Abstract
Rationale
Research documents a reciprocal impact of anxiety on working memory (WM), although its strength and direction depend on factors like task difficulty. A better understanding of these factors may generate insights into cognitive mechanisms of action involved in anxiety, culminating into treatment implications. By blocking the physiological effects of anxiety, propranolol might also block anxiety interference on WM. Conversely, by improving task-directed attention, methylphenidate might reduce anxiety, or, alternatively, by improving cognitive efficiency and free up processing resources to compute anxiety.
Objectives
To investigate the interplay between induced anxiety and WM, we pharmacologically manipulated either anxiety or cognition, using single doses of 40 mg propranolol (PRO), 20 mg methylphenidate (MPH), or placebo (PLA). In this double-blind parallel-group design study, 60 healthy volunteers (20/drug group) performed a verbal WM task under three loads, 1-, 2- and 3-back, and in two conditions, threat of shock and safety. Startle electromyography (EMG) was used to measure anxiety.
Results
Findings were twofold: (1) MPH blocked anxiety interference only on the 3-back WM performance, while PRO or PLA had no effects on anxiety-WM interference, and (2) drugs had no effects on anxiety, but, after controlling for baseline anxiety, MPH enhanced anxiety-potentiated startle during the 3-back task.
Conclusions
These findings support that MPH-related improvement of cognitive efficiency permits anxiety to be processed and expressed. In conclusion, MPH may be a useful tool to investigate the mechanisms of interaction between anxiety and WM, particularly those under catecholaminergic control.
Keywords: Fear-potentiated startle, Stimulant, Cognition, Limited resources theory, Dopamine, Catecholamine
Introduction
Anxiety disorders are the most prevalent of all mental health disorders (Kessler et al. 2009), and they carry heavy psychological, social, and economic costs (Beddington et al. 2008). Although treatment of these disorders continue to improve, the relatively low rate of remission and high rate of relapse call for a better understanding of the psychological and physiological constituents of these disorders to devise improved or novel treatment strategies. Next to the emotional pain, the deleterious cognitive impact of anxiety contributes substantially to the economic burden (e.g., lowering job performance) and the reduced quality of life associated with these disorders. Indeed, anxious individuals typically complain of distractibility and poor concentration, while population-based studies report deficits in executive function and episodic memory across anxiety disorders (Airaksinen et al. 2005). A large literature documents the deleterious influence of anxiety on cognition (Eysenck et al. 2007), and conversely the influence of cognition in down-regulating anxiety (McRae et al. 2009; Van Dillen and Koole 2007). This brings up two key questions: (1) does reducing anxiety improve cognition, and (2) does improving cognition reduce anxiety. The present study is a proof-of-concept investigation that uses a pharmacological approach to examine the interaction between anxiety induced by threat of shock and performance of a working memory task. Specifically, the objective was to explore whether (1) the cognitive enhancer methylphenidate can reduce induced anxiety during a working memory task and (2) the anxiolytic propranolol can improve cognitive performance during induced - anxiety.
One of the most popular theories used to explain the interactions between emotion and cognition is competition for resources. Broadly, this theory posits that cognitive resources are limited, and, consequently, emotional and cognitive stimuli compete for resources in order to be processed (e.g., Bishop 2009; Eysenck and Calvo 1992; King and Schaefer 2011). If resources are sufficient, both types of stimuli are processed. But, if insufficient, then only the cognitive or the emotional stimuli are fully processed, based on prioritization. Hence, based on competition and prioritization, if anxiety prevails, performance will be impaired, but if cognition prevails, then anxiety will be reduced. The more difficult is a task, the more resources it will engage. Similarly, the more anxious a subject is, the more resources will be devoted to the processes involved in processing anxiety (e.g., worrisome thoughts).
Working memory (WM) is particularly salient because of its distinct role in anxiety. Indeed, worry, a cardinal symptom of anxiety, consumes WM resources, which, in turn, may become less available for other tasks also requiring WM (Eysenck et al. 2007). Conversely, WM tasks are especially efficient at reducing anxiety (Vytal et al. 2012). In the present study, a WM task is selected based on both its relevance to anxiety, and the consistent findings generated by our laboratory. These findings evidence a linear reduction of anxiety induced by unpredictable threat of shock while performing a WM task of increasing difficulty (WM load) (Patel et al. 2016; Vytal et al. 2012). Presently, our goal is to examine (1) whether improving cognition pharmacologically will improve focus on the WM task and, in turn, down-regulate induced anxiety, and (2) whether reducing anxiety pharmacologically will improve performance during induced anxiety.
Methylphenidate (MPH) appears to be ideal to manipulate the cognitive component for two reasons. First, clinical and empirical evidence demonstrate cognitive improvement under MPH (for review, see Berridge and Arnsten 2013), which is stronger on more complex tasks (for review Bagot and Kaminer 2014; Linssen et al. 2014). Second, studies generally have failed to evidence reliable MPH effects on anxiety (Bagot and Kaminer 2014; Hermens et al. 2007). MPH's effects are attributed primarily to its dopaminergic action through blocking the dopa-mine transporter (DAT), thereby increasing extracellular dopamine concentration (Volkow et al. 2001). A similar, albeit weaker, effect is also noted on noradrenergic function (for review, see Berridge and Arnsten 2013).
Propranolol hydrochloride (PRO) is used as an anxiolytic medication (see review, Steenen et al. 2016), with minimal effects on cognition (for review, see Fogari and Zoppi 2004). PRO reduces the physiological effects of anxiety, such as changes in heart rate, blood pressure, respiration rate, and skin conductance, through blocking peripheral and central beta-adrenergic receptors (Steenen et al. 2016). It has the advantage of not being sedative like benzodiazepines and of being active acutely, unlike selective serotonin reuptake inhibitors (SSRIs). PRO also reduces startle potentiation during prolonged threat periods (Grillon et al. 2004; Walker and Davis 2002). While fear conditioning, as well as other types of emotional memory, can be impacted by PRO (Rodriguez-Romaguera et al. 2015), little evidence supports an effect of PRO on non-emotional memory, including WM.
In the present study, MPH, a cognitive enhancing agent, and PRO, an anxiolytic agent, are employed to assess the interactions between anxiety and WM in healthy adults. Anxiety is manipulated using a well-validated threat-of-shock paradigm (Grillon and Baas 2003), and performance is assessed using a WM task previously used in our laboratory (Patel et al. 2016; Vytal et al. 2012). The within-subject manipulation of anxiety (threat vs. safe condition) and WM load (1-back, 2-back, and 3-back) permits testing the following hypotheses. (1) MPH will facilitate the engagement of more cognitive resources by the WM task, reducing the availability of resources needed to attend to the threat of shock. Thus, MPH will improve accuracy across all conditions, and decrease anxiety under threat. MPH will have its strongest facilitatory effect in the threat condition and 3-back WM, given the increasing efficacy of MPH on more difficult tasks (for review, see Bagot and Kaminer 2014; Linssen et al. 2014). (2) PRO will reduce anxiety and, in so doing, attenuate anxiety's interference with WM performance. This effect will be especially evident at lower WM loads, when threat interference is more prominent (Vytal et al. 2012). In other words, if cognition is boosted via task difficulty, anxiety should be reduced. And if anxiety is reduced, performance should be improved.
Methods
Participants
A parallel-group design was used in this double-blind, placebo-controlled study. Sixty healthy adults (age, mean = 26.2 years, SD = 6.6 years) were randomized to receive placebo (PLA), methylphenidate 20 mg (MPH), or propranolol 40 mg (PRO) using a randomization scheme established by the NIH pharmacy. All subjects gave written informed consent approved by the NIMH Institutional Review Board and received compensation for their participation.
On the screening day, trained clinical staff assessed participants via medical history and physical exam, as well as the Structured Clinical Interview for DSM-IV (First 2002). Participants also provided a self-report on their trait anxiety using the trait subscale of the Spielberger State-Trait Anxiety Inventory (STAI-t, Spielberger 1983). Then, startle reactivity was assessed with nine startle stimuli. Inclusion criteria included: (1) no past or current psychiatric disorders, (2) no history of a psychiatric disorder in any first-degree relatives, (3) no medical condition interfering with the objectives of the study as established by a physician, and (4) no use of tobacco, illicit drugs, or psychoactive medications as per history (confirmed by a negative urine screen). Additionally, subjects were excluded for: (1) prior exposure to propranolol or methylphenidate, (2) IQ <80, (3) current use of psychotropic medication, (4) pregnancy, (5) positive toxicology screen, and (6) poor startle reactivity (no startle blink on any of the nine startle stimuli during habituation). Four (n = 4) subjects were excluded for poor startle reactivity during screening.
Anxiety self-report, vital signs, and cortisol level
As indicated in Table 1 (study timeline), the state subscale of the Spielberger State-Trait Anxiety Inventory (STAI-s, Spielberger 1983) was collected at different time points of the study to examine changes in self-report of subjective anxiety. Vital signs (blood pressure and heart rate) were recorded at several time points to monitor changes in cardiovascular function associated with MPH and PRO. Cortisol samples were collected after ensuring that subjects did not eat or drink for at least 20 min prior to sample collection. Subjects placed SalivaBio Oral Swabs (Salimetrics, State College, PA) in the pocket of their cheek or under their tongue for 2 min. The swab was then placed in the swab storage tube and frozen.
Table 1.
Timeline of events
| Time (min) | Events |
|---|---|
| T-30 | Subject arrival |
| T-25 | Vitals#1, STAI-s#1, Cortisol#1 |
| T-15 | Habituation#1 |
| T-5 | Shock workup |
| T | Drug administration |
| T + 80 | Vitals #2, STAI-s #2, Cortisol #2 |
| Habituation#2 | |
| Practice WM task | |
| T + 90 | Threat run 1 |
| T + 100 | Threat run 2 |
| STAI-s#3 | |
| T + 115 | Threat run 3 |
| T + 125 | Threat run 4 |
| Vitals#3, STAI-s#4, Cortisol#3 | |
| T + 140 | Adverse events checklist and debriefing |
| T+ 150 | Subject discharged |
Once arrived at the NIH Clinical Center, subjects completed a Spielberger State Anxiety Inventory (STAI-s). Vital signs and saliva for a cortisol sample were taken. Then, subjects were habituated to the startle stimuli, and the shock level was adjusted to be uncomfortable, but not painful, according to subject report. Following these procedures, subjects ingested 40 mg propranolol, 20 mg methylphenidate, or placebo. Eighty minutes later, STAI-s, vital signs, saliva sample, and habituation were repeated. Subjects were given working memory task (n-back) instructions and practiced each difficulty level. Then, the n-back task began. Subjects filled out a STAI-s mid-way through the task. After the task, the last STAI-s, vital signs, and saliva sample were taken. Adverse events were checked and subjects were discharged
Drug
Participants received a single dose of PLA, 40 mg PRO, or 20 mg MPH in identical-appearing capsules. The PRO dose was selected based on its reported effectiveness in reducing performance anxiety in adults as a starting dose (Elman et al. 1998; Faigel 1991) and its minimal side effects in experimental contexts (Beversdorf et al. 2002; Hermans et al. 2011). The MPH dose was based on the lowest effective dose on cognitive function (Mehta et al. 2000; Moeller et al. 2014; Pauls et al. 2012). The 90-min interval between dosing and cognitive testing was informed by the peak plasma levels, 0.3 to 4.4 h for immediate-release MPH (Novartis Pharmaceuticals) and 1–4 h for PRO (UpToDate), as well as previous studies of methylphenidate (Mehta et al. 2000; Nandam et al. 2014) and propranolol (Müller et al. 2005).
Potential side effects were monitored using a 21-item (0, not present, 3, severe) instrument of clinician-read, subject-endorsed, physical and mental symptoms (e.g., somnolence, nausea, dizziness, headache).
Stimuli, apparatus
Stimuli were presented using the Presentation Software package (version 14.6, Neurobehavioral Systems, Berkeley, CA) via a standard 19-in. LCD monitor. Same levels of n-back or threat/safe conditions never followed one another. Letters were in both upper and lowercase to reduce reliance on perceptual information. Approximately 35% of trials were targets (i.e., “same” responses), in keeping with ratios used in previous n-back research (Braver et al. 1997; Ragland et al. 2002).
Shock
Shocks were used to define periods of threat vs. safe during the performance of the memory task (Robinson et al. 2013; Vytal et al. 2012; Vytal et al. 2013). Shocks were delivered on the non-dominant wrist via 6-mm Ag/AgCl electrodes, using the SHK module of the Psychlab system (Contact Precision Instruments, London, UK). Prior to the experiment, a standard shock workup procedure was conducted to determine individual shock intensity.
To minimize their effects on performance, shocks were not delivered during 75% of the threat blocks. In addition, to minimize their effects on startle (discussed below), shocks preceded startle probes by at least 16 s and followed startle probes with a mean latency of approximately 2 s.
Acoustic startle probe
The startle probe was a 40 ms burst of a 103-dB white noise delivered over headphones by the TN-WN module of the Psychlab system. Prior to the experiment, the subject received nine presentations of the white noise to habituate the startle reflex (i.e., eyeblink reflex).
Eyeblink startle reflex
The white noise probe elicited a startle reflex, which was measured via EMG activity of the eyeblink reflex recorded with 6-mm Ag/AgCl electrodes placed over the orbicularis oculi muscle of the left eye (Blumenthal et al. 2005). EMG was recorded at 1000 Hz and analyzed using the Psychlab version 7 software. The EMG signal was bandpass filtered (30–500 Hz), rectified, and smoothed with a 20-ms time constant. The peak startle/eyeblink magnitude was determined for 20–100 ms after white noise onset. These scores were then transformed to z-scores and converted to t - scores for each subject in order to reduce large inter-individual differences of the overall magnitude of startle reflex (Blumenthal et al. 2005).
Procedure
A timeline of the study is shown in Table 1. Subjects arrived at the clinic and were seated in a comfortable reclining chair. Vital signs, STAI-s ratings, and cortisol saliva samples were collected (Vitals#1, STAI-s#1, Cortisol#1). Recording electrodes for the startle reflex were placed over the orbicularis oculi, and headphones were adjusted over the subjects’ ears for white noise delivery (Habituation#1). Then, the setup for shock delivery was completed by placing electrodes on the internal side of the non-dominant wrist and determining shock level (Shock workup). At this point, the drug was administered. Approximately 80 min later, a second set of vitals, STAI-s, and cortisol saliva sample were collected (Vitals#2, STAI-s#2, Cortisol#2). Participants underwent a second habituation (Habituation#2) and practiced the n-back WM task. Finally, 90 min post-drug administration, the actual testing began.
Working memory task
The WM task consisted of single letters presented sequentially (Fig. 1). Participants were asked to press one of two buttons: same (“s”) or different (“d”), as described in other studies (Vytal et al. 2013). Three levels of difficulties were tested: 1-, 2-, and 3-back. Accordingly, participants were instructed to indicate whether the letter currently displayed was the same as the letter presented 1-, 2-, or 3 letters back.
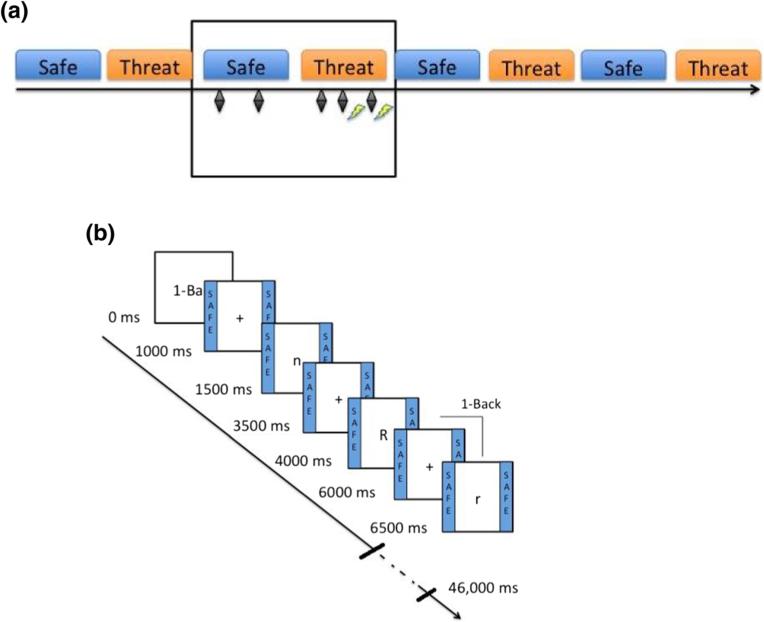
Fig. 1.
Schematic of the WM task. a) Illustration of a run. There were two conditions, threat and safe. Subjects were informed that under the threat condition, they could receive electric shocks at any time (illustrated here as yellow lightning bolts), while during the safe condition they would not receive shocks. A total of eight shocks (2 per run; 0 or 2 per threat block) were delivered during the task. To minimize sensitization effects of the shocks on startle, shocks preceded startle probes (represented here as black diamonds) by at least 16 s and followed startle probes with a mean latency of approximately 2 s. b) Illustration of a 1-back WM block. One-back blocks occurred during each threat and safe condition. Both uppercase and lowercase letters (18 in each block) were presented to reduce reliance on perceptual information. Letters were shown for 500 ms each, separated by 2000 ms intertrial intervals (ITIs). Subjects were asked to indicate on a keyboard whether each letter was the same (“s”) or different (“d”) from the letter they had just seen (1 letter back). For the 2-back, subjects compared the letter to the one they had seen 2 letters prior, and for the 3-back, the one they had seen 3 letters prior. For all levels, approximately 35 % of letters were targets (i.e., “same” responses). (Color figure online)
The task was organized in 4 runs, 8 blocks per run (4 safe blocks and 4 threat blocks presented alternatively) (Fig. 1a), 18 sequential letters per block (Fig. 1b). Each block (threat or safe) featured a single task (1-, 2- or 3-back). Participants were told of the two conditions, “Threat,” during which shocks could be delivered, or “Safe,” during which no shock could be administered.
Between the 2nd and 3rd run, subjects completed the STAI-s (STAI-s#3). After the final run, measures and samples were collected again (Vitals#3, STAI-s#4, Cortisol#3), and adverse events were assessed.
Data analysis
The effects of drug (PLA, MPH, PRO), condition (threat, safety), and load (1-, 2-, 3-back) on cognitive performance (accuracy and reaction time) and startle were analyzed with three-way repeated measures analyses of variance (rANOVAs). A score for anxiety-potentiated startle (startle during threat minus startle during safe) was also computed for analysis.
Cortisol samples were shipped on dry ice to the Arizona State University Institute for Interdisciplinary Salivary Bioscience Research (IISBR) for analysis. After thawing, samples were centrifuged at 3000 rpm for 15 min to remove mucins. Cortisol levels were determined using ELISA technology, and all measurements were done by immunoassay using an individual 96-well format. Cortisol samples were assayed in duplicate using a highly sensitive enzyme immunoassay (Salimetrics, Carlsbad, CA). The test uses 25 μL of saliva per determination and has a lower limit of sensitivity of 0.007 μg/dL, a standard curve range from 0.012 to 3.0 μg/dL, an average intra-assay coefficient of variation of less than 10%, and an average inter-assay coefficient of variation less than 15%. Data were subjected to a log transformation, then analyzed using a two-way rANOVA to examine effects of drug and time.
Vital signs and STAI-s ratings were also analyzed using two-way rANOVAs for the effects of drug and time. An alpha of 0.05 was used for all statistical tests. Greenhouse-Geisser corrections (GG-ε) were used for main effects and interactions involving factors with more than two levels.
Results
Sample characteristics
Groups (PLA, MPH, PRO) did not differ on demographics, trait anxiety (STAI-t), shock intensity (mA), or retrospective rating of shock discomfort (Table 2). Because anxiety state can modulate responses to threat, we tested correlations of STAI-t scores with performance and startle measures. STAI-t scores did not correlate with performance measures (p > 0.7), but showed a trend for a positive correlation with anxiety-potentiated startle, i.e., higher STAI-t, higher anxiety-potentiated startle (F(1,38) = 3.65, p = 0.06). Therefore, STAI-t was used as a covariate of nuisance for the startle analyses.
Table 2.
Demographics (mean (SE)) of the three drug groups (methylphenidate 20 mg [MPH], placebo [PLA], and propranolol 40 mg [PRO])
| MPH (n = 20) | PLA (n = 20) | PRO (n = 20) | |
|---|---|---|---|
| Age | 25.95 (1.33) | 26.45 (1.46) | 26.25 (1.70) |
| Sex (m/f) | 10/10 | 10/10 | 10/10 |
| IQ | 119.37 (2.76) | 113.95 (2.48) | 117.9 (2.07) |
| STAI-t | 27.95 (1.40) | 27 (1.54) | 30.65 (1.36) |
| Shock (mA) | 207.00 (11.43) | 176.90 (15.59) | 184.59 (6.80) |
| Shock discomfort | 7.58 (0.22) | 7.28 (0.33) | 6.80 (0.26) |
Samples were group-matched on age, sex, IQ, and trait anxiety (STAI-t). In addition, groups did not statistically differ on shock intensity or on retrospective ratings of shock discomfort (scale of 1–10)
WM performance
Accuracy and reaction time (RT) were each analyzed using a three-way, condition (safe, threat) × load (1-, 2-, 3-back) × drug (PLA, PRO, MPH) rANOVA. Results are presented in Table 3 and Fig. 2 for accuracy (percent-correct) and Fig. 3 for RT (ms).
Table 3.
Significant effects of the three-way (condition × load × drug) rANOVA on accuracy of the working memory task
| ACCURACY | Condition | Load | Drug |
|---|---|---|---|
| Main effects | F = 22.41, df(1,57), p < 0.001 | F = 106.81, df(2,114), p < 0.001 | F = 2.76, df(2,57), p = 0.07 |
| × condition | F = 5.92, df(2,114), p < 0.01 | F = 1.80, df(2,57), p = 0.17 | |
| × load | F = 1.20, df(4,114), p = 0.32 | ||
| × condition × load | F = 3.38, df(4,114), p = 0.01 |
The three-way interaction of load × condition × drug and the two-way interaction of load × condition were statistically significant. Main effects were statistically significant for condition (safe vs. threat) and load (1-, 2-, or 3-back), but not for drug (methylphenidate, propranolol, or placebo).
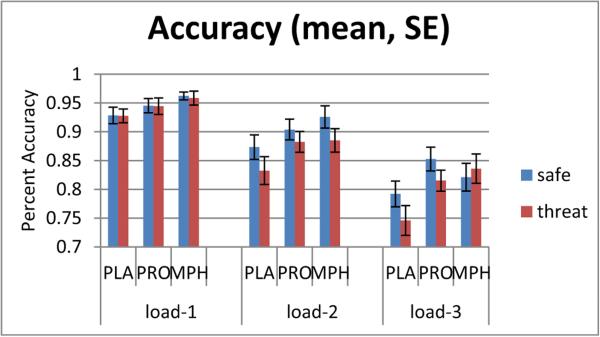
Fig. 2.
Accuracy during threat and safe conditions. Drug condition affected accuracy only at the level of highest difficulty of the working memory task (load-3). The y-axis shows percent accuracy. The working memory task (n-back) was performed at easy (load-1), medium (load-2), and hard levels (load-3). There was a significant difference among drug groups (placebo vs. propranolol vs. methylphenidate) only at the hard level (load-3), when the methylphenidate group was the only group failing to show a threat-induced impairment in accuracy. PLA placebo, PRO propranolol, MPH methylphenidate
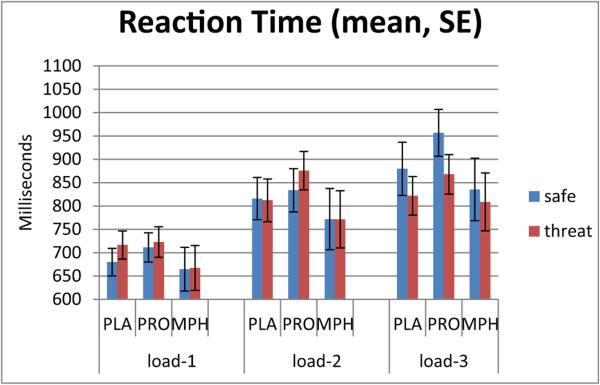
Fig. 3.
Reaction time during threat and safe conditions. Drug condition affected reaction time in the placebo and propranolol, but not methylphenidate, groups. The y-axis shows reaction time (ms), and the x-axis shows drug groups clustered by working memory task (n-back) difficulty: easy (load-1), medium (load-2), and hard (load-3). In the placebo and propranolol groups, threat increased reaction time in the easy level, or low load, but shortened reaction time in the hard level, or high load. Threat did not modulate reaction time in the methylphenidate group. PLA placebo, PRO propranolol, MPH methylphenidate
Accuracy
The rANOVA revealed a significant three-way interaction (F(4,114) = 3.38, p = 0.01), as well as other interactions and main effects, all subsumed under the three-way interaction (Table 3). There was also a trend of a main effect of drug on accuracy (F(2,57) = 2.76, p = 0.07), where accuracy was highest with MPH, intermediate with PRO, and lowest with PLA (MPH > PLA: F(1,38) = 4.36, p = 0.04). To better understand the nature of the three-way interaction, we decomposed it by load.
The decomposition by load was done by running a two-way (condition × drug) rANOVA for each load level. Only load - 3 was sensitive to drug effects, revealing a significant drug × condition interaction (F(2,57) = 6.64, p =0.003) and a trend for a main effect of drug (F(2,57) = 2.69, p = 0.08), where accuracy was significantly lower with PLA relative to either PRO or MPH, which were not different from one another. No significant drug effects emerged for load - 1 or load - 2 (all p > 0.10).
The significant drug × condition interaction at load - 3 reflected the following. The MPH group showed no significant threat vs. safe difference in accuracy (F(1,19) = 1.73, p = 0.20), while the PLA and PRO groups showed significantly lower accuracy during threat vs. safe (PLA: F(1,19) = 9.25, p = 0.01; PRO: F(1,19) = 10.48, p < 0.01) (Fig. 2). In other words, MPH blocked the deleterious effects of threat on accuracy at load - 3. Post hoc tests showed that the threat effects on accuracy in the MPH group were significantly different from those of either the PLA or PRO group, which did not differ from one another (post hoc Tukey's studentized range (HSD) test, p < 0.05).
Reaction time
The three-way rANOVA of RT also revealed a significant three-way interaction (F(4,114) = 3.03, p < 0.05). Here again, only the three-way interaction will be addressed.
The decomposition by load, as done with accuracy, revealed no significant effects of drug or drug × condition in any of the load levels. Therefore, we decomposed the rANOVA by drug to better capture the meaning of the three-way interaction.
For the decomposition by drug, a two-way (condition × load) rANOVA was conducted for each drug group separately. The condition × load interaction was significant for the PLA (F(2,38) = 6.25, p < 0.01) and the PRO (F(2,38) = 10.82, p < 0.001) group, but not for the MPH group. In both PLA and PRO, threat had the strongest effect on load - 3, shortening RT (threat–safe: PLA: t(19) = −3.04, p < 0.01; PRO: t(19) = −3.86, p = 0.001) (Fig. 3). RT was either unaffected or lengthened in load - 1 and load - 2.
Taken together, these analyses suggest that RT under MPH is not modulated by threat, in contrast to RT under PLA or PRO, which were similar to each other.
Startle EMG
The distribution of anxiety-potentiated startle scores by drug and load are shown in Fig. 4. The condition × load × drug rANOVA failed to show any effects of drug, either as a main effect or in interaction.
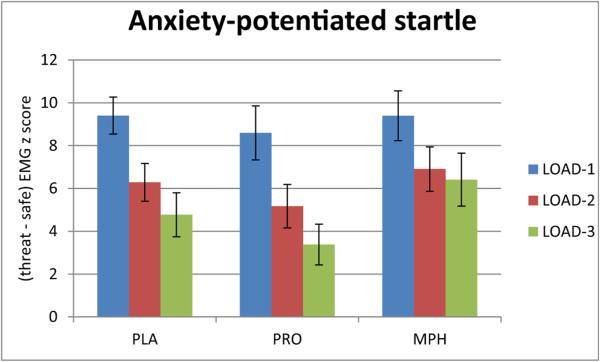
Fig. 4.
Threat-induced changes in startle (anxiety-potentiated startle). Mean and standard error of the [threat minus safe] differences of startle EMG z-scores (anxiety-potentiated startle). The three-way rANOVA of group × condition × load failed to show any effects of drug, either as a main effect or an interaction. However, the examination of load-3 using a two-way rANOVA revealed a trend for a significant condition × drug interaction (F(2,56) = 3.0, p = 0.06). This interaction was due to the strongest anxiety-potentiated startle response in the MPH group, and weakest in the PRO group
However, the main effects of the repeated factors (condition and load) and their interaction were highly significant (load: F(2,114) = 11.99, p < 0.001; condition: F(1,57) = 190.57, p < 0.001; load × condition: F(2,114) = 23.42, p < 0.001). There was a strong potentiation of startle by threat in all three drug groups, as well as a reduction of the anxiety-potentiated startle (threat minus safe) with increasing load.
As an exploratory analysis, and to follow up on the specific drug effect on the load - 3 performance (see above), anxiety-potentiated startle was examined selectively during load - 3. Findings revealed a trend for a significant condition × drug interaction (F(2,56) = 3.0, p = 0.06). This interaction was due to the strongest anxiety-potentiated startle response in the MPH group, and weakest in the PRO group.
Drug effects on cortisol, vital signs, and STAI-s
Drug effects were assessed on five measures: cortisol, systolic blood pressure, diastolic blood pressure, heart rate, and STAI-s (Table 4). Two-way (drug × time) ANOVAs examined the drug effects on the changes of these measures across three time points: pre-drug, post-drug, and post-study.
Table 4.
Descriptives (mean (SE)) of Cortisol, vital signs, and STAI-s
| PLA |
PRO |
MPH |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-drug | Post-drug | Post-study | Pre-drug | Post-drug | Post-study | Pre-drug | Post-drug | Post-study | |
| CORT | 0.18 (0.02) | 0.14 (0.02) | 0.11 (0.01) | 0.17 (0.02) | 0.18 (0.01) | 0.16 (0.02) | 0.18 (0.02) | 0.20 (0.04) | 0.19 (0.03) |
| SBP | 114.80 (2.20) | 114.70 (2.20) | 115.10 (2.57) | 112.75 (2.55) | 107.15 (2.50) | 107.75 (2.50) | 114.00 (2.30) | 118.70 (2.05) | 117.80 (1.94) |
| DBP | 68.40 (1.86) | 66.40 (2.03) | 68.55 (2.06) | 65.65 (1.36) | 66.20 (5.13) | 60.50 (1.57) | 66.20 (1.72) | 66.55 (1.63) | 108.25 (41.01) |
| HR | 74.35 (4.29) | 75.01 (2.88) | 72.45 (2.64) | 66.35 (3.89) | 64.60 (1.88) | 60.05 (1.72) | 72.80 (2.34) | 73.60 (2.29) | 75.75 (3.03) |
| STAI-s | 25.50 (1.27) | 36.20 (2.82) | 33.25 (2.38) | 25.70 (0.88) | 35.75 (1.58) | 32.90 (1.50) | 25.75 (1.30) | 36.40 (2.58) | 34.15 (2.90) |
Measures of serum cortisol (CORT), systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), and state anxiety (STAI-s) were collected three times, pre-drug (placebo, propranolol 40 mg, methylphenidate 20 mg), post-drug, and post-study. Only systolic blood pressure and heart rate showed a significant drug effect
PLA placebo, PRO propranolol, MPH methylphenidate
Cortisol (F(4,112) = 0.92, p = 0.46) and diastolic blood pressure (F(4,114) = 1.22, p = 0.30) did not show any significant drug effects. Regarding systolic blood pressure, the two-way ANOVA revealed a significant time × drug effect (F(4,114) = 5.06, p < 0.001). This interaction reflected an increase in systolic blood pressure with MPH and a decrease with PRO. Regarding heart rate, drug had a significant main effect (F(2,57) = 6.13, p < 0.01), according to which heart rate was significantly decreased in the PRO group relative to the other two groups. STAI-s exhibited a main effect of time across all drugs (F(2,112) = 29.79, p < 0.001), increasing from pre- to post-drug significantly, but remaining unchanged between post-drug administration and post-study. Drug had no effects on changes in STAI-s across the study.
Discussion
The aim of the present study was to test whether improving cognition could reduce anxiety, and, conversely, whether reducing anxiety could improve cognition. Specifically, we manipulated orthogonally and within subjects both cognitive load (working memory task: 1-, 2-, 3-back) and anxiety state (safe and threat conditions), and we used methylphenidate (MPH) to strengthen cognitive performance and propranolol (PRO) to decrease anxiety.
MPH was selected as a way to prioritize cognitive resources and, at the highest task load, to increase available resources to optimally perform the WM task, at the expense of processing threat. Accordingly, predictions regarding MPH were threefold: (1) relative to PLA, MPH would improve accuracy across conditions. This was partly supported by the trend of a main effect indicating best accuracy under MPH. No significant drug differences were found on RT. (2) MPH would decrease the deleterious impact of threat on WM, particularly at the highest WM load, given the increasing efficacy of MPH as tasks become harder (Mehta et al. 2000). Findings supported this prediction. MPH blocked the interfering effects of threat on the 3-back performance. Finally, (3) the MPH-related strengthening of cognitive function would reduce the subjective and physiological measures of anxiety. This was not the case. In fact, exploratory analyses suggested the opposite. Anxiety-potentiated startle was highest in the MPH vs. the PLA or PRO groups during the most difficult task (3-back WM). Furthermore, the more improved was the 3-back accuracy by threat, the stronger was the anxiety-potentiated startle.
PRO was selected as a way to reduce anxiety (Grillon et al. 2004; Walker and Davis 2002) and, in turn, to free resources for cognitive performance. Findings revealed that PRO failed to influence anxiety or cognitive performance. This negative finding could be due to insufficient dose amount (40 mg single dose) (Dooley 2015) or time of administration (90 min prior to task) (Müller et al. 2005), or it could be specific to the experimental design, i.e., PRO has no effect on anxiety induced by threat of shock.
The most interesting finding concerns the effects of MPH, specifically the combination of the blockade of threat interference on high-load WM performance together with an increase in anxiety measures. This finding can be interpreted under the framework of the Limited Resources Theory (Eysenck and Calvo, 1992). According to this theory, there is a limited capacity of the brain to process stimuli. Therefore, when this limit is reached, only the most salient processes will be carried on. In other words, competition and prioritization determine the selection of processes to be acted upon. For example, resources can be prioritized towards processing cognitive performance at the expense of processing anxiety, or, vice versa, processing anxiety at the expense of processing cognitive performance. Accordingly, two possible interpretations of MPH effects are possible.
The first possibility is that MPH decreased the amount of resources required to perform the WM task by making it more “efficient” and, in turn, freeing resources to process threat. Under this assumption, while threat would interfere with WM performance during PLA, threat during MPH would not interfere with WM performance, but anxiety would be processed and expressed during the threat condition. This was true at the highest cognitive load, when MPH is known to be most effective (for review, see Bagot and Kaminer 2014; Linssen et al. 2014). Indeed, both accuracy and RT during load - 3 showed no deleterious effects of threat with MPH, in contrast to PRO or PLA (Figs. 2 and 3). The second possibility is that MPH increased the limits of cognitive capacity, which could, in turn, fully process both cognitive and threat processes. This interpretation challenges the Limited Resources Theory, unless we can speculate that cognitive capacity does not always function at “full capacity” and divides its resources before reaching its limits.
One way to test these hypotheses would be to examine these effects at the neural level using functional neuroim-aging. Increased efficiency would be reflected as reduced activation of the WM neural network, particularly within the dorsolateral prefrontal cortex (DLPFC), and increased threat processing would be accompanied by increased activation in limbic areas, such as the amygdala. The alternative interpretation of increased overall cognitive capacity would be associated with increased activation in both DLPFC and limbic systems.
Finally, a substantial contribution of this study concerns the refinement of the effects of threat on WM load, independently of drug effects. Across all three groups (n = 60), threat decreased accuracy across loads, but also had a dichotomous effect on reaction time, speeding reaction time at high cognitive load, but tending to slow it down at lower loads. These results are inconsistent with the model put forward by Eysenck according to which anxiety preferentially impairs performance “efficiency,” reflected in reaction time, over performance “effectiveness,” indexed by accuracy (Eysenck and Calvo 1992; Eysenck et al. 2007). However, Eysenck's model is based on individual differences in trait anxiety, not on changes in state anxiety. The influence of anxiety on performance in individuals prone to anxiety could be different from that of heightened state anxiety in response to a threat. Eysenck's model assumes a speed-accuracy trade-off. There was no speed-accuracy trade-off in the current study, as evidenced by the absence of correlation of threat-related changes between RT and accuracy (not shown in results but available upon request). The speeding effect of threat at high cognitive load might reflect the combination of enhanced cognitive and emotional arousal, while, at low cognitive load, a slowing of RT might reflect the preponderance of the inhibitory effect typically associated with threat (Robinson et al. 2013). This would suggest the possibility of dissociating these two components of threat (arousal and inhibition).
Overall, this study presents a number of strengths and limitations. Regarding strengths, anxiety was evoked and measured using a reliable, validated strategy (Grillon and Baas 2003). In addition, both anxiety and task difficulty were manipulated orthogonally in a within-subject design. Regarding limitations, the use of single dose and between-group design was suboptimal for the full interpretation of the drug effects. While the advantage of between-group design is to avoid learning effects, which are particularly strong for WM tasks (Beckmann et al. 2007), the disadvantage is that the possibility of a drug-independent group effect cannot be discarded. Here, the groups consisted of well-characterized healthy adults, who were group-matched on demographics and baseline levels of anxiety. The best strategy for ruling out an independent group effect would be to replicate these findings. This can be done while also extending the design to multiple doses and to various times of administration, allowing, in the same vein, to test more comprehensively the effects of PRO.
In conclusion, our hypotheses with regards to the interplay of anxiety and cognition were partially confirmed with MPH. Although cognitive enhancement with MPH prevented the deleterious effect of induced anxiety on high-load task performance, improved cognition did not reduce anxiety. This pharmacological manipulation of the catecholaminergic system yields interesting hypotheses regarding specific dynamics of the limited cognitive resources theory, which (1) need to be examined in future work and (2) could lead to refining pharmacological anxiety treatment approaches that manipulate cognitive resources.
Acknowledgments
Funding Financial support of this study was provided by the Intramural Research Program of the National Institute of Mental Health, ZIAMH002798.
Footnotes
Compliance with ethical standards
Disclosure The authors report no conflicts of interest.
References
- Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. J Psychiatr Res. 2005;39:207–214. doi: 10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Bagot SK, Kaminer Y. Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: a systematic review. Addiction. 2014;109:547–557. doi: 10.1111/add.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann B, Holling H, Kuhn J-T. Reliability of verbal-numerical working memory tasks. Personal Individ Differ. 2007;43:703–714. [Google Scholar]
- Beddington J, Cooper CL, Field J, Goswami U, Huppert FA, Jenkins R, Jones HS, Kirkwood TB, Sahakian BJ, Thomas SM. The mental wealth of nations. Nature. 2008;455:1057–1060. doi: 10.1038/4551057a. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Arnsten AF. Psychostimulants and motivated behavior: arousal and cognition. Neurosci Biobehav Rev. 2013;37:1976–1984. doi: 10.1016/j.neubiorev.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, White DM, Chever DC, Hughes JD, Bornstein RA. Central [beta]-adrenergic modulation of cognitive flexibility. Neuroreport. 2002;13:2505–2507. doi: 10.1097/00001756-200212200-00025. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Dooley TP. Treating anxiety with either beta blockers or antiemetic antimuscarinic drugs: a review. Mental Health Family Med. 2015;11:89–99. [Google Scholar]
- Elman MJ, Sugar J, Fiscella R, Deutsch TA, Noth J, Nyberg M, Packo K, Anderson RJ. The effect of propranolol versus placebo on resident surgical performance. Trans Am Ophthalmol Soc. 1998;96:283–291. [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: the processing efficiency theory. Cognit Emot. 1992;6:409–434. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Faigel HC. The effect of beta blockade on stress-induced cognitive dysfunction in adolescents. Clin Pediatr (Phila) 1991;30:441–445. doi: 10.1177/000992289103000706. [DOI] [PubMed] [Google Scholar]
- First MB. The DSM series and experience with DSM-IV. Psychopathology. 2002;35:67–71. doi: 10.1159/000065121. [DOI] [PubMed] [Google Scholar]
- Fogari R, Zoppi A. Effect of antihypertensive agents on quality of life in the elderly. Drugs Aging. 2004;21:377–393. doi: 10.2165/00002512-200421060-00003. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Morgan CA, 3rd, Charney DS, Davis M. Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology. 2004;175:342–352. doi: 10.1007/s00213-004-1819-5. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernández G. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Cooper NJ, Clark CR, Debrota D, Clarke SD, Williams LM. An integrative approach to determine the best behavioral and biological markers of methylphenidate. J Integr Neurosci. 2007;6:105–140. doi: 10.1142/s0219635207001441. [DOI] [PubMed] [Google Scholar]
- Husain M, Mehta MA. Cognitive enhancement by drugs in health and disease. Trends Cogn Sci. 2011;15:28–36. doi: 10.1016/j.tics.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Ustün TB, Wang PS. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18:23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R, Schaefer A. The emotional startle effect is disrupted by a concurrent working memory task. Psychophysiology. 2011;48:269–272. doi: 10.1111/j.1469-8986.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- Linssen AM, Sambeth A, Vuurman EF, Riedel WJ. Cognitive effects of methylphenidate in healthy volunteers: a review of single dose studies. Int J Neuropsychopharmacol. 2014;17:961–977. doi: 10.1017/S1461145713001594. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JDE, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. J Cogn Neurosci. 2009;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Tobbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Honorio J, Tomasi D, Parvaz MA, Woicik PA, Volkow ND, Goldstein RZ. Methylphenidate enhances executive function and optimizes prefrontal function in both health and cocaine addiction. Cereb Cortex. 2014;24:643–653. doi: 10.1093/cercor/bhs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, Mottweiler E, Bublak P. Noradrenergic blockade and numeric working memory in humans. J Psychopharmacol. 2005;19:21–28. doi: 10.1177/0269881105048888. [DOI] [PubMed] [Google Scholar]
- Nandam LS, Hester R, Bellgrove MA. Dissociable and common effects of methylphenidate, atomoxetine and citalopram on response inhibition neural networks. Neuropsychologia. 2014;56:263–270. doi: 10.1016/j.neuropsychologia.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Patel N, Vytal K, Pavletic N, Stoodley C, Pine DS, Grillon C, Ernst M. Interaction of threat and verbal working memory in adolescents. Psychophysiology. 2016;53:518–526. doi: 10.1111/psyp.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls AM, O'Daly OG, Rubia K, Riedel WJ, Williams SC, Mehta MA. Methylphenidate effects on prefrontal functioning during attentional-capture and response inhibition. Biol Psychiatry. 2012;72:142–149. doi: 10.1016/j.biopsych.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Post TW, editor. Propranolol: Drug Information. Waltham, MA: [July 29, 2016]. UpToDate. UpToDate. [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Chan R, Gur RE. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370–379. [PMC free article] [PubMed] [Google Scholar]
- Ritalin/Ritalin SR [package insert] Novartis Pharmaceuticals; East Hanover, NJ: Dec, 2013. [Google Scholar]
- Robinson OJ, Krimsky M, Grillon C. The impact of induced anxiety on response inhibition. Front Hum Neurosci. 2013;7:1–5. doi: 10.3389/fnhum.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Do-Monte FH, Tanimura Y, Quirk GJ, Haber SN. Enhancement of fear extinction with deep brain stimulation: evidence for medial orbitofrontal involvement. Neuropsychopharmacology. 2015;40:1726–1733. doi: 10.1038/npp.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto, CA: 1983. [Google Scholar]
- Steenen SA, van Wijk AJ, van der Heijden GJ, van Westrhenen R, de Lange J, de Jongh A. Propranolol for the treatment of anxiety disorders: systematic review and meta-analysis. J Psychopharmacol. 2016;30:128–139. doi: 10.1177/0269881115612236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen LF, Koole SL. Clearing the mind: a working memory model of distraction from negative mood. Emotion. 2007;7:715–723. doi: 10.1037/1528-3542.7.4.715. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding YS, Gatley SJ, Gifford A, Franceschi D. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal K, Cornwell B, Arkin N, Grillon C. Describing the interplay between anxiety and cognition: from impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology. 2012;49:842–852. doi: 10.1111/j.1469-8986.2012.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal KE, Cornwell BR, Letkiewicz AM, Arkin NE, Grillon C. The complex interaction between anxiety and cognition: insight from spatial and verbal working memory. Front Hum Neurosci. 2013;7:93. doi: 10.3389/fnhum.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology. 2002;159:304–310. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]