Abstract
Background
Muscle quantity at intensive care unit (ICU) admission has been independently associated with mortality. In addition to quantity, muscle quality may be important for survival. Muscle quality is influenced by fatty infiltration or myosteatosis, which can be assessed on computed tomography (CT) scans by analysing skeletal muscle density (SMD) and the amount of intermuscular adipose tissue (IMAT). We investigated whether CT-derived low skeletal muscle quality at ICU admission is independently associated with 6-month mortality and other clinical outcomes.
Methods
This retrospective study included 491 mechanically ventilated critically ill adult patients with a CT scan of the abdomen made 1 day before to 4 days after ICU admission. Cox regression analysis was used to determine the association between SMD or IMAT and 6-month mortality, with adjustments for Acute Physiological, Age, and Chronic Health Evaluation (APACHE) II score, body mass index (BMI), and skeletal muscle area. Logistic and linear regression analyses were used for other clinical outcomes.
Results
Mean APACHE II score was 24 ± 8 and 6-month mortality was 35.6%. Non-survivors had a lower SMD (25.1 vs. 31.4 Hounsfield Units (HU); p < 0.001), and more IMAT (17.1 vs. 13.3 cm2; p = 0.004). Higher SMD was associated with a lower 6-month mortality (hazard ratio (HR) per 10 HU, 0.640; 95% confidence interval (CI), 0.552–0.742; p < 0.001), and also after correction for APACHE II score, BMI, and skeletal muscle area (HR, 0.774; 95% CI, 0.643–0.931; p = 0.006). Higher IMAT was not significantly associated with higher 6-month mortality after adjustment for confounders. A 10 HU increase in SMD was associated with a 14% shorter hospital length of stay.
Conclusions
Low skeletal muscle quality at ICU admission, as assessed by CT-derived skeletal muscle density, is independently associated with higher 6-month mortality in mechanically ventilated patients. Thus, muscle quality as well as muscle quantity are prognostic factors in the ICU.
Trial registration
Retrospectively registered (initial release on 06/23/2016) at ClinicalTrials.gov: NCT02817646.
Keywords: Intensive care unit, Computed tomography, CT, Muscle, Muscle quality, Myosteatosis, Skeletal muscle density, Intermuscular adipose tissue, Mortality, Outcome
Background
Muscle wasting is a severe complication of critical illness [1]. Puthucheary et al. reported a steady decrease in skeletal muscle mass of almost 20% during the first 10 days of intensive care unit (ICU) admission [2]. Loss of muscle has been associated with longer duration of mechanical ventilation and higher ICU and hospital mortality [3–5]. If patients survive, they exhibit long-term functional disability with a great impact on quality of life for as long as 5 to 8 years after admission [6–8]. However, many patients already have a low muscle quantity upon admission to the ICU. In two retrospective studies as much as 60–70% of patients had low muscle quantity as assessed on computed tomography (CT) scans on ICU admission, and low muscle quantity at ICU admission was associated with a higher mortality [9, 10].
Not only the quantity, but also the quality of muscle seems important [11]. Along with a decline in muscle mass, fatty infiltration of muscles or myosteatosis has been identified as a possible cause of loss of muscle quality [11]. Myosteatosis can be apparent within muscle fibres and evaluated on CT scans by measuring skeletal muscle density (SMD), or between muscle fibres and evaluated on CT scans by measuring the amount of adipose tissue between muscles (also termed intermuscular adipose tissue or IMAT). A lower SMD was associated with increased lipid infiltration in muscle biopsies and poor clinical outcomes in non-ICU populations [12–14]. Additionally, a recent study in critically ill patients using ultrasound of the quadriceps muscle found that not only a decrease in muscle quantity but also increased muscle echogenicity was related to a decrease in muscle function [15]. An increased amount of IMAT as assessed on CT scans has been associated with decreased muscle function and increased (systemic) inflammation in non-ICU populations [16, 17]. The aim of the present study was to investigate if muscle quality, as assessed by CT-derived SMD and IMAT, is associated with mortality independently of muscle quantity and severity of illness. We hypothesized that low SMD and high IMAT at ICU admission are associated with a poor outcome, independent of the quantity of muscle and severity of illness.
Methods
Patients and data
This is a retrospective analysis of CT-derived muscle quality at a single time point at ICU admission in critically ill patients admitted to a mixed medical-surgical ICU of a university hospital from September 2003 to April 2013. Patients were included if they were aged 18 years or older, stayed in the ICU for at least 4 days, required mechanical ventilation during their ICU stay, and had an abdominal CT scan made 1 day before or up to 4 days after admission to the ICU. Patients were excluded if the CT scan was not eligible for analysis, or if data on body weight or height or the Acute Physiological, Age, and Chronic Health Evaluation (APACHE) II score was missing. By searching the hospital information system for any patients meeting inclusion criteria, we expanded our previously reported cohort of ICU patients [9].
Patient data including age, sex, weight, height, admission diagnosis, APACHE II score, length of ventilation (LOV), ICU length of stay (ICU-LOS) and hospital length of stay (hospital-LOS), discharge destination, and ICU and hospital mortality was obtained from the ICU patient data management system (Metavision; IMDsoft, Tel-Aviv, Israel) and the hospital information system (Mirador; iSOFT Nederland BV, Leiden, The Netherlands). If mortality data were not registered, these were collected from the civil registry or from the general practitioner.
CT scan analysis
The precision of single slice CT scan analysis at the third lumbar vertebra (L3) level is high (inter- and intra-observer variability less than 2% in healthy volunteers) [18]. Both skeletal muscle area (r = 0.83–0.99; p < 0.01) and IMAT (r = 0.39–0.61; p < 0.05) at this level are closely related to whole body skeletal muscle and IMAT volumes as assessed by magnetic resonance imaging (MRI) [19–21].
CT scans made 1 day before to 4 days after ICU admission for diagnostic purposes were imported from the hospital radiology system and stored on a secure computer system. Scans were analysed using Slice-O-matic versions 4.3 and 5.0 (TomoVision, Montreal, QC, Canada) by two trained and certified investigators (WGPML and IMD, trained by the Cross Cancer Institute, Edmonton, AB, Canada) who had frequent consultation with each other if there was any doubt about eligibility, landmarking, or analysis.
The CT scans were analysed for eligibility and rejected if the scan quality was too low for analysis or if they contained artefacts, or if muscle was cut off due to windowing. Landmarking was performed by identifying the L3 and isolating the CT slice that depicted the whole vertebra the best. A bony landmark was used to ensure reproducibility and consistency between patients.
Different tissues were identified using boundaries in Hounsfield Units (HU) set to –29 to +150 for muscle, –190 to –30 for IMAT and subcutaneous adipose tissue, and –150 to –50 for visceral adipose tissue [22]. SMD was assessed by the mean radiological muscle attenuation of all muscle visible at the L3 level, measured in HU. The HU scale is a radiological scale describing the density of tissues on CT scans [23]. Lower mean muscle attenuation indicates less dense muscle tissue with more lipid infiltration, e.g. lower SMD, while a higher mean muscle attenuation indicates denser muscle tissue with less lipid infiltration, e.g. higher SMD [14]. IMAT was assessed by identifying all visible adipose tissue within muscle fascia in cm2 [22]. Previously found ICU-specific optimal cut-off points related to hospital mortality were used to define low skeletal muscle area: below 170 cm2 for male patients and below 110 cm2 for female patients [9]. See Fig. 1 for an example of CT scan analysis.
Fig. 1.
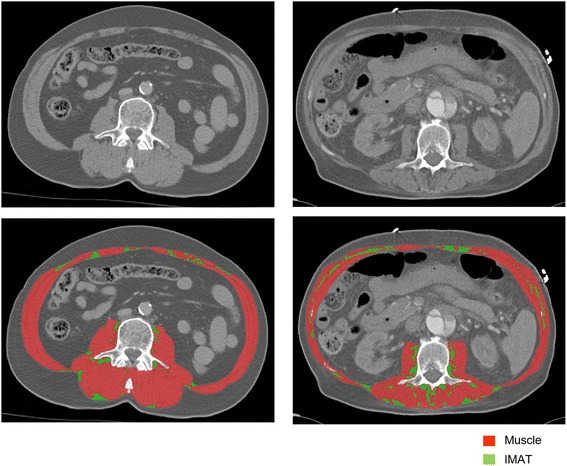
Example of CT scan analysis. This image shows CT scans at the level of lumbar vertebra 3 of two patients both un-analysed (upper row) and analysed (lower row). The analysed images show muscle tissue (red) and intermuscular adipose tissue (IMAT, green). The patient on the left has more muscle (165 vs. 120 cm2), less IMAT (10 vs. 19.5 cm2), and higher mean skeletal muscle density (42 vs. 18 Hounsfield Units) than the patient on the right
Because muscle quality is important for dealing with recovery after ICU and hospital discharge, we chose 6-month mortality as the primary endpoint. Secondary endpoints were the odds of being discharged from the hospital to home, length of ventilation, and ICU and hospital LOS in survivors.
Statistics
Independent sample t tests were used to compare survivors and non-survivors for normally distributed continuous variables, and Mann-Whitney U tests for non-normally distributed continuous variables. Fisher exact and Chi2 tests with post-hoc Bonferroni analysis were used to compare survivors and non-survivors for categorical variables. Kaplan-Meier plots were made to visualize the effect of SMD and IMAT (divided into two groups based on the median) on 6-month mortality, with log-rank tests to compare the survival curves of the two groups. Cox regression analysis was used to evaluate the association between SMD or IMAT (as continuous variables) and 6-month mortality. After univariable analyses, APACHE II score was added to the models to adjust for severity of illness (model 2). In the second adjusted model, body mass index (BMI), and skeletal muscle area were included as well (model 3). Age is included in the APACHE II score and was therefore not separately included in the adjusted models. Additionally, we performed analyses on the subgroup of patients with available data on visceral and subcutaneous adipose tissue in which BMI was substituted with visceral and subcutaneous adipose tissue as a measure of total body fatness (model 4).
Logistic and linear regression analyses were used to evaluate the association between SMD or IMAT and the secondary outcome measures discharge to home, LOV, ICU-LOS, and hospital-LOS in survivors. LOV, ICU-LOS, and hospital-LOS were non-normally distributed and positively skewed; therefore, the analysis was performed on the natural logarithm of the variables. By re-transforming by using the inverse, the influence of a given predictor was calculated as a percentage change in outcome.
IBM SPSS Statistics 22 (IBM Corp, Armonk, NY, USA) was used for statistical analysis. Values are reported as mean ± standard deviation (SD) or median and 25–75% interquartile range (IQR). All statistical tests were two-sided. A p < 0.05 was considered statistically significant.
Results
A total of 13,434 patients were admitted to the ICU during the study period with a mean APACHE II score of 17.4 ± 9.2. Six hundred and seventy-eight patients fulfilled inclusion criteria and had their CT scans imported from the radiology system to be analysed for eligibility. CT scans that were found not to be eligible were due to artefacts (78 scans), muscle cut-off (50 scans), or low quality (47 scans). Finally, 491 patients (72%) with complete clinical data and good quality CT scans were included for the statistical analysis. However, due to windowing or artefacts, visceral and/or subcutaneous adipose tissue could not be analysed in 154 patients. We therefore performed subgroup analyses that included visceral and subcutaneous adipose tissue in a subgroup of 337 patients (50%). Figure 2 is the consort diagram showing the inclusion process.
Fig. 2.
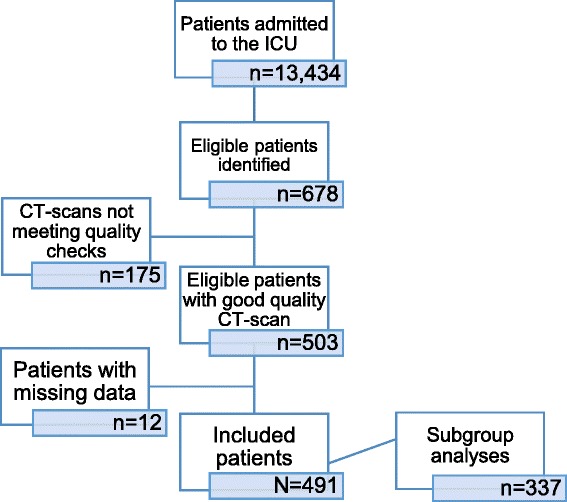
Consort diagram showing the inclusion process. CT computed tomography, ICU intensive care unit
Patient characteristics
Patient characteristics are presented in Table 1 for 6-month survivors and non-survivors. Outcome measures are presented separately in Table 2. CT scans were mostly made on the day of admission to the ICU. Three hundred and twelve (64.7%) patients had a low skeletal muscle area at ICU admission. Six-month mortality was 35.6%. Non-survivors were older (67 ± 14 vs. 55 ± 18 years; p < 0.001), had a lower BMI (24.6 ± 4.3 vs. 25.5 ± 4.4 kg/m2; p = 0.042), higher APACHE II score (27 ± 8 vs. 22 ± 8; p < 0.001), and were more often medical patients (62% vs. 43%; p < 0.001) than survivors.
Table 1.
Patient characteristics of all patients and comparison between survivors and non-survivors
| All patients N = 491 |
Survivors1
(n = 299) |
Non-survivors1
(n = 165) |
P value survivors vs. non-survivors | ||||
|---|---|---|---|---|---|---|---|
| Mean/median/n | SD/IQR/% | Mean/median/n | SD/IQR/% | Mean/median/n | SD/IQR/% | ||
| Age, years | 58 | ±18 | 55 | ±18 | 67 | ±14 | <0.001 |
| Sex, male, n (%) | 305 | 62% | 191 | 64% | 93 | 56% | 0.135 |
| BMI, kg/m2 | 25.2 | ±4.3 | 25.5 | ±4.4 | 24.6 | ±4.3 | 0.042 |
| Underweight2, n (%) | 19 | 4.1% | 11 | 3.7% | 8 | 4.8% | 0.291 |
| Normal weight2, n (%) | 238 | 51.3% | 145 | 48.5% | 93 | 56.4% | |
| Overweight2, n (%) | 158 | 34.1% | 108 | 36.1% | 50 | 30.3% | |
| Obesity2, n (%) | 49 | 10.6% | 35 | 11.7% | 14 | 8.5% | |
| APACHE II score | 24 | ±8 | 22 | ±8 | 27 | ±8 | <0.001 |
| Admission category, n (%) | 0.001 | ||||||
| Medical | 248 | 50.5% | 130 | 43% | 102 | 62% | |
| Surgical | 243 | 49.5% | 169 | 57% | 63 | 38% | |
| Admission diagnosis, n (%) | <0.001 | ||||||
| Cardiovascular | 32 | 6.5% | 18a | 6.0% | 14a | 8.5% | |
| Metabolic/renal | 15 | 3.1% | 8a | 2.7% | 6a | 3.6% | |
| Neurologic | 41 | 8.4% | 19a | 6.4% | 16a | 9.7% | |
| Post-resuscitation | 28 | 5.7% | 16a | 5.4% | 11a | 6.7% | |
| Post-surgery | 149 | 30.3% | 95a | 31.8% | 50a | 30.3% | |
| Respiratory insufficiency | 68 | 13.8% | 40a | 13.4% | 25a | 15.2% | |
| Sepsis | 31 | 6.3% | 14a | 4.7% | 15a | 9.1% | |
| Trauma | 94 | 19.1% | 74a | 24.7% | 13b | 7.9% | |
| Other | 33 | 6.7% | 15a | 5.0% | 15a | 9.1% | |
| Length of hospital stay before ICU admission, days | 0 | 0–4 | 0 | 0–4 | 0 | 0–6 | 0.166 |
| Time from ICU admission to CT scan, days | 0 | 0–1 | 0 | 0–1 | 0 | 0–1 | 0.277 |
| Skeletal muscle area, cm2 | 136.5 | ±39.0 | 143.5 | ±38.9 | 120.3 | ±33.0 | <0.001 |
| Skeletal muscle index, cm2/m2 | 44.8 | ±11.0 | 46.6 | ±10.6 | 40.4 | ±9.9 | <0.001 |
| Low skeletal muscle area3, n (%) | 312 | 63.5% | 163 | 54.5% | 137 | 83.0% | <0.001 |
| SMD, HU | 29.9 | ±11.7 | 31.4 | ±11.7 | 25.1 | ±9.4 | <0.001 |
| IMAT, cm2 | 13.6 | 8.4–24.3 | 13.3 | 7.9–23.2 | 17.1 | 10.5–27.1 | 0.004 |
| Visceral adipose tissue, cm2 (n = 337) | 96.7 | 49.3–170.6 | 95.8 | 50.9–178.1 | 108.1 | 54.1–177.5 | 0.593 |
| Subcutaneous adipose tissue, cm2 (n = 337) | 132.7 | 90.2–182.4 | 133.7 | 89.8–189.2 | 127.7 | 95.7–176.2 | 0.440 |
1Survivors and non-survivors 6 months after ICU admission
2WHO categories: underweight, BMI <18.5; normal weight: BMI 18.5–24.9; overweight: BMI 25–29.9; obesity: BMI ≥30 [42]
3Defined by skeletal muscle area: <170 cm2 for males and <110 cm2 for females [9]
a, bValues in the same row not sharing the same superscript letter are significantly different in a post-hoc Bonferroni analysis
Values in bold indicate statistically significant p values
APACHE Acute Physiological, Age, and Chronic Health Evaluation, BMI, body mass index, CT computed tomography, HU Hounsfield Units, ICU intensive care unit, IMAT intermuscular adipose tissue, IQR interquartile range, SD standard deviation, SMD skeletal muscle density
Table 2.
Primary and secondary outcome measures
| n | % | Days | IQR | |
|---|---|---|---|---|
| Six-month mortality | 165 | 35.6% | ||
| ICU mortality | 84 | 17.1% | ||
| Hospital mortality | 132 | 26.9% | ||
| Length of ventilation | 11 | 6–20 | ||
| ICU length of stay | 13 | 7–23 | ||
| Hospital length of stay | 35 | 19–59 | ||
| Destination after discharge | ||||
| Home | 144 | 40.7% | ||
| Other hospital | 80 | 22.6% | ||
| Nursing home | 76 | 21.5% | ||
| Rehabilitation unit | 45 | 12.7% | ||
| Other | 9 | 2.5% |
ICU intensive care unit, IQR interquartile range
Mean SMD at ICU admission was 29.9 ± 11.7 HU. Median IMAT at ICU admission was 13.6 (8.4–24.3) cm2, comprising 9.1% of total tissue within muscle fascia (skeletal muscle area plus IMAT) at the L3 level. Non-survivors had a lower skeletal muscle area (120.3 ± 33.0 vs. 143.5 ± 38.9 cm2; p < 0.001), lower SMD (25.1 ± 9.4 vs. 31.4 ± 11.7 HU; p < 0.001), and more IMAT (17.1 (10.5–27.1) vs. 13.3 (7.9–23.2) cm2; p = 0.004) than survivors.
Association between muscle quality and 6-month mortality
Mortality was significantly higher in patients with low muscle quality with SMD values below the median or IMAT values above the median (Fig. 3).
Fig. 3.
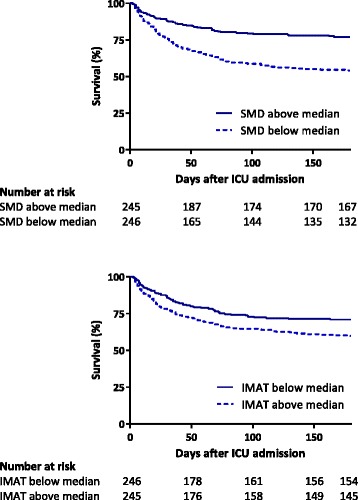
Kaplan-Meier plots. These graphs illustrate mortality for groups below and above median skeletal muscle density (SMD) (29.2 Hounsfield Units) and median intermuscular adipose tissue (IMAT) (13.6 cm2). ICU intensive care unit
Cox regression analysis showed that higher SMD was associated with lower 6-month mortality (hazard ratio (HR) per 10 HU, 0.640; 95% confidence interval (CI), 0.552–0.742; p < 0.001; Table 3). This association was still apparent when SMD was adjusted for the confounders APACHE II score, BMI, and skeletal muscle area (HR per 10 HU, 0.774; 95% CI, 0.643–0.931; p = 0.006).
Table 3.
Cox regression: association between skeletal muscle density or intermuscular adipose tissue and mortality
| Univariable N = 491 |
Model 2 N = 491 |
Model 3 N = 491 |
Model 4 (n = 337) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6-month mortality | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value |
| SMD (per 10 HU) | 0.640 | 0.552–0.742 | <0.001 | 0.703 | 0.605–0.818 | <0.001 | 0.774 | 0.643–0.931 | 0.006 | 0.728 | 0.571–0.928 | 0.010 |
| IMAT (per 10 cm2) | 1.153 | 1.042–1.277 | 0.006 | 1.092 | 0.980–1.217 | 0.110 | 1.092 | 0.966–1.236 | 0.159 | 1.244 | 1.048–1.476 | 0.012 |
Model 2: adjusted for APACHE II score
Model 3: adjusted for APACHE II score, skeletal muscle area, and BMI
Model 4 (subgroup analysis): adjusted for APACHE II score, skeletal muscle area, visceral adipose tissue, and subcutaneous adipose tissue
Values in bold indicate statistically significant p values
APACHE Acute Physiological, Age, and Chronic Health Evaluation, CI confidence interval, HR hazard ratio, HU Hounsfield Units, IMAT intermuscular adipose tissue, SMD skeletal muscle density
Cox regression analysis showed that higher IMAT was associated with higher 6-month mortality (HR per 10 cm2, 1.153; 95% CI, 1.042–1.277; p = 0.006). However, when adjusted for APACHE II score alone or the confounders APACHE II score, BMI, and skeletal muscle area the association between IMAT and 6-month mortality was not significant (HR per 10 cm2, 1.092; 95% CI, 0.966–1.236; p = 0.159).
Analyses in the subgroup with visceral and subcutaneous adipose tissue
Additional Cox regression analyses were performed in the subgroup of patients with available data on visceral and subcutaneous adipose tissue (n = 337, Table 3). Patients in this subgroup were significantly different from patients in whom visceral and/or subcutaneous adipose tissue could not be analysed. They were younger (56 vs. 64 years; p < 0.001), more often male (66 vs. 55%; p = 0.021), and had a lower BMI (24.8 vs. 25.9 kg/m2; p = 0.026). In this subgroup, we found both SMD (HR per 10 HU, 0.623; 95% CI 0.524–0.739; p < 0.001) and IMAT (HR per 10 cm2, 1.245; 95% CI, 1.106–1.401; p < 0.001) were significantly associated with 6-month mortality. In multivariable analyses both SMD (HR per 10 HU, 0.728; 95% CI, 0.571–0.928; p = 0.010) and IMAT (HR per 10 cm2, 1.244; 95% CI, 1.048–1.476; p = 0.012) remained significantly associated with 6-month mortality, adjusted for APACHE II score, skeletal muscle area, and visceral and subcutaneous adipose tissue.
Secondary outcome measures in survivors
Higher SMD was significantly associated with shorter hospital-LOS after adjustment for APACHE II score, BMI, and skeletal muscle area (Table 4). After re-transformation we found that 10 HU higher SMD was associated with a 14% shorter hospital-LOS. IMAT was not associated with hospital LOS. Neither SMD nor IMAT were significantly associated with the odds of being discharged to home, LOV, or ICU-LOS.
Table 4.
Logistic and linear regression: association between skeletal muscle density or intermuscular adipose tissue and secondary outcomes
| Univariable | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR/B | 95% CI | P value | OR/B | 95% CI | P value | OR/B | 95% CI | P value | |
| Discharge to home | |||||||||
| SMD (per 10 HU) | 1.039 | 0.864 to 1.250 | 0.683 | 0.990 | 0.816 to 1.200 | 0.915 | 0.926 | 0.718 to 1.195 | 0.556 |
| IMAT (per 10 cm2) | 0.886 | 0.741 to 1.059 | 0.182 | 0.912 | 0.761 to 1.093 | 0.317 | 0.884 | 0.715 to 1.093 | 0.254 |
| Length of ventilation | |||||||||
| SMD (per 10 HU) | –0.038 | –0.107 to 0.032 | 0.292 | –0.003 | –0.075 to 0.069 | 0.936 | –0.018 | –0.111 to 0.075 | 0.705 |
| IMAT (per 10 cm2) | 0.050 | –0.014 to 0.115 | 0.126 | 0.029 | –0.036 to 0.094 | 0.384 | 0.026 | –0.049 to 0.101 | 0.499 |
| Length of ICU stay | |||||||||
| SMD (per 10 HU) | –0.051 | –0.123 to 0.020 | 0.158 | –0.020 | –0.092 to 0.053 | 0.598 | –0.032 | –0.128 to 0.063 | 0.506 |
| IMAT (per 10 cm2) | 0.064 | –0.003 to 0.130 | 0.059 | 0.043 | –0.023 to 0.110 | 0.199 | 0.041 | –0.036 to 0.119 | 0.292 |
| Length of hospital stay | |||||||||
| SMD (per 10 HU) | –0.123 | –0.192 to –0.054 | 0.001 | –0.112 | –0.184 to –0.041 | 0.002 | –0.134 | –0.228 to –0.040 | 0.005 |
| IMAT (per 10 cm2) | 0.075 | 0.010 to 0.140 | 0.023 | 0.065 | –0.001 to 0.131 | 0.052 | 0.064 | –0.012 to 0.141 | 0.100 |
Model 2: adjusted for APACHE II score
Model 3: adjusted for APACHE II score, skeletal muscle area, and BMI
Discharge to home results are given as OR; length of ventilation, ICU, and hospital stay are given as B values
Values in bold indicate statistically significant p values
APACHE Acute Physiological, Age, and Chronic Health Evaluation, B beta coefficient, CI confidence interval, HU Hounsfield Units, ICU intensive care unit, IMAT intermuscular adipose tissue, OR odds ratio, SMD skeletal muscle density
Discussion
This retrospective study in mechanically ventilated patients admitted to the ICU for 4 days or longer shows that low skeletal muscle quality at ICU admission, as assessed by skeletal muscle density on CT scans, is associated with higher 6-month mortality independent of muscle quantity, APACHE II score, and BMI. A lower SMD was also associated with a longer hospital stay in survivors. This is the first study investigating the relation between CT-derived markers for muscle quality and outcome in ventilated critically ill patients. Intermuscular adipose tissue was also associated with mortality but not independently, suggesting that SMD is a stronger marker of muscle quality for 6-month mortality or that IMAT is better represented by confounders than SMD.
Muscle quality and quantity
Previously we have found that low muscle quantity as assessed by skeletal muscle area on CT scans at ICU admission is a risk factor for hospital mortality, independent of sex and APACHE II score [9]. These findings were in line with a study by Moisey et al. in elderly injured ICU patients, who found low skeletal muscle area to be associated with higher mortality and less ventilator-free and ICU-free days [10]. In the present study, we found that the quality of muscle appeared to be important for survival in addition to quantity.
The APACHE II score is the best validated prognostic ICU score for hospital mortality incorporating age, comorbidities, and acute illness. However, it appears that, independently of APACHE II score, a poor health status as reflected by low muscle quantity and quality (whether due to inactivity, comorbidity, or high age) are important prognostic markers. Unfortunately, the updated APACHE III and IV scores were not available for all patients.
Of interest, IMAT was independently associated with 6-month mortality in a subgroup, but not in the entire cohort. The patients in the subgroup were younger, more often male, and had a lower BMI. Apparently, visceral tissue on CT scans can more often not be analysed in older patients with high BMI, mostly because a part of the scan is often cut-off in the windowing process.
Causes and consequences of myosteatosis
Previous studies have shown that inactivity, as seen in pre-existing illness and advancing age, can cause an increase in myosteatosis (as seen by a decrease in SMD and an increase in IMAT) and that these changes are associated with decreased muscle strength [24–26]. During inactivity there is a decrease in lipoprotein lipase activity, the rate-limiting enzyme in triglyceride metabolism, which hydrolyses triglycerides into lipoproteins [27, 28]. Additionally, during bed rest a decrease in 3-hydroxyacyl-CoA-dehydrogenase concentration is seen, which impairs the muscle’s ability to metabolize free fatty acids to acyl-CoA [29, 30]. Finally, denervation causes an increase in malonyl-CoA concentrations, which in turn inhibits the rate-limiting enzyme responsible for transporting acyl-CoA into the mitochondria [31]. These altered metabolic mechanisms associated with inactivity decrease the ability of muscles to oxidise lipids and promotes a shift in muscle fuel utilisation from lipids towards glucose, causing accumulation of lipids in the muscle [26, 32]. Manini et al. found that 4 weeks of lower limb immobilisation in healthy adults caused an increase in IMAT and a loss in muscle strength independent of a decrease in muscle mass [26]. Their findings support the idea that myosteatosis is related to decreased muscle quality.
Adipose tissue has been noted as a major endocrine organ. To date, hundreds of adipokines, cytokines secreted by adipose tissue, have been identified [33]. Myosteatosis is associated with an upregulation of macrophage and T-cell expression [34]. These inflammatory cells produce pro-inflammatory cytokines such as tumour necrosis factor-alpha (TNFα) and interleukin-6 (IL-6) [35] which mediate contractile dysfunction [36, 37] and create a low-grade inflammatory environment in which the metabolic syndrome, cardiovascular disease, and insulin resistance are prone to develop [16, 17, 34].
Muscle wasting and long-term outcome
Previous studies have shown that muscle wasting as occurring during critical illness has a large impact on survival, successful weaning from ventilation, and long-term functioning [3–8, 38]. Herridge et al. found functional disability in survivors of acute respiratory distress syndrome as much as 5 years after admission to the ICU [7] and Iwashyna et al. found functional limitations up to 8 years after severe sepsis [8]. A decrease in muscle quality as assessed by CT scans has been described in 15 patients in a small substudy of the EPaNIC trial where a substantial decrease in skeletal muscle area and SMD, and an increase in IMAT developing over a 7-day period during the early stage of critical illness was found [39]. In two observational studies including 136 and 115 patients requiring at least 5 and 7 days of mechanical ventilation, respectively, muscle weakness acquired during critical illness was associated with increased ICU and hospital mortality [3, 38]. Our study found that low muscle quality present at the beginning of critical illness was already associated with poor outcome, before the devastating effects of critical illness on muscle wasting.
Strengths and limitations
Our study has strengths and limitations. This is the first study up to now investigating the relation between muscle quality assessed with CT scans and clinical outcomes in a large group of critically ill ventilated patients. However, we only included patients who had a CT scan made and the resulting selection bias might limit the generalizability of our findings to the overall ICU population. The APACHE II score of the study population was higher than the overall ICU population, all patients were ventilated, and had an ICU length of stay of at least 4 days, indicating that the study patients were severely ill. Low muscle quality at admission likely has greater impact in the more severely ill patients, because the effect of additional critical illness-related muscle wasting is greater in this population.
Muscle quality is typically defined as muscle strength per unit of muscle mass or cross-sectional area. However, measuring muscle strength in ventilated critically ill patients is not feasible. Therefore, we used SMD and IMAT as proxy markers for muscle quality [40]. To date, SMD on CT scans has been related to myosteatosis [14, 23]. However, in recent studies in ICU patients using ultrasound, a relation between ultrasound echogenicity and myonecrosis in muscle biopsies has been found [2, 41]. Changes in SMD on CT scans might therefore not only reflect myosteatosis, but also myonecrosis. A prospective study using CT scans and muscle biopsies will have to further elucidate which changes in muscle are reflected by SMD in ICU patients.
A further limitation to our study is its observational design, precluding any deduction of causality. In addition, the complexity of critical illness may obscure residual confounding. Finally, the focus of our study was the prediction of long-term mortality at ICU admission, e.g. whether muscle quality at admission is a predictor of long-term mortality independent of muscle mass and of the best validated predictive score (APACHE). Further studies are needed to determine the risk factors for poor muscle quality and to determine the additional impact of ICU-acquired weakness on long-term mortality.
Conclusions
Low skeletal muscle quality at ICU admission, as assessed by skeletal muscle density on CT scans, is associated with higher 6-month mortality in mechanically ventilated patients, independent of muscle quantity, APACHE II score, and BMI. Low muscle quality was also associated with longer hospital length of stay in survivors. Therefore, muscle quality appears to be as important for outcome as muscle quantity. Future intervention studies, including nutrition and early exercise, should not only focus on preventing further deterioration of muscle quantity, but also of muscle quality.
Acknowledgements
We thank Ronald Driessen from the Department of Intensive Care Medicine for his contribution in the collection of data.
Funding
A research grant provided by Baxter Healthcare was used for acquisition of CT scan analysis software and for a part of CT scan analysis. The funding source was not involved in any aspect of the design of the study, nor in collection, analysis, and interpretation of data, nor in manuscript preparation.
Availability of data and materials
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
WGPML, HMO-vS and PJMW designed research; WGPML and IMD collected data; ARJG and SNS provided essential resources; WGPML, PJMW, HMO-vS, and JWRT analysed the data; WGPML, HMO-vS, and PJMW wrote the paper; WGPML had primary responsibility for final content. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All images in this manuscript are entirely unidentifiable and do not include any personal details, therefore no consent for publication was obtained.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the VU University Medical Center (identification number 2012/243). The need for informed consent was waived because of the retrospective nature of the study using only data obtained from standard care.
Abbreviations
- APACHE
Acute Physiological, Age, and Chronic Health Evaluation
- BMI
Body mass index
- CI
Confidence interval
- CT
Computed tomography
- Hospital-LOS
Hospital length of stay
- HR
Hazard ratio
- HU
Hounsfield Units
- ICU
Intensive care unit
- ICU-LOS
ICU length of stay
- IMAT
Intermuscular adipose tissue
- LOV
Length of ventilation
- SMD
Skeletal muscle density
Contributor Information
Wilhelmus G. P. M. Looijaard, Phone: +31 20 4443325, Email: w.looijaard@vumc.nl
Ingeborg M. Dekker, Email: i.dekker2@vumc.nl
Sandra N. Stapel, Email: s.stapel@vumc.nl
Armand R. J. Girbes, Email: arj.gribes@vumc.nl
Jos W. R. Twisk, Email: jwr.twisk@vumc.nl
Heleen M. Oudemans-van Straaten, Email: h.oudemans@vumc.nl
Peter J. M. Weijs, Email: p.weijs@vumc.nl
References
- 1.Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19:274. doi: 10.1186/s13054-015-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 3.Ali NA, O'Brien JM, Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 4.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 5.De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 6.Wieske L, Dettling-Ihnenfeldt DS, Verhamme C, Nollet F, van Schaik IN, Schultz MJ, et al. Impact of ICU-acquired weakness on post-ICU physical functioning: a follow-up study. Crit Care. 2015;19:196. doi: 10.1186/s13054-015-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 8.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weijs PJ, Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18:R12. doi: 10.1186/cc13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moisey LL, Mourtzakis M, Cotton BA, Premji T, Heyland DK, Wade CE, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17:R206. doi: 10.1186/cc12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 12.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 13.Antoun S, Lanoy E, Iacovelli R, Albiges-Sauvin L, Loriot Y, Merad-Taoufik M, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer. 2013;119:3377–3384. doi: 10.1002/cncr.28218. [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985) 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 15.Parry SM, El-Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 2015;30:1151. doi: 10.1016/j.jcrc.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:309570. doi: 10.1155/2014/309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrinol Metab. 2012;23:391–398. doi: 10.1016/j.tem.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald AJ, Greig CA, Baracos V. The advantages and limitations of cross-sectional body composition analysis. Curr Opin Support Palliat Care. 2011;5:342–349. doi: 10.1097/SPC.0b013e32834c49eb. [DOI] [PubMed] [Google Scholar]
- 19.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 20.Schweitzer L, Geisler C, Pourhassan M, Braun W, Gluer CC, Bosy-Westphal A, et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr. 2015;102:58–65. doi: 10.3945/ajcn.115.111203. [DOI] [PubMed] [Google Scholar]
- 21.Ruan XY, Gallagher D, Harris T, Albu J, Heymsfield S, Kuznia P, et al. Estimating whole body intermuscular adipose tissue from single cross-sectional magnetic resonance images. J Appl Physiol (1985) 2007;102:748–754. doi: 10.1152/japplphysiol.00304.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 23.Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014;210:489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baracos V, Kazemi-Bajestani SM. Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int J Biochem Cell Biol. 2013;45:2302–2308. doi: 10.1016/j.biocel.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85:377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 27.Zderic TW, Hamilton MT. Physical inactivity amplifies the sensitivity of skeletal muscle to the lipid-induced downregulation of lipoprotein lipase activity. J Appl Physiol (1985) 2006;100:249–257. doi: 10.1152/japplphysiol.00925.2005. [DOI] [PubMed] [Google Scholar]
- 28.Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol. 2003;551:673–682. doi: 10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hikida RS, Gollnick PD, Dudley GA, Convertino VA, Buchanan P. Structural and metabolic characteristics of human skeletal muscle following 30 days of simulated microgravity. Aviat Space Environ Med. 1989;60:664–670. [PubMed] [Google Scholar]
- 30.Ferretti G, Antonutto G, Denis C, Hoppeler H, Minetti AE, Narici MV, et al. The interplay of central and peripheral factors in limiting maximal O2 consumption in man after prolonged bed rest. J Physiol. 1997;501(Pt 3):677–686. doi: 10.1111/j.1469-7793.1997.677bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagenmakers AJ. A malonyl-CoA fuel sensing mechanism in muscle: effects of insulin, glucose and denervation. Clin Nutr. 1996;15:144–145. doi: 10.1016/S0261-5614(96)80041-0. [DOI] [PubMed] [Google Scholar]
- 32.Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824s–8s. [DOI] [PubMed]
- 33.Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012;6:91–101. doi: 10.1002/prca.201100052. [DOI] [PubMed] [Google Scholar]
- 34.Khan IM, Perrard XY, Brunner G, Lui H, Sparks LM, Smith SR, et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int J Obes (Lond) 2015;39:1607–1618. doi: 10.1038/ijo.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DE, Kehlenbrink S, Lee H, Hawkins M, Yudkin JS. Getting the message across: mechanisms of physiological cross talk by adipose tissue. Am J Physiol Endocrinol Metab. 2009;296:E1210–E1229. doi: 10.1152/ajpendo.00015.2009. [DOI] [PubMed] [Google Scholar]
- 36.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166:479–484. doi: 10.1164/rccm.2202005. [DOI] [PubMed] [Google Scholar]
- 37.Wilcox P, Osborne S, Bressler B. Monocyte inflammatory mediators impair in vitro hamster diaphragm contractility. Am Rev Respir Dis. 1992;146:462–466. doi: 10.1164/ajrccm/146.2.462. [DOI] [PubMed] [Google Scholar]
- 38.Sharshar T, Bastuji-Garin S, Stevens RD, Durand MC, Malissin I, Rodriguez P, et al. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med. 2009;37:3047–3053. doi: 10.1097/CCM.0b013e3181b027e9. [DOI] [PubMed] [Google Scholar]
- 39.Casaer MP, Langouche L, Coudyzer W, Vanbeckevoort D, De Dobbelaer B, Guiza FG, et al. Impact of early parenteral nutrition on muscle and adipose tissue compartments during critical illness. Crit Care Med. 2013;41:2298–2309. doi: 10.1097/CCM.0b013e31828cef02. [DOI] [PubMed] [Google Scholar]
- 40.McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan. 2014;3:9. doi: 10.1186/2046-2395-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puthucheary ZA, Phadke R, Rawal J, McPhail MJ, Sidhu PS, Rowlerson A, et al. Qualitative ultrasound in acute critical illness muscle wasting. Crit Care Med. 2015;43:1603–1611. doi: 10.1097/CCM.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Obesity: preventing and managing the global epedemic: report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-253. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.


