Abstract
Objective
In Botswana, a 36-month course of isoniazid treatment of latent tuberculosis (TB) infection [isoniazid preventive therapy (IPT)] was superior to 6-month IPT in reducing TB and death in persons living with HIV (PLHIV), having positive tuberculin skin tests (TSTs) but not in those with negative TST. We examined the cost-effectiveness of IPT in Botswana, where antiretroviral therapy (ART) is widely available.
Design
Using a decision-analytic model, we determined the incremental cost-effectiveness of strategies for reducing TB and death in 10,000 PLHIV over 36 months.
Methods
IPT for 6 months and provision of ART if CD4+ lymphocyte count <250 cells per microliter (2011 Botswana policy) was compared with 6 alternative strategies that varied the use of IPT, TST, and ART for CD4+ count thresholds, including CD4+ <350 and <500 cells per microliter.
Results
Botswana policy, 2011 was dominated by most other strategies. IPT of 36 months for TST-positive PLHIV with ART for CD4+ <250 cells per microliter resulted in 120 fewer TB cases for an additional cost of $1612 per case averted and resulted in 80 fewer deaths for an additional $2418 per death averted compared with provision of 6-month IPT to TST-positive PLHIV who received ART for CD4+ <250 cells per microliter, the next most effective strategy. Alternative strategies offered lower incremental effectiveness at higher cost. These findings remained consistent in sensitivity analyses.
Conclusions
A strategy of treating PLHIV who have positive TST with 36-month IPT is more cost effective for reducing both TB and death compared with providing IPT without a TST, providing only 6-month IPT, or expanding ART eligibility without IPT.
Keywords: cost, cost-effectiveness, tuberculosis, HIV infection, antiretroviral therapy, antituberculosis therapy
INTRODUCTION
Antiretroviral therapy (ART) improves survival and reduces the incidence of tuberculosis (TB) by approximately 65% in persons living with HIV (PLHIV) while reducing the risk of onward HIV transmission to partners. However, in countries with high TB incidence, PLHIV receiving ART continue to suffer a high incidence of TB1–3 even when ART is initiated at higher CD4+ lymphocyte counts.4,5 Isoniazid preventive therapy (IPT) reduces TB in PLHIV both before and after ART initiation.6 Based on a retrospective review of program data from Brazil and South Africa and results from two clinical trials, ART in combination with IPT additively reduces the risk of TB.7–10
A limitation of IPT is that it benefits only PLHIV who are tuberculin skin test (TST) positive,6 but program challenges with the administration and reading of the test have prevented strong recommendations to use TST.11,12 Provision of IPT to all PLHIV regardless of TST may result in an inefficient use of program resources because 67%–80% of PLHIV seen at HIV care centers in TB-endemic countries are TST negative.13–16 A further limitation of IPT is that in high TB incidence settings, the standard 6-month course loses its benefit within 6–18 months after the IPT stops.17,18 Continuing IPT up to 36 months has recently been shown to benefit TST-positive PLHIV, including those receiving ART,7,19 and World Health Organization (WHO) now recommends a 36-month course of IPT where possible.12
Policy makers in countries with high TB incidence have options for reducing mortality and morbidity in PLHIV, including earlier initiation of ART and the provision IPT with or without a TST for various durations. A key challenge is selecting the most appropriate combination of strategies to maximize health benefits, given available resources. Researchers have modeled the cost-effectiveness of expanding ART eligibility on survival20 and TB incidence,21 the cost-effectiveness of providing IPT based on use of TST,22,23 and the comparative cost-effectiveness of providing ART versus treating latent TB infection.24 No model to date compares the cost-effectiveness of policies that concurrently vary ART eligibility criteria, the duration of IPT, and the use of TST. We developed a decision-analytic model to assist policy makers in selecting cost-effective interventions for responding to the TB-HIV syndemic using recent evidence of the impact of ART and IPT on preventing TB and death.
Our analysis focused on Botswana for several reasons: (1) 80% of people with TB disease are coinfected with HIV in Botswana, (2) primary epidemiological, efficacy, and cost data are available from a recently completed clinical trial on 6-month versus 36-month IPT for PLHIV (hereafter, “the Trial”),7 and (3) country-specific ART cost data were available from a recently published study.25
METHODS
We developed a decision-analytic model to assess the outcomes, costs, and cost-effectiveness of strategies for reducing TB disease and all-cause mortality over a 3-year analytic horizon in a cohort of 10,000 PLHIV presenting to HIV care clinics in Botswana. The model was constructed in TreeAge Pro 2011 (TreeAge, Williamstown, MA).
We calculated costs and outcomes for 7 strategies (Table 1). Each strategy used a combination of eligibility criteria for ART initiation (based on CD4+ lymphocyte count thresholds), provision of IPT, and use of TST. ART initiation was considered at CD4 <250 cells per microliter, CD4 <350 cells per microliter, or CD4 <500 cells per microliter. If provided, IPT duration was 6 months or 36 months. For the use of TST, we considered 3 scenarios: provision of IPT to all (without conducting TST), provision of IPT to only those with a positive TST, or a targeted approach in which those with negative TST received IPT for 6 months and those with positive TST received IPT for 36 months. Although eligibility for ART during most of the trial was based on CD4 <200 cells per microliter, the modeled “2011 policy” reflects the policy in Botswana toward the end of the trial: TST was not performed, PLHIV received 6-month IPT, and those with CD4 <250 cells per microliter were eligible for ART.
TABLE 1.
Strategy Descriptions
| Strategy Name | Description |
|---|---|
| ALL_H6 | 2011 policy in Botswana—provide all PLHIV with 6-mo IPT and initiate ART at CD4 <250 cells/µL |
| ALL_H36 | Provide all PLHIV with 36-mo IPT and initiate ART at CD4 <250 cells/µL |
| TST_H6 | Provide only TST-positive PLHIV with 6-mo IPT and initiate ART at CD4 <250 cells/µL |
| TST_H36 | Provide only TST-positive PLHIV with 36-mo IPT and initiate ART at CD4 <250 cells/µL |
| TST_H6H36 | Provide all PLHIV with 6-mo IPT, extend duration to 36 mo for TST-positives, and initiate ART at CD4 <250 cells/µL |
| ART_350 | Discontinue IPT and increase ART initiation threshold for PLHIV from CD4 <250 cells/µL to <350 cells/µL |
| ART_500 | Discontinue IPT and increase ART initiation threshold for PLHIV from CD4 <250 cells/µL to <500 cells/µL |
There were 2 primary outcomes of interest: the expected number of new TB cases (incidence) and the expected number of deaths from TB and other causes (all-cause mortality). For each strategy, we assumed that CD4 counts were known for PLHIV entering the model and that persons with CD4 counts below the specified threshold initiated continuous ART; all patients received TB screening at baseline (month 0) using a standard clinical symptom screen consistent with Botswana’s national guidelines26,27; and PLHIV diagnosed with TB disease were treated according to WHO directly observed therapy short-course (DOTS) guidelines28 (Supplementary Digital Content, http://links.lww.com/QAI/A727). False positives—those incorrectly classified as positive for TB disease at screening—were excluded from the sample of persons eligible for IPT as they would not likely be identified as truly negative before completing DOTS. False negatives—those incorrectly classified as TB disease-free at screening—were assumed to receive the same clinical care as true negatives; however, we assumed they would be correctly diagnosed and begin TB treatment within 3 months of screening. Deaths were tabulated for the entire cohort. After each 12-month period, the pool of PLHIV without TB disease or death was adjusted downward to account for those who developed TB or died in the previous period.
Efficacy and Other Epidemiological Parameters
The model uses primary data and key results from the trial. The randomized, double-blind, placebo-controlled trial enrolled adults with HIV infection who attended one of 8 government clinics that provided ART and IPT in Gaborone and Francistown, Botswana, between November 26, 2004 and July 20, 2006.7 Required inputs unavailable from trial data were abstracted from the literature or imputed, when necessary.
TB disease prevalence at baseline by CD4 strata and expected number of PLHIV requiring hospitalizations for TB and isoniazid toxicity was estimated using trial data and published sources.1,29 For each CD4 stratum, we used published data to estimate the number of PLHIV falsely classified positive or negative for TB disease given prevalence and assumptions about screening sensitivity and specificity.1,29,30 For each CD4 stratum, we estimated rates of TST positivity separately for those with and without prevalent TB based on abstracted data from published materials.14,16,31–34
We used unadjusted rates of incident TB and death from the trial’s intent-to-treat analysis to determine the efficacies of ART and IPT, ie, no adjustment was made for losses to follow-up or nonadherence to study medication or ART. We derived model estimates of TB incidence and death rates using higher thresholds for the ART initiation literature (Table 2). In model scenarios based on initiating ART at higher CD4 cell counts, we accounted for reductions in incident TB disease and death because of higher median CD4 counts in the eligible portion of the cohort.1 In trial data, the reduction in TB and death due to IPT varied according to duration (6 or 36 months), the progression of HIV disease, the concomitant provision of ART, and TST status. We accounted for each of these factors in the model. In strategies in which IPT was provided without TST results, rates of TB and death by TST status from the trial were averaged and weighted by the observed number of TST positives (0.25) and TST negatives (0.75). Further information on the derivation of key efficacy parameters can be found in the Supplementary Digital Content, http://links.lww.com/QAI/A727.
TABLE 2.
Model Parameter Values and Data Sources
| Epidemiologic and Intervention Effect Inputs | Base Value | Range | Source | ||
|---|---|---|---|---|---|
| Distribution of cohort by selected CD4 categories | |||||
| <250 cells/µL | 0.40 | 0.20–0.50 | Trial data | ||
| <350 cells/µL | 0.60 | 0.40–0.70 | |||
| <500 cells/µL | 0.80 | 0.60–0.90 | |||
|
Base Value Below CD4 Threshold* |
Range |
Base Value Above CD4 Threshold* |
Range | Sources | |
| TB disease prevalence at baseline | |||||
| CD4 250 cells/µL | 0.11 | 0.05–0.16 | 0.05 | 0.02–0.07 | 3,29,45 |
| CD4 350 cells/µL | 0.10 | 0.05–0.15 | 0.03 | 0.02–0.05 | 3,29,45 |
| CD4 500 cells/µL | 0.09 | 0.04–0.13 | 0.01 | 0.00–0.02 | 3,29,45 |
| TB screening† | |||||
| Proportion correctly identified with TB disease at baseline |
|||||
| CD4 250 cells/µL | 0.96 | 0.79–0.98 | 0.83 | 0.63–0.95 | Imputed from45 |
| CD4 350 cells/µL | 0.95 | 0.78–0.97 | 0.80 | 0.65–0.94 | |
| CD4 500 cells/µL | 0.90 | 0.73–0.92 | 0.75 | 0.60–0.85 | |
| Proportion not requiring anti-TB treatment at baseline | |||||
| CD4 250 cells/µL | 0.98 | 0.80–1.00 | 0.99 | 0.80–1.00 | Assumption |
| CD4 350 cells/µL | 0.98 | 0.80–1.00 | 0.99 | 0.80–1.00 | |
| CD4 500 cells/µL | 0.99 | 0.80–1.00 | 1.00 | 0.80–1.00 | |
| TST positivity | |||||
| CD4 250 cells/µL | |||||
| Proportion TST-positive with active TB | 0.52 | 0.42–0.62 | 0.73 | 0.59–0.87 | 31,33 |
| Proportion TST-positive without active TB | 0.17 | 0.15–0.23 | 0.30 | 0.24–0.36 | 13,14,16,32,34 |
| TB incidence rates‡ | |||||
| CD4 200 cells/µL | |||||
| TST-positive PLHIV | 0.18 | 0.07–0.49 | 0.03 | 0.02–0.05 | Trial data |
| TST-negative PLHIV | 0.03 | 0.01–0.08 | 0.01 | 0.01–0.02 | |
| PLHIV (TST status unknown) | 0.07 | 0.03–0.18 | 0.02 | 0.01–0.03 | |
| Mortality rates‡ | |||||
| CD4 200 cells/µL, TB-negative PLHIV | |||||
| TST-positive | 0.05 | 0.01–0.36 | 0.02 | 0.01–0.04 | Trial data |
| TST-negative | 0.05 | 0.02–0.12 | 0.01 | 0.00–0.02 | |
| TST status unknown | 0.05 | 0.02–0.18 | 0.01 | 0.01–0.02 | |
|
Base Value on ART |
Range |
Base Value Not on ART |
Range | Sources | |
| Mortality rates∥ | |||||
| TB-positive PLHIV | |||||
| Prevalent cases identified at baseline | 0.05 | 0.01–0.20 | 0.15 | 0.05–0.30 | 46–48 |
| Incorrectly classified for active TB at baseline | |||||
| False negatives—IPT/delayed TB treatment | 0.27 | 0.05–0.35 | 0.35 | 0.10–0.40 | Supplementary§ Digital Content |
| False negatives—No IPT/delayed TB treatment | 0.27 | 0.05–0.35 | 0.35 | 0.10–0.40 | Supplementary§ Digital Content |
| False positives—TB treatment | 0.02 | 0.01–0.15 | 0.01 | 0.00–0.10 | 49–53 |
|
Base Value Below CD4 Threshold* |
Range |
Base Value Above CD4 Threshold* |
Range | Source | |
| Reduction in TB incidence due to ART initiation at higher CD4 compared to threshold CD4 200 cells/µL‡ |
|||||
| CD4 250 cells/µL | 0.21 | 0.04–0.32 | 0.20 | 0.04–0.30 | 1,54 |
| CD4 350 cells/µL | 0.26 | 0.05–0.38 | 0.50 | 0.10–0.75 | 1,14,54–59 |
| CD4 500 cells/µL | 0.35 | 0.07–0.52 | 0.65 | 0.13–0.97 | 1,54,56,57,60 |
| Reduction in mortality due to ART initiation at higher | |||||
| CD4 compared to threshold CD4 200 cells/µL‡ | |||||
| CD4 250 cells/µL | 0.22 | 0.04–0.34 | 0.18 | 0.04–0.27 | 53,61 |
| CD4 350 cells/µL | 0.28 | 0.06–0.41 | 0.54 | 0.11–0.81 | 49–51,53,61 |
| CD4 500 cells/µL | 0.44 | 0.09–0.66 | 0.71 | 0.14–1.07 | 53,61 |
| Reduction in TB incidence after provision of ART‡ | |||||
| CD4 250 cells/µL | 0.59 | 0.12–0.86 | — | — | Supplementary§ Digital Content |
| CD4 350 cells/µL | 0.56 | 0.13–0.88 | — | — | |
| CD4 500 cells/µL | 0.49 | 0.10–0.74 | — | — | |
| Reduction in mortality after provision of ART‡ | |||||
| CD4 250 cells/µL | 0.62 | 0.12–0.93 | — | — | Supplementary§ Digital Content |
| CD4 350 cells/µL | 0.58 | 0.15–0.87 | — | — | |
| CD4 500 cells/µL | 0.45 | 0.09–0.68 | — | — | |
| Reduction (increase) in TB incidence after provision of IPT‡ |
|||||
| CD4 200 cells/µL, 6-mo course | |||||
| TST-positive | 0.21 | 0.00–0.30 | 0.21 | 0.00–0.30 | Trial data |
| TST-negative | 0.05 | 0.00–0.10 | 0.05 | 0.00–0.10 | Trial data |
| TST status unknown | 0.16 | 0.00–0.20 | 0.12 | 0.00–0.20 | Trial data |
| CD4 200 cells/µL, 36-mo course | |||||
| TST-positive | 0.77 | 0.39–0.91 | 0.88 | 0.74–0.95 | Trial data |
| TST-negative | 0.31 | 0.58–0.10 | 0.30 | 0.23–0.35 | Trial data |
| TST status unknown | 0.41 | 0.23–0.42 | 0.56 | 0.47–0.61 | Trial data |
| Reduction (increase) in mortality after provision of IPT‡ | |||||
| CD4 200 cells/µL, 6-mo course | |||||
| TST-positive | 0.09 | 0.00–0.15 | 0.09 | 0.00–0.15 | Trial data |
| TST-negative | 0.00 | 0.00–0.01 | 0.00 | 0.00–0.00 | Trial data |
| TST status unknown | 0.02 | 0.01–0.04 | 0.04 | 0.00–0.10 | Trial data |
| CD4 200 cells/µL, 36-mo course | |||||
| TST-positive | 0.06 | 0.10–0.00 | 0.77 | 0.63–0.87 | Trial data |
| TST-negative | 0.71 | 1.08–0.38 | 0.47 | 0.64–0.34 | Trial data |
| TST status unknown | 0.56 | 0.99–0.22 | 0.13 | 0.08–0.20 | Trial data |
| Cost Inputs∥ | Base Value | Range | Source | ||
| Annual ART | |||||
| Clinical care and laboratory services | 371 | 186–557 | 25 | ||
| Antiretroviral drugs | 230 | 115–345 | 62 | ||
| Total ART | 601 | 301–902 | |||
| TB treatment | |||||
| Pharmacist observation (10 minutes daily) | 322 | — | 27 | ||
| Standard 182 d DOTS treatment | 10 | — | 27 | ||
| Total TB treatment | 332 | 166–498 | 27 | ||
| IPT | |||||
| Nurse time (15 min per visit) | 2.66 | — | 27 | ||
| Isoniazid per month | 0.50 | — | 27 | ||
| Pyridoxine (B6) per month | 0.17 | — | 27 | ||
| Total 6-mo IPT | 20 | 10–29 | |||
| Total 36-mo IPT | 117 | 59–176 | |||
| TST | |||||
| Protein purified derivative per dose | 6.50 | — | 12 | ||
| Nurse time and medical supplies | 4.60 | — | Trial data | ||
| Required infrastructure investments per person | 2.03 | — | 36 | ||
| Total TST | 13 | 7–20 | |||
| Hospitalization | |||||
| Inpatient cost per day | 190 | — | 36 | ||
| Total hospitalization for TB (21 d) | 3999 | 1999–5998 | |||
| Total hospitalization for IPT toxicity (10 d) | 1904 | 952–2856 | |||
TB disease prevalence at baseline CD4 250 cells/µL, CD4 350 cells/µL, and CD4 500 cells/µL.
PLHIV below the specified threshold were assumed to also receive ART; PLHIV above the specified threshold did not receive ART over the period.
Data imputed for CD4 <250 and CD4 <500.
Calculated using intent-to-treat trial data and estimates from the published literature. All values presented are per annum; rate reductions averaged over 36 months.
Detailed derivation of these estimates can be found in the Supplementary Digital Content, http://links.lww.com/QAI/A727.
All costs are reported per patient and have been inflated to 2010 US dollars using the Medical Care Consumer Price Index provided by the US census bureau.
Cost Inputs
We adopted a Botswana health system perspective and included relevant health care utilization costs (Table 2). We did not include out-of-pocket costs incurred by individuals, productivity loss from illness or death, or costs that were identical under each strategy. The time frame and analytic horizon for the analysis were 36 months. The total cost of each strategy was calculated by multiplying the total number of individuals using each service (ie, TST, IPT, ART, TB treatment, and hospitalizations) by the associated unit cost and summing across all services. All costs have been adjusted to 2010 US dollars.35
Cost per patient for TST consisted of health care personnel labor, average cost per dose of purified protein derivative-TST, medical supplies, and necessary investment costs, including health worker training and refrigeration for purified protein derivative. Labor cost for administration and reading of TST was calculated as nurse time devoted to patient testing multiplied by the median wage rate. Cost per patient of both IPT and TB treatment was calculated as the sum of drug costs and health worker time for administration and monitoring of therapy (assuming monthly follow-up visits for IPT and daily pharmacist observation for DOTS).27 The cost per patient for hospitalization was calculated by multiplying the average number of inpatient days (21 for TB disease and 10 for isoniazid toxicity) by the average hospitalization cost per day.36
The annual cost per patient of clinical care for ART (clinic visits and laboratory monitoring) was taken from the President’s Emergency Plan for AIDS Relief Botswana ART cost study.25 Annual per-patient antiretroviral costs were calculated using drug prices and regimen distribution data provided by the Supply Chain Management System for President’s Emergency Plan for AIDS Relief. We assumed that 3% of patients required second-line ART. All PLHIV received similar care before initiating ART. To account for costs associated with ART initiation, we applied only the incremental cost of antiretrovirals and added intensity of clinical care required to monitor patients receiving ART. For each strategy, we adjusted the total cost of ART upward to account for the proportion of the cohort above the specified CD4 threshold that would become eligible and initiate ART over the 36-month period.37 ART costs were also adjusted downward for patients who died within the analytic horizon, as the health system would not incur costs for the full 36 months of ART.
Cost-Effectiveness Analysis
We conducted cost-effectiveness analysis separately for each outcome measure. We evaluated the expected number of TB cases, deaths, and total cost for each strategy and ranked them in order of increasing effectiveness. We computed the incremental cost-effectiveness ratios (ICERs) for each outcome by taking the difference in total costs divided by the difference in cases or deaths when comparing one strategy to the next most effective. The final ICERs excluded strategies that were both less effective and more costly than others (ie, absolutely dominated strategies). Strategies with higher ICERs than more effective alternatives (ie, extended dominated) were similarly excluded.38
Sensitivity Analysis
We varied cost inputs by 50% above and below their base values in the sensitivity analysis. To identify influential model parameters, we conducted tornado analyses for each outcome across a range of arbitrary willingness-to-pay (WTP) thresholds.39 In the absence of well-documented WTP thresholds for each outcome measure, we considered a wide range of values of WTP. For influential model parameters, we conducted additional univariate sensitivity analysis to determine whether the rank of optimal strategies would change at any value from the specified range.
RESULTS
Expected outcomes (incident TB and all-cause mortality), total costs, and ICERs are presented in Table 3.
TABLE 3.
ICERs Per TB Case or Death Averted
| Strategy Alternatives | Incident TB Cases* |
Incremental Cases Averted |
Total Cost of Program |
Incremental Cost of Program |
ICER Per Case Averted |
|
|---|---|---|---|---|---|---|
| TST_H6 | Provide only TST-positive PLHIV with 6-mo IPT, ART <250 cells/µL |
318 | — | $5,874,660 | — | — |
| TST_H36 | Provide only TST-positive PLHIV with 36-mo IPT, ART <250 cells/µL |
198 | 120 | $6,068,082 | $193,422 | $1612 |
| TST_H6H36 | Provide 6-mo IPT for TST-negatives, 36-mo IPT for TST-positives, ART <250 cells/µL |
171 | 27 | $6,244,913 | $176,831 | $6549 |
|
Total Deaths† |
Incremental Deaths Averted |
Total Cost of Program |
Incremental Cost of Program |
ICER Per Death Averted |
||
| TST_H6 | Provide only TST-positive PLHIV with 6-mo IPT, ART <250 cells/µL |
301 | — | $5,874,660 | — | — |
| TST_H36 | Provide only TST-positive PLHIV with 36-mo IPT, ART <250 cells/µL |
221 | 80 | $6,068,082 | $193,422 | $2,418 |
| TST_H6H36 | Provide 6-mo IPT for TST-negatives, 36-mo IPT for TST-positives, ART <250 cells/µL |
218 | 3 | $6,244,913 | $176,831 | $58,944 |
| ART500 | Discontinue IPT and increase ART initiation threshold to CD4 <500 cells/µL |
171 | 47 | $9,896,548 | $3,651,635 | $77,694 |
The current strategy ALL_H6 was dominated in the analysis and was not included in the final ICERs. ALL_H6 resulted in 321 total incident cases, 290 total deaths and $5,937,863 in total program costs.
Total number of new TB cases expected over the analytic horizon; excludes those diagnosed with TB at baseline.
Total number of deaths expected over the analytic horizon, including TB and all other causes.
Extended dominance occurs when the ICER of an alternative is greater than the ICER of a more effective strategy, indicating an inefficient use of resources.
Absolute dominance occurs when an alternative is both less effective and more costly than other alternatives.
Incident TB
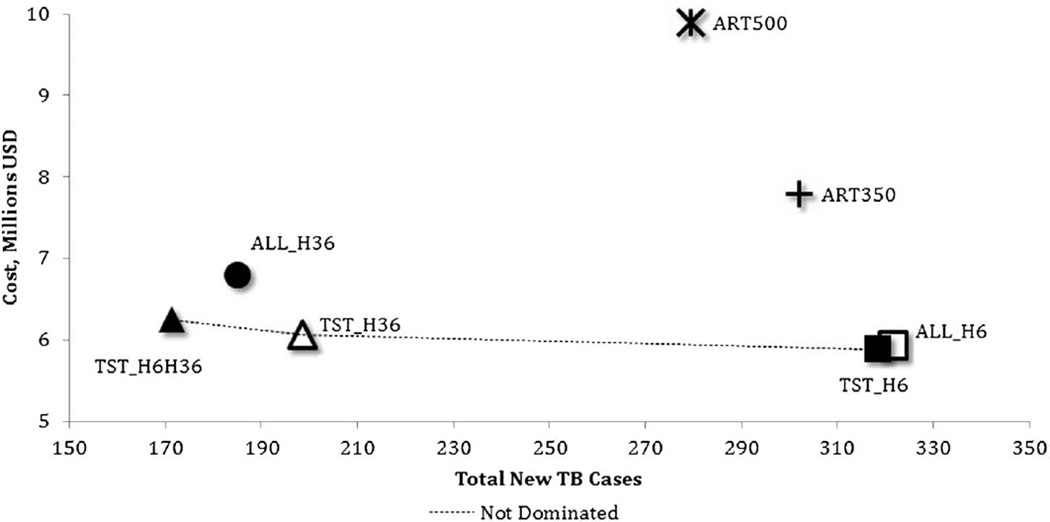
Considering incident TB cases averted as the primary outcome, 4 strategies were both less effective and more costly than other alternatives (Fig. 1) and were excluded from the incremental analysis. These included providing IPT to PLHIV for 6 months (2011 policy, ALL_H6), providing IPT to all PLHIV for 36 months (ALL_H36), and both strategies that increased the threshold for ART initiation without the provision of IPT (ART350 and ART500).
FIGURE 1.
Expected cost and incident TB among 7 strategies in 10,000 PLHIV in Botswana.
Treating only PLHIV with a positive TST with 6-month IPT and initiating ART at CD4 <250 cells per microliter (TST_H6) were the least costly but least effective of the remaining strategies (Table 3, upper panel). Comparatively, extending IPT to 36 months for TST-positives (TST_H36) resulted in 120 fewer cases for an additional $1612 per case averted when compared with the next most effective strategy (TST_H6). The strategy of providing TST-negatives with a 6-month course of IPT and TST-positives with a 36-month course (TST_H6H36) resulted in 27 fewer cases for an additional $6549 per case averted compared with the next most effective alternative (TST_H36).
Four parameter categories accounted for at least 85% of variation in model results across all WTP thresholds in the sensitivity analysis. At a WTP threshold of $10,000, the per-patient cost of ART and percent of the cohort below CD4 250 cells per microliter accounted for 55% and 30% of the variation, respectively. At a $1,000,000 WTP threshold, the effect of ART and the effect of higher CD4 count had a greater impact, accounting for 64% and 27% of the variation. The rank-order of undominated strategies remained consistent at all values. Additional detail on sensitivity analysis results is in the Supplementary Digital Content, http://links.lww.com/QAI/A727.
All-Cause Mortality
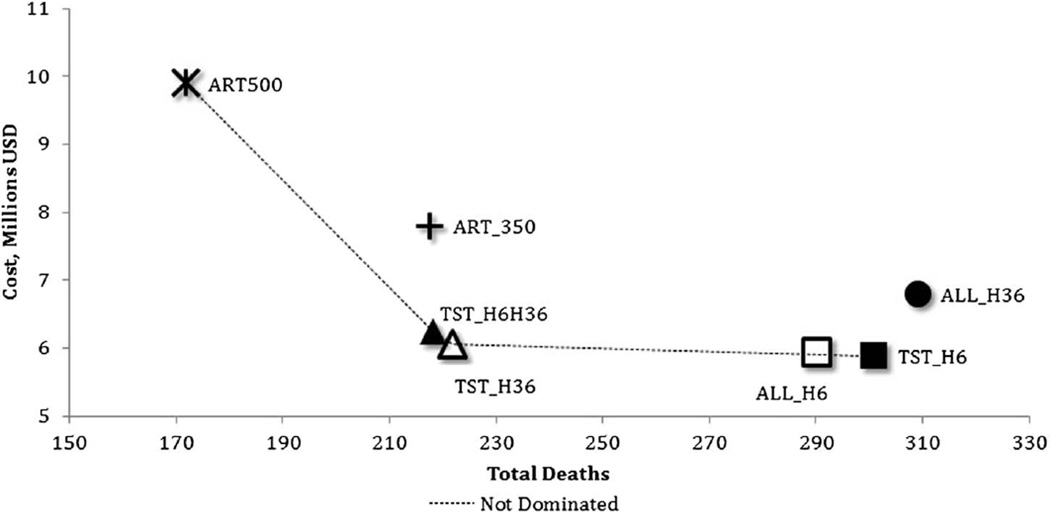
Considering mortality as the primary outcome, providing 36-month IPT to all PLHIV (ALL_H36) was less effective than all other strategies and more costly than all alternatives that included IPT and was excluded from the incremental analysis (Fig. 2). Two strategies—providing 6-month IPT to all PLHIV and initiating ART at CD4 <250 cells per microliter (2011 Botswana policy, ALL_H6) and increasing the CD4 threshold for ART initiation to <350 cells per microliter without IPT (ART350)—had higher ICERs than more effective alternatives and were also excluded. Similar to the results for incident TB, providing 6-month IPT for TST-positives only (TST_H6) was the least effective and least costly alternative of those not dominated.
FIGURE 2.
Expected cost and all-cause mortality among 7 strategies in 10,000 PLHIV in Botswana.
Compared to providing 6 months of IPT, extending the duration of IPT to 36 months for TST-positives (TST_H36) resulted in 80 fewer deaths for an additional $2418 per death averted compared with the next most effective strategy (TST_H6) (Table 3, lower panel). Adding 6-month IPT for TST-negatives (TST_H6H36) resulted in 3 fewer deaths at a cost of $58,944 per death averted when compared with the next most effective alternative (TST_H36). Discontinuing IPT and increasing the threshold for ART initiation to CD4 <500 cells per microliter (ART500) were both the most effective and most costly alternative, resulting in 47 fewer deaths than TST_H6H36 at an additional $77,694 per death averted (in comparison with TST_H6H36) among the undominated strategies.
From our sensitivity analysis with mortality as the outcome, we found that 4 parameter categories accounted for more than 90% of variation across all WTP thresholds. Per-patient cost of ART, percent of the cohort below CD4 250 cells per microliter, effect of ART, and effect of initiation of ART at higher CD4 count were the same 4 parameters that accounted for most of the variation when incident TB was the outcome. There was no change in the rank-order of undominated strategies; however, ART500 becomes dominated when the effect of ART is reduced by 28% or more or the effect of higher threshold CD4 count is reduced by 11% or more. Conversely, TST_H6H36 is dominated when the effect of ART is increased by 18% or more, or the effect of higher threshold CD4 count is increased by 10% or more. TST_H6H36 is also dominated when more than 47% of the cohort has a CD4 count <250 cells per microliter or the annual cost per patient of ART (inclusive of drugs) is less than $271.
DISCUSSION
Our cost-effectiveness analysis of several strategies for the prevention of TB and death in PLHIV in Botswana suggests that strategies that use TST in conjunction with continuous IPT and ART are superior to providing 6-month IPT and ART for PLHIV with CD4 <250 cells per microliter, which was the current strategy at the time of this study. Treating TST-positive PLHIV with 36-month IPT is more cost-effective than providing IPT without a TST, providing only 6-month IPT or expanding ART eligibility with no IPT. Our results are not surprising given the benefits of continuous IPT demonstrated in the trial. Although we cannot conclude definitively which strategy is most cost effective—an assessment that requires an understanding of the WTP for additional TB cases or deaths averted—we can submit that strategies including the provision of 36 months of IPT for TST-positive PLHIV resulted in the highest number of TB cases and deaths averted for the least cost. These findings remain pertinent to the current situation in Botswana: in 2014, the Ministry of Health policy is to provide ART at a threshold of 350 cells per microliter and 6 months of IPT without the use of the TST.
Although the model results clearly favor strategies that include provision of IPT, the importance of ART should not be downplayed. Both ART and IPT have significant impacts on TB incidence, whereas death is reduced by ART and may be reduced by IPT in PLHIV with positive TSTs.7,40 Our analysis showed that although death and TB incidence declined in PLHIV receiving ART at higher CD4 levels, early ART strategies were either far more costly or dominated by those that added continuous IPT for persons with positive TST while providing ART at the lower CD4 <250 cells per microliter threshold. Because there are other important reasons to provide early ART not captured by this model (for example, impact on HIV transmission), IPT should be considered a cost-effective adjunct to ART. Despite living in a setting with high TB incidence, PLHIV who were TST-negative have been observed to have a steady ~1% per annum rate of TB despite provision of ART for CD4 <200 cells per microliter and 6-month IPT.7 Strategies that provided IPT to all PLHIV including TST-negative PLHIV were dominated by strategies that targeted IPT to TST-positive PLHIV.
Based on our results, TST is a critical component for allocating resources efficiently. In other settings, approximately 3-quarters of PLHIV screened in TB-endemic settings were documented as TST-negative7,13,41,42 and are unlikely to benefit from IPT. Additionally, they may be subjected to the adverse effects of IPT. Our results are congruent with findings from other TST studies. In a voluntary counseling and testing center setting in Uganda, TST before 6 months of IPT was found to be cost effective compared with not using TST.22 A recent example from a Thai program concluded that limiting IPT to TST-positive PLHIV receiving ART was practical and effective.43 Program managers and WHO officials remain concerned that TST is difficult to implement in resource-constrained settings. Given the cost-effectiveness of IPT provision based on TST, programs should include plans to mitigate operational challenges, including training networks that enable outreach workers to read TST results consistently, in a system including routine quality checks.
Resistance to anti-TB drugs is a concern and could impact interpretation of our results in 2 ways: accidental provision of IPT to persons with active TB disease may lead to isoniazid resistance and high rates of latent isoniazid-resistant TB infection may influence the effectiveness of preventive therapies. No increased risk of isoniazid resistance was observed in the 2000-person cohort followed in the Botswana trial. However, our findings reflect the effectiveness of IPT in Botswana at the time of the trial and might not extend to situations with higher background rates of latent isoniazid-resistant infection; this may be a subject for future investigation.
A key strength of our model is the stability of results in sensitivity analysis. When we varied the CD4 range of the enrolled cohort, the cost-effectiveness rank order of alternative strategies was constant. The stability provides a sense of generalizability for places where there are higher or lower proportions of TST-positivity, as TST-positivity rates are higher among PLHIV with higher CD4 counts. The priority order of the 7 strategies did not change when varying model parameters to their upper and lower bounds across a range of WTP thresholds, indicating results may be generalizable to other low-income and middle-income settings with similar TB-HIV burden.
Limitations of our analysis include a possible underestimation of the mortality benefit of IPT given that 2 reports have shown reductions in mortality in PLHIV receiving 6 months IPT in ART programs.40,44 We did not consider the risk of multidrug-resistant TB (ie, resistance to isoniazid and rifampin) or the associated costs of second-line or third-line anti-TB therapies. We did not consider how TB prevention in the cohort might affect TB transmission, nor did we include other possible approaches, such as implementation of infection control practices that might also reduce the risk of TB infection. Our choice of a 3-year analytic horizon reduces the complexity of the model; however, it does not allow us to account for outcomes related to the likelihood of developing TB after IPT is discontinued at 36 months. We focused on prevention of TB and all-cause mortality, whereas early provision of ART may also reduce HIV transmission, lifetime health care costs, and productivity loss. Although the perspective of our analysis allows us to focus on the direct costs of diagnosis and treatment, it does not account for the potential benefits to society resulting from averting TB-related morbidity and mortality. Our analysis addresses epidemiologic and economic considerations for a middle-income country with high TB incidence and may not be generalizable to other settings.
In TB-endemic settings in which ART is already provided for CD4 <250 cells per microliter, a policy of treating TST-positive PLHIV with 36-month IPT is more cost effective than providing IPT without a TST, providing only 6-month IPT, or expanding ART eligibility with no IPT. Compared with ART initiation at higher CD4 thresholds, the strategy of treating TST-positive PLHIV with 36-month IPT is the most cost-effective approach to reduce TB disease and death.
Acknowledgments
All coauthors were directly involved in creating the analytic framework, gathering and incorporating data, building the model, and writing the manuscript. We extend our appreciation to Nong Shang, PhD, for his statistical expertise in deriving base case parameters from the Botswana IPT trial.
Footnotes
Preliminary results were presented at the American Thoracic Society Annual Conference in Denver, Colorado (2011).
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
REFERENCES
- 1.Lawn SD, Myer L, Edwards D, et al. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23:1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 3.Were W, Moore D, Ekwaru P, et al. A simple screening tool for active tuberculosis in HIV-infected adults receiving antiretroviral treatment in Uganda. Int J Tuberc Lung Dis. 2009;13:47–53. [PubMed] [Google Scholar]
- 4.Fox MP, Sanne IM, Conradie F, et al. Initiating patients on antiretroviral therapy at CD4 cell counts above 200 cells/microl is associated with improved treatment outcomes in South Africa. AIDS. 2010;24:2041–2050. doi: 10.1097/QAD.0b013e32833c703e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 8.Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384:682–690. doi: 10.1016/S0140-6736(14)60162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golub JE, Pronyk P, Mohapi L, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23:631–636. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preventive therapy against tuberculosis in people living with HIV. Wkly Epidemiol Rec. 1999;74:385–398. [PubMed] [Google Scholar]
- 12.WHO. Guidelines for Intensified Tuberculosis Case-finding and Isoniazid Preventive Therapy for People Living with HIV in Resource-constrained Settings. Geneva, Switzerland: ANNEXES; 2011. [Google Scholar]
- 13.Kerkhoff AD, Kranzer K, Samandari T, et al. Systematic review of TST responses in people living with HIV in under-resourced settings: implications for isoniazid preventive therapy. PLoS One. 2012;7:e49928. doi: 10.1371/journal.pone.0049928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miranda A, Morgan M, Jamal L, et al. Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995–2001. PLoS One. 2007;2:e826. doi: 10.1371/journal.pone.0000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosimaneotsile B, Mathoma A, Chengeta B, et al. Isoniazid tuberculosis preventive therapy in HIV-infected adults accessing antiretroviral therapy: a Botswana Experience, 2004–2006. J Acquir Immune Defic Syndr. 2010;54:71–77. doi: 10.1097/QAI.0b013e3181c3cbf0. [DOI] [PubMed] [Google Scholar]
- 16.Mugisha B, Bock N, Mermin J, et al. Tuberculosis case finding and preventive therapy in an HIV voluntary counseling and testing center in Uganda. Int J Tuberc Lung Dis. 2006;10:761–767. [PubMed] [Google Scholar]
- 17.Johnson JL, Okwera A, Hom DL, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. AIDS. 2001;15:2137–2147. doi: 10.1097/00002030-200111090-00009. [DOI] [PubMed] [Google Scholar]
- 18.Quigley MA, Mwinga A, Hosp M, et al. Long-term effect of preventive therapy for tuberculosis in a cohort of HIV-infected Zambian adults. AIDS. 2001;15:215–222. doi: 10.1097/00002030-200101260-00011. [DOI] [PubMed] [Google Scholar]
- 19.Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365:11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walensky RP, Wood R, Ciaranello AL, et al. Scaling up the 2010 World Health Organization HIV Treatment Guidelines in resource-limited settings: a model-based analysis. Plos Med. 2010;7:e1000382. doi: 10.1371/journal.pmed.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams BG, Granich R, De Cock KM, et al. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci U S A. 2010;107:19485–19489. doi: 10.1073/pnas.1005660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrestha RK, Mugisha B, Bunnell R, et al. Cost-effectiveness of including tuberculin skin testing in an IPT program for HIV-infected persons in Uganda. Int J Tuberc Lung Dis. 2006;10:656–662. [PubMed] [Google Scholar]
- 23.Shrestha RK, Mugisha B, Bunnell R, et al. Cost-utility of tuberculosis prevention among HIV-infected adults in Kampala, Uganda. Int J Tuberc Lung Dis. 2007;11:747–754. [PubMed] [Google Scholar]
- 24.Currie CS, Floyd K, Williams BG, et al. Cost, affordability and cost-effectiveness of strategies to control tuberculosis in countries with high HIV prevalence. BMC Public Health. 2005;5:130. doi: 10.1186/1471-2458-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menzies NA, Berruti AA, Berzon R, et al. The cost of providing comprehensive HIV treatment in PEPFAR-supported programs. AIDS. 2011;25:1753–1760. doi: 10.1097/QAD.0b013e3283463eec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agizew TB, Arwady MA, Yoon JC, et al. Tuberculosis in asymptomatic HIV-infected adults with abnormal chest radiographs screened for tuberculosis prevention. Int J Tuberc Lung Dis. 2010;14:45–51. [PubMed] [Google Scholar]
- 27.Samandari T, Bishai D, Luteijn M, et al. Costs and consequences of additional chest x-ray in a tuberculosis prevention program in Botswana. Am J Respir Crit Care Med. 2011;183:1103–1111. doi: 10.1164/rccm.201004-0620OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Treatment of Tuberculosis: Guidelines for National Programmes. Geneva, Switzerland: 2010. [Google Scholar]
- 29.Shah S, Demissie M, Lambert L, et al. Intensified tuberculosis case finding among HIV-Infected persons from a voluntary counseling and testing center in Addis Ababa, Ethiopia. J Acquir Immune Defic Syndr. 2009;50:537–545. doi: 10.1097/QAI.0b013e318196761c. [DOI] [PubMed] [Google Scholar]
- 30.Cain KP, McCarthy KD, Heilig CM, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362:707–716. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 31.Cobelens FG, Egwaga SM, van Ginkel T, et al. Tuberculin skin testing in patients with HIV infection: limited benefit of reduced cutoff values. Clin Infect Dis. 2006;43:634–639. doi: 10.1086/506432. [DOI] [PubMed] [Google Scholar]
- 32.Mwinga A, Hosp M, Godfrey-Faussett P, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS. 1998;12:2447–2457. doi: 10.1097/00002030-199818000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Rangaka MX, Diwakar L, Seldon R, et al. Clinical, immunological, and epidemiological importance of antituberculosis T cell responses in HIV-infected Africans. Clin Infect Dis. 2007;44:1639–1646. doi: 10.1086/518234. [DOI] [PubMed] [Google Scholar]
- 34.Vitek E, Gusseinova N, Laricheva N, et al. Factors associated with positive tuberculin skin test results among HIV-infected persons in Orel Oblast, Russia. Int J Tuberc Lung Dis. 2009;13:829–835. [PubMed] [Google Scholar]
- 35.BLS. [Accessed January 11, 2013];Bureau of Labor Statistics: CPI for Medical Services. Available at: http://www.bls.gov/cpi/data.htm.
- 36.Public Service Management Directive No. 1 of 2008. Adjustment of Salary Scales and Review of Allowances. DP 2/5 XI (115) Botswana, Africa: 2008. [Google Scholar]
- 37.Duvignac J, Anglaret X, Kpozehouen A, et al. CD4+ T-lymphocytes natural decrease in HAART-naive HIV-infected adults in Abidjan. HIV Clin Trials. 2008;9:26–35. doi: 10.1310/hct0901-26. [DOI] [PubMed] [Google Scholar]
- 38.Drummond M. Methods for the Economic Evaluation of Health Care Programs. 3rd. New York, NY: Oxford University Press; 2005. [Google Scholar]
- 39.Haddix AC, Teutsch SM, Corso PS. Prevention Effectiveness: A Guide to Decision Analysis and Economic Evaluation. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 40.Durovni B, Saraceni V, Moulton LH, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster-randomised trial. Lancet Infect Dis. 2013;13:852–858. doi: 10.1016/S1473-3099(13)70187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Garcia ML, Valdespino-Gomez JL, Garcia-Sancho C, et al. Underestimation of Mycobacterium tuberculosis infection in HIV-infected subjects using reactivity to tuberculin and anergy panel. Int J Epidemiol. 2000;29:369–375. doi: 10.1093/ije/29.2.369. [DOI] [PubMed] [Google Scholar]
- 42.Lugada ES, Watera C, Nakiyingi J, et al. Operational assessment of isoniazid prophylaxis in a community AIDS service organisation in Uganda. Int J Tuberc Lung Dis. 2002;6:326–331. [PubMed] [Google Scholar]
- 43.Khawcharoenporn T, Apisarnthanarak A, Manosuthi W, et al. Isoniazid preventive therapy and 4-year incidence of pulmonary tuberculosis among HIV-infected Thai patients. Int J Tuberc Lung Dis. 2012;16:336–341. doi: 10.5588/ijtld.11.0402. [DOI] [PubMed] [Google Scholar]
- 44.Charalambous S, Grant AD, Innes C, et al. Association of isoniazid preventive therapy with lower early mortality in individuals on antiretroviral therapy in a workplace programme. AIDS. 2010;24(suppl 5):S5–S13. doi: 10.1097/01.aids.0000391010.02774.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cain KP, McCarthy KD, Heilig CM, et al. [Accessed January 21, 2014];An algorithm for tuberculosis screening and diagnosis in people with HIV. Available at: http://www.nejm.org/doi/suppl/10.1056/NEJMoa0907488/suppl_file/nejm_cain_707sa1.pdf. Supplemetary Appendix.
- 46.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Sadr WM, Perlman DC, Denning E, et al. A review of efficacy studies of 6-month short-course therapy for tuberculosis among patients infected with human immunodeficiency virus: differences in study outcomes. Clin Infect Dis. 2001;32:623–632. doi: 10.1086/318706. [DOI] [PubMed] [Google Scholar]
- 49.Egger M, May M, Chêne G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 50.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 51.May M, Sterne JA, Sabin C, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21:1185–1197. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palella FJ, Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–626. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 53.When To Start C, Sterne JA, May M, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta A, Wood R, Kaplan R, et al. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7:e34156. doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 56.Collaboration HC. Impact of antiretroviral therapy on tuberculosis incidence among HIV-positive patients in high-income countries. Clin Infect Dis. 2012;54:1364–1372. doi: 10.1093/cid/cis203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Girardi E, Sabin CA, d’Arminio Monforte A, et al. Incidence of Tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis. 2005;41:1772–1782. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- 58.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 59.Moreno S, Jarrin I, Iribarren JA, et al. Incidence and risk factors for tuberculosis in HIV-positive subjects by HAART status. Int J Tuberc Lung Dis. 2008;12:1393–1400. [PubMed] [Google Scholar]
- 60.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schim van der Loeff MF, Jaffar S, Aveika AA, et al. Mortality of HIV-1, HIV-2 and HIV-1/HIV-2 dually infected patients in a clinic-based cohort in the Gambia. AIDS. 2002;16:1775–1783. doi: 10.1097/00002030-200209060-00010. [DOI] [PubMed] [Google Scholar]
- 62.Quantimed, Pharmaceutical Quantification and Cost Estimation Tool. Arlington, TX: Center for Pharmaceutical Management; 2009. [Google Scholar]