals of Cleveland. After recruitment and written consent, a research assistant obtained baseline demographic and medical data from the official medical records and conducted the series of self-report questionnaires and neuropsychological testing (Visit 1). At Visit 2, research assistants installed the electronic pillbox in patients’ homes which collected adherence data for 21-days.
Data Analyses
The characteristics of participants were summarized using mean ± standard deviation for continuous variables (e.g., age, percent daily medication adherence) and frequencies and percentages for categorical variables (e.g., sex, non-adherent status). Correlations were calculated to determine the relationship between all medication bins used and ranged from 0.85–0.87. For studying association between medication adherence (highly skewed variable) and cognitive function after controlling for several covariates, we used median regression. Measurements of several variables, including attention, executive function, and memory, were missing for a wide variety of reasons (e.g., attrition, colorblindness, refusal, fatigue, etc.). Specific missingness for each variable was 8.3% for attention (n = 31), 9.1% for executive function (n = 34), and 4.7% for memory (n = 17). We assume that the missing data follow the missing at random probability mechanism (i.e., missing values do not carry any extra information about why they are missing than what is already available in the observed data). The median regressions,36 a robust regression procedure, were performed on the 20 imputed datasets separately and the results are combined. We generated the imputed datasets by simulating from a (approximate) Bayesian posterior predictive distribution of the missing data. We applied predictor selection methodology in studying the association between various covariates (demographic characteristics, medical psychosocial factors) and medication adherence (i.e., percent of days that the patient was compliant with their medication regimen). Finally, the association between cognitive function and medication adherence was examined while adjusting for covariates that are associated with the outcome using a multivariable median regression model. Covariates in the final analyses met the following two criteria: 1) the variable had clinical significance to the primary variables (i.e., cognitive function and/or medication adherence) and 2) when entered as the single predictor, the variable was associated with medication adherence using a statistically significant p-value of less than 0.15.37 Covariates meeting these criteria were used in the multivariable regression models with one of the cognitive function measures (attention, executive function or memory) as the primary predictor. To correct for testing of multiple, correlated cognitive outcomes (mean r = .49), a partial Bonferroni correction was applied using the SISA program with the following input parameters: Alpha = .05, N of Tests = 3, Correlation = .49, and Degrees of freedom = 308.38 Using these parameters, a p-value < .028 is required for significance. All the analyses were performed using software Stata 13.0 (StataCorp, LP, College Station, TX).
Results
Sample Characteristics
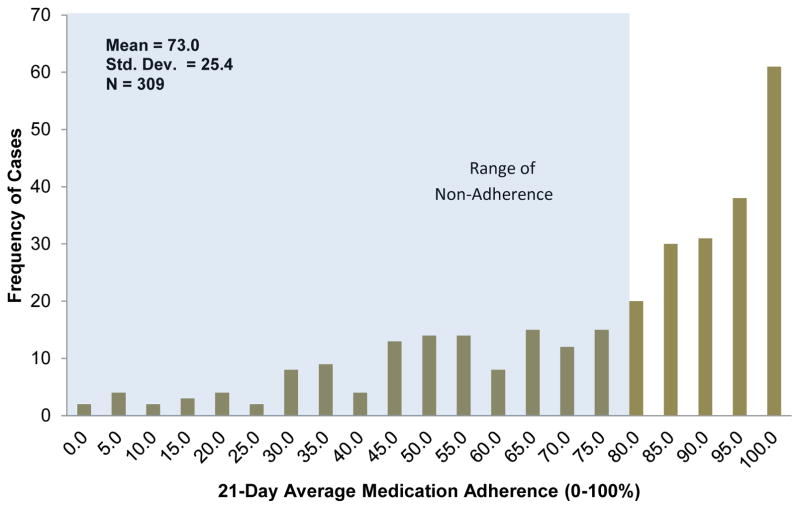
The sample was predominantly older (68.7 ± 9.7 years), white (72.0%), male (59.7%), and had Class II or III self-reported HF severity (84.9%) and a high school education level or higher (87.3%; Table 1). Most patients were cognitively intact (T-scores ≥ 35); mean composite scores for each cognitive domain and individual test are presented (Table 1). At the group level, patients exhibited an average medication adherence of 73.0% (SD = 25.4), and the majority (53.4%) of the sample was adherent (with ≥80% adherence) (Figure 2). However, 46.6% of patients were considered non-adherent (< 80% adherence) with nearly 12% of patients adhering to 50% or less of their daily medications (Figure 2; Table 1).
Table 1.
Characteristics of Participants (max N = 309)
| Total Sample M ± SD or N(%) |
Adherent Max n = 165 |
Non-adherent Max n = 144 |
|
|---|---|---|---|
| Demographic, Medical, and Psychosocial Factors (Possible Range) | |||
| Age | 68.5 ± 9.6 | 69.6 ± 8.9 | 67.2 ± 10.2* |
| Female | 122 (39.5) | 62 (37.6) | 60 (41.7) |
| Race-ethnicity | |||
| Caucasian | 227 (73.5) | 131 (79.4) | 96 (66.7)* |
| African American | 79 (25.7) | 33 (20.0) | 46 (31.9)* |
| American Indian | 2 (0.6) | 0 (0.0) | 2 (1.4) |
| Asian/Pacific Islander | 1 (0.3) | 1 (0.6) | 0 (0.0) |
| Education Level | |||
| 8th Grade or Less | 5 (1.6) | 2 (1.2) | 3 (2.1) |
| 9–11th Grade | 29 (9.4) | 12 (7.3) | 17 (11.8) |
| High School | 88 (28.5) | 50 (30.3) | 38 (26.4) |
| Technical or Trade School | 31 (10.0) | 17 (10.3) | 14 (9.7) |
| Some College | 85 (27.5) | 43 (26.1) | 42 (29.2) |
| Bachelor’s Degree | 42 (13.6) | 26 (15.8) | 16 (11.1) |
| Master’s Degree | 29 (9.4) | 15 (9.1) | 14 (9.7) |
| SES z-score | 0.09 ± 4.2 | 0.8 ± 4.1 | −0.7 ± 4.3* |
| Charlson Comorbidity Index† (0-no max) | 3.3 ± 1.7 | 3.3 ± 1.6 | 3.3 ± 1.8 |
| Medication Regimen Complexity Index (1.5-no max) | 22.6 ± 12.5 | 21.8 ± 12.1 | 23.6 ± 13.0 |
| Self-reported HF Severity at Baseline (NYHA) | |||
| Class I | 29 (9.4) | 18 (10.9) | 11 (7.6) |
| Class II | 77 (24.9) | 46 (27.9) | 31 (21.5) |
| Class III | 188 (60.8) | 98 (59.4) | 90 (62.5) |
| Class IV | 15 (4.9) | 3 (1.8) | 12 (8.3)* |
| Depressive Symptoms (PHQ-9) (0–27) | 4.5 ± 4.9 | 3.8 ± 4.5 | 5.3 ± 5.2* |
| Anxiety Symptoms (PROMIS-Anxiety) (5–35) | 13.0 ± 5.3 | 12.4 ± 5.1 | 13.6 ± 5.5 |
| Social Support (MSPSS) (12–84) | 69.1 ± 14.5 | 71.2 ± 12.0 | 66.7 ± 16.6* |
| Medical Term Recognition Test (METER) (0–40) | 35.5 ± 6.1 | 36.1 ± 5.1 | 34.7 ± 7.0* |
| Global Cognition Impaired (3MS < 90) (0–100) | 89 (29.0) | 48 (29.1) | 41 (28.9) |
| Cognitive Function T-scores (20–80) | |||
| Attention Composite | 44.9 ± 7.4 | 46.0 ± 7.1 | 43.7 ± 7.5* |
| Trails A | 42.8 ± 10.2 | 43.3 ± 9.9 | 42.1 ± 10.4 |
| Letter-Number Sequencing | 47.3 ± 10.4 | 49.1 ± 10.2 | 45.2 ± 10.2* |
| Stroop Color | 45.8 ± 9.5 | 46.7 ± 8.8 | 44.7 ± 10.2 |
| Stroop Word | 43.4 ± 9.5 | 44.8 ± 9.0 | 41.7 ± 9.7* |
| Executive Function Composite | 46.3 ± 7.9 | 47.3 ± 7.8 | 45.1 ± 7.9* |
| Trails B | 41.9 ± 11.9 | 44.2 ± 11.3 | 39.2 ± 12.1* |
| Frontal Assessment Battery | 51.0 ± 8.2 | 51.5 ± 8.3 | 50.4 ± 8.0 |
| Stroop Color-Word | 45.5 ± 10.1 | 45.8 ± 10.2 | 45.1 ± 9.9 |
| Memory Composite | 47.7 ± 8.0 | 48.9 ± 8.0 | 46.3 ± 7.7* |
| Hits | 48.9 ± 9.0 | 49.6 ± 9.2 | 48.0 ± 8.7 |
| Short Delay | 45.4 ± 11.0 | 47.5 ± 10.8 | 43.0 ± 10.9* |
| Long Delay | 47.1 ± 9.6 | 48.6 ± 9.8 | 45.4 ± 8.9* |
| Learning Over Time | 49.2 ± 11.1 | 49.9 ± 11.1 | 48.5 ± 11.0 |
| Medication Adherence Variables | |||
| Average 21-Day Adherence (0–100%) | 73.0 ± 25.4 | 92.1 ± 6.4 | 51.0 ± 20.7* |
| Patients Not Adherent (< 80% Adherence) | 144 (46.6) | -- | -- |
Note. SES = socioeconomic status. HF = heart failure. NYHA = New York Heart Association. PHQ-9 = Patient Health Questionnaire-9. PROMIS-Anxiety = Patient Reported Outcomes Measurement Information System Anxiety Scale. MSPSS = Multidimensional Scale of Perceived Social Support. Means and standard deviations are presented for continuous variables. Sample size and percentages are presented for categorical variables.
Most common comorbidities reported on the Charlson and corresponding % of participants: myocardial infarction (51.3%), diabetes (44.1%), and chronic obstructive pulmonary disease; COPD (27.4%).
T-test or chi-square difference test between adherent and non-adherent groups significant at p < .05
Figure 2. 21-Day Average Medication Adherence among Patients with Heart Failure.
Note. Shaded region depicts range of non-adherence (<80% adherence). Percentage of patients who were in range non-adherence = 46.6% (N = 144).
Cognitive Function Predicting Medication Adherence
Composite higher attention, executive function, and memory scores were all significantly associated with greater medication adherence in the unadjusted regression analysis (Table 2). After adjusting for age, minority status, SES, HF severity, depression, and social support, there was no longer an association between attention and medication adherence (β = 0.34, p = .131) (Table 3). After adjusting for these covariates, the relationship between reduced executive function and poorer medication adherence was not significant (β = 0.24 p = .075) (Table 3). The relationship between reduced memory and poorer medication adherence remained significant in the adjusted analysis (β = 0.51, p = .008) (Table 3).
Table 2.
Unadjusted Median Regressions of Demographic, Medical, Psychosocial, and Cognitive Factors Predicting 21-Day Medication Adherence (% of Days Adherent)
| Factors | β (95%CI) | SE | p |
|---|---|---|---|
| Demographic/Medical | |||
| Age | 0.37 (−0.02, 0.75) | 0.20 | .064 |
| Race-ethnicity | −13.75 (−21.97, −5.5) | 7.18 | .001* |
| SES | 1.26 (0.49, 2.03) | 0.39 | .001* |
| NYHA Class II | −5.75 (−16.61, 5.11) | 5.52 | .298 |
| NYHA Class III | −10.00 (−19.89, −0.11) | 5.03 | .048* |
| NYHA Class IV | −41.25 (−56.98, −25.52) | 4.66 | .000* |
| Psychosocial | |||
| Depression (PHQ-9) | −1.54 (−2.22, −0.85) | 0.35 | .000* |
| Social Support (MSPSS) | 0.33 (0.04, 0.61) | 0.14 | .024* |
| Cognitive Domains | |||
| Attention | 0.61 (0.15, 1.06) | 0.23 | .009* |
| Executive Function | 0.52 (0.20, 0.85) | 0.17 | .002* |
| Memory | 0.85 (0.36, 1.34) | 0.25 | .001* |
Note. SE = standard error. SES = socioeconomic status. NYHA = New York Heart Association. PHQ-9 = Patient Health Questionnaire-9. MSPSS = Multidimensional Scale of Perceived Social Support.
Significant at p < .05
Table 3.
Adjusted Median Regressions of Cognitive Function Predicting 21-Day Medication Adherence (% of Days Adherent)
| Cognitive Domainsa | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Attention | Executive Attention | Memory | |||||||
|
| |||||||||
| Variables | β (95%CI) | SE | p | β (95%CI) | SE | p | β (95%CI) | SE | p |
| Demographic/Medical | |||||||||
| Age | 0.27 (−0.06, 0.59) | 0.17 | .110 | 0.35 (0.09, 0.60) | 0.13 | .009* | 0.17 (−0.16, 0.50) | 0.17 | .319 |
| Race-ethnicity | −5.05 (−13.20, 3.10) | 4.14 | .224 | −4.57 (−10.96, 1.82) | 3.24 | .160 | −6.80 (−15.33, 1.74) | 4.33 | .118 |
| SES | 0.41 (−0.40, 1.23) | 0.41 | .320 | 0.46 (−0.17, 1.10) | 0.32 | .152 | 0.57 (−0.27, 1.41) | 0.43 | .182 |
| Self-reported NYHA | |||||||||
| Class II | −6.50 (−17.69, 4.69) | 5.69 | .254 | −5.36 (−14.14, 3.41) | 4.46 | .230 | −9.21 (−20.81, 2.39) | 5.89 | .119 |
| Class III | −6.18 (−16.67, 4.32) | 6.33 | .248 | −5.52 (−13.70, 2.66) | 4.15 | .185 | −7.95 (−18.78, 2.89) | 5.50 | .150 |
| Class IV | −23.65 (−40.48, −6.82) | 8.55 | .006* | −23.64 (−36.70, −10.57) | 6.63 | .001* | −18.92 (−36.1, −1.75) | 8.72 | .031* |
| Psychosocial | |||||||||
| Depression (PHQ-9) | −0.55 (−1.25, 0.15) | 0.36 | .124 | −0.48 (−1.03, 0.08) | 0.28 | .090 | −0.62 (−1.34, 0.08) | 0.36 | .081 |
| Social Support (MSPSS) | 0.19 (−0.03, 0.41) | 0.11 | .098 | 0.16 (−0.02, 0.34) | 0.09 | .087 | 0.21 (−0.02, 0.44) | 0.12 | .071 |
| Cognitive Domains | |||||||||
| Attention | 0.34 (−0.12, 0.57) | 0.22 | .131 | -- | -- | -- | -- | -- | -- |
| Executive Function | -- | -- | -- | 0.24 (0.02, 0.51) | 0.14 | .075 | -- | -- | -- |
| Memory | -- | -- | -- | -- | -- | -- | 0.51 (0.14, 0.89) | 0.19 | .008* |
Note. SES = socioeconomic status. NYHA = New York Heart Association. PHQ-9 = Patient Health Questionnaire. MSPSS = Multidimensional Scale of Perceived Social Support.
Each cognitive domain was examined in a separate model.
Significant at p < .05
Demographic, Medical, and Psychosocial Predictors of Medication Adherence
Several of the demographic, medical, and psychosocial variables were significantly associated with medication adherence (Table 2). Among demographic variables, white race (β = −13.75, p = .001) and higher SES (β = 1.26, p = .001) were significantly associated with greater medication adherence while older age was not significant (β = 0.37, p = .064). There was no association between gender or education and medication adherence. Among medical variables, individuals in NYHA class III (β = −10.00, p = .048) and NYHA class IV (β = −41.25, p = .000) had significantly poorer medication adherence. There was no association between medication adherence and NYHA class II status, comorbidity index, or medication regimen complexity. Among psychosocial factors, greater depressed mood was associated with poorer adherence to medication (β = −1.54, p < .001) while greater social support score was associated with greater adherence to medication (β = 0.33, p = .024). There was no association between anxiety or health literacy and medication adherence.
Discussion
The current study examined the relationship between cognitive function and objectively-monitored medication adherence in a community sample of adults with HF. Although the majority of our sample (53%) met or exceeded the recommended adherence level of 80%, nearly half (47%) of patients failed to meet this level. Indeed, one of every five patients had adherence levels of 50% or less. Extrapolating this figure to the national rates (5.1 million) suggests that nearly one million of patients with HF are taking only half of their medications or less. Importantly, poorer performance on all three cognitive domains predicted poorer objectively-monitored medication adherence when examined in unadjusted models. The effect of poorer memory on adherence remained after the adjustment of medical and psychosocial factors known to predict adherence (e.g., depression, social support, and disease severity level). For every eight-point decrease in patients’ standardized memory scores at baseline, adherence rates dropped by 13% points. For a patient taking 10 doses per day, this drop in adherence would translate to missing nine doses/week or nearly 40 doses/month. In contrast, attention and executive function were no longer significant.
Whether alone or in fully adjusted models, poorer memory function predicted worse adherence to objectively-monitored medication adherence. Memory may be a more potent predictor than attention and executive function because it is among last cognitive domains to be impacted by the disease process,39 and thus deficits in the memory domain might reflect greater severity of physical and cognitive impairment. Memory function may also be more relevant to medication taking behavior than attention or executive functions, given its role in a person’s ability to retain information and directions related to his/her medication prescriptions and dosing regimens.
Our results are consistent with those of the few studies that have examined the relationship between cognitive function and objectively-monitored medication adherence in HF.9,10 The results also parallel those indicating that lower cognitive function is associated with poorer treatment adherence in the domains of self-reported adherence18 and self-care behaviors.40 Importantly, similar to our results, Hajduk et al. also reported that memory was the only domain of cognitive function associated with poor self-care in HF.41 Taken together, the clinical implications of the findings from our study and others are that cognitive domains, especially memory function, should be examined in patients with HF to identify those who may be at risk for poorer treatment adherence, especially related to medication-taking behaviors.
The current findings are subject to several limitations. First, the monitoring period of 21 days was relatively short and may have resulted in the high levels of adherence observed due to measurement reactivity, although the range of 21-day adherence was wide (1.25% to 100%) and the standard deviation was large (22%) which suggests adequate variability in adherence rates. Second, we did not ask patients to keep a diary detailing reasons for missing certain doses given potential measurement reactivity of the diary, but this information could be helpful in recording intentional missing of doses (e.g., at provider’s recommendation). Next, the generalizability of these findings to samples with other characteristics (e.g., preserved ejection fraction, younger, higher SES, diastolic HF) may be limited. Our screening procedures excluded NYHA Class IV HF for safety reasons, but each patient’s self-reported HF symptoms were re-assessed during the study and some were judged to have Class IV HF. Thus, a more severely ill population may have had more cognitive impairment. In addition, our findings may not generalize to the hospitalized HF population, a group typically exhibiting higher rates of cognitive impairment. Thus, future studies should certainly include more diverse samples to confirm whether our results will replicate amongst patients with different medical or demographic characteristics. Another significant limitation is the quality of our NYHA assessment, which could have been enhanced by utilizing a validated interview,42 reassessing the medical record, or contacting providers directly. Relatedly, the dearth of objective measurement of HF severity or vascular disease (e.g. stenosis, white matter hyperintensities) is problematic and should encourage future studies to determine the degree to which physiologic heart or brain damage is related to cognition and medication adherence. The examination of cerebrovascular damage and perfusion is especially warranted given that non-adherent patients were twice as likely to report a history of CVA or TIA compared to adherent patients (13% vs. 6%) and may have hypoperfusion.43 Lastly, these data cannot determine whether better medication adherence preserves cognitive function in HF.44 Certainly, better medication adherence would be expected to predict better outcomes,14 but this possibility needs to be tested in prospective trials in which medication adherence and cognitive function are measured at multiple time points over an extended period.
Conclusions
To briefly summarize, we found that cognitive impairment, and memory in particular, is associated with objectively-monitored medication adherence in patients with HF. Given the substantial number of patients who were non-adherent and the known association between medication non-adherence and poorer HF outcomes, these findings highlight the importance of considering cognitive function in the management of patients with HF. Such considerations might include standard screening of cognitive impairment and added intervention for those with identified deficits. Future studies are needed which examine the link from cognitive impairment and poor medication adherence to actual hard clinical outcomes, such as hospitalization and mortality.
Clinical Perspective.
Heart failure patients with memory impairment may be at risk for poorer adherence to medications. For every eight-point decrease in patients’ standardized memory scores at baseline, adherence rates dropped by 13% points. For a patient taking 10 doses per day, this drop in adherence could translate to missing nearly 40 doses/month. Practitioner responses to these findings may include: 1) utilizing brief cognitive screening methods (e.g., Montreal Cognitive Assessment; MoCA) to identify cognitively at-risk patients, 2) referring patients for comprehensive neuropsychological testing if indicated by abnormal cognitive screener results, and 3) implementing tailored interventions to compensate for poorer medication adherence, such as eliciting caregiver support or scheduling more frequent follow-up visits to prevent symptom exacerbation due to non-adherence.
Acknowledgments
The authors thank all members of the Heart ABC team for their technical assistance on this project.
Sources of Funding
This work was supported by the National Heart, Lung, and Blood Institute at the NIH [R01 HL096710-01A1].
Footnotes
Disclosures
None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics—2015 Update: A Report From the American Heart Association. Circulation. 2015 Jan 27;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Bennett SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, Chui M, Deer M, Murray MD. Comparison of Quality of Life Measures in Heart Failure. Nursing Research. 2003;52:207–216. doi: 10.1097/00006199-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in Heart Failure: A Meta-Analytic Review of Prevalence, Intervention Effects, and Associations With Clinical Outcomes. Journal of the American College of Cardiology. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 4.Seo Y, Roberts BL, Piña I, Dolansky M. Predictors of motor tasks essential for daily activities among persons with heart failure. Journal of cardiac failure. 2008;14:296–302. doi: 10.1016/j.cardfail.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Gure TR, Blaum CS, Giordani B, Koelling TM, Galecki A, Pressler SJ, Hummel SL, Langa KM. Prevalence of cognitive impairment in older adults with heart failure. Journal of the American Geriatrics Society. 2012;60:1724–1729. doi: 10.1111/j.1532-5415.2012.04097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauvé MJ, Ding Y, Kim J, Sloan R, Jaynes H. Cognitive deficits in chronic heart failure. Nursing Research. 2010;59:127. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogels R, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: A systematic review of the literature. European Journal of Heart Failure. 2007 May 1;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Cameron J, Pressler SJ, Ski CF, Thompson DR. Cognitive impairment in heart failure: towards a consensus on screening. European Journal of Heart Failure. 2013;16:235–237. doi: 10.1002/ejhf.12. [DOI] [PubMed] [Google Scholar]
- 9.Hawkins LA, Kilian S, Firek A, Kashner TM, Firek CJ, Silvet H. Cognitive impairment and medication adherence in outpatients with heart failure. Heart & Lung: The Journal of Acute and Critical Care. 2012;41:572–582. doi: 10.1016/j.hrtlng.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Riegel B, Lee CS, Ratcliffe SJ, De Geest S, Potashnik S, Patey M, Sayers SL, Goldberg LR, Weintraub WS. Predictors of objectively measured medication nonadherence in adults with heart failure. Circulation: Heart Failure. 2012;5:430–436. doi: 10.1161/CIRCHEARTFAILURE.111.965152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masoudi FA, Baillie CA, Wang Y, Bradford WD, Steiner JF, Havranek EP, Foody JM, Krumholz HM. The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998–2001. Archives of internal medicine. 2005;165:2069–2076. doi: 10.1001/archinte.165.18.2069. [DOI] [PubMed] [Google Scholar]
- 12.Mastromarino V, Casenghi M, Testa M, Gabriele E, Coluccia R, Rubattu S, Volpe M. Polypharmacy in heart failure patients. Current heart failure reports. 2014;11:212–219. doi: 10.1007/s11897-014-0186-8. [DOI] [PubMed] [Google Scholar]
- 13.Volpe M, Chin D, Paneni F. The challenge of polypharmacy in cardiovascular medicine. Fundamental & clinical pharmacology. 2010;24:9–17. doi: 10.1111/j.1472-8206.2009.00757.x. [DOI] [PubMed] [Google Scholar]
- 14.Choudhry NK, Glynn RJ, Avorn J, Lee JL, Brennan TA, Reisman L, Toscano M, Levin R, Matlin OS, Antman EM. Untangling the relationship between medication adherence and post–myocardial infarction outcomes: Medication adherence and clinical outcomes. American Heart Journal. 2014;167:51–58. e55. doi: 10.1016/j.ahj.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald AA, Powers JD, Ho PM, Maddox TM, Peterson PN, Allen LA, Masoudi FA, Magid DJ, Havranek EP. Impact of medication nonadherence on hospitalizations and mortality in heart failure. Journal of Cardiac Failure. 2011;17:664–669. doi: 10.1016/j.cardfail.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Riegel B, Knafl GJ. Electronically monitored medication adherence predicts hospitalization in heart failure patients. Patient preference and adherence. 2014;8:1. doi: 10.2147/PPA.S54520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Committee NYHAC, Association NYH. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. Little: Brown Medical Division; 1979. [Google Scholar]
- 18.Alosco ML, Spitznagel MB, van Dulmen M, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Hughes J, Rosneck J. Cognitive function and treatment adherence in older adults with heart failure. Psychosomatic Medicine. 2012;74:965–973. doi: 10.1097/PSY.0b013e318272ef2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng E, Chui H. The Modified Mini-Mental State Examination (3MS) Can J Psychiatry. 1987;41:114–121. [PubMed] [Google Scholar]
- 20.McDowell I, Kristjansson B, Hill G, Hebert R. Community screening for dementia: The mini mental state exam (MMSE) and modified mini-mental state exam (3MS) compared. Journal of clinical epidemiology. 1997;50:377–383. doi: 10.1016/s0895-4356(97)00060-7. [DOI] [PubMed] [Google Scholar]
- 21.Blais MA, Baity MR. A comparison of two mental status examinations in an inpatient psychiatric sample. Assessment. 2005;12:455–461. doi: 10.1177/1073191105281441. [DOI] [PubMed] [Google Scholar]
- 22.Golden JC. Stroop Color and Word Test. Chicago, IL: Stoelting; 1978. [Google Scholar]
- 23.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and motor skills. 1958;8:271–276. [Google Scholar]
- 24.Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 25.Abood DA, Black DR, Birnbaum RD. Nutrition education intervention for college female athletes. Journal of nutrition education and behavior. 2004;36:135–139. doi: 10.1016/s1499-4046(06)60150-4. [DOI] [PubMed] [Google Scholar]
- 26.Lezak MD. Neuropsychological Assessment. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 27.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo’s older americans normative studies: Updated AVLT norms for ages 56 to 97. Clinical Neuropsychologist. 1992 Jun 01;6:83–104. [Google Scholar]
- 28.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Archives of clinical neuropsychology. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 29.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB A frontal assessment battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 30.Roux AVD, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. New England Journal of Medicine. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.George J, Phun Y-T, Bailey MJ, Kong DC, Stewart K. Development and validation of the medication regimen complexity index. Annals of Pharmacotherapy. 2004;38:1369–1376. doi: 10.1345/aph.1D479. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:1–7. [Google Scholar]
- 34.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, Group PC. Item Banks for Measuring Emotional Distress From the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, Anxiety, and Anger. Assessment. 2011 Sep 1;18:263–283. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawson K, Gunstad J, Hughes J, Spitznagel M, Potter V, Waechter D, Rosneck J. The METER: A Brief, Self-Administered Measure of Health Literacy. Journal of general internal medicine. 2010 Jan 01;25:67–71. doi: 10.1007/s11606-009-1158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Little RJ, Rubin DB. Statistical analysis with missing data. John Wiley & Sons; 2014. [Google Scholar]
- 37.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. Springer Science & Business Media; 2011. [Google Scholar]
- 38.Uitenbroek DG. SISA Bonferroni Correction. [Accessed March 15, 2015];Simple Interactive Statistical Analysis (SISA) Binomial. 1997 http://www.quantitativeskills.com/sisa/calculations/bonfer.htm.
- 39.O’Brien JT. Vascular cognitive impairment. American Journal of Geriatric Psych. 2006;14:724–733. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- 40.Cameron J, Worrall-Carter L, Page K, Riegel B, Lo SK, Stewart S. Does cognitive impairment predict poor self-care in patients with heart failure? European Journal of Heart Failure. 2010;12:508–515. doi: 10.1093/eurjhf/hfq042. [DOI] [PubMed] [Google Scholar]
- 41.Hajduk AM, Lemon SC, McManus DD, Lessard DM, Gurwitz JH, Spencer FA, Goldberg RJ, Saczynski JS. Cognitive impairment and self-care in heart failure. Clinical epidemiology. 2013;5:407. doi: 10.2147/CLEP.S44560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubo SH, Schulman S, Starling RC, Jessup M, Wentworth D, Burkhoff D. Development and validation of a patient questionnaire to determine New York Heart Association classification. Journal of cardiac failure. 2004;10:228–235. doi: 10.1016/j.cardfail.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Alosco ML, Brickman AM, Spitznagel MB, Garcia SL, Narkhede A, Griffith EY, Raz N, Cohen R, Sweet LH, Colbert LH. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congestive Heart Failure. 2013;19:E29–E34. doi: 10.1111/chf.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alosco ML, Spitznagel MB, Cohen R, Sweet LH, Josephson R, Hughes J, Rosneck J, Gunstad J. Better adherence to treatment recommendations in heart failure predicts improved cognitive function at a one-year follow-up. Journal of clinical and experimental neuropsychology. 2014;36:956–966. doi: 10.1080/13803395.2014.957167. [DOI] [PMC free article] [PubMed] [Google Scholar]