Abstract
Background
Complete surgical resection of primary tumors is critical for long-term control of high-grade osteosarcoma. Uniform assessment of the extent of surgical resection is important in clinical trials, though the accuracy of this reporting has been poorly studied.
Methods
We conducted a retrospective cohort study of patients 5–40 years of age with newly diagnosed high-grade resectable osteosarcoma treated as part of the AOST0331 clinical trial at Children’s Oncology Group institutions. The extent of surgical resection of the primary tumor was graded as wide or radical by the treating institution. Central assessment of the extent of resection by two orthopedic oncologists was compared with institutional assessment by reviewing pathology and operative reports.
Results
We included 956 patients who had data available for central review. The extent of resection reported by treating institutions was 536/956 (56%) radical and 420/956 (44%) wide. The extent of resection assessed by central review was 162/956 (17%) radical and 794/956 (83%) wide. The overall discordance rate for the cohort was 43%.
Conclusions
Institutional reports of radical resection in high-grade osteosarcoma significantly over-estimate the proportion of patients undergoing radical resection. This highlights the need for centralized review and improved accuracy of reporting of the extent of resection.
Keywords: cooperative group trial, osteosarcoma, surgical margins
INTRODUCTION
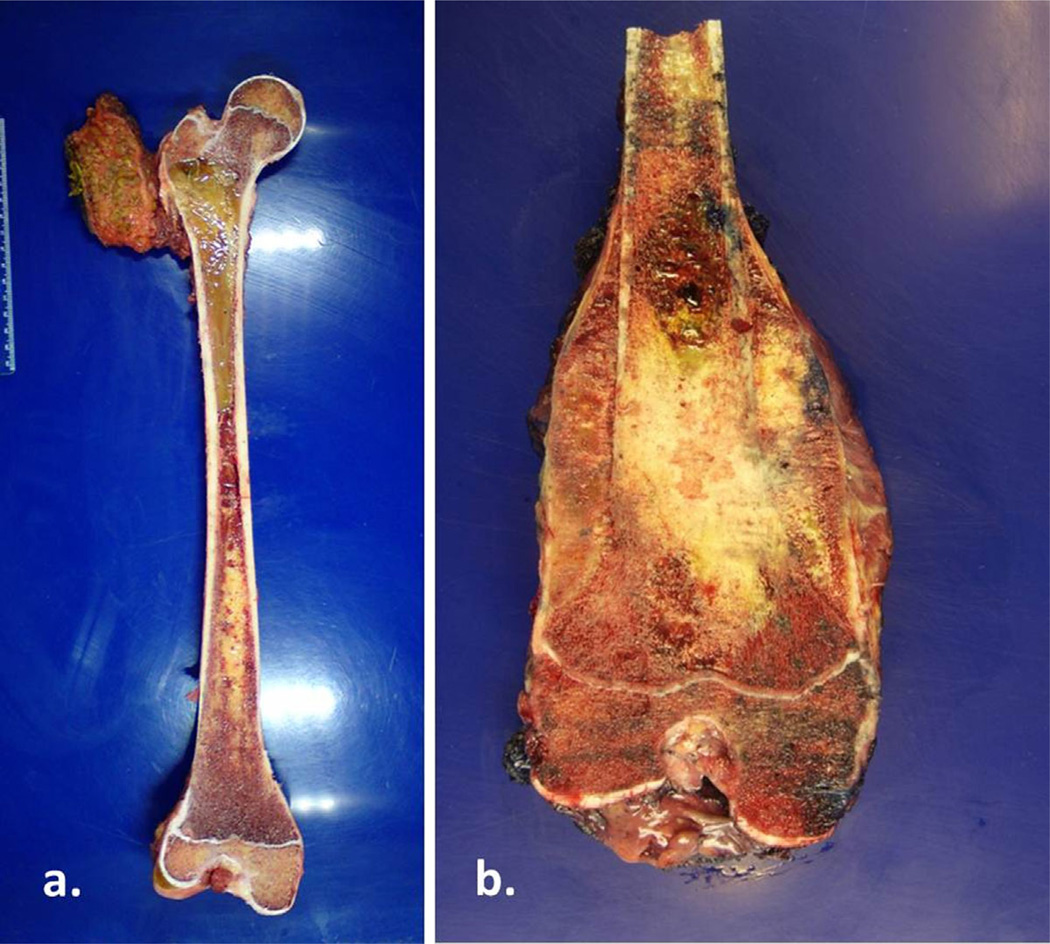
Surgical oncologic classifications are commonly used to convey the extent of surgical interventions in treating patients with bone and soft tissue sarcomas. Most sarcoma treatment centers use the following surgical classification system described by Enneking et al. [1]: intralesional, marginal, wide, and radical. In intralesional excisions, the tumor is violated and removed in a piecemeal fashion, whereas in marginal excisions the tumor is removed through the so-called reactive zone (the inflammatory area around the pseudocapsule). Intralesional and marginal excisions are inadequate surgical procedures for the removal of sarcomas because tumor cells are likely to be left behind. The goal of sarcoma excision is to achieve a negative margin via wide or radical resection. Wide resection refers to removing the involved part of the bone with a cuff of normal tissue, whereas radical resection refers to removing the entire bone or compartment containing the tumor (Fig. 1). With current imaging capabilities and effective adjuvant therapy, most sarcomas can be safely resected by wide excision.
Fig. 1.
(a) Radical resection of a femoral Ewing’s sarcoma. The tumor involved nearly the entire intramedullary portion of the femur, necessitating removal of the entire bony compartment. (b) Wide excision of a distal femur for osteosarcoma. Only a portion of the femur was removed to achieve a negative margin.
Wide and radical resections of bone sarcoma are distinct oncologic surgical procedures and hence potential differences in margin status. Accurate reporting of resection status in the context of clinical trials is important because inaccurate reporting may compromise subsequent data interpretation. The collective clinical impression is that with current imaging and effective adjuvant therapy, local control for osteosarcoma is equally effective with wide or radical excision. However, no prospective studies have been performed to prove this. Furthermore, retrospective data suggest that even in the modern era of therapy, marginal and intralesional resections increase the risk of recurrence [2]. Therefore, accurate data distinguishing margin type is an essential component of clinical trials to ensure that the effect of margin status on outcome can be evaluated. The accuracy of this reporting has been poorly studied, and the few studies on this topic show that errors are more common when reported by those unfamiliar with the surgical techniques [3,4].
To better understand the accuracy of the reported extent of surgical resection in osteosarcoma, we leveraged data from the largest cooperative group clinical trial in high-grade osteosarcoma to date. We describe the concordance rate between institutional assessment and central review of wide versus radical resection. We focused on this distinction rather than accuracy of reporting marginal versus intralesional resections because these are not considered oncologically adequate.
METHODS
Patients
All cases were derived from patients treated as part of the Children’s Oncology Group (COG) AOST0331: A Randomized Trial of the European and American Osteosarcoma Study Group to Optimize Treatment Strategies for Resectable Osteosarcoma Based on Histologic Response to Pre-Operative Chemotherapy. This multi-center international randomized trial accrued patients with newly diagnosed high-grade osteosarcoma from 2005 to 2011. Patients 5–40 years of age were eligible if the treating institution deemed all sites of disease, including metastatic disease, resectable. The trial examined whether the addition of ifosfamide/etoposide or interferon improved event-free survival in patients with standard response (≥10% viable tumor) or good response (<10% viable tumor) to neoadjuvant chemotherapy. All patients enrolled in the study were administered standard preoperative chemotherapy consisting of doxorubicin, cisplatin, and high-dose methotrexate (MAP therapy). Definitive surgical resection was performed after two cycles. Treating institutions reported extent of surgical resection, and histologic response to chemotherapy was assessed according to the method of Huvos [5]. Patients with >90% tumor necrosis were then randomized to continue with MAP treatment or MAP plus pegylated interferon alpha. Patients with <90% tumor necrosis were randomized to continue MAP therapy or MAP plus ifosfamide and etoposide.
This clinical trial included patients treated at COG centers and patients treated by European cooperative groups. The analytical cohort for the current report included only patients enrolled in the clinical trial at COG centers. Included in this analysis were patients reported by the treating institution to have undergone a wide or radical resection (defined below). Patients who did not undergo surgical resection of the primary tumor for any reason (e.g., early death or withdrawal from the study) were excluded from this analysis. Patients who had resection before the start of chemotherapy were not eligible for the clinical trial and therefore not included in this analysis.
All patients (or caregivers) provided informed consent for clinical trial participation. Each center’s institutional review board approved the trial. This retrospective analysis used only de-identified data derived from the clinical trial and therefore did not require additional institutional review board approval.
Study Design and Statistical Analysis
We performed a retrospective study of patients with available data on extent of resection in AOST0331. At least one of two orthopedic oncologists (C.D.M. or R.L.R.) reviewed the surgical and pathology reports of patients considered to have undergone a wide or radical resection of the primary tumor on the basis of the treating institution assessment. Wide resection was defined as partial bone excision, and radical resection was defined as removal of the entire involved bone.
The extent of resection based on the central review was tabulated against the institutional report of the extent of resection. Using McNemar’s test, we tested the hypothesis that the probability of a disagreement in assessment of radical according to central review and wide according to institutional review was equal to the probability of a disagreement in assessment of wide according to central review and radical according to institutional review.
RESULTS
Of the 1,160 patients enrolled in the clinical trial at COG institutions, data on the extent of resection were available for 977 patients. Of these 977 patients, 21 had undergone marginal or intralesional surgery and were not analyzed. The remaining 956 patients were reported to have undergone a wide or radical resection and formed the analytical cohort for this report. Clinical features for these 956 patients are shown in Table I.
TABLE I.
Characteristics of 956 Patients Who Underwent Tumor Resection for High-Grade Osteosarcoma
| Patients (N = 956) | ||
|---|---|---|
| Characteristics | N | % |
| Age at enrollment (years) | ||
| Median (range) | 14 (5–40) | |
| Sex | ||
| Male | 565 | 59.1 |
| Female | 391 | 40.9 |
| Race | ||
| White | 691 | 72.3 |
| Black | 129 | 13.5 |
| Asian | 34 | 3.5 |
| Hawaiian, Pacific Islander, NOS | 1 | 0.1 |
| American Indian, Aleutian, or Eskimo | 11 | 1.2 |
| Other | 58 | 6.1 |
| Unknown | 32 | 3.4 |
| Primary site | ||
| Lower extremity | ||
| Femur | 467 | 48.8 |
| Non-femur | 309 | 32.3 |
| Upper extremity | 126 | 13.2 |
| All other sites | 54 | 5.7 |
| Metastasis present at time of enrollment | ||
| No | 794 | 83.1 |
| Yes | 150 | 15.7 |
| Unknown | 12 | 1.2 |
The reported extent of resection by the institution was 536/956 (56%) radical resections and 420/956 (44%) wide excisions. In contrast, central review of the extent of resection data showed 162/956 (17%) radical resections and 794/956 (83%) wide excisions (Table II). Three hundred ninety-four (74%) patients initially classified as having undergone radical resection by the treating institutions were reclassified as having undergone wide resection.
TABLE II.
Concordance of Institutional Versus Central Review of Radical Versus Wide Resection in 956 Patients With Newly Diagnosed Osteosarcoma
| Central review | |||
|---|---|---|---|
| Institutional review | Radical | Wide | Total |
| Radical | 142 | 394 | 536 |
| Wide | 20 | 400 | 420 |
| Total | 162 | 794 | 956 |
The overall discordance rate for the entire cohort was 43%. There was significant evidence (P < 0.001) to reject the null hypothesis that the two types of disagreement (wide resection erroneously classified as radical and radical resection erroneously classified as wide) were equally probable. Of the 414 patients for which the institutional assessment did not agree with central review, 95% constituted cases where the institutional assessment was radical resection and the central review was wide resection.
DISCUSSION
Before the advent of effective systemic therapy for controlling microscopic disease, radical resections were routinely performed for patients with primary bone tumors. The success of chemotherapy combined with advanced imaging techniques allowed orthopedic oncologists to remove less bone and still achieve oncologically acceptable margins and results. Although we do not know if there is a different rate of local disease control between wide and radical resection, the compromised vernacular in reporting implies a different treatment than was actually performed, and thus downstream oncologic inferences are potentially corrupted.
In this review of COG trial AOST0331, the discordance rate of reported oncologic surgical classification (wide vs. radical) was 43%. Most of the inconsistencies were in patients categorized as having undergone radical resection, with 74% of those reclassified as wide resection. Only 5% of those classified as wide excision were reclassified as radical.
Although the exact reason for this high rate of discordance cannot be definitively identified, it is important to explore potential reasons. Most osteosarcomas are removed by wide excision. However, there is no Current Procedural Terminology (CPT) code for this operation. For the surgical removal of a bone malignancy, all CPT codes currently use the description of “radical resection” regardless of the amount of bone removed and anatomic site. It is possible that the greater number of patients reclassified as wide from radical reflects the influence and limits of the CPT coding system. In addition, most data submission by treating institutions is performed by non-physician research personnel supervised by oncologists. Our results indicate a need for increased educational efforts targeting those personnel responsible for data entry. Another possible intervention may be to encourage surgical oncologists to state the true extent of resection when dictating the name of the operation, as opposed to a reflection of the CPT code.
Distinguishing between these two oncologic surgical procedures is important for understanding oncologic outcomes for several reasons. First, it is unclear from the literature whether differences in survival exist between these two groups. “Skip metastases” refer to lesions within the same bone that are radiographically and histologically separate from the primary tumor and are well known to occur in patients with osteosarcoma [6]. Skip metastases are associated with high local recurrence and an inferior prognosis as reflected by their classification as American Joint Committee on Cancer Stage III compared with Stage II bone tumor. For patients who have true radical resections, an unrecognized skip metastasis would be removed, eliminating a source of recurrence. It is possible that whole-compartment resection renders a superior local control option. Second, because of the potential difference in local tumor control between wide and radical margins, subsequent data analysis is problematic, particularly in assessing the relation of margins and local recurrence [2,7–9]. Unfortunately, the literature has failed to make these distinctions because most studies report “wide” and “radical” interchangeably to imply a negative margin. Critical evaluation of outcomes is only as accurate as the data provided. Third, it is important for the oncologic community to use a common language when describing local tumor control. Facilitating clearer communication will facilitate our understanding of the strengths and limitations of the data reported in these studies.
Our analysis is notable for a particular strength. We leveraged the largest clinical trial conducted to date in high-grade osteosarcoma. Nearly 1,000 centrally reviewed patients were included in this analysis. Access to pathology reports and operative notes allowed confirmation of the extent of resection. It is important to note that our European colleagues did not note the divergent classification of “radical” versus “wide” resection in their central review. However, European patients have not yet undergone central review by orthopedic oncologists as we had done through the COG mechanism.
Although this does not appear to be a reporting problem unique to COG studies, we aim to use this example to raise awareness among our colleagues about the importance of accurate data reporting for all bone sarcomas, including Ewing sarcoma and chondrosarcoma. Providing better training to those responsible for submitting data and encouraging accurate dictation of surgical procedures may decrease the incidence of erroneous data entry. This would improve our ability to evaluate, in the context of prospective clinical trials, how margin status and surgical approach affect outcome.
Abbreviations
- COG
Children’s Oncology Group
- CPT
Current Procedural Terminology
- MAP
preoperative chemotherapy consisting of doxorubicin, cisplatin and high-dose methotrexate
REFERENCES
- 1.Enneking W, Dunham W, Gebhardt M, et al. A system for the classification of skeletal resections. Chir Organi Mov. 1990;75:217–240. [PubMed] [Google Scholar]
- 2.Gherlinzoni F, Picci P, Bacci G, et al. Limb sparing versus amputation in osteosarcoma. Correlation between local control, surgical margins and tumor necrosis: Istituto Rizzoli experience. Ann Oncol. 1992;3:S23–S27. doi: 10.1093/annonc/3.suppl_2.s23. [DOI] [PubMed] [Google Scholar]
- 3.Randall RL, Bruckner JD, Papenhausen MD, et al. Errors in diagnosis and margin determination of soft-tissue sarcomas initially treated at non-tertiary centers. Orthopedics. 2004;27:209–212. doi: 10.3928/0147-7447-20040201-14. [DOI] [PubMed] [Google Scholar]
- 4.Trovik CS, Skjeldal S, Bauer H, et al. Reliability of margin assessment after surgery for extremity soft tissue sarcoma: The SSG experience. Sarcoma. 2012;2012:290698. doi: 10.1155/2012/290698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: Selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.Kager L, Zoubek A, Kastner U, et al. Skip metastases in osteosarcoma: Experience of the cooperative osteosarcoma study group. J Clin Oncol. 2006;24:1535–1541. doi: 10.1200/JCO.2005.04.2978. [DOI] [PubMed] [Google Scholar]
- 7.Picci P, Sangiorgi L, Bahamonde L, et al. Risk factors for local recurrences after limb-salvage surgery for high-grade osteosarcoma of the extremities. Ann Oncol. 1997:899–903. doi: 10.1023/a:1008230801849. [DOI] [PubMed] [Google Scholar]
- 8.Sluga M, Windhager R, Lang S, et al. The role of surgery and resection margins in the treatment of Ewing’s sarcoma. Clin Orthop. 2001:394–399. doi: 10.1097/00003086-200111000-00051. [DOI] [PubMed] [Google Scholar]
- 9.Trovik CS. Local recurrence of soft tissue sarcoma. A Scandinavian sarcoma group project. Acta Orthop Scand Suppl. 2001;72:1–31. [PubMed] [Google Scholar]