Abstract
Objective
Adults and adolescents with major depressive disorder (MDD) show a blunted neural response to rewards. Depression has been validated in children as young as age 3; however, it remains unclear whether blunted response to reward is also a core feature of preschool-onset depression. If so, this would provide further validation for the continuity of the neural correlates of depression across the life span and would identify a potential target for treatment in young children.
Method
Fifty-three 4–7-year-old children with depression and 25 psychiatrically healthy 4–7-year-old children completed a simple guessing task in which points could be won or lost on each trial while event-related potentials (ERPs) were recorded. Psychiatric diagnosis was established using a preschool version of the Kiddie Schedule for Affective Disorders and Depression.
Results
Young children with depression showed a reduced differentiation between response to gains and losses, and this finding was driven by a blunted response to reward (i.e., the reward positivity [RewP]). These findings held even when controlling for co-occurring attention-deficit/hyperactivity disorder, oppositional defiant disorder, and generalized anxiety disorder. The RewP did not vary as a function of depression severity within the group with depression.
Conclusion
Similar to adults and adolescents with depression, preschoolers with depression display reductions in responsivity to rewards as indexed by the RewP. These findings provide further evidence for continuity in the neural mechanisms associated with depression across the lifespan, and point to altered reward sensitivity as an early-emerging potential target for intervention in preschool-onset depression.
Clinical trial registration information
A Randomized Controlled Trial of PCIT-ED for Preschool Depression; http://clinicaltrials.gov/; NCT02076425.
Keywords: preschool depression, ERP, RewP, reward, children
INTRODUCTION
Major depressive disorder (MDD) is one of the most widespread and costly mental illnesses.1 MDD is associated with substantial social burden, as it reduces quality of life and function among individuals who suffer from this illness, and is also associated with increased rates of suicide and self-harm.2 As such, identifying precursors or early indicators associated with the earliest onset of MDD has received growing research attention.3–9 Preschool-onset depression is now recognized to be associated with alterations in later childhood brain development10 and to be a precursor for later childhood and adolescent MDD.5, 11 If reliable and effective preventative interventions can be developed and validated for this population at an early age, it could minimize both the personal and societal burdens of depression and its long-term deleterious effects. To accomplish this goal, it is imperative that we advance our understanding of the pathophysiology of depression in early childhood.
Recent findings suggest that deficits in reward processing—blunted neural and psychological responses to obtaining reward—may be a characteristic associated with risk for depression that emerges early in life and sustains throughout adolescence and into adulthood.12–15 Further, blunted reward responding may be an important predictor of risk for the development of depression in adolescence.16–19 As such, blunted reward responding may also be an important treatment target for children even earlier in development. Thus, the goal of the current study is to examine whether children with preschool-onset depression also show altered neural responses to reward, as a key precursor to understanding whether this domain of function may be a potential focus of intervention among children with depression early in life.
Two complementary approaches that have helped to delineate alterations in task-related neural activation associated with the processing of reward in depression are functional magnetic resonance imaging (fMRI) and event-related potentials (ERPs). The fMRI literature has focused on response to reward in a network that includes the dorsal and ventral striatum, the orbital frontal cortex, and the anterior cingulate.20, 21 ERP research has focused on the Reward Positivity (RewP), which is a frontocentral deflection occurring 250–550ms post feedback that indexes responses to positive (e.g., wins) outcomes in a range of tasks using reward, such as guessing, and/or gambling.22 Prior literature indicates that the RewP correlates with subcortical regions of the brain such as the ventral striatum and the dorsal anterior cingulate.23–25
A growing body of literature suggests that adults with depression show altered behavioral responses to reward in a variety of domains,15, 26 including reduced impact of reward on adaptive behavior27, 28 and less willingness to expend effort for rewards.29–31 Additionally, adults with MDD also consistently show a reduction in reward circuit activation in fMRI studies of reward processing, especially in the striatum.15,26,32 Further, a number of studies show a reduction in RewP amplitude among adults with MDD33,34 and in association with higher self-reported depression.35 Similarly, adolescents and school-aged children with MDD show behavioral evidence of reduced responsiveness to reward,26,36 including evidence for an association between elevated anhedonic symptoms and reduced behavioral responses to rewards in 7–10-year-old children.37 Further, both children and adolescents with depression also showed altered neural responses to reward, including reduced task-related activation to reward processing in the striatum13,38 and a blunted RewP amplitude.39,40 Importantly, prospective studies have found that a blunted ventral striatal response to reward anticipation in previously healthy adolescents predicted increased depression two years later.18 Along similar lines, work has also shown that a reduced RewP in never-depressed adolescent girls (15–17 years) predicted later MDD and increased depressive symptoms, even when controlling for baseline depressive symptoms.16,41
Thus, reduced neural response to rewards has been robustly demonstrated among adults, adolescents, and school-aged children with or at risk for depression; moreover, evidence suggests that reduced reward responding predicts the emergence or worsening of depression over time.
However, little is known about the nature of reward processing in young children (< age 7) with symptoms of depression. There are several reasons why it is critical to determine whether altered responsivity to reward is also present in even younger children with depression. First, it would provide added evidence for the similaritiy of depressive features across the lifespan and would be consistent with the hypothesis that some of the same neural and behavioral mechanisms are associated with depression whether it emerges very early in life, during school age or adolescence, or as an adult. This would provide important guidance for studies examining the genetic and environmental mechanisms that may contribute to depression across the lifespan. Second, if young children with depression also show blunted reward processing, it is possible that these neural markers may guide the development of novel intervention and prevention strategies. Because response to reward and experiences of positive affect are a highly salient developmental feature of early childhood when pleasure in activities and play is a central theme, early childhood may be an ideal time to intervene to target and enhance this neural response. Thus, the goal of the current study was to determine whether young children with preschool-onset major depression (PO-MDD) show evidence of blunted neural responses to reward. It is important to note that we used PO-MDD throughout the manuscript but children in the PO-MDD group could be up to 7 years old.
Given the very large literature demonstrating the feasibility of using ERPs with preschool-aged children, the current study used a developmentally appropriate guessing task to examine the RewP in response to rewards in young children with preschool-onset depression. Based upon the findings from older children and adolescents discussed above, we hypothesized that: (1) treatment-naive children with PO-MDD would show significantly smaller responses to reward (i.e., RewP) than typically developing same-age peers, and (2) among children with PO-MDD, children who are more severely depressed would show significantly smaller responses to reward (i.e., RewP).
METHOD
Participants
The current study included a total of N = 84 children between the ages of 4 and 7. Child participants with depression were recruited from a larger ongoing randomized controlled trial (RCT) study (PCIT-ED [Parent-Child Interaction Therapy-Emotion Development]) for PO-MDD. Caregivers and their children were invited to participate in the current ERP study during their initial visit for their baseline assessment as part of the parent study. All children in the group with depression met developmentally modified DSM criteria for an acute episode of MDD at the time of their ERP. Exclusion criteria for both depressed and healthy children included current enrollment in psychotherapy, current use of psychiatric medication for mood disorders, or diagnosis of autism spectrum disorder, as well as neurological disorders, head injury, or severe developmental delay. An additional recruitment effort was launched to attain typically developing children with no current or prior diagnosis (based on parent report) of MDD for use as a healthy comparison group. Children in the healthy comparison group were recruited from similar geographical areas and matched as closely as possible, at the group level, to the depressed group of children on age, gender, parental income, and ethnicity. ERP data was collected from children prior to being randomized to a treatment group (i.e., 16-week treatment vs. 16-week waitlist) and prior to the commencement of any therapy sessions. All children were recruited from a large metropolitan area and its surrounding cities. Table 1 provides demographic data as well as diagnostic group differences on key demographic and clinical variables.
Table 1.
Demographic and Clinical Characteristics of Participants
| Healthy Control Children (n=25) |
PO-MDD (n=53) |
Group Comparison | |
|---|---|---|---|
| Age in Years; Mean(SD) | 5.63 (1.02) | 5.50 (.85) | t(82) = .61, p = .54 |
| Gender (% Males) | 64 | 66 | χ2(1) = .034, p = .85 |
| Ethnicity | |||
| % White | 72 | 82 | |
| %Black | 16 | 12 | |
| %Other | 12 | 6 | χ2(2) = 1.08, p = .58 |
| Parental income; Mean(SD) | $70K ($26K) | $66K ($34K) | t(51.31)= .37 p = .69 |
| Maternal BDI score; Mean(SD) | 4.04(4.35) | 10.17(7.10) | t(71.14)= −4.83, p < .001. |
| N of K-SADS MDD Symptoms Endorsed; Mean(SD) |
0.60(.87) | 5.66(1.57) | t(76.26) = −18.88, p < .001 |
| Comorbidities | |||
| %ADHD | 0 | 30 | ns |
| %GAD | 0 | 20 | |
| %ODD | 0 | 52 |
Note: n=2 missing data for parental income of healthy control participants. ADHD = attention-deficit/hyperactivity disorder; BDI = Beck Depression Inventory; GAD = generalized anxiety disorder; K-SADS = Kiddie Schedule for Affective Disorders; MDD = major depressive disorder; PO-MDD = preschool-onset major depression; ODD = oppositional defiant disorder.
Of the total 84 included in the study (n = 27 healthy and n = 57 PO-MDD), n = 6 children (n = 2 healthy controls) were excluded because they had <50% usable ERP segments (≤ 30 usable trials out of 60 total) in the reward and/or loss condition. Thus, the final sample included in analyses was n = 53 children with depression and n = 25 healthy control participants.
Behavioral and Diagnostic Measures
Preschool Depression
PO-MDD diagnosis was determined using the Kiddie Schedule for Affective Disorders and Depression-Early Childhood Version (K-SADS-EC),42 administered to the child’s primary caregiver by a research clinician trained to reliability. To be diagnosed with PO-MDD, the parent had to report that the child met four or more of the DSM criteria for major depression with a 2-week duration in the last month.43 In addition to generating a categorical diagnosis for MDD, depression severity scores were created for each child by summing the total of 9 possible core DSM symptoms used to assess MDD. For the current sample, interviewer intraclass correlation coefficient for MDD severity scores averaged .96, 95%CI: .89 – .99 and Kappa for MDD diagnosis averaged .91.
Comorbid Diagnoses
The K-SADS also assess symptoms for a number of other Axis I disorders. The three that were most frequently co-occurring in the children with PO-MDD were oppositional defiant disorder (ODD: 52%), attention-deficit/hyperactivity disorder (ADHD: 30%) and generalized anxiety disorder (GAD: 20%). Thus, we accounted for the presence of these comorbidities in analyses.
Preschoolers’ Behavioral Activation/Inhibition
The Behavioral Activation and Inhibition Scales (BIS/BAS) were administered to the primary caregivers of preschool participants. Extant literature indicates that the BIS/BAS is a valid and reliable measure of children’s appetitive motives (BAS), in which the goal is to move toward something desired, as well as aversive motives (BIS), in which the goal is to move away from something unpleasant.44 We examined the following subscales, BAS Drive, BAS Reward Responsiveness, and BIS, using the recently revised and validated scoring.45
Preschoolers’ Emotion Regulation
The Emotion Regulation Checklist – Preschool Version (ERC-PV) was administered to the primary caregivers reporting on preschool participants. The measure targets such processes as affective liability, intensity, valence, flexibility, and situational appropriateness.46
Maternal Depression
The Beck Depression Inventory (BDI) was administered to mothers of preschool participants. The BDI measures severity of depression through self-report of symptoms of depression such as hopelessness and irritability, cognitions such as guilt, and physical symptoms such as fatigue.47,48
Task and Materials
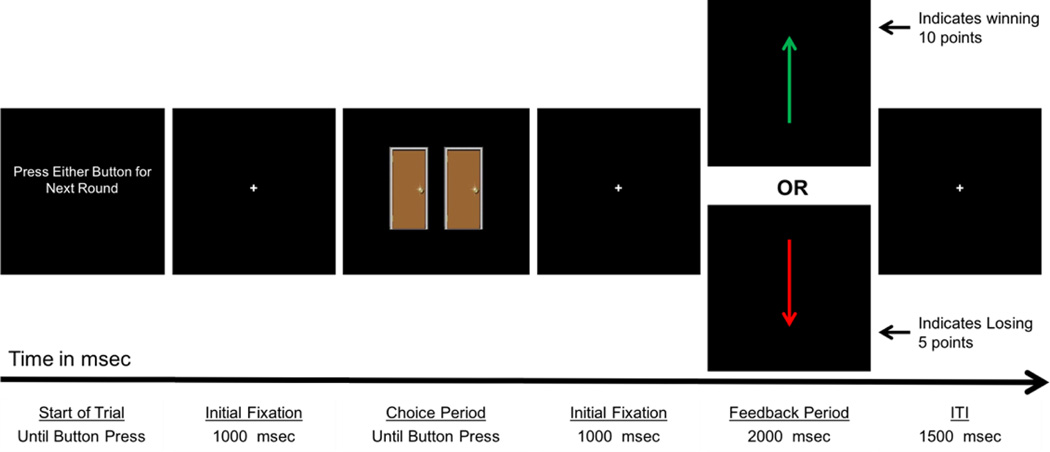
Children were asked to complete a guessing game. The guessing game was a modified version of the Doors Guessing Task (see Figure 1) used in numerous previous studies of older children, adolescents, and adults with depression.33,40,49–51 Although still analogous to “gambling” tasks used with older samples, the modified version uses varying level of prizes (e.g., poor prize = a single yellow pencil; a great prize = a doll or nerf toys) instead of the typical monetary incentives. That is, young children do not have a clear conception of money and its relative quantities; however, at very young ages, children reactive positively to prizes and gifts. Prior to the task, children were shown 3 boxes of toys: each box had increasingly appealing toys (e.g., least appealing box included standard pencils, and the most appealing box included toys such as dolls and nerf toys). Children were told that when they guessed the correct door, they would see a green up arrow, and if they guessed the wrong door, they would see a red arrow pointing down. The experimenter explained that the box of toys the children could pick from depended on the total number points they earned by guessing doors with either green vs red arrows behind them. Children were told that green arrows were worth 10 pts and red arrows were only worth 5 pts and that the more points they received the better the box they could choose from. The experiment explained that the computer would keep track of the number of green and red arrows and their total number of points.
Figure 1.
Timing of event-related potential (ERP) doors reward task. Note: ITI = intertrial interval.
The task was administered on a computer, using Presentation (Neurobehavioral Systems, Inc., Albany, California, USA) software to control the presentation and timing of all stimuli. Prior to the task, the experimenter first showed children three containers of prizes, each increasing in attractiveness to the child and in amount of points required to obtain a prize. The experimenter told the children that if they received a certain number of points in the subsequent task, they could receive a prize from one of the containers. This exchange was designed to encourage the child to engage in the task and to make it relevant to the children. During the task, participants were shown a graphic displaying two doors horizontally adjacent and were told to select a door to open (the graphic occupied approximately 6” of the visual field vertically and 8” horizontally). Participants were instructed to respond using a Logitech Gamepad F310 game controller by pressing a specific button on the left of the controller to choose the left door, or a specific button on the right of the controller to choose the right door. Following each choice, a 1,000 ms fixation cross was presented, and then a feedback stimulus appeared on the screen informing the children whether they lost or gained points. A green upward arrow indicated a correct guess, and a red downward arrow indicated an incorrect guess. All cues and feedback were presented against a black background and occupied approximately 3” of the visual field vertically and 1” horizontally. A fixation mark (+) was presented prior to the onset of each stimulus.
The order and timing of all stimuli were as follows: (i) the text “Click for the next round” was presented until the participant presses a button, (ii) a fixation mark was presented for 1000 ms, (iii) the graphic of two doors was presented until a choice was made (iv), a fixation mark was presented for 1000 ms, (v) a feedback arrow was presented for 2000 ms, and finally (vi) a fixation mark was presented for 1500 ms. The timing of the task can be found in Figure 1. Participants received negative feedback on exactly 50% of the trials, and positive feedback on exactly 50% of the trials.
Procedure
Following a brief description of the experiment, electroencephalography (EEG) sensors were attached while participants watched a movie of their choice. To familiarize children with the procedure and to increase ther interest in participating in the task, they were given a practice block containing four trials. The actual experiment involved 60 trials (3 blocks of 20) that were presented in random order.
Psychophysiological Recording and Data Reduction
The EEG was recorded using a BrainVision ActiCHamp recording system and actiCAP active electrodes (Brain Products GmbH, Munich, Germany). The electrodes were mounted in an elastic cap using a subset of the International 10/20 System sites (FP1, F3, F7, FC1, FC5, FT9, C3, T7, CP1, CP5, TP9, P3, P7, O1, Fz, Cz, Pz, Oz, FP2, F4, F8, FC2, FC6, FT10, C4, T8, CP2, CP6, P4, P8, TP10, O2). A ground electrode was located at FPz. The EEG data were recorded and referenced to Cz. The horizontal electrooculogram (EOG) was recorded as the voltage between electrodes placed lateral to the external canthi and was used to measure horizontal eye movements. The vertical EOG was recorded from electrodes placed above and below the left eye and was used to detect blinks and vertical eye movements. An electrode on the forehead served as the ground for the EOG signals. The EEG and EOG were digitized at 500 Hz with 24 bits of resolution.
Offline analysis was performed using Brain Vision Analyzer software (Brain Products GmbH, Munich, Germany). EEG data were re-referenced offline to the average of TP9 and TP10 (located adjacent to the mastoids) and band-pass filtered with cutoffs at .1 and 30 Hz. The EEG was segmented for each trial, beginning 200 ms before feedback onset and continuing for 1000 ms. The EEG for each trial was corrected for blinks and eye movements using the method developed by Gratton et al.52 Specific intervals for individual channels were rejected in each trial using a semi-automated procedure, with physiological artifacts identified by the following criteria: a voltage step of more than 50.0 µV between sample points, a voltage difference of 300.0 µV within a trial, and a maximum voltage difference of less than .50 µV within 100 ms intervals. At the initial start of the doors task the mean impendence level for the entire sample at cite PZ was 6.80 kΩ with SD = 6.05 kΩ and a maximal impendence value = 30 kΩ and a mode of 2.00 kΩ.
Data Analysis
Stimulus-locked ERPs were averaged separately for each type of feedback (reward or loss) and the activity in the 200 ms window prior to stimulus onset served as the baseline. Based on visual inspection of the overall grand average ERP, we measured the mean amplitude between 250 ms to 550 ms at electrode site Pz separately for reward and loss trials. We defined the RewP as the difference between the mean amplitude on gain minus loss trials. Our primary analysis was a repeated measures analysis of variance (ANOVA) comparing the two groups across the two conditions (reward and loss). We also conducted a univariate analysis of covariance (ANCOVA) to examine whether the hypothesized effect of PO-MDD on RewP was significant when covarying for children’s concurrent diagnosis of ODD, ADHD, and GAD. To further understand the source of any significant group difference in RewP, we used regression to create two residual scores.53–55 The first was the residuals from a regression using the mean amplitude of the loss condition to predict the mean amplitude of the reward condition. This residual score reflected variation in the reward responses not predicted by the loss response. The second was the residuals from a regression using the mean amplitude of the reward condition to predict the mean amplitude of the loss condition. This residual reflected variation in loss responses not associated for by reward responses.
We then used Pearson’s product moment correlations to examine the relations between children’s depression severity, as well as their manifestations of anhedonia as reported by caregivers, BIS-BAS scores and the RewP (reward minus loss) and the reward residual score. All statistical analyses were performed using SPSS (24.0; SPSS Inc., Chicago, Illinois, USA).
RESULTS
Demographic and Clinical Characteristics
Table 1 summarizes demographic and clinical information for the groups. Results from t-tests indicated no significant diagnostic group differences in relation to children’s age or parental income. Further, Chi-Square results showed that diagnostic groups did not differ in relation to gender, age, or ethnicity. As expected, results indicated that diagnostic groups differed on depression severity measured by the KSADS. Maternal depression, as measured by the BDI, also differed significantly between depressed and healthy preschool groups.
Differences in Neural Response to Reward (RewP) Between Young Children With Depression and Healthy Young Peers
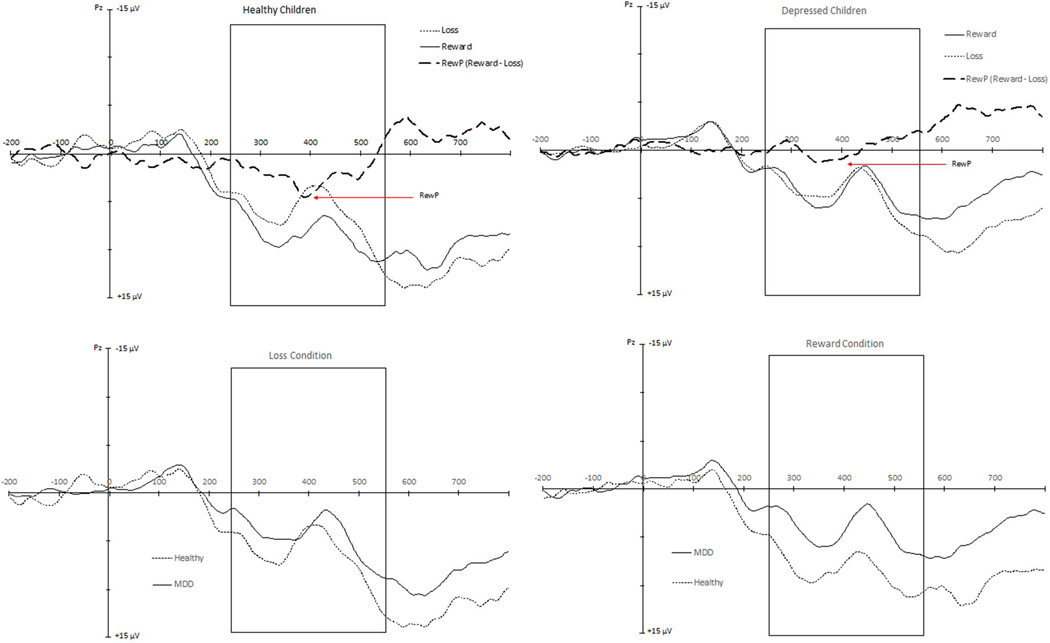
A repeated measured ANOVA was conducted to test for diagnostic group differences between the reward and loss condition mean amplitudes. Results indicated that the main effect of condition (reward vs. loss) approached statistical significance, F(1,76) = 2.95 p = .09. More importantly, the results indicated a significant condition by diagnostic group interaction effect, F(1,76) = 7.39, p = .008, Willks Lambda = .91 (see Figures 2 and 3). Specifically, children in the PO-MDD group (M = 4.03, SD = 7.11) had a smaller response to reward trials compared to healthy children (M = 8.53, SD = 6.16), t(76) = 2.72, p = .008, but the PO-MDD (M = 4.52, SD = 7.00) and healthy children (M = 6.34, SD = 7.43) did not differ in response to loss trials, t(76) = 1.05, p = 30 (see Table 2 for descriptive ERP values). Within-group analyses indicated that there was a significant effect of condition within healthy control children, F(1,24) = 7.96 p = .009, but not within the PO-MDD group, F(1,52) = .75 p = .39.
Figure 2.
Stimulus-locked event-related potentials in Pz region to feedback indicating reward and loss, shown separately in healthy participants and those with depression. Note: MDD = major depressive disorder; RewP = reward positivity.
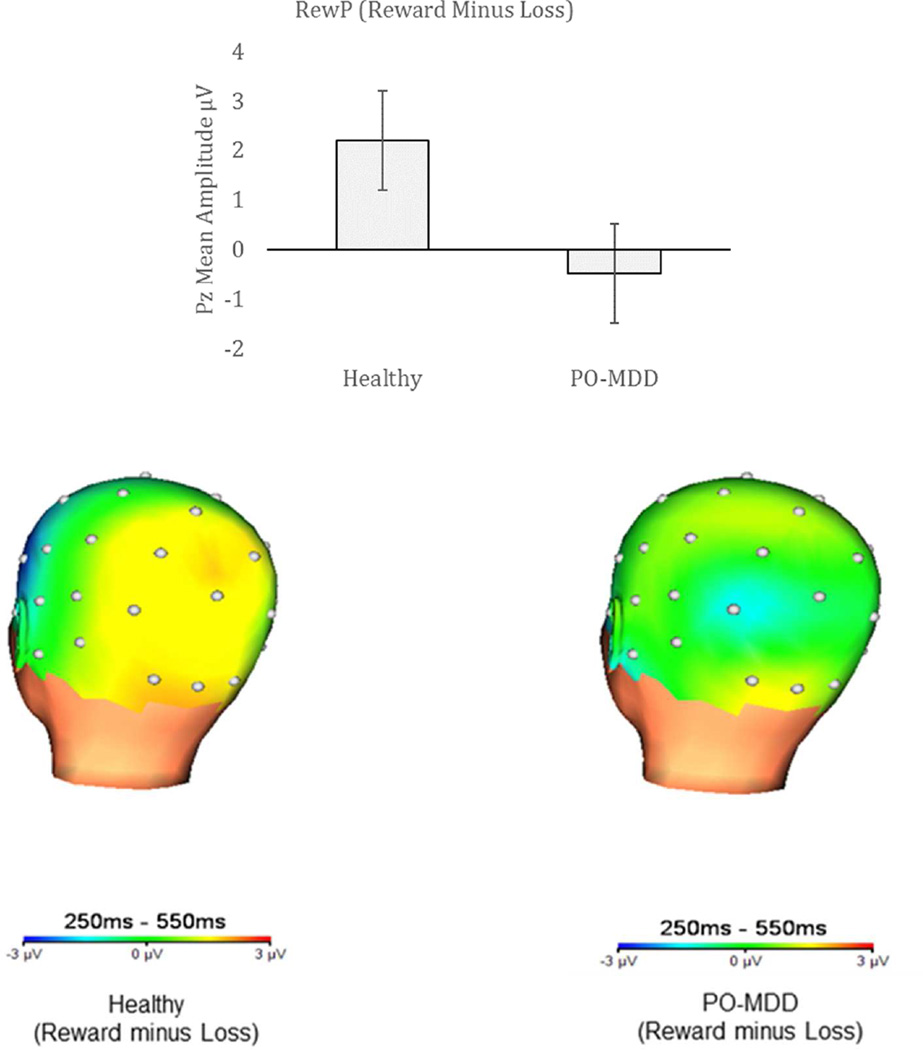
Figure 3.
Mean amplitudes of the reward positivity (RewP), calculated by response to reward minus loss, in both healthy participants and those with depression. Note: Respective head maps indicate areas of activation. PO-MDD = preschool-onset major depression.
Table 2.
Descriptive for Raw and Residualized Event-Related Potential (ERP) Mean Scores During Win and Loss Condition
| ERP Descriptives | Healthy | PO-MDD |
|---|---|---|
| Raw PZ Win; Mean(SD); Range | 8.53(6.16); 26.66 | 4.03(7.11); 35.59 |
| Residualized PZ Win; Mean(SD); Range | 1.89(3.40); 13.21 | −1.04(3.99); 20.81 |
| Raw PZ Loss; Mean(SD); Range | 6.34(7.43); 28.87 | 4.52(7.00); 29.30 |
| Residualized PZ Loss; Mean(SD); Range | −1.02(4.18); 13.70 | .64(3.94); 19.94 |
Note: PO-MDD = preschool-onset major depression.
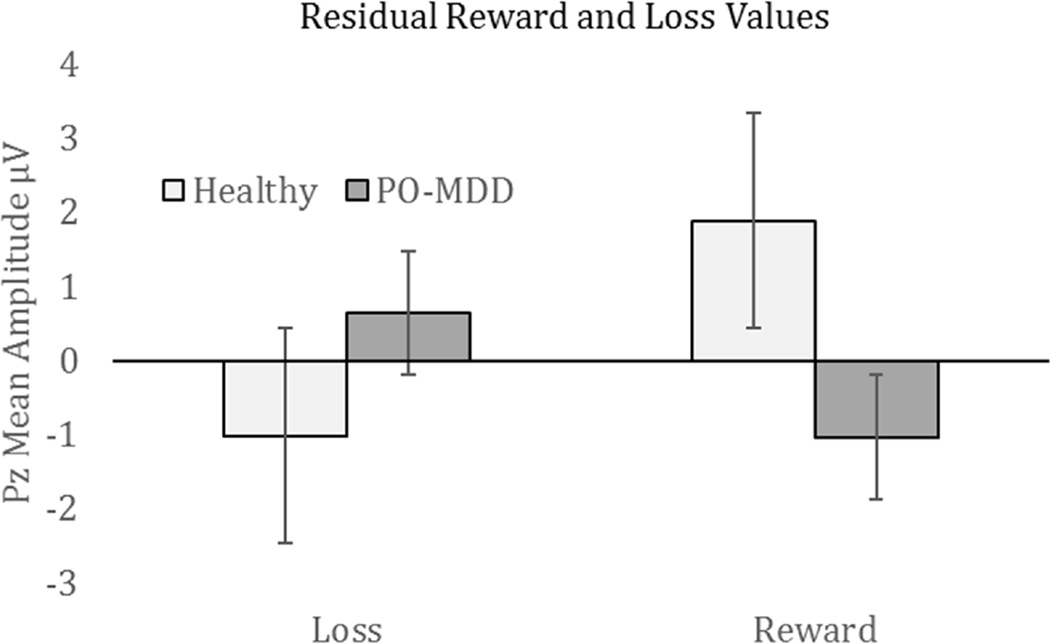
To further understand the source of the group differences in RewP, we examined the diagnostic group differences in the residualized reward and loss scores. As shown in Figure 4, the children with MDD showed a significant reduction compared to the healthy controls in the residualized reward scores, t(76) = 3.16, p = .002, but did not differ significantly in the residualized loss scores, t(76) = −1.70), p = .09.
Figure 4.
Residual loss and gain responses in both healthy participants and those with depression. Note: PO-MDD = preschool-onset major depression.
RewP Amplitudes in Young Children With Depression When Covarying for GAD, ODD, and ADHD
We used a repeated measures ANCOVA to test for the effect of PO-MDD on response to reward and loss when covarying for children’s concurrent diagnosis of GAD, ODD, and/or ADHD. Results indicated that children in the PO-MDD group still showed a group × condition interaction with a significantly smaller RewP when covarying for concurrent GAD, ODD, and ADHD, F(1,73) = 7.37, p = .008. Again, when conducting separate follow-up analyses using reward and loss residual scores, the groups did not differ on loss response, F(1,73) = 4.02, p = .05. However, children in the PO-MDD group showed a significantly smaller response to reward compared to controls when covarying for co-morbid GAD, ODD, and ADHD, F(1,73) = 8.79, p = .004.
Individual Differences in RewP and MDD Severity, BIS-BAS, Emotion Regulation, and Maternal Depression
Results from bivariate correlations indicated that in the total sample of children, greater depression severity was associated with significantly reduced neural response to reward, r = −.20, p = .04. However, follow-up within-group analyses indicated no significant association between depression severity and neural response to win trials within the healthy (r = −.24, p = .13) or depressed groups (r = −.06, p = .32). An additional series of bivariate correlations was performed within the children with depression to relate the RewP to the BIS/BAS scores, emotion regulation, and maternal depression. There were no significant correlations between children with depression’s RewP amplitudes and their BIS/BAS scores, their emotion regulation scores, or maternal depression (all rs < |.17|, all ps > .12). We also examined correlational analyses using children residualized reward scores. Again results indicated no significant associations with any of the individual difference variables (all rs < |.18|, all ps > .10).
DISCUSSION
We found that preschool-aged children with depression showed a reduced RewP in an age-appropriate reward task compared to healthy control children. This group difference was driven by a blunted response to reward in depressed compared to healthy preschoolers. However, there were no significant relationships between individual differences in RewP within the healthy or depressed group in relation to emotion functioning or depression severity. These findings provide evidence for similar reward-related neural dysfunction in depression as early as age 4, and add further evidence for the importance of reward processing in understanding the pathophysiology of depression across the lifespan and beginning early in childhood.
The reduction in RewP in young children with depression is consistent with findings from adolescent and adult samples, showing reductions in RewP are associated with current depression,33,34,39,40 and predict later emergence/worsening of depression.16,41 Further, we found that the reduction in RewP among preschool-aged children with depression held even when controlling for comorbid ADHD, ODD, and GAD diagnoses, suggesting that the group differences in RewP were not accounted for by these comorbid diagnoses and were specific to depression. Within-group findings also demonstrated that in healthy children, brain activity related to reward feedback was significantly greater than activity elicited by negative feedback. In contrast, but consistent with expecations, brain activity elecited by reward and loss feedback did not differ in magnitude in the group of young children with depression. These findings contribute to the growing literature suggesting that both behavioral and neural alterations in response to rewards are a consistent characteristic of depression at varying developmental periods15,26 that may also serve as a risk factor for future episodes.19
At one level, our results are consistent with the idea that anhedonia (reduced response to pleasure) is a core feature of MDD, which should be related to reduced neural responses to reward. However, in the current study, we did not find a relationship between individual differences in neural responses to gains and depression severity (including anhedonia symptoms in particular), BIS-BAS responses, or emotion regulation within our sample of preschoolers with depression. On average, our sample of children with PO-MDD had a high level of depression severity and anhedonia (i.e., approximately 85% of the children with depression exhibited anhendonia) without a great deal of variance. Thus, there may not have been sufficient variation in the current sample to detect these effects. Additionally, because of the age of the children, we needed to primarily rely on parent reports of anhedonia, which may not fully capture this trait in preschool children. Future studies could address this issue by including an observational measure of anhedonic symptoms in children to supplement parent report.
Interestingly, we did not find any evidence for increased response to loss in preschoolers with MDD as compared to healthy controls. There is some evidence in the literature that elevated negative mood associated with depression relates to increased behavioral measures of loss avoidance in children (7–10 years) at high risk for depression37 as well as stronger responses to loss in the striatum among high-risk children; however, these studies were conducted with an older sample.19 Other studies in the literature that have found evidence for increased loss responses associated with depression or risk for depression have also worked with older children or adults.56–59 Thus, it is possible that an enhanced response to loss emerges later in the course of development in children with depression or at risk for depression.12 It is also possible that the absence of increased brain activity in relation to loss feedback was due to differences in the task used across studies. For example, in an fMRI study examining reward processing in relation to depressive symptomatology in at-risk children ages 7 to 10, authors found blunted response to reward was associated with greater risk for MDD, but they also found the stronger deactivation to loss was an even stronger predictor of risk group.19 It may be that young children in the current sample did not react to loss as one might expect because they knew that the negative feedback was related to poorer prize choices but would not result in losing a prize all together. It is also possible that negative feedback is simply less salient to younger children as this is a developmental period in which gratification and reward seeking are especially strong motivators. A study with a wider range of ages that spans from preschool through adolescence will be needed to test this hypothesis.
This study had a number of limitations that are important to consider. As noted above, our measures of individual differences in depression, approach and avoidance motivation, and emotion regulation were based on parent report rather than direct child observation. It is possible that there was bias in this reporting and/or a greater difficulty in reporting more nuanced internalizing symptoms of depression such as experiences of pleasure or loss. An additional limitation and direction for future research would be the inclusion of a third comparison group that included preschoolers who are symptomatic with a form of psychopathology other than depression (i.e., anxiety, disruptive disorder). Further, although the use of ERP to measure RewP is becoming increasingly common and has many advantages, this method also has limitations. ERPs have excellent temporal resolution for measuring real-time neural responses, are straightforward to use in young populations, and provide an important measure of reward response that is not dependent on self-report. However, ERPs do not provide the same spatial resolution as measures such as fMRI, and thus we cannot localize our finding to specific brain regions (e.g., ventral striatum). Nonetheless, prior studies integrating ERP and fMRI have suggested that the RewP signal correlates with fMRI responses to reward in the striatum and the dorsal anterior cingulate cortex.23–25 Finally, our rewards scenario was an abstracted situation that was experienced in a laboratory setting, and thus may not fully generalize to real-life situations in which children might encounter rewarding or disappointing feedback.
In summary, this is the first study to our knowledge to show that preschoolers with depression showed reduced ERP response to rewards and lack of differentiation between rewards and losses (reduced RewP) as compared to healthy controls. This is the youngest sample in which the RewP has been examined in relation to individual differences, and adds to the literature on the value of using ERP measures to understand neural function in young children and in relation to psychopathology. These findings provide important evidence for diminished response to rewarding outcomes as a potentially key mechanism associated with depression across the lifespan by demonstrating its atypical neural actiation in the youngest sample with depression studied to date.
Study findings underscore the importance of clinical attention to alterations in reward response as a core area of emotional impairment in early childhood depression. These findings of continuity in reward response deficits in depression as early as the preschool period suggest that interventions targeting behavioral and/or neural mechanisms of reward processing may be an important avenue for early interventions in depression. Study findings point to the potential utility of focusing on upregulation of responses to rewards as a potential pathway for intervention in already depressed young children, or as a means to prevent the occurrence of depression in individuals at risk for this often debilitating illness.
Acknowledgments
This work was supported by the National Institutes of Health [1R01MH098454-01A1 to J.L.L. and 3R01MH098454-03S1 to Co-PIs J.L.L. and D.M.B]; Dr. Hajcak’s work on this project was supported by grant 3R01MH098454-03S1.
The authors thank the study families and participants who have generously given their time and effort to this study.
Dr. Luby has received royalties from Guilford Press. Dr. Barch has served as a consultant for Pfizer, Amgen, Roche, and Takeda, and has a contract to analyze imaging data for Pfizer. She has received grant or research support from the NIMH, the National Institute for Drug Abuse, and the National Institutes of Health (NIH) Blueprint. Drs. Belden, Hajcak, Kappenman and Mss.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Irvin, Kelly, and Karlow report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Andy C. Belden, Washington University in St. Louis, St. Louis, MO..
Kelsey Irvin, Washington University in St. Louis, St. Louis, MO..
Greg Hajcak, Stony Brook University, Stony Brook, NY..
Emily S. Kappenman, University of California at Davis, Center for Mind and Brain..
Danielle Kelly, Washington University in St. Louis, St. Louis, MO..
Samantha Karlow, Washington University in St. Louis, St. Louis, MO..
Joan L Luby, Washington University in St. Louis, St. Louis, MO..
Deanna M. Barch, Washington University in St. Louis, St. Louis, MO..
References
- 1.Robins LN, Regier D. Psychiatric disorders in america: The Epidemiologic Catchment Area Study. New York: Free Press; 1991. pp. 328–366. [Google Scholar]
- 2.Donohue JM, Pincus HA. Reducing the societal burden of depression: a review of economic costs, quality of care and effects of treatment. Pharmacoeconomics. 2007;25(1):7–24. doi: 10.2165/00019053-200725010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Luby J. Depression in preschool-age children: current evidence. The Brown University Child and Adolescent Behavior Letter. 2007;23(1–6) [Google Scholar]
- 4.Luby J, Belden A. Defining and validating bipolar disorder in the preschool period. Development and psychopathology. 2006;18(4):971–988. doi: 10.1017/S0954579406060482. [DOI] [PubMed] [Google Scholar]
- 5.Luby JL, Belden AC, Pautsch J, Si X, Spitznagel E. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J Affect Disord. 2009;112:111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luby JL, Heffelfinger AK, Mrakotsky C, et al. The clinical picture of depression in preschool children. J Am Acad Child Adolesc Psychiatry. 2003;42:340–348. doi: 10.1097/00004583-200303000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Kujawa A, Proudfit GH, Klein DN. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. J Abnorm Psychol. 2014;123:287–297. doi: 10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kujawa AJ, Torpey D, Kim J, et al. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. J Abnorm Child Psychol. 2011;39:125–135. doi: 10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bufferd SJ, Dougherty LR, Carlson GA, Rose S, Klein DN. Psychiatric disorders in preschoolers: continuity from ages 3 to 6. Am J Psychiatry. 2012;169:1157–1164. doi: 10.1176/appi.ajp.2012.12020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luby JL, Belden AC, Jackson JJ, et al. Early Childhood Depression and Alterations in the Trajectory of Gray Matter Maturation in Middle Childhood and Early Adolescence. JAMA Psychiatry. 2016;73(1):31–38. doi: 10.1001/jamapsychiatry.2015.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luby JL, Gaffrey MS, Tillman R, April LM, Belden AC. Trajectories of Preschool Disorders to Full DSM Depression at School Age and Early Adolescence: Continuity of Preschool Depression. Am J Psychiatry. 2014;171(7):768–776. doi: 10.1176/appi.ajp.2014.13091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luking KR, Pagliaccio D, Luby JL, Barch DM. Reward Processing and Risk for Depression Across Development. Trends Cogn Sci. 2016;20:456–468. doi: 10.1016/j.tics.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes EE, Dahl RE. Research Review: altered reward function in adolescent depression: what, when and how? Journal of child psychology and psychiatry, and allied disciplines. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forbes EE, Goodman SH. Reward function: a promising but (still) underexamined dimension in developmental psychopathology. J Abnorm Psychol. 2014;123(2):310–313. doi: 10.1037/a0036494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Admon R, Pizzagalli DA. Dysfunctional Reward Processing in Depression. Curr Opin Psychol. 2015;4:114–118. doi: 10.1016/j.copsyc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg A, Liu H, Hajcak G, Shankman SA. Blunted neural response to rewards as a vulnerability factor for depression: Results from a family study. J Abnorm Psychol. 2015;124:878–889. doi: 10.1037/abn0000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stringaris A, Vidal-Ribas Belil P, Artiges E, et al. The Brain's Response to Reward Anticipation and Depression in Adolescence: Dimensionality, Specificity, and Longitudinal Predictions in a Community-Based Sample. Am J Psychiatry. 2015;172:1215–1223. doi: 10.1176/appi.ajp.2015.14101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luking KR, Pagliaccio D, Luby JL, Barch DM. Depression Risk Predicts Blunted Neural Responses to Gains and Enhanced Responses to Losses in Healthy Children. J Am Acad Child Adolesc Psychiatry. 2016;55:328–337. doi: 10.1016/j.jaac.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverman MH, Jedd K, Luciana M. Neural networks involved in adolescent reward processing: An activation likelihood estimation meta-analysis of functional neuroimaging studies. Neuroimage. 2015;122:427–439. doi: 10.1016/j.neuroimage.2015.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Proudfit GH. The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology. 2015;52(4):449–459. doi: 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- 23.Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Human brain mapping. 2011;32:2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. NeuroImage. 2011;57:1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 25.Becker MP, Nitsch AM, Miltner WH, Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. J Neurosci. 2014;34:3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. J Abnorm Psychol. 1994;103:460–466. doi: 10.1037//0021-843x.103.3.460. [DOI] [PubMed] [Google Scholar]
- 28.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121:553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XH, Huang J, Zhu CY, et al. Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. 2014;220:874–882. doi: 10.1016/j.psychres.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 31.Yang XH, Huang J, Lan Y, et al. Diminished caudate and superior temporal gyrus responses to effort-based decision making in patients with first-episode major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:52–59. doi: 10.1016/j.pnpbp.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151:531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 33.Foti D, Carlson JM, Sauder CL, Proudfit GH. Reward dysfunction in major depression: multimodal neuroimaging evidence for refining the melancholic phenotype. Neuroimage. 2014;101:50–58. doi: 10.1016/j.neuroimage.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu WH, Wang LZ, Shang HR, et al. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 2014;53:213–220. doi: 10.1016/j.neuropsychologia.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological psychology. 2009;81(1):1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biol Psychiatry. 2007;61(5):633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Luking KR, Pagliaccio D, Luby JL, Barch DM. Child Gain Approach and Loss Avoidance Behavior: Relationships With Depression Risk, Negative Mood, and Anhedonia. J Am Acad Child Adolesc Psychiatry. 2015;54(8):643–651. doi: 10.1016/j.jaac.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olino TM, McMakin DL, Morgan JK, et al. Reduced reward anticipation in youth at high-risk for unipolar depression: A preliminary study. Dev Cogn Neurosci. 2014;8:55–64. doi: 10.1016/j.dcn.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bress JN, Smith E, Foti D, Klein DN, Hajcak G. Neural response to reward and depressive symptoms in late childhood to early adolescence. Biol Psychol. 2012;89:156–162. doi: 10.1016/j.biopsycho.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bress JN, Meyer A, Hajcak G. Differentiating anxiety and depression in children and adolescents: evidence from event-related brain potentials. J Clin Child Adolesc Psychol. 2015;44:238–249. doi: 10.1080/15374416.2013.814544. [DOI] [PubMed] [Google Scholar]
- 41.Bress JN, Meyer A, Proudfit GH. The stability of the feedback negativity and its relationship with depression during childhood and adolescence. Dev Psychopathol. 2015;27:1285–1294. doi: 10.1017/S0954579414001400. [DOI] [PubMed] [Google Scholar]
- 42.Gaffrey MS, Luby JL. Kiddie Schedule for Affective Disorders and Schizophrenia - Early Childhood Version (K-SADS-EC) St. Louis: Washington University School of Medicine; 2012. [Google Scholar]
- 43.Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Hessler M, Spitznagel E. Modification of DSM-IV criteria for depressed preschool children. Am J Psychiatry. 2003;160:1169–1172. doi: 10.1176/appi.ajp.160.6.1169. [DOI] [PubMed] [Google Scholar]
- 44.Carver CS, White TL. Behavioral inhbition, behavioral activation and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 45.Pagliaccio D, Luking KR, Anokhin AP, et al. Revising the BIS/BAS Scale to study development: Measurement invariance and normative effects of age and sex from childhood through adulthood. Psychol Assess. 2016;28(4):429–442. doi: 10.1037/pas0000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shields AM, Cicchetti D, Ryan RM. The development of emotional and behavioral self-regulation and social competence among maltreated school-age children. Dev Psychopathol. 1994;6:57–75. doi: 10.1017/S0954579400005885. [DOI] [PubMed] [Google Scholar]
- 47.Beck AT, Steere RA. Beck Depression Inventory Manual. 1987 [Google Scholar]
- 48.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 49.Bress JN, Smith E, Foti D, Klein DN, Hajcak G. Neural response to reward and depressive symptoms in late childhood to early adolescence. Biol Psychol. 2012;89:156–162. doi: 10.1016/j.biopsycho.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foti D, Kotov R, Klein DN, Hajcak G. Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. J Abnorm Child Psychol. 2011;39:913–924. doi: 10.1007/s10802-011-9503-9. [DOI] [PubMed] [Google Scholar]
- 51.Nelson BD, Perlman G, Hajcak G, Klein DN, Kotov R. Familial risk for distress and fear disorders and emotional reactivity in adolescence: an event-related potential investigation. Psychol Med. 2015;45(12):2545–2556. doi: 10.1017/S0033291715000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 53.Hajcak G, Moser JS, Holroyd CB, Simons RF. It's worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiol. 2007;44:905–912. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 54.Dunning JP, Hajcak G. Error-related negativities elicited by monetary loss and cues that predict loss. Neuroreport. 2007;18:1875–1878. doi: 10.1097/WNR.0b013e3282f0d50b. [DOI] [PubMed] [Google Scholar]
- 55.Holroyd CB, Larsen JT, Cohen JD. Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology. 2004;41:245–253. doi: 10.1111/j.1469-8986.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 56.Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67(4):380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCabe C, Woffindale C, Harmer CJ, Cowen PJ. Neural processing of reward and punishment in young people at increased familial risk of depression. Biological psychiatry. 2012;72:588–594. doi: 10.1016/j.biopsych.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 58.Beevers CG, Worthy DA, Gorlick MA, Nix B, Chotibut T, Todd Maddox W. Influence of depression symptoms on history-independent reward and punishment processing. Psychiatry Res. 2013;207:53–60. doi: 10.1016/j.psychres.2012.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maddox WT, Gorlick MA, Worthy DA, Beevers CG. Depressive symptoms enhance loss-minimization, but attenuate gain-maximization in history-dependent decision-making. Cognition. 2012;125:118–124. doi: 10.1016/j.cognition.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]