ABSTRACT
Membrane type 1-matrix metalloproteinase (MT1-MMP) functions as a signaling molecules in addition to a transmembrane metalloprotease, which degrades interstitial collagens and extracellular matrix components. This review focuses on the multifunctional roles of MT1-MMP as a signaling molecule in vascular responses to pro-atherosclerotic stimuli in the pathogenesis of cardiovascular diseases. First, the lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1)-MT1-MMP signaling axis contributes to endothelial dysfunction, which is mediated via small GTP-binding protein RhoA and Rac1 activation. Second, MT1-MMP plays a crucial role in reactive oxygen species (ROS) generation through the activation of receptor for advanced glycation end products (AGEs) in smooth muscle cells, indicating that MT1-MMP may be a therapeutic target for diabetic vascular complications. Third, MT1-MMP is involved in RhoA/Rac1 activation and Ca2+ signaling in the mechanism of thrombin-stimulated endothelial dysfunction and oxidant stress. Fourth, the inhibition of the MT1-MMP/Akt signaling pathway may be an attractive strategy for treating endothelial disordered hemostasis in the development of vascular diseases linked to TNF-α-induced inflammation. Fifth, MT1-MMP through RAGE induced RhoA/Rac1 activation and tissue factor protein upregulation through NF-κB phosphorylation in endothelial cells stimulated by high-mobility group box-1, which plays a key role in the systemic inflammation. These findings suggest that the MT1-MMP-mediated signaling axis may be a promising target for treating atherosclerosis and subsequent cardiovascular diseases.
Keywords: MT1-MMP, endothelial dysfunction, atherosclerosis, cardiovascular disease
INTRODUCTION
Matrix metalloproteinase (MMP) and membrane-type MMPs (MT-MMPs), a large family of zinc-dependent endopeptidases, are the fibrinolysins responsible for degrading a variety of extracellular matrix (ECM) components1,2). MT-MMPs were identified as multifunctional enzymes for modulating the bioactivity of transmembrane receptors, which was anchored to the cell membrane instead of being soluble1-3). MT-MMPs have been well known to serve as important enzymes engaged by tumor cells in the mechanisms of metastasis4). All MT-MMPs act at the cell surface, and membrane type 1-MMP (MT1-MMP), an activator of pro-MMP-2, was localized predominantly in human invasive breast carcinomas and spontaneously metastasizing melanoma cell lines, indicating that MT1-MMP may play a key role in the promotion of tumor cell invasion, metastasis, and angiogenesis5,6). Degradation of the vascular ECM by MT-MMPs is critical for smooth muscle cell (SMC) migration, plaque instability, and consequent hypercoagulability in the pathogenesis of atherosclerosis and aortic aneurysms7,8). Recent studies of bone marrow transplantation in MT1-MMP2/2 mice have demonstrated that macrophage-derived MT1-MMP plays a role in the pathogenesis of plaque stability9,10). MT1-MMP may be a key MMP responsible for effecting postinfarction cardiac ECM remodeling and cardiac dysfunction11). MT-MMPs also play important roles for remodeling ECM during vascular injury, cell recruitment to the vessel wall, endothelial cell growth, and angiogenesis12,13). Proinflammatory molecules including tumor necrosis factor (TNF)-α and oxidized, low-density lipoprotein (ox-LDL) upregulate MT1-MMP expression in vascular SMCs and macrophages in human atherosclerotic plaques, and MT1-MMP activated by proinflammatory molecules affects ECM remodeling in the pathology of atherosclerosis14,15). MT1-MMP regulates MMP-2 expression and angiogenesis-related functions in human umbilical vein endothelial cells16). Rajavashisth et al. also reported that activation of endothelial cells by inflammatory cytokines and/or ox-LDL increases MT1-MMP expression17). Schneider et al. reported that MT1-MMP deficiency in bone marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques18). To our knowledge, MT1-MMP affects various cellular functions not only as a pericellular protease but also as a signaling molecule in both physiological and pathological settings19-21).
Here, we focus our investigations on multifunctional roles of MT1-MMP as a signaling molecule in vascular responses to atherosclerotic stimuli and suggest that MT1-MMP-mediated signaling pathways investigated may be a therapeutic target for cardiovascular disease.
INTERACTIVE IMPACT OF MT1-MMP AND LOX-1
Lectin-like ox-LDL receptor-1 (LOX-1) with a type II membrane protein structure has been identified as a major endothelial receptor for ox-LDL in endothelial cells (ECs)22). Atherosclerotic stimuli including ox-LDL induce the downregulation of endothelial nitric oxide synthase (eNOS) mediated via LOX-1, which is associated with the activation of small GTP-binding protein RhoA23), and subsequent ox-LDL-reduced nitric oxide (NO) production contributes to impaired endothelial function24,25). MT1-MMP has been shown to be a key effector molecule during NO-induced endothelial migration and tube formation, indicating that MT1-MMP is a potential therapeutic target for NO-associated vascular disorders26). Small GTP-binding protein Rac1, a component of nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) is activated by ox-LDL, and Rac1 activation subsequently increases reactive oxygen species (ROS) generation, which is implicated in the initiation and progression of atherosclerosis27-29). Molecular links between MT1-MMP and small GTPases—in particular Rho and Rac—have been explored in cell migration as well as molecular synthesis30,31). The formation of a complex of LOX-1 with MT1-MMP was detected by fluorescent immunostaining and immunoprecipitation, and molecular interaction between MT1-MMP and LOX-1 contributes to the RhoA-dependent eNOS protein synthesis and EC invasion, and Rac1-mediated NADPH oxidase activity and ROS generation32) (Fig. 1). The tissue inhibitor of metalloproteinases-2 (TIMP-2) inhibits MT-MMPs, which is an MMP-2 activator, and TIMP-2 binds to the catalytic domain of the cell surface receptor, MT1-MMP in the dimer, and to the hemopexin-like domain of proMMP-233-37). Our findings showed that selective siRNA-mediated suppression of MT1-MMP and TIMP-2 markedly attenuated rapid RhoA and Rac1 activation caused by ox-LDL mediated via LOX-1, which forms a complex with MT1-MMP, confirming that a complex formed with MT1-MMP and LOX-1 plays an integral role in ox-LDL-mediated signaling pathways in ECs32).
Fig. 1.
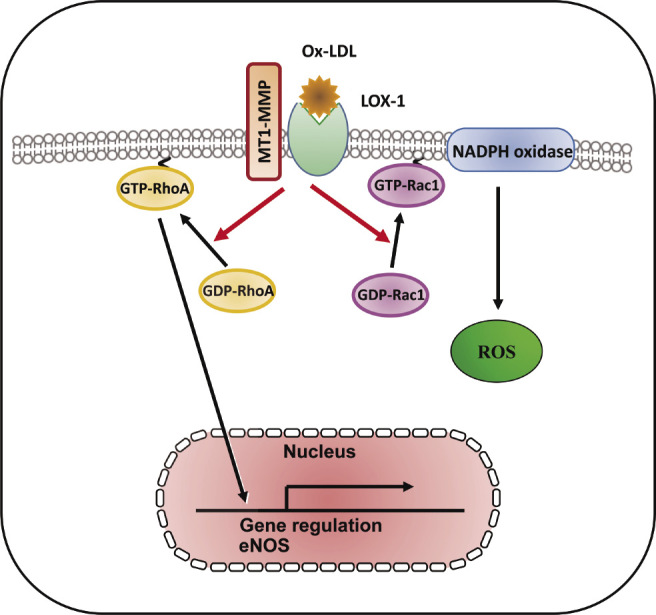
LOX-1-MT1-MMP axis plays a crucial role in RhoA and Rac1 activation signaling pathways in ox-LDL stimulation. In cultured human aortic ECs, surface expression of MT1-MMP is an essential mediator of rapid RhoA and Rac1 activation through the activation of LOX-1 by ox-LDL. The formation of a complex of LOX-1 with MT1-MMP contributed to RhoA-dependent endothelial NO synthase, Rac1-mediated NADPH oxidase activity, and subsequent ROS generation. The LOX-1-MT1-MMP signaling axis plays an important role in ox-LDL-mediated signaling pathways in ECs32).
INVOLVEMENT OF MT1-MMP IN RAGE ACTIVATION SIGNALING PATHWAYS
An advanced glycation end products (AGEs)/receptor for AGE (RAGE) signaling pathways involved in the pathogenesis of microvascular and macrovascular diabetic complications38-40). Ligands which bind to RAGE were accumulated and increased RAGE expression enhanced in accelerated diabetic atherosclerotic lesions41,42). RAGE expression is increased in non-diabetic subjects with premature coronary artery disease43). AGEs induce C-reactive protein expression in hepatoma cells by suppressing Rac1 activation44). AGEs induced Rac1 and p47 (phox), NADPH oxidase activation, resulting in subsequent increased ROS generation and NF-κB phosphorylation-related, redox-sensitive molecular expression in SMCs47) (Fig. 2). Geranylgeranyl transferase I (GGTase I) through the small GTPases RhoA and Rac1 activation is involved in vascular injury with endothelial dysfunction, which contributes to ROS generation and vascular NO production45,46). AGEs induced GGTase I activity, Rac1-p47(phox) activation, NADPH oxidase activity, ROS generation, and molecular expression in SMCs (Fig. 2), and RAGE was found to form a complex with MT1-MMP in both cultured SMCs and the aortae of diabetic rats47). Our findings show the role of MT1-MMP in the AGE/RAGE-triggered signaling pathways and the molecular interaction between RAGE and MT1-MMP in SMCs, suggesting that MT1-MMP may be a novel therapeutic target for diabetic vascular complications47).
Fig. 2.
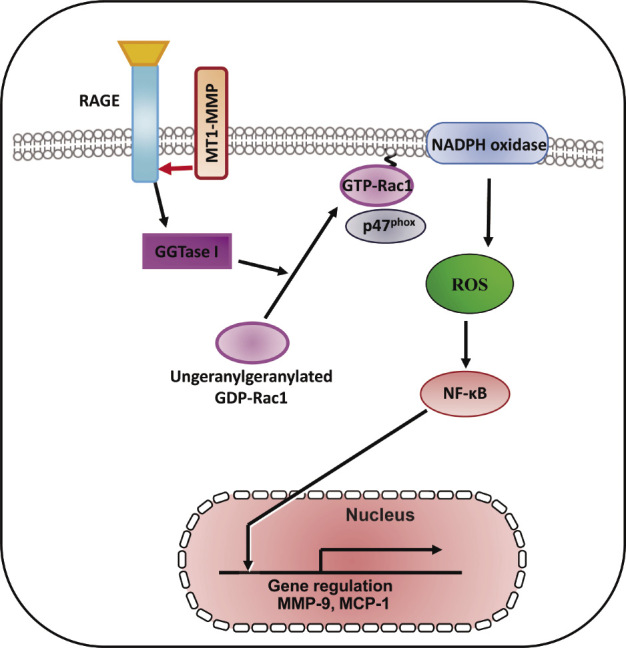
Therapeutic targeting RAGE/MT1-MMP/Rac1 axis in redox-sensitive signaling pathway in diabetic vascular complications. The schematic diagram shows that MT1-MMP is involved in AGEs/RAGE-dependent, redox-sensitive signaling pathways in cultured SMCs. The MT1-MMP/RAGE complex modifies this pathway and the blockade of RAGE/MT1-MMP axis are candidates for therapeutic strategy. These findings identify the attractive therapeutic targeting for RAGE/MT1-MMP/Rac1 in diabetic vascular complications47).
ROLES OF MT1-MMP IN RHOA/RAC1-DEPENDENT SIGNALING PATHWAYS IN THROMBIN STIMULATION
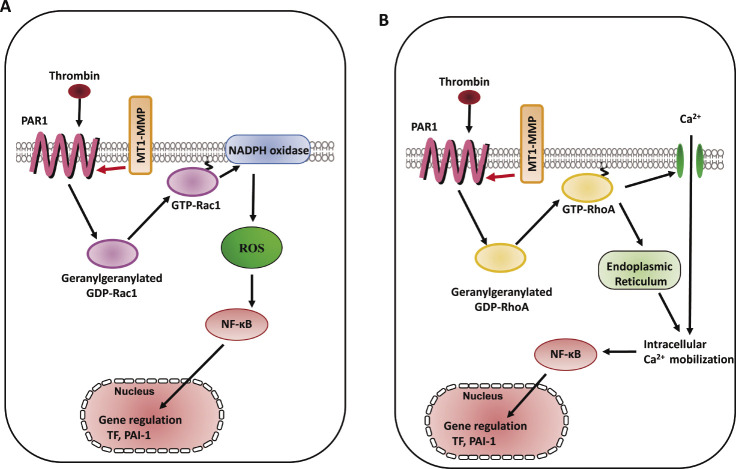
Thrombin regulates the cascade of Rac1/NADPH oxidase-dependent ROS generation, which modulates NO synthesis including eNOS protein expression48,49). Thrombin exerts vascular responses mediated via a family of G protein-coupled receptors, protease-activated receptors (PARs) as well as the process of hemostasis including coagulation, platelet aggregation, and thrombus formation50). Thrombin signaling is mediated by a family of G protein-coupled PARs, and the prototype for this family, PAR1, is activated when thrombin cleaves its N-terminal exodomain at a specific site51). PAR1 is the primary receptor that mediates active responses in atherosclerosis52). Red wine polyphenolic compounds strongly inhibit thrombin-induced matrix invasion of SMCs, and this effect is associated with a direct inhibition of MT1-MMP53). Thrombin upregulates MT1-MMP expression via PI3K and Rac1 activation in cord blood hematopoietic stem/progenitor cells (CB HSPC), and, subsequently, MT1-MMP contributes to the priming of the homing-related responses of CB HSPC54). The interaction between MT1-MMP and PAR1 in the membrane of ECs contributes to the suppression of thrombin-induced endothelial dysfunction via Rac1-dependent NADPH activation including ROS generation and the expression of both TF and the plasminogen activator inhibitor type-1 (PAI-1)60) (Fig. 3A). Silencing of MT1-MMP suppresses sphingosine-1-phosphate-triggered Ca2+ mobilization in glioblastoma cells55). It is known that expression of TF expression is regulated in part by NF-κB phosphorylation56). We previously reported that RhoA-dependent NF-κB phosphorylation and RhoA-related Ca2+ signaling mediate TF and PAI-1 expression in monocyte adhesion to ECs and that there is a cross-talk between Ca2+ signaling and Rac1-dependent ROS generation57,58). Ca2+ influx via transient receptor potential canonical (TRPC) channels induces NF-κB phosphorylation in ECs59). Our study revealed that molecular interaction between MT1-MMP and PAR1 regulated thrombin-triggered TF and PAI-1 overexpression through RhoA-associated Ca2+ signaling and NF-κB phosphorylation in ECs60) (Fig. 3B). Our findings suggest that MT1-MMP mediates thrombin-triggered RhoA and Rac1 activation and Ca2+ signaling in ECs, suggesting that thrombin-triggered MT1-MMP-related signaling may be a target for endothelial dysfunction and oxidant stress in the pathogenesis of cardiovascular diseases60).
Fig. 3.
Schema of MT1-MMP involvement in thrombin-triggered signaling pathways in ECs. A. RhoA activation involved in thrombin-triggered [Ca2+]i increase and TF and PAI-1 expression in ECs, whereas Rac1 activation induced thrombin-triggered ROS generation and TF and PAI-1 expression60). B. MT1-MMP mediates thrombin-triggered RhoA and Rac1 activation, resulting in the downstream events including Ca2+ signaling, NADPH oxidase activity, ROS generation, and TF and PAI-1 expression. MT1-MMP contributes to the RhoA/Ca2+ and Rac1/NADPH oxidase-dependent signaling pathways in thrombin-induced vascular responses in ECs60).
IMPACT OF MT1-MMP ON AKT PHOSPHORYLATION IN TNF-α STIMULATION
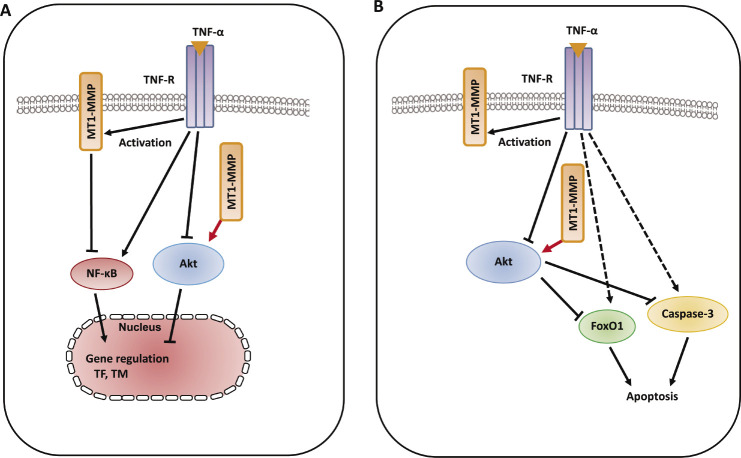
Phosphorylation of protein kinase B (Akt) at two key sites, the activation loop and the hydrophobic motif, activates the kinase and promotes endothelial proliferative dysfunction, leading to apoptosis of ECs, and regulates the balance between cell survival61,62). The Akt signaling pathway is also associated with various cellular processes including coagulation and inflammation63). Activation of phospho-inositide 3-kinase (PI3K) and its downstream target Akt is essential for TNF-α-induced NF-κB activation as well as decreased TNF-α-induced adhesion molecule expression and monocyte adhesion, which are linked to the development of vascular diseases and induce inflammatory responses in ECs64). Granulocyte colony-stimulating factor increased MT1-MMP protein and activity in human hematopoietic cells in a PI3K/Akt-dependent manner, indicating the molecular interaction between MT1-MMP and Akt65). Inflammatory cytokines such as TNF-α are master regulators of vascular proatherogenic changes, the action of which has been linked to endothelial dysfunction in many pathophysiological conditions29,66). TNF-α-induced inflammatory responses and procoagulant activity have been implicated in the pathogenesis of vascular diseases67,68). TNF-α induced the increased levels of TF antigen and activity and reduced the antigen levels of thrombomodulin (TM), which directly blocks the interaction between thrombin and the procoagulant protein substrates in the pathogenesis of vascular disease69,70). MT1-MMP possesses transmembrane and cytoplasmic domains in addition to extracellular domains, and the cytoplasmic domain of MT1-MMP has an important role in cell invasion and proliferation, indicating that MT1-MMP functions as a signaling molecule71,72). TIMP-2 and MT1-MMP siRNA enhanced the increased levels of TF antigen and activity, and further reduced the decreased levels of TM antigen in TNF-α-stimulated ECs, indicating that MT1-MMP may be critical for the modulation of procoagulant states in TNF-α stimulation. Additionally, TIMP-2 and MT1-MMP siRNA inhibited the decrease in Akt phosphorylation in TNF-α-stimulated ECs73). A specific pharmacological inhibitor of Akt and Akt siRNA enhanced the TNF-α-induced changes of TF antigen and activity as well as TM antigen in ECs, indicating that MT1-MMP modulates Akt signaling pathways in TNF-α-stimulated ECs73). MT1-MMP siRNA also inhibited TNF-α-induced NF-κB phosphorylation. MT1-MMP binds to Akt within the cytoplasm of TNF-α-treated ECs. These findings suggest that molecular interaction between MT1-MMP and Akt contributes to changes in TNF-α-induced TF and TM expression changes in ECs73) (Fig. 4A). Forkhead box protein O1 (FoxO1) is a transcription factor that contributes to physiological processes including Akt-dependent cell proliferation, apoptosis, and insulin signaling74). TNF-α induced FoxO1 activation via Akt phosphorylation, which acts as a master switch to control cell cycle arrest and apoptosis75,76). Moreover, MT1-MMP regulates EC apoptosis through Akt-mediated phosphorylation of a forehead transcription factor, FoxO1, as well as the activation of caspase-3 in TNF-α stimulation, indicating that the MT1-MMP/Akt signaling axis plays a critical role in the mechanism(s) of endothelial apoptosis73) (Fig. 4B). The MT1-MMP/Akt signaling axis modulates TNF-α-stimulated procoagulant activity and endothelial apoptosis in ECs, suggesting that MT1-MMP is a potential signaling molecule for treating endothelial disordered hemostasis in the development of vascular diseases linked to TNF-α-induced inflammation73).
Fig. 4.
Schematic diagram describing the mechanisms of MT1-MMP/Akt signaling axis in TNF-α-dependent procoagulant activity and apoptosis of ECs. A. MT1-MMP in the cytoplasm of ECs forms a complex with Akt in the intracellular signaling pathways in TNF-α-stimulated ECs. The interaction between MT1-MMP and Akt regulates TNF-α-induced changes in TF and TM expression in ECs73). B. The interaction between MT1-MMP and Akt contributes to endothelial apoptosis through FoxO1 phosphorylation as well as caspase-3 activation. MT1-MMP plays a crucial role of MT1-MMP in Akt-dependent signaling pathways in TNF-α stimulation73).
INTERACTION OF MT1-MMP AND RAGE IN HMGB-1 STIMULATION
A proinflammatory cytokine, high-mobility box group 1 (HMGB-1), which is derived from both injured endothelium and activated macrophages/monocytes, contributes to the progression of atherosclerosis and other cardiovascular diseases77). RAGE is a multiligand receptor, which binds to AGEs as well as other high-affinity ligands such as HMGB-178). Statin suppresses vascular inflammation and atherosclerosis in ApoE−/− mice by downregulation of the HMGB-1-RAGE axis in atherosclerotic plaques79). Our previous report has demonstrated that the interaction between MT1-MMP and RAGE induced NADPH oxidase-dependent ROS generation and NF-κB phosphorylation in SMCs47). The binding of MT1-MMP to RAGE also occurred in human ECs80). MMPs are inflammatory mediators linking inflammation with angiogenesis and vascular remodeling81). The addition of HMGB-1 to ECs activated the rapid activation of MT1-MMP80). TIMP-2 and MT1-MMP siRNA prevented the GTP/GDP exchange of small GTP protein RhoA in ox-LDL-stimulated ECs37). The RhoA/Rho kinase pathway was upstream of the NF-κB-dependent pathway, which is required for TF upregulation57,82). Our results clarified that MT1-MMP suppressed HMGB-1-induced TF upregulation via RAGE, RhoA, and NF-κB signaling in ECs80) (Fig. 5). NADPH oxidase in AGE/RAGE-mediated generation of ROS enhanced the expression levels of TF upon stimulation with AGE83). Rac1 activation-dependent ROS production induced thrombin-induced upregulation of TF, which is one of the redox-sensitive, signaling-dependent molecules60). Our findings suggest that HMGB-1-stimulated Rac1 activation induces NADPH oxidase-mediated ROS generation and TF upregulation80) (Fig. 5). Toll-like receptor (TLR)-2 and TLR-4 are expressed in vascular ECs and function as the receptors for HMGB-184). Further studies are needed to examine the role of TLRs in HMGB-1-dependent TF synthesis. Our findings suggest that HMGB-1 activates MT1-MMP through RAGE, leading to RhoA/Rac1 activation and NF-κB phosphorylation, resulting in TF antigen upregulation and eNOS antigen downregulation in the pathogenesis of the progression of endothelial dysfunction-dependent atherosclerosis80).
Fig. 5.
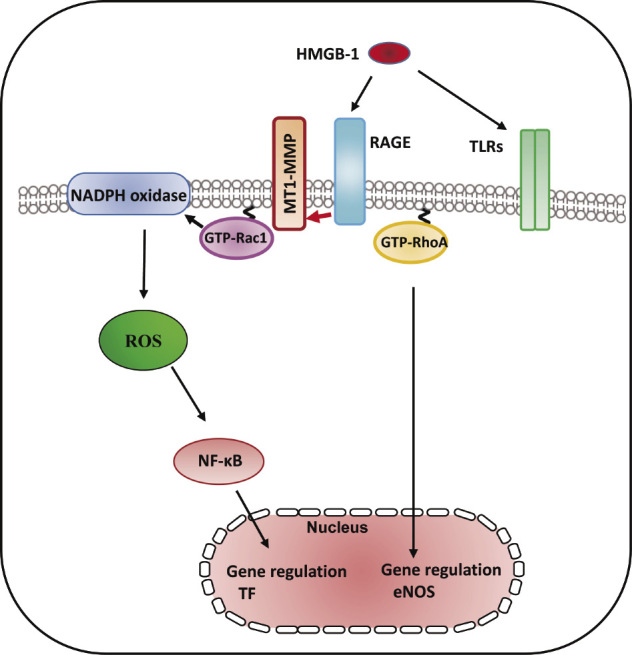
RAGE/MT1-MMP signaling axis modified HMBG-1-mediated TF expression through RhoA and Rac1 activation and NF-κB phosphorylation. HMGB-1 induced RhoA/Rac1 activation and NF-kB phosphorylation in cultured human aortic ECs. HMGB-1 increased the activity of MT1-MMP, which resulted in subsequent activation of RAGE leading to RhoA/Rac1 activation and NF-κB phosphorylation in ECs, indicating that MT1-MMP was involved in vascular inflammation and might be an effective target for treating atherosclerosis80).
CONCLUDING REMARKS
In this review, we summarized several features of MT1-MMP as regulators of vascular signaling, which contribute to initiation and progression of atherosclerosis. MT1-MMP is a signaling molecule of vascular proatherogenic changes induced by several proinflammatory and proatherogenic stimuli, which linked to impaired vascular cell function in the pathology of atherosclerosis and subsequent cardiovascular disease32,47,60,73,80).
ACKNOWLEDGEMENTS
The authors thank Drs. Koichi Sugimoto, Masashi Kamioka, Atsuya Ando, and Toshiyuki Ishibashi for their contributions to this work.
FUNDING STATEMENT
This work was supported by Fukushima Medical University Research Project and Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (22790718). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell, 141: 52-67, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato H, Seiki M. Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem, 119: 209-215, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Itoh Y, Seiki M. MT1-MMP: An enzyme with multidimensional regulation. Trends Biochem Sci, 29: 285-289, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer, 2: 161-174, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann UB, Eggert AA, Blass K, Bröcker EB, Becker JC. Expression of matrix metalloproteinases in the microenvironment of spontaneous and experimental melanoma metastases reflects the requirements for tumor formation. Cancer Res, 63: 8221-8225, 2003. [PubMed] [Google Scholar]
- 6.Ueno H, Nakamura H, Inoue M, et al. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res, 57: 2055-2060, 1997. [PubMed] [Google Scholar]
- 7.Rajavashisth TB, Xu XP, Jovinge S, et al. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation, 99: 3103-3109, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Carrell TW, Burnand KG, Wells GM, Clements JM, Smith A. Stromelysin-1 (matrix metalloproteinase-3) and tissue inhibitor of metalloproteinase-3 are overexpressed in the wall of abdominal aortic aneurysms. Circulation, 105: 477-482, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Schneider F, Sukhova GK, Aikawa M, Canner J, et al. Matrix-metalloproteinase-14 deficiency in bone-marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques. Circulation, 117: 931-939, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Xiong W, Knispel R, MacTaggart J, Greiner TC, Weiss SJ, Baxter BT. Membrane-type 1 matrix metalloproteinase regulates macrophage-dependent elastolytic activity and aneurysm formation in vivo. J Biol Chem, 284: 1765-1771, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenig GC, Rowe RG, Day SM, et al. MT1-MMP-dependent remodeling of cardiac extracellular matrix structure and function following myocardial infarction. Am J Pathol, 180: 1863-1878, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm, 2013: 928315, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravanti L, Kähäri VM. Matrix metalloproteinases in wound repair (review). Int J Mol Med, 6: 391-407, 2000. [PubMed] [Google Scholar]
- 14.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci USA, 48: 1014-1022, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajavashisth TB, Xu XP, Jovinge S, et al. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation, 99: 3103-3109, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Chang KW, Yang HY, Lin PW, Chen SU, Huang YL. MT1-MMP regulates MMP-2 expression and angiogenesis-related functions in human umbilical vein endothelial cells. Biochem Biophys Res Commun, 437: 232-238, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Rajavashisth TB, Liao JK, Galis ZS, et al. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J Biol Chem, 274: 11924-11929, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Schneider F, Sukhova GK, Aikawa M, et al. Matrix-metalloproteinase-14 deficiency in bone-marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques. Circulation, 117: 931-939, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Lehti K, Allen E, Birkedal-Hansen H, et al. An MT1-MMP-PDGF receptor-beta axis regulates mural cell investment of the microvasculature. Genes Dev, 19: 979-991, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol, 206: 1-8, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Langlois S, Gingras D, Béliveau R. Membrane type 1-matrix metalloproteinase (MT1-MMP) cooperates with sphingosine 1-phosphate to induce endothelial cell migration and morphogenic differentiation. Blood, 103: 3020-3028, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature, 386: 73-77, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation, 97: 1129-1135, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature, 362: 801-809, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev, 84: 1381-148, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Genís L, Gonzalo P, Tutor AS, et al. Functional interplay between endothelial nitric oxide synthase and membrane type 1 matrix metalloproteinase in migrating endothelial cells. Blood, 110: 2916-2923, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow SE, Hshu YC, Wang JS, Chen JK. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol (1985), 102: 1520-1527, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Cominacini L, Pasini AF, Garbin U, et al. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem, 275: 12633-12638, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Gregg D, Rauscher FM, Goldschmidt-Clermont PJ. Rac regulates cardiovascular superoxide through diverse molecular interactions: more than a binary GTP switch. Am J Physiol Cell Physiol, 285: C723-C734, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Hoshino D, Tomari T, Nagano M, Koshikawa N, Seiki M. A novel protein associated with membrane-type 1 matrix metalloproteinase binds p27(kip1) and regulates RhoA activation, actin remodeling, and matrigel invasion. J Biol Chem, 284: 27315-27326, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirvaikar N, Marquez-Curtis LA, Ratajczak MZ, Janowska-Wieczorek A. Hyaluronic acid and thrombin upregulate MT1-MMP through PI3K and Rac-1 signaling and prime the homing-related responses of cord blood hematopoietic stem/progenitor cells. Stem Cells Dev, 20: 19-30, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Sugimoto K, Ishibashi T, Sawamura T, et al. LOX-1-MT1-MMP axis is crucial for RhoA and Rac1 activation induced by oxidized low-density lipoprotein in endothelial cells. Cardiovasc Res, 84: 127-136, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Koka V, Wang W, Huang XR, et al. Advanced glycation end products activate a chymase-dependent angiotensin II-generating pathway in diabetic complications. Circulation, 113: 1353-1360, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlassara H, Fuh H, Donnelly T, Cybulsky M. Advanced glycation endproducts promote adhesion molecule (VCAM-1, ICAM-1) expression and atheroma formation in normal rabbits. Mol Med, 1: 447-456, 1995. [PMC free article] [PubMed] [Google Scholar]
- 35.Forbes JM, Yee LT, Thallas V, et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes, 53: 1813-1823, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med, 4: 1025-1031, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Zang P, Feng B, Qian Q. Atorvastatin inhibits the expression of RAGE induced by advanced glycation end products on aortas in healthy Sprague-Dawley rats. Diabetol Metab Syndr, 6: 102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stawowy P, Meyborg H, Stibenz D, et al. Furin-like proprotein convertases are central regulators of the membrane type matrix metalloproteinase-pro-matrix metalloproteinase-2 proteolytic cascade in atherosclerosis. Circulation, 111: 2820-2827, 2005. [DOI] [PubMed] [Google Scholar]
- 39.El Bedoui J, Oak MH, Anglard P, Schini-Kerth VB. Catechins prevent vas-cular smooth muscle cell invasion by inhibiting MT1-MMP activity and MMP-2 expression. Cardiovasc Res, 67: 317-325, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Zucker S, Drews M, Conner C, et al. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP). J Biol Chem, 273: 1216-122, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Butler GS, Butler MJ, Atkinson SJ, et al. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem, 273: 871-880, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Strongin AY, Collier I, Bannikov G, et al. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem, 270: 5331-5338, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Mahajan N, Malik N, Bahl A, Dhawan V. Receptor for advanced glycation end products (RAGE) and its inflammatory ligand EN-RAGE in non-diabetic subjects with pre-mature coronary artery disease. Atherosclerosis, 207: 597-602, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida T, Yamagishi S, Nakamura K, et al. Pigment epithelium-derived factor (PEDF) inhibits advanced glycation end product (AGE)-induced C-reactive protein expression in hepatoma cells by suppressing Rac-1 activation. FEBS Lett, 580: 2788-2796, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Ohkawara H, Ishibashi T, Sakamoto T, et al. Thrombin-induced rapid geranylgeranylation of RhoA as an essential process for RhoA activation in endothelial cells. J Biol Chem, 280: 10182-10188, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Zuckerbraun BS, Barbato JE, Hamilton A, Sebti S, Tzeng E. Inhibition of geranylgeranyltransferase I decreases generation of vascular reactive oxygen species and increases vascular nitric oxide production. J Surg Res, 124: 256-263, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Kamioka M, Ishibashi T, Ohkawara H, et al. Involvement of membrane type 1-matrix metalloproteinase (MT1-MMP) in RAGE activation signaling pathways. J Cell Physiol, 226: 1554-1563, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res, 68: 26-36, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Ohkawara H, Ishibashi T, Saitoh S, et al. Preventive effects of pravastatin on thrombin-triggered vascular responses via Akt/eNOS and RhoA/Rac1 pathways in vivo. Cardiovasc Res, 88: 492-501, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature, 407: 258-264, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost, 3: 1800-1814, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Martorell L, Martínez-González J, Rodríguez C, Gentile M, Calvayrac O, Badimon L. Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb Haemost, 99: 305-315, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Oak MH, El Bedoui J, Anglard P, Schini-Kerth VB. Red wine polyphenolic compounds strongly inhibit pro-matrix metalloproteinase-2 expression and its activation in response to thrombin via direct inhibition of membrane type 1-matrix metalloproteinase in vascular smooth muscle cells. Circulation, 110: 1861-1867, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Shirvaikar N, Marquez-Curtis LA, Ratajczak MZ, Janowska-Wieczorek A. Hyaluronic acid and thrombin upregulate MT1-MMP through PI3K and Rac-1 signaling and prime the homing-related responses of cord blood hematopoietic stem/progenitor cells. Stem Cells Dev, 20: 19-30, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Fortier S, Labelle D, Sina A, Moreau R, Annabi B. Silencing of the MT1-MMP/G6PT axis suppresses calcium mobilization by sphingosine-1-phosphate in glioblastoma cells. FEBS Lett, 582: 799-804, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Mackman N. Regulation of the tissue factor gene. FASEB Journal, 9: 883-889, 1995. [DOI] [PubMed] [Google Scholar]
- 57.Ishibashi T, Sakamoto T, Ohkawara H, et al. Integral role of RhoA activation in monocyte adhesion-triggered tissue factor expression in endothelial cells. Arterioscler Thromb Vasc Biol, 23: 681-687, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Sakamoto T, Ishibashi T, Sugimoto K, et al. RhoA-dependent PAI-1 gene expression induced in endothelial cells by monocyte adhesion mediates geranylgeranyl transferase I and Ca2+ signaling. Atherosclerosis, 193: 44-54, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Thippegowda PB, Singh V, Sundivakkam PC, Xue J, Malik AB, Tiruppathi C. Ca2+ influx via TRPC channels induces NF-kappaB-dependent A20 expression to prevent thrombin-induced apoptosis in endothelial cells. Am J Physiol Cell Physiol, 298: C656-664, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ando K, Ishibashi T, Ohkawara H, et al. Crucial role of membrane type 1 matrix metalloproteinase (MT1-MMP) in RhoA/Rac1-dependent signaling pathways in thrombin- stimulated endothelial cells. J Atheroscler Thromb, 18: 762-773, 2011. [DOI] [PubMed] [Google Scholar]
- 61.Hemmings BA. Akt signaling: linking membrane events to life and death decisions. Science, 275: 628-623, 1997. [DOI] [PubMed] [Google Scholar]
- 62.Grehan JF, Levay-Young BK, Fogelson JL, François-Bongarçon V, Benson BA, Dalmasso AP. IL-4 and IL-13 induce protection of porcine endothelial cells from killing by human complement and from apoptosis through activation of a phosphatidylinositide 3-kinase/Akt pathway. J Immunol, 175: 1903-1910, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol, 24: 1963-1969, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Kim CW, Lee TH, Park KH, Choi SY, Kim J. Human lactoferrin suppresses TNF-α-induced intercellular adhesion molecule-1 expression via competition with NF-κB in endothelial cells. FEBS Lett, 586: 229-234, 2012. [DOI] [PubMed] [Google Scholar]
- 65.Vagima Y, Avigdor A, Goichberg P, et al. MT1-MMP and RECK are involved in human CD34+ progenitor cell retention, egress, and mobilization. J Clin Invest, 119: 492-503, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jander S, Sitzer M, Wendt A, et al. Expression of tissue factor in high-grade carotid artery stenosis: association with plaque destabilization. Stroke, 32: 850-854, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Moons AHM, Levi M, Peters RJG. Tissue factor and coronary artery disease. Cardiovasc Res, 53: 313-325, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Steffel J, Lüscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation, 113: 722-731, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res, 88: 877-887, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol, 24: 1374-1383, 2004. [DOI] [PubMed] [Google Scholar]
- 71.Lehti K., Valtanen H, Wickström SA, Lohi J, Keski-Oja J. Regulation of membrane-type-1 matrix metalloproteinase activity by its cytoplasmic domain. J Biol Chem, 275: 15006-15013, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Moss NM, Wu YI, Liu Y, Munshi HG, Stack MS. Modulation of the membrane type 1 matrix metalloproteinase cytoplasmic tail enhances tumor cell invasion and proliferation in three-dimensional collagen matrices. J Biol Chem, 284: 19791-19799, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohkawara H, Ishibashi T, Sugimoto K, Ikeda K, Ogawa K, Takeishi Y. Membrane type 1-matrix metalloproteinase/Akt signaling axis modulates TNF-α-induced procoagulant activity and apoptosis in endothelial cells. PLoS One, 9: e105697, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alikhani M, Alikhani Z, Graves DT. FOXO1 functions as a master switch that regulates gene expression necessary for tumor necrosis factor-induced fibroblast apoptosis. J Biol Chem, 280: 12096-12102, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta, 1813: 1978-1986, 2011. [DOI] [PubMed] [Google Scholar]
- 76.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell, 96: 857-868, 1999. [DOI] [PubMed] [Google Scholar]
- 77.Li W, Sama AE, Wang H. Role of HMGB1 in cardiovascular diseases. Curr Opin Pharmacol, 6: 130-135, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeVerse JS, Bailey KA, Jackson KN, Passerini AG. Shear stress modulates RAGE-mediated inflammation in a model of diabetes-induced metabolic stress. Am J Physiol Heart Circ Physiol, 302: H2498-H2508, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu M, Yu Y, Jiang H, et al. Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE(−/−) mice by downregulating the HMGB1-RAGE axis. Acta Pharmacol Sin, 34: 830-836, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sugimoto K, Ohkawara H, Nakamura Y, Takuwa Y, Ishibashi T, Takeishi Y. Receptor for Advanced Glycation End Products - Membrane Type1 Matrix Metalloproteinase Axis Regulates Tissue Factor Expression via RhoA and Rac1 Activation in High-Mobility Group Box-1 Stimulated Endothelial Cells. PLoS One, 9: e114429, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm, 2013: 928315, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakakuki T, Ito M, Iwasaki H, et al. Rho/Rho-kinase pathway contributes to C-reactive protein-induced plasminogen activator inhibitor-1 expression in endothelial cells. Arterioscler Thromb Vasc Biol, 25: 2088-2093, 2005. [DOI] [PubMed] [Google Scholar]
- 83.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab, 280: E685-E694, 2001. [DOI] [PubMed] [Google Scholar]
- 84.Furlani D, Donndorf P, Westien I, et al. HMGB-1 induces c-kit+ cell microvascular rolling and adhesion via both toll-like receptor-2 and toll-like receptor-4 of endothelial cells. J Cell Mol Med, 16: 1094-1105, 2112. [DOI] [PMC free article] [PubMed] [Google Scholar]