Abstract
The shedding of the old exoskeleton that occurs in insects at the end of a molt (a process called ecdysis) is typically followed by the expansion and tanning of a new one. At the adult molt, these postecdysial processes include expanding and hardening the wings. Here we describe recent advances in understanding the neural and hormonal control of wing expansion and hardening, focusing on work done in Drosophila where genetic manipulations have permitted a detailed investigation of postecdysial processes and their modulation by sensory input. To place this work in context, we briefly review recent progress in understanding the neuroendocrine regulation of ecdysis, which appears to be largely conserved across insect species. Investigations into the neuroendocrine networks that regulate ecdysial and postecdysial behaviors, will provide insights into how stereotyped, yet environmentally-responsive, sequences are generated, as well as into how they develop and evolve.
Keywords: Ecdysis, Bursicon, Wing Expansion, Drosophila, Neuroendocrine, Neuroethology
I. Introduction
Insects wear their skeletons on the outside of their bodies. While this adaptation shields soft tissues and prevents dehydration, it presents severe challenges to growth. To grow, insects must periodically molt to shed their exoskeletons and form new ones. The fact that they flawlessly and repeatedly perform this feat during development belies its complexity, as it requires first digesting the exoskeleton from the current stage and producing one for the next. Shedding the remains of the old exoskeleton during ecdysis involves first breaking the links between it and the body, and then extricating not only the body—which may have multiple, articulated appendages--but also the cuticular linings of the old trachea and parts of the gut. During this delicate process insects are extremely vulnerable, covered only by the new exoskeleton, which is typically soft and permeable. Therefore, insect survival generally requires the rapid hardening and melanization of the new exoskeleton in expanded form. At the adult molt, wings, if present, must also be expanded prior to imminent hardening. Thus, successful passage from one developmental stage to the next requires a precise concatenation of multiple behavioral and physiological events that occur during and after the shedding of the old cuticle. The behavioral aspects of this progression are collectively referred to as the “ecdysis sequence.”
Over the course of the last century, the ecdysis sequences of numerous insects have been characterized in detail (for general reviews see 47, 64). For a given species at a given developmental stage, the ecdysis sequence is usually quite stereotyped, though it may include mechanisms for pausing or extending the performance of behavioral steps in response to sensory input. Although the ecdysis sequences of different species exhibit considerable diversity, investigation of the underlying hormonal and neural mechanisms of their regulation has revealed considerable similarity at the molecular and cellular levels. The effort to understand the hormonal orchestration of ecdysis sequences has been a rich source of insight into how nervous systems generate and organize their motor output into coherent steps to achieve adaptive goals.
A relatively comprehensive model of the neuroendocrine basis of larval ecdysis in the tobacco hornworm, Manduca sexta, emerged from work carried out in the closing decades of the last century (19). More recent work has focused on understanding the regulation of ecdysis sequences in Drosophila, which offers a broader palette of tools for molecular genetic manipulation. While results from Drosophila have revealed both similarities and differences in the regulation of ecdysis between the fly and Manduca, they have specifically deepened our understanding of the control of postecdysial behaviors. This is due in part to the molecular genetic characterization in Drosophila of the hormone bursicon--which is now recognized to regulate both postecdysial physiology and behavior--and in part to a shift in focus to the adult molt, where ecdysis and postecdysis behavior assumes what is perhaps its most spectacular form: following its emergence from the pupal case the metamorphosed adult completes its transformation by expanding its new wings. Adult ecdysis in Drosophila also offers an exemplar of how plasticity can be introduced into the execution of a stereotyped motor program because the onset of wing expansion can be delayed--sometimes for hours depending on environmental conditions (43).
In this review, we focus on the recent work in Drosophila, with particular attention to studies of the neuroendocrine network underlying wing expansion. Because the structure and function of this network can only be meaningfully understood in the context of the broader network governing the entire adult ecdysis sequence in Drosophila, we also briefly review what is known about the core molecular and cellular substrates of ecdysis sequences and their regulation, incorporating recent results from Drosophila and other insects.
II- Insect ecdysis
1- Behavioral components of ecdysis sequences
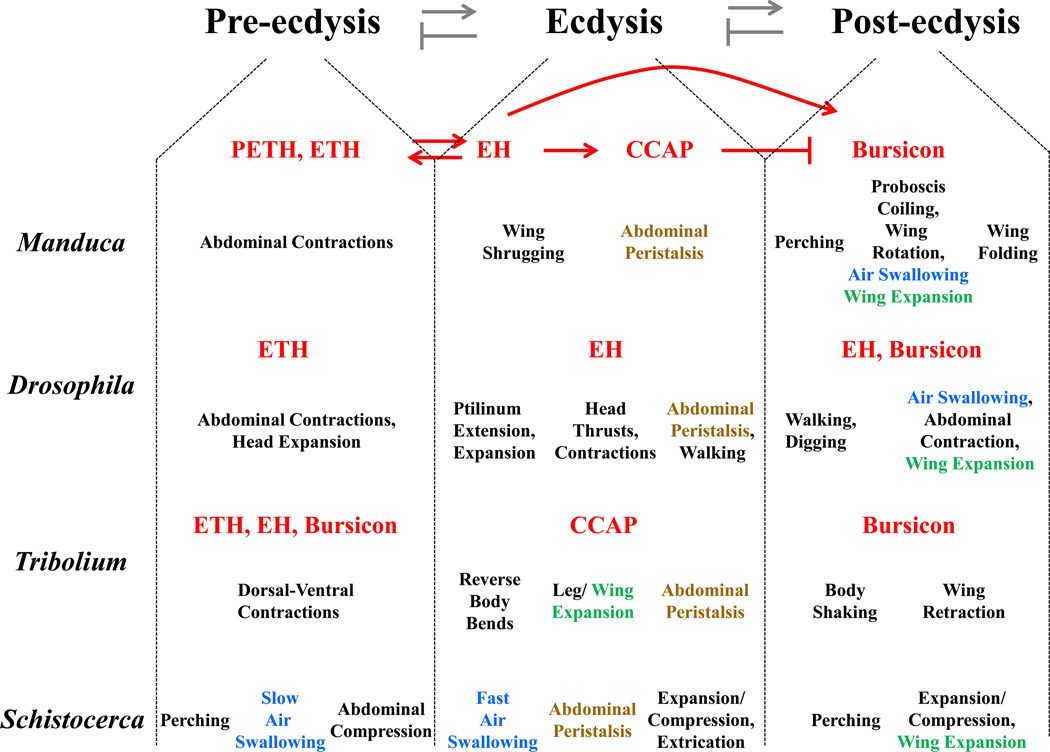
The behavioral sequences used by insects to replace the exoskeleton of one developmental stage with a new one are typically divided into three major phases (Fig. 1). The first, called pre-ecdysis, consists of preparatory behaviors, which likely function to loosen the old exoskeleton; this phase may be accompanied by the swallowing of air (or sometimes water), which raises internal pressure and stretches the old exoskeleton. Pre-ecdysis is followed shortly by the ecdysial phase (ecdysis proper), during which the old exoskeleton is shed. This phase may also be aided by the ingestion of air to expand the new exoskeleton and help rupture the old, but always includes a burst of anterior-directed peristalses that serve two purposes. On the one hand, they cause pressure to be exerted on the anterior region of the old cuticle, leading it to split (or open further, for some species) along a dorsal seam, partially freeing the animal from its straitjacket. On the other hand, these anterior-directed peristalses help push the animal out of the old exoskeleton. Depending on the stage and body plan, this phase may also include an ordered series of individual motor programs for extricating the animal’s various appendages and trachea. This phase results in the complete freeing of the insect, and is followed by the postecdysial phase during which the new exoskeleton is expanded, hardened, and pigmented. The mechanical means of expansion, as noted before, typically involves air-swallowing, which is often accompanied by abdominal contractions and serves to swell the body to its greatest size and/or expand the wings before they are permanently hardened.
Figure 1. Adult Ecdysis Sequences of Several Insects.
The behaviors and hormones (red) associated with the canonical phases of the adult ecdysis sequence are shown for the hawkmoth (Manduca), fruitfly (Drosophila), red flour beetle (Tribolium), and locust (Schistocerca). Interactions between the key hormones as established for Manduca are shown above that sequence. While abdominal peristalsis (brown) is invariably associated with ecdysis proper for all sequences, another critical motor pattern, namely air swallowing (blue), may occur at different phases, as does wing expansion (green). Similarly, there is a common requirement for ETH at pre-ecdysis, but the phases at which other hormones are required appears to vary and their actions may overlap. Arrows indicate activation; blocked lines indicate inhibition. Based on data from sources referenced in the text.
Although the subdivision of the ecdysial sequence into these three phases is convenient, we caution that it hides a number of complexities. First, the phases are rarely discrete and it can be difficult, if not impossible, to decide where the motor subroutines of one phase end and those of the next start. Furthermore, phases may overlap: for instance, in the bug, Rhodnius prolixus, air swallowing begins during pre-ecdysis at the adult molt, and expansion of the body and wings (i.e. postecdysis) proceeds concurrently with the shedding of the old exoskeleton (i.e. ecdysis) (1). Second, a chronologically sequential view of ecdysis sequences can imply that each neuropeptide hormone involved in the control of a sequence (see below) functions only during a specific phase, when in fact it is more likely that several neuropeptides cooperatively cause each phase of motor programs, with some neuropeptides contributing to several phases. For example in the red flour beetle, Tribolium, signaling by the neurohormone bursicon is required to modulate the strength of pre-ecdysis behaviors at adult emergence as well as to insure proper postecdysial behavior and wing expansion (2, Fig. 1). Perhaps because of these various complexities, there is little agreement regarding the precise definition of each phase in different insects, which complicates comparative studies. Nonetheless, and despite the behavioral differences observed between species, investigation of the hormonal basis of ecdysis sequences has revealed considerable commonality in the signaling molecules and cell groups that participate in this process across different insect species.
2- The neural and hormonal control of ecdysis sequences
The endocrine control of ecdysis has been extensively reviewed elsewhere, and here we endeavor to provide only a brief overview, focusing on some recent advances. Readers interested in comprehensive reviews may refer to those cited above, as well as (52, 62).
Our primary understanding of the endocrine mechanisms that control ecdysis sequences stems from work done in Lepidoptera (especially the moth, Manduca sexta). More recently, strong contributions have come from work using insects amenable to genetic manipulations, principally Drosophila melanogaster, but also the beetle, Tribolium castaneum, and the silk moth, Bombyx mori. Four hormones have been implicated in the control of Manduca ecdysis sequences: Ecdysis Triggering Hormones (including Pre-ecdysis Triggering Hormone, PETH, and Ecdysis Triggering Hormone, ETH), Eclosion Hormone (EH), Crustacean Cardioactive Peptide (CCAP) and bursicon. Other factors may also play a role, including kinins, myoinhibitory peptides (MIPs), FMRFamide (31, 32), and corazonin (30), but the extent to which they do so in different insects, as well as their exact roles, are poorly understood.
Pre-ecdysis
In Manduca, the initial trigger of the ecdysis sequence is the release of ETH (and PETH) from peripheral endocrine “Inka” cells. Through a reciprocal positive feedback loop between ETH and centrally produced EH, both neuropeptides are then massively released, providing an unambiguous endocrine signal that irreversibly commits the animal to ecdysis. Interestingly, ecdysis normally occurs only when the old cuticle can be shed and the new one is ready. This critical coordination of ecdysis with the stage of progression through the molt is accomplished at least in part by modulating the sensitivity of the nervous system to ETH, so that it responds only when the molting hormones fall below a threshold level that is reached at the very end of the molt.
In Manduca, the neuropeptide corazonin first signals the fall in molting hormones (30). This is not the case in Drosophila, where another, as yet unknown, endocrine signal may participate in setting ecdysis in motion at the correct developmental time. In addition to molt-associated factors, other inputs can influence the exact time of occurrence of ecdysis. These include proprioceptive inputs, as occurs in species such as crickets, where the start of ecdysis can be delayed until a suitable perch is found. Another important input is the circadian clock, which, depending on species and stage, can restrict ecdysis to a specific time of day. How this is accomplished is best understood in the bug, Rhodnius prolixus, where circadian regulation of molting hormone production causes the molt to end only within a permitted interval (49).
Ecdysis
Following the surge of ETH, the accompanying release of EH within the central nervous system (CNS) is believed to cause CCAP release; CCAP then turns on the ecdysis motor program. The responses of isolated CNS preparations to CCAP support this interpretation, in that this neuropeptide can turn on the fictive ecdysial motor program when applied to an isolated CNS in vitro; it also shuts off the pre-ecdysis motor program if added after an ETH challenge (24). Following ecdysis, the secretion of bursicon from a subset of CCAP-expressing neurons in the abdominal ganglia whose activity is delayed by descending inhibition, regulates the postecdysial phase (52, 53).
This sequential model, in which ETH, EH, CCAP, and bursicon are released more or less stepwise and act largely independently to generate different components of the ecdysis sequence, has considerable explanatory power (Fig. 1). However, it has been challenged by recent studies that suggest greater complexity. First, in Manduca (31), as well as Drosophila (32), the ETH receptor (ETHR) is expressed not only in the neurons that produce EH, but also in those that express CCAP and bursicon and in neurons that express other neuropeptides implicated in the control of the pupal ecdysis sequence. The staggered release of these neuropeptides is thus somehow accomplished by the simultaneous activation by ETH of all these neurons. A mechanism in which inhibitory delay pathways are co-activated to regulate the pattern of release has been proposed, and anatomical lesions provide some evidence for such pathways, but none has been identified at the cellular level (23, 63).
In addition, recent results from Drosophila suggest that different neuropeptides may exert overlapping, rather than strictly sequential, actions. Thus, flies lacking EH- and CCAP-expressing neurons have ecdysis sequence defects that are more severe than those seen in animals lacking either the EH or the CCAP neurons alone(8). Similarly, flies lacking the neuropeptides CCAP and bursicon are more impaired than those lacking either neuropeptide alone (33). Non-sequential models of neuropeptide action are also consistent with the observation that some peptides appear to participate in more than one phase of an ecdysis sequence, as described above for bursicon signaling in Tribolium (2).
Recent findings also suggest that the mechanisms that regulate ecdysis sequences may vary across species, with the same neuropeptide playing different roles in different insects. For example, PETH, which causes only pre-ecdysis in Manduca, can trigger the entire ecdysial sequence in the silkmoth, Bombyx mori (65).
Postecdysis
The sequential model described above was largely developed to explain the larval ecdysis sequence in Manduca, which does not end in overt expansion of the new cuticle and does not depend critically on bursicon function. The inclusion of bursicon in the model is based instead on evidence that implicates this neurohormone in controlling wing expansion at adult ecdysis (53). Postecdysis in the adult is, in fact, quite different from that in the caterpillar, as is the ecdysis phase, which includes, in addition to the core behavior of anterior-directed peristalses other motor components, such as the “shrugging” that accompanies emergence (29, 53). These observations raise the question of whether the hormonal control of the ecdysis sequence remains the same across developmental stages.
Although considerable evidence indicates conserved roles for both ETH and EH across developmental stages in Manduca (19), the patterns of expression of both CCAP and bursicon within the nervous system change, suggesting modified roles of these neuropeptides in ecdysis sequence control (11, 20). Studies from Drosophila provide more direct evidence for such changes in function. It has been demonstrated that genetically ablating CCAP-expressing neurons in the fly causes only minor defects at larval ecdyses, but causes severe defects at pupal ecdysis (41). This developmentally acquired sensitivity to CCAP neuron ablation is conferred by the addition of late-differentiating CCAP-expressing neurons just prior to pupation. Unlike embryonically differentiating CCAP-expressing neurons, this late-differentiating set is necessary for pupal ecdysis (57). A similar set of neurons has been described in Manduca (12), suggesting that this mechanism may be conserved.
As indicated by this brief discussion, research on Manduca has produced a useful model of the neuroendocrine basis of an ecdysis sequence and of how it might progressively generate distinct behavioral phases. The control of the postecdysial phase is the least well-developed element of this model and our emerging understanding of how this phase is controlled, at least at adult ecdysis in Drosophila, will be taken up in the next section. As should be clear from the foregoing, however, numerous challenges remain in the effort to achieve a complete understanding of the neural and hormonal determinants of ecdysis sequences in general. These will be discussed in the final section of this review.
III. Insect postecdysis
1. Postecdysial behavior and wing expansion
In insects with a rigid exoskeleton the postecdysial phase invariably requires expansion of the new cuticle prior to hardening. As noted above, expansion entails swallowing air or water to increase internal hydrostatic pressure so that the new cuticle hardens at a size that can accommodate further growth. After adult ecdysis, winged insects must likewise expand and harden the cuticle of the thorax, in order to provide the rigid supports for flight. More importantly, the wing pads themselves, which have formed during development, must be expanded to their full size and hardened to permit flight. Infusion of hemolymph into the wing pads to expand them relies on the same increase in internal pressure.
The often spectacular nature of wing expansion and the fact that insects perform it while sedentary—rendering it readily observable—have made this process a commonly-observed display of postecdysial behavior. Although details of the behavior—and in some cases even the underlying motor patterns—have been described in numerous insects, the hormonal and neural basis of wing expansion has been most extensively studied in flies (for a comprehensive review, see 14), with most of our current knowledge derived from recent work in Drosophila.
Drosophila is an attractive model for the study of wing expansion for two reasons. The first is that the powerful genetic tools available in this organism make possible selective alterations of hormonal and cellular function at the level of single genes and neurons (48, 59). The second reason is behavioral. As described in greater detail below, the program for wing expansion in the fruitfly is under the control of not only of hormonal factors, but also environmental conditions: flies in adverse environments will delay expansion to search for better circumstances (43). Just as in the circadian gating of eclosion described above, the ability to delay wing expansion in Drosophila is an example in which the nervous system integrates intrinsic and extrinsic signals to make a simple choice between two behavioral states (i.e. exploration vs. expansion). Postecdysial behavior in Drosophila thus affords an opportunity to study both the neuroendocrine mechanisms used to elaborate a behavioral sequence, and the plasticity in these mechanisms that allow for behavioral choices within the sequence, so that the animal can respond flexibly to environmental circumstances.
2. Brief History of Investigations of Postecdysial Processes
Over the course of the last century, adult ecdysis and wing expansion have attracted the interest of several disciplines, including developmental biology, endocrinology, and neuroethology. Developmentally-oriented biologists interested in the mechanisms of growth and metamorphosis first established the basic phenomenology of adult emergence early in the 20th century in largely descriptive studies that combined observation with simple manipulations, such as Eidmann’s demonstration that pricking the air-filled gut of Drosophila obscura prevents expansion of the wings (17). Fraenkel’s 1935 investigation of wing expansion and cuticle tanning in the blowfly, Calliphora, set the stage for endocrinological investigations (21). This study demonstrated that blowflies will delay wing expansion and cuticle tanning in response to certain environmental manipulations, but that they will immediately initiate these processes upon brief exposure to ether, an indication that their execution is inhibited during the delay period. Fraenkel and Hsiao (22), and independently Cottrell (10), subsequently established that postecdysial processes--with a principal focus on cuticle tanning—required a blood-borne factor. The appearance of this “darkening factor” in the hemolymph occurred only after eclosion and was delayed in response to environmental perturbations. Perhaps most excitingly, the factor—or one sufficiently similar that it induced tanning in blowflies—could be detected in the blood of insects of other orders at the time of ecdysis. Fraenkel and Hsiao (22) called this conserved hormone, “bursicon.”
Although it took an additional four decades to establish the molecular identity of bursicon, bioassays for this hormone showed that tanning activity was localized to the nervous system in a variety of insects, with the largest reservoir invariably found in the abdominal ganglia. The timecourse of bursicon depletion from this region suggested that it was the principal source of bursicon released into the hemolymph (40, 51). Because decapitation or neck ligation immediately after eclosion prevented such release, it seemed likely to require a signal from the head, and more refined brain lesions performed by Fraenkel and Hsiao (22) on the fleshfly, Sarcophaga, demonstrated that release of bursicon was indeed under neural control.
The bursicon-containing blood of newly eclosed insects was also used to characterize bursicon’s physiological functions and mechanism of action (for review of this work see 45). Several lines of evidence indicated that bursicon acted to tan the cuticle by elevating cAMP in the epidermis (13). Additionally, various investigators demonstrated that bursicon-bioactive blood acted not only to initiate the post-expansion processes of melanization and sclerotization, but also to increase the extensibility of the cuticle prior to expansion (10, 46)--a result that has been confirmed more recently with synthetic bursicon (11). Despite a strong correlation between bursicon release into the hemolymph and wing expansion, injections of bursicon-containing hemolymph were generally ineffective in inducing wing expansion, and the role of bursicon in behavioral control remained speculative. Tellingly, the authoritative review by Denlinger and Zdarek (14) of behavioral studies done in higher Diptera mentions bursicon briefly and only in the context of cuticle tanning.
In parallel with the work in flies, the endocrine study of ecdysis in Lepidoptera was spurred by the ground-breaking discovery of the “eclosion hormone” (i.e. EH) by Truman and Riddiford (54) and the subsequent intensive investigation of ecdysis in the tobacco hornworn, Manduca.(for comprehensive review see 19). The results of this work, which focused initially on adult ecdysis, strongly paralleled those obtained in flies, suggesting substantial conservation of the underlying mechanisms. As in flies, Manduca released bursicon into the hemolymph from sites in the abdominal ganglia after eclosion, and release could be delayed by environmental perturbation. In addition, bursicon release could be delayed by confinement and was under descending neural control. Two distinct components of control were identified, one located in the subesophageal ganglia and the other in the brain (53). The brain was shown to be the source of EH, and direct evidence that EH participated in regulating bursicon release came from the finding that bursicon bioactivity localized to cells in the abdominal ganglia that were EH targets (18). The dependence of bursicon release on EH thus provided an elegant mechanism for guaranteeing that expansion and hardening of the new cuticle would strictly follow shedding of the old one.
It was subsequently found that homologs of the neurons targeted by EH, which included neurons that expressed CCAP in addition to bursicon, could be found in diverse insect species, where they likewise responded to this hormone (20). This finding again underscored the generality of the regulatory mechanisms involved, and as indicated in the preceding section, numerous lines of evidence suggested that the hormonal regulation of ecdysis in Manduca was similar at all molts, and led to the proposal that it was conserved in many, if not all insects. A generalized model emerged for the control of ecdysis sequences by the hormones EH, ETH, CCAP, and bursicon, which provided an elegant neuroendocrine basis for the temporal progression of these sequences.
A key feature of the work in Manduca, with its elaboration of a detailed cellular as well as molecular model of ecdysis control, was that it broadened the emphasis of ecdysis research from studies of development and hormones to the investigation of the neural basis of behavior. An explicitly neuroethological agenda also motivated the characterization of the adult ecdysis sequences of two other insects in the late 1970’s, namely the cricket, Teleogryllus oceanicus, and the desert locust, Schistocera gregaria. The adult ecdysis sequences of these two insects and the neurophysiological correlates of the underlying motor patterns were comprehensively characterized by Carlson for the cricket (5) and Hughes for the locust (27). These studies, which represent the gold standard in the ecdysis field for quantitative behavioral analysis, revealed the importance of sensory feedback mechanisms in providing checkpoint control for the progression of the sequence and in regulating the duration of specific behavioral steps. Of particular interest from the standpoint of postecdysial behavior was the observation that wing expansion in both insects depends on the extrication of a specific body part from the old exoskeleton: the head, in the case of the locust (28), and the cerci in the cricket (4). The identity of the relevant sensory neurons that signal the completion of extrication remains unknown and despite the richness of behavioral and neurophysiological detail in the original analyses of locust and cricket ecdysis sequences, neither analysis led to subsequent similarly detailed investigation of the cellular and molecular substrates of behavior, outside of several preliminary studies of the neural and hormonal basis of air swallowing, which in both animals commences prior to ecdysis (6, 61).
Beginning in the late 1990’s, the investigation of ecdysis sequences began to benefit from the power of genetic tools available in Drosophila, which was adopted as a new model organism for ecdysis studies. Research on postedysial processes in Drosophila during the last decade is the principal focus of the next section.
3. Genetic and Cellular Investigation of the Bursicon Pathway in Wing Expansion and Tanning
Postecdysial behavior in Drosophila
Although the ecdysis sequences of all developmental stages have been studied in Drosophila, postecdysis has mostly been studied following adult ecdysis (also called adult emergence or eclosion). Unlike the ecdysis sequences of the soft-bodied larva, which do not terminate with an evident expansion or tanning of the cuticle, the adult ecdysis sequence must conclude with the expansion and hardening of both the adult cuticle and the wings if the fly is to successfully survive and reproduce in the wild. The behaviors that follow eclosion are therefore directed at facilitating this conversion to the hard-bodied adult form, and in this respect the Drosophila adult ecdysis sequence resembles that of many other insects. In other ways, however, the Drosophila adult ecdysis sequence is unusual.
Unlike many other insects, including most Diptera, adult Drosophila do not rupture their pre-adult integument with the aid of swallowed air, but use instead a specialized, balloon-like structure in the head, called the ptilinum. The emerging insect repeatedly contracts thoracic and abdominal muscles to force hemolymph into the ptilinum, which rhythmically expands, thrusting open the anterior end of the pupal case. This creates an exit path for the adult, which initially moves forward by ecdysial peristalsis and then by walking, as the legs become free.
The uncoupling of ecdysis from wing and cuticle expansion in Drosophila and other cyclorraphous Diptera makes possible the second unusual feature of the adult ecdysis sequence in these flies, which is the intercalation of an environmentally-sensitive exploratory phase between ecdysis and expansion. That is, depending on the conditions under which they eclose, these flies may perform an extended locomotor search for an appropriate perch on which to initiate expansion. If the fly is in a spacious container and is unperturbed, it often simply perches after eclosing and shortly thereafter initiates expansion. However, if the fly is confined or perturbed, it will continue to walk about.
In addition to walking, exploring flies that encounter gaps in the walls of their chamber, engage in a behavior called “digging,” which consists of two motor patterns. In the fleshfly, one digging pattern is identical to ecdysial peristalsis; the other is a variant of this behavior that is useful for moving obstacles and includes bouts of ptilinum extension (44). Digging behavior is also observed in the fruitfly, and in both blowflies and fruitflies the duration of the exploratory phase correlates inversely with the size of the confining chamber. In a chamber only several times longer than the length of the fly, all Drosophila will eventually initiate expansion, though often after a delay of hours (43). Even under the extreme condition of burial in sand, about 25% of blowflies will also initiate wing expansion and tanning after extended digging (9).
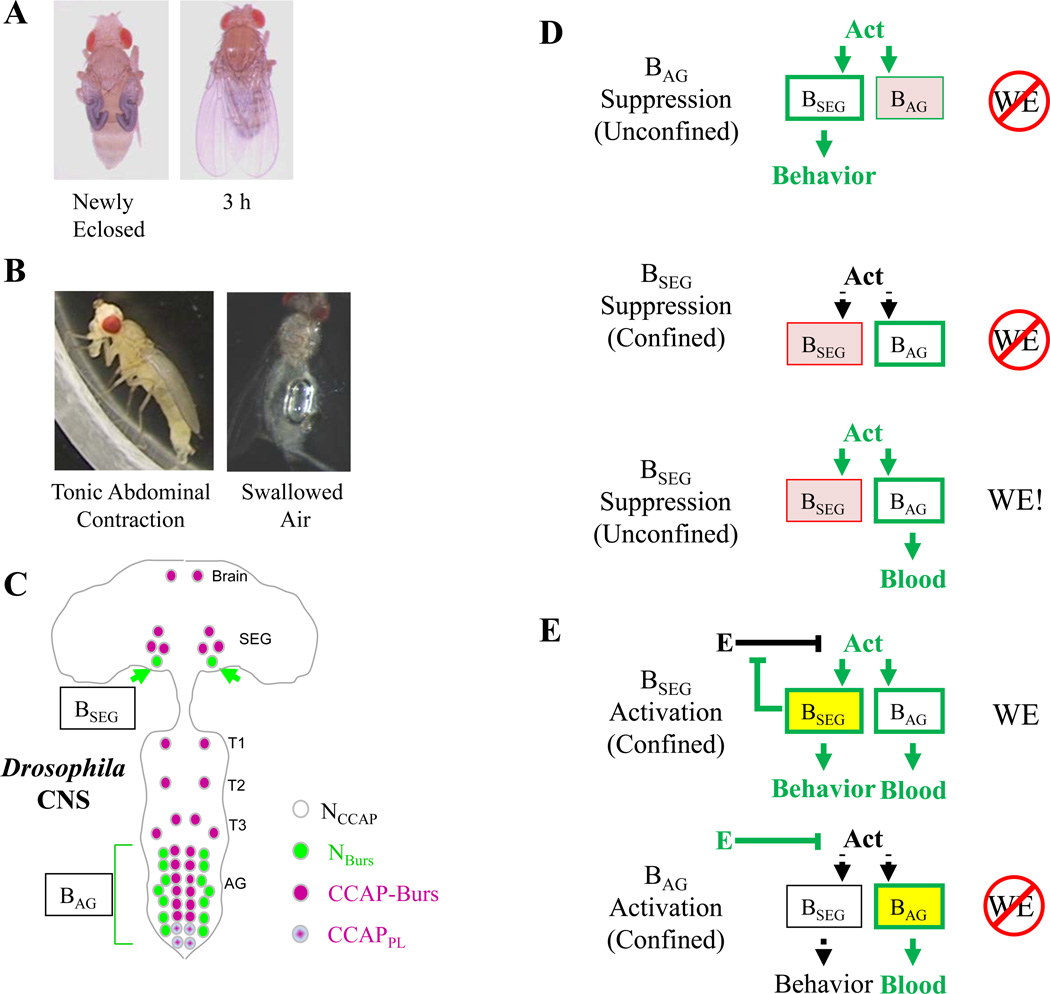
The exploratory phase ends when the fly selects a perch and then remains relatively sedentary, engaging in intermittent bouts of grooming. In Drosophila, this sedentary phase shifts to a phase of active expansion with the initiation of two motor patterns. The first is the activation of the pharyngeal pump and the swallowing of air into the gut, usually accompanied by an extension of the proboscis; the second is a slowly developing extension and downward flexion of the abdomen (43, Fig. 2A,B). The tonic abdominal contraction typically lasts about 15 min and is accompanied by expansion of the thorax and wings. The wing pads unfold and are briefly groomed by the hindlegs at which time they become angled slightly to the side and then slowly return to the midline as the phase ends. Pigmentation of the cuticle then develops slowly after this time and it is generally well-advanced within three hours after wing expansion is completed (Fig. 2A).
Figure 2. Control of Postecdysial Processes by Bursicon in Drosophila.
(A) Comparison of wing morphology and cuticle tanning in a newly eclosed vs. three hour old adult. (B) Left, the tonic abdominal contraction and extended proboscis characteristic of a fly in the process of expanding its wings. Right, the exposed bubble of swallowed air in the gut of a fly that has just expanded. (C) Distribution of subsets of CCAP-expressing neurons (NCCAP) in the nervous system of the fly. NBurs, the subset of NCCAP that robustly expresses bursicon; CCAP-Burs, the subset that does not express bursicon; CCAP-PL, a subset that is required for pupal ecdysis and wing expansion as described by Ververysta and Allan (PNAS, 2012). (D) The effects of targeted suppression of either the BAG (top) or BSEG (middle, bottom) in either confined or unconfined environments. Red indicates selective suppression of activity; green indicates which cells and pathways are active in the indicated environment. “Act” represents the putative “activation network” responsible for coordinate activation of the two sets of bursicon-expressing neuron, as described in the text. In confined environments, this network is initially inhibited (indicated by black). The consequences of the manipulation on behavior, bursicon secretion into the blood, and wing expansion are indicated. As described in the text, flies will generally expand their wings in unconfined environments when the BSEG are suppressed, despite the impairment of normal wing expansion behaviors. (E)The effects of targeted activation (yellow) of either the BSEG (top) or BAG (bottom) in confined environments. As described in the text, BSEG activation under these circumstances robustly induces wing expansion and all associated behavioral and neuroendocrine processes, perhaps by suppressing inhibitory environmental input (E) to the activation network as suggested by Luan et al. (J Neurosci, 2012). The interactions between E, Act, and the BSEG constitute a positive feedback loop, which may mediate the decision to expand in that as a fly moves into a better environment, inhibition from E will attenuate and activity of the Act network will increase, thus stimulating the BSEG. Increasing BSEG activity will further suppress E and the cycle will tend to robustly drive the behavioral transition to wing expansion.
SEG, subesophageal ganglion; T1-T3, thoracic neuromeres; AG, abdominal ganglion; BSEG, bursicon-expressing neurons of the SEG; BAG, bursicon-expressing neurons of the AG
The postecdysial behavioral patterns and accompanying physiological changes just outlined above for Drosophila have been described in general terms in several studies (3, 34, 43), and are broadly similar to previous such descriptions for larger cyclorraphous Diptera (14). An the exception is the mechanism of abdominal constriction, which in blowflies and fleshflies involves rhythmic longitudinal abdominal pulses, rather than tonic contraction. Despite this difference, many of the conclusions drawn from studies carried out in blowflies and fleshflies are likely to apply to the fruitfly as well.
In the larger flies, changes in hydrostatic pressure caused by air swallowing and abdominal contractions have been measured directly, and experimental abolition of air swallowing and abdominal contraction have confirmed the requirement of these motor patterns for expansion (see 14). In addition, the responses of flies to direct manipulations of internal pressure at different times relative to wing expansion have assayed changes in cuticle plasticity (10), and behavioral and pharmacological manipulations have been used to examine the availability of various motor patterns at different points in the ecdysis sequence. Finally, it has been shown in Sarcophaga, and is likely to be true in Drosophila, that muscles in the ptilinum are kept contracted during expansion to prevent hemolymph from being pushed into the head rather than the thorax and wings (60).
Genetic dissection of the bursicon pathway in Drosophila
Drosophila, by virtue of its sequenced genome, large collection of characterized mutants, and amenability to genetic manipulation, played a critical role in facilitating the molecular identification and characterization of bursicon. This work has been reviewed elsewhere (25), and we briefly summarize here only the chief results. Following the heroic purification of bursicon bioactive material from nearly 3000 cockroach nervous systems, five short tryptic peptide fragments were obtained, four with sequences showing credible homology to predicted coding sequences within a previously unidentified gene from Drosophila. This gene was originally called bursicon, and has since been called burs and bursicon-α; we refer to it here as burs. Five strains of fly with deficits in wing expansion and adult cuticle tanning that were generated in an earlier mutagenesis screen were found to have mutations in this gene (15).
Identification of the burs gene led, in turn, to the identification of a second, homologous gene (i.e. partner-of-bursicon, pburs, and bursicon-β-- here called pburs), which had a sequence motif similar to that of the fifth tryptic peptide from the cockroach (36). Null mutant alleles of this gene were likewise found to cause wing expansion and cuticle tanning deficits and failed to genetically complement an existing wing expansion mutant known as pupal1 (33). In addition, the protein products of burs and pburs were shown to heterodimerize to form, bursicon, which binds the G-Protein Coupled Receptor encoded by a Drosophila gene known as rickets (rk) (36, 38). Consistent with the earlier identification of cAMP as a second messenger of bursicon signaling, binding of the bursicon heterodimer to the RK protein stimulated cAMP production in heterologous cells. Flies with mutations in the rk gene also had expansion and tanning deficits, and Baker and Truman (3) presciently predicted from their work on these mutants that rk might encode the gene encoding the bursicon receptor. Importantly, this latter work demonstrated that rk mutants, in addition to not tanning, exhibited significant deficits in postecdysial behavior, failing to either swallow air or execute the sustained contraction of the abdomen characteristic of the expansional phase.
Identification and characterization of the neural substrates of bursicon action
During the interval that Drosophila has been used for the genetic characterization of bursicon, and more generally of ecdysis, it has also grown in power as a model system for analyzing the neural circuitry underlying behavior. Increasingly sophisticated genetic techniques available in the fruitfly allow precise targeting of transgene expression to neurons of interest to alter their properties (56). Such manipulations have been used to identify neural substrates of ecdysis and wing expansion. The effort to map the neuroendocrine network underlying wing expansion, in particular, has produced results that support and extend the conclusions drawn from the analysis of mutants in the bursicon signaling pathway.
A starting point in the dissection of the wing expansion network was the study by Park et al. (41), in which a transgene encoding the cell death gene reaper was specifically targeted to CCAP-expressing neurons to ablate them during development. This manipulation resulted in substantial death at pupal ecdysis, and all the animals that survived to adulthood showed severe defects in wing expansion and post-eclosion tanning. Subsequent analysis showed that CCAP was dispensable for the production of both phenotypes, a result that is surprising given the requirement for CCAP in adult ecdysis in Tribolium (2) and its ability to induce ecdysial, and terminate pre-edysial, motor output in isolated Manduca larval CNS preparations (24). However, null mutant alleles for both CCAP and pburs in Drosophila (33)--as well as targeted ablation of both CCAP- and EH-expressing neurons (8)--suggests that CCAP may have redundant functions with other factors in ecdysis in the fruitfly.
While CCAP is not required for wing expansion in the fly, bursicon, as noted above, clearly is, and the observation that ablation of CCAP-expressing neurons impairs wing expansion derives from the fact that both bursicon subunits are expressed in a subset of CCAP-expressing neurons in the newly eclosed adult (35, Fig. 2C). This subset consists of two anatomically distinct groups, one comprised of a pair of neurons in the labial neuromere of the subesophageal ganglion (and occasionally a second pair in the maxillary neuromere), and the other comprised of seven pairs of neurons in the abdominal neuromeres (42). The abdominal neurons are segmental homologs, and the entire cell group are likely to be homologous to previously described neurons in Manduca, designated as “Cell 27” homologs (20). In Drosophila, the bursicon-expressing subsets of CCAP-expressing neurons have been variably named, but we adopt here the nomenclature of Luan et al. (35) in collectively calling the 7 pairs in the abdominal ganglion the “BAG,” and the subesophageal pair, the “BSEG.” Interestingly, the co-localization of bursicon and CCAP in segmentally homologous neurons in abdominal ganglia appears to be broadly conserved in insect orders and has even been found in crustacea where shedding of the old carapace is accompanied by a surge in CCAP and bursicon in the hemolymph (58).
Selective inhibition of electrical activity of either the CCAP-expressing neurons, or of only the subset that co-expresses bursicon, gives rise to phenotypes similar to those obtained by CCAP cell ablation (35, 42). In both cases, release of bursicon into the hemolymph is blocked and tonic abdominal contraction and air swallowing are eliminated in most or all animals. As in blowflies and fleshflies, bursicon release in Drosophila begins during the relatively sedentary phase prior to the onset of tonic abdominal contraction (43). The duration of this phase is sensitive to the level of suppression of CCAP neuron activity, with graded increases in suppression resulting in incrementally longer delays in the onset of expansional behaviors. This evidence is thus broadly consistent with the conclusion that bursicon is critical for initiating the behavioral aspects of wing expansion.
The behavioral changes that support wing expansion are necessarily mediated by the nervous system, whereas the somatic changes that allow the wings to expand (e.g. cuticle plasticization) must be mediated by blood-borne hormone. That the blood-borne hormone is released from the BAG, consistent with results from previously studied insects, is confirmed by several lines of evidence. First, the BAG, unlike the BSEG, project their axons to the periphery through the abdominal nerves, which contain the bulk of bursicon immunoreactivity. Extensive loss of bursicon-immunoreactivity in these nerves correlates with the appearance of bursicon in the hemolymph and with wing expansion and tanning (42). Also, selective electrical suppression of the BAG--a type of manipulation that can currently only be performed in Drosophila--inhibits bursicon release into the hemolymph, without however blocking the execution of the wing expansion motor program (Fig. 2D, top). Under these circumstances, wing expansion is impaired, likely due to ineffective plasticization of the wing cuticle by blood-borne bursicon (34).
Selective knockdown of pburs expression in the BAG by driving a pburs RNAi transgene in these neurons similarly causes wing expansion failure, without inhibiting behavior. Interestingly, both electrical suppression and pburs RNAi knockdown in the BAG can cause execution of the wing expansion motor program twice, something that has not been described in wildtype flies. This suggests that the circuitry regulating wing expansion behavior receives feedback either from the BAG themselves, or from peripheral sensors that monitor the success of bursicon release and/or expansion.
In contrast to the effects of BAG suppression, selective inhibition of electrical activity in the BSEG results in partial or complete loss of the two expansional motor patterns (34), indicating that the behavioral circuitry itself is regulated by the BSEG. Consistent with such a role, the processes of these neurons ramify widely in the central nervous system (42). Interestingly, the effects of BSEG suppression on wing expansion are profoundly dependent upon the fly’s environmental situation. Under conditions of confinement, suppression of the BSEG completely eliminates both tonic abdominal contraction and air swallowing and all flies fail to expand (34, Fig. 2D, middle). In contrast, flies allowed to expand under relatively unconfined conditions will expand their wings, but their behavior remains abnormal (Fig. 2D, bottom). Air swallowing is absent or severely reduced in most flies, and no flies tonically contract their abdomens. Instead, they execute a range of abdominal movements, which in conjunction with wing grooming by the hindlegs often successfully opens and flattens the wings. In these flies, bursicon is also appropriately released into the hemolymph by the BAG, and the cuticle tans normally after expansion. However, neither tanning, expansion, nor execution of the alternate behavioral routine for wing expansion is observed when flies are subjected to even mild perturbation.
Towards a working model for the network controlling postecdysial behavior in the fly
The results of the suppression experiments just described have a number of interesting implications for the control of wing expansion in the fly. First, the fact that the BAG can release bursicon when the BSEG are suppressed, coupled with the observation that wing expansion behaviors are executed when the BAG are suppressed, suggests that the two sets of bursicon-expressing neurons are independently controlled. Because both sets of neurons are jointly activated under normal circumstances, they are presumably coordinately regulated by a common upstream activating network (Fig. 2D; “Act”). Second, the sensitivity to perturbation and confinement of both the neuroendocrine release of bursicon and behavioral output in BSEG-suppressed flies suggests that the BSEG have a role in modulating environmental input to the wing expansion circuitry.
Luan et al. (34) have speculated that, in addition to activating the motor patterns that normally support wing expansion, the BSEG also negatively regulate environmentally-sensitive sensory signals that inhibit expansion in adverse environments. Such signals (Fig. 2E; “E”) must necessarily feed into the common network that coordinately activates both the BSEG and BAG. The implication of this model (Fig. 2E, top), then is that the BSEG participate in a positive feedback loop to regulate their own activity. An attractive feature of this model is that it provides a rationale for how the wing expansion decision is made, as described more fully in the legend of Fig. 2. Consistent with this model, direct and selective activation of the BSEG induces full wing expansion under environmental conditions that normally inhibit it (Fig. 2E, top), while selective activation of the BAG induces only bursicon release into the hemolymph (34, Fig. 2E, bottom).
The nature of the postulated “activating network” remains an open question. Obvious candidates for neurons within this pathway are those that regulate eclosion itself, which immediately precedes the postecdysial sequence. Although the hormone(s) directly responsible for eclosion in the fly have not been identified, manipulations of the EH-expressing neurons have been shown to cause wing expansion deficits, and McNabb et al. (37) have suggested that these neurons participate in activating the bursicon-expressing neurons. This proposal is consistent with the observation that in most insects EH appears to activate CCAP-expressing neurons (20). However, Drosophila appears to be an anomaly in this regard and currently there is only negative evidence for regulation of bursicon-expressing neurons by EH. The recent identification of an EH receptor from Oriental fruitflies (7), which has an ortholog in Drosophila, may help resolve this issue. It is also possible that CCAP-expressing neurons that do not express bursicon regulate the activity of the bursicon-expressing neurons. This idea is a feature in models of bursicon release in Manduca (52).
Finally, ETH also plays a known role in activating bursicon-expressing neurons at pupal ecdysis (32) and is capable of inducing the adult ecdysis sequence in Drosophila. Given ETH’s apparent master role in organizing the behavioral steps in ecdysis sequences (64), its participation in activating the bursicon-expressing neurons at adult ecdysis is likely. Because ETH release is expected to long precede bursicon release, however, the mechanism by which it might exert a delayed effect on activating bursicon-expressing neurons remains to be clarified.
IV. Summary and Future Directions
Progress over the last 10 years has made it clear that the hormone bursicon critically regulates not only postecdysial cuticle tanning, but also the behaviors that cause wing expansion in Drosophila. The neurons that produce this hormone in the fly and their functional roles in differentially regulating the somatic and behavioral aspects of postecdysial processes have been largely elucidated. However, as noted above, we do not yet understand how the bursicon-expressing neurons are regulated, nor how they control the motor output that underlies expansion. The recent development of a tool for selectively identifying and manipulating the activity of neurons that express bursicon’s receptor, RK (16), promises to help identify and characterize the downstream targets of the bursicon neurons. These targets probably include neurons that either directly or indirectly modulate the pharyngeal pump as well as motor neurons responsible for tonic changes in abdominal posture. More interestingly, bursicon targets in the central nervous system should include neurons that modulate sensory pathways that mediate environmental inhibition of the wing expansion network. As yet, the sensory systems that mediate environmental cues and the circuitry that inhibits wing expansion and promotes environmental search all remain to be identified. Indeed, one of the most interesting open questions from a neurobiological standpoint is how the decision between engaging in postecdysial search versus perching and expansion is adjudicated. Because search employs motor patterns that are also used in ecdysis, it may be that postecdysial search represents an extension of the ecdysis phase into the postecdysial period. This presents the interesting possibility that in the fly, the termination of ecdysis has been made dependent on environmental signals rather than hormonal or proprioceptive cues as appears to be the case in many other insects.
The general question of how the circuitry underlying the adult ecdysis sequence in Drosophila compares to circuitry underlying other ecdysis sequences is also one of major interest. As indicated above, the broad conservation of both the hormones and cells involved in insect ecdysis suggest that the neuroendocrine networks that mediate ecdysis sequences will share common features; recent evidence suggests that this conservation may extend not only to crustacea (58) but also chelicerates (55). The full complement of shared regulatory factors is likely not yet known, but genetic approaches, such as mutagenesis screens in Drosophila or RNAi screens in Tribolium provide the means to discover more.
At the same time, the incredible diversity of ecdysis sequences themselves indicates that their genetic and neural components are unlikely to be exactly the same. This is true for ecdysis sequences at different stages within a single species as well as for ecdysis sequences of different insects at equivalent developmental stages. As noted above, recent work in Drosophila already has shown how the addition of CCAP-expressing neurons to the ecdysis circuit between the larval and pupal molts accommodates the changing needs of the animal as it undergoes metamorphosis. Similarly, comparative studies offer clues about the differing roles of ecdysis hormones in different animals. Although bursicon was initially identified as the insect “tanning hormone,” RNAi knockdown studies from both Tribolium and the silkmoth indicate that it plays an essential role in wing expansion in these insects, but not in tanning (2, 26). Whether bursicon always regulates the same motor patterns involved in expansion is unclear. One of the greatest sources of diversity in insect ecdysis sequences is when expansion (via air swallowing) is initiated (see Fig. 1). Although current evidence suggests that expansion is mediated by bursicon in the fly, evidence from locusts (61), where expansion is initiated prior to ecdysis, and from Manduca (39), implicates the action of other hormones.
Understanding how the neuroendocrine networks that govern ecdysis sequences are modified in the course of development and evolution to serve the needs of animals that diverge widely in body, habitat, and behavior offers a unique opportunity to comprehensively analyze the essential problems in behavior that were enunciated over a half-century ago by Tinbergen (50). Tinbergen stated that a complete understanding of an innate behavior required determining its selective advantage, neurophysiological basis, development, and evolution. The selective advantage of ecdysis is obvious in an animal that needs to molt to grow, and, as an adult, often benefits from wings to find food and mates. As for understanding the neurophysiological control of ecdysis, we already have a reasonably comprehensive model for this process in the tobacco hornworm. For the fruitfly, we can anticipate models of comparable sophistication soon for the larval, pupal, and adult molts, thus offering the opportunity to understand how the ecdysis network in a single animal alters in form and function over development. Finally, comparative studies, made increasingly possible by the availability of complete genomic sequences and RNAi technology, should help elucidate their similarities and differences in the use of conserved factors to mediate components of ecdysis sequences in insects of diverse orders. This should provide a means of correlating variations in life history and behavior with differences in hormonal control and ecdysis network topology. The comparative approach thus promises rich insights into the way in which evolutionary processes have sculpted ecdysis networks in response to the constraints of genome and environment. In short, ecdysis studies have reached a level of maturity at which they are poised to spread their wings and fly.
Summary List Highlighting Central Points.
Postecdysial behavior in Drosophila is characterized by two environmentally-sensitive behavioral programs: an exploratory locomotor program and a wing expansion program.
The wing expansion program, which is inhibited in adverse environments, is governed by the hormone bursicon, which was originally identified as the insect “tanning hormone.”
Bursicon governs both the somatic and behavioral changes underlying wing expansion in the fruitfly.
Bursicon’s somatic actions are mediated by seven bilateral pairs of neurons located in the abdominal ganglion, which release the hormone into the hemolymph, while its behavioral actions are mediated by a single pair of neurons located in the subesophageal ganglion, which ramify broadly within the central nervous system.
The subesophageal pair of bursicon-expressing neurons also plays an essential role in modulating the inhibitory environmental inputs that delay wing expansion.
Neurons that secrete eclosion hormone and crustacean cardioactive peptide (but not bursicon) also are necessary for normal wing expansion in the fly.
In some insect species, bursicon is required for wing expansion, but not necessarily for cuticle tanning.
Acknowledgments
Funding: BHW is supported by the Intramural Research Program of the National Institute of Mental Health (Project 1-ZIA-MH002800-10)). JE funded by FONDECYT grant #1111023 and Centro Interdisciplinario de Neurociencia de Valparaiso Millenium grant.
Mini-Glossary of Key Terms
- Ecdysis
the behavioral act of shedding the exoskeleton of the previous developmental stage. This is distinct from “molting,” which also includes the physiological processes of dissolving the old cuticle (i.e. apolysis) and secreting the new one. In Diptera the exchange of cuticle at the transition from the last larval instar to the pupal stage is called “pupal ecdysis,” even though the last larval cuticle is kept as a protective casing rather than shed. This is because the behaviors and neuropeptides that mediate this transition are similar to those found at pupal ecdysis in other insect orders and because the larval trachea are, in fact, cast off at this time. Adult ecdysis is also called eclosion
- Ecdysis Sequence
the sequence of motor programs used by an insect to shed its cuticle and (often) to then expand and harden a new one. The nature and order of motor programs varies considerably depending on the specific developmental transition and insect species, but ecdysis sequences are generally quite stereotyped for any given molt in a given species
- Cuticle
Extracellular layer that covers the insect epidermis. It is made up of chitin (poly N-Acetylglucosamine) and crosslinked proteins, whose exact composition and chemical modifications determine the elasticity and flexibility of the exoskeleton. During the molt the majority of the cuticle is digested and recycled, with the exception of the outermost layer which is shed as the exuvia at ecdysis
- Tanning
a term used to describe the combined processes of sclerotizing (or hardening) and melanizing (or pigmenting) the new cuticle. In flies and many other insects, these processes can be induced by the hormone bursicon
- Pharyngeal pump
Also called the cibarial pump, this is the set of muscles located at the base of the proboscis that in adult Drosophila controls the ingestion of food, and during postecdysial expansion controls the swallowing of air. The rhythmic activity of the pharyngeal pump is presumed to be under the control of an as yet unidentified central pattern generator
- Wing expansion
Several synonymous terms have been used to describe the post-ecdysial unfolding of the wings by adult insects, including “inflation,” “spreading,” and perhaps most poetically, “unfurling.” We prefer here the neutral term “expansion,” because it indicates that this process is part of the more general expansion of the adult cuticle. It also avoids the potential confusion of the commonly used term “inflation,” since hemolymph and not air is the primary medium that fills the wings to unfold them
- Classification of insects used for the study of ecdysis sequences
order Diptera: Calliphora, blow-fly; Sarcophaga, flesh-fly; Drosophila, fruitfly; order Lepidoptera: Manduca, tobacco hornworm/hawkmoth; Bombyx mori, silk moth; order Hemiptera: Rhodnius, kissing bug; order Coleoptera: Tribolium, red flour beetle; order Blattodea: Periplaneta, cockroach. Order Orthoptera: Schistocerca, desert locust; Teleogryllus, cricket
Important Acronyms Used
- CCAP
crustacean cardioactive peptide
- EH
eclosion hormone
- ETH
ecdysis triggering hormone
- PETH
pre-ecdysis triggering hormone
- CNS
Central nervous system
- BSEG
A pair of bursicon-expressing neurons in the subesophageal ganglion
- BAG
Seven pairs of bursicon-expressing neurons in the abdominal ganglion
- rk
rickets, the gene encoding the bursicon receptor
Footnotes
Posted with permission from the Annual Review of Entomology, Volume 59 © 2014 by Annual Reviews, http://www.annualreviews.org.
Literature Cited
- 1.Ampleford EJ, Steel CGH. THE BEHAVIOR OF RHODNIUS-PROLIXUS (STAL) DURING THE IMAGINAL ECDYSIS. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1982;60:168–174. [Google Scholar]
- 2. Arakane Y, Li B, Muthukrishnan S, Beeman RW, Kramer KJ, Park Y. Functional analysis of four neuropeptides, EH, ETH, CCAP and bursicon, and their receptors in adult ecdysis behavior of the red flour beetle, Tribolium castaneum . Mech Dev. 2008;125:984–995. doi: 10.1016/j.mod.2008.09.002. Function of EH, CCAP, and bursicon in Tribolium at adult ecdysis determined by RNAi knockdown
- 3.Baker JD, Truman JW. Mutations in the Drosophila glycoprotein hormone receptor, rickets, eliminate neuropeptide-induced tanning and selectively block a stereotyped behavioral program. J Exp Biol. 2002;205:2555–2565. doi: 10.1242/jeb.205.17.2555. [DOI] [PubMed] [Google Scholar]
- 4.Carlson JR. Imaginal Ecdysis Of Cricket (Teleogryllus-Oceanicus) .1. Organization Of Motor Programs And Roles Of Central And Sensory Control. Journal Of Comparative Physiology. 1977;115:299–317. [Google Scholar]
- 5. Carlson JR, Bentley D. Ecdysis - Neural Orchestration Of A Complex Behavioral Performance. Science. 1977;195:1006–1008. doi: 10.1126/science.841322. One of several papers by Carlson beautifully characterizing the adult ecdysis sequence in the cricket
- 6.Carlson JR, Ogara BA. THE ECDYSIS OF THE CRICKET, TELEOGRYLLUS-OCEANICUS - GENERATION OF THE PHARYNGEAL AIR-SWALLOWING MOTOR PROGRAM BY THE ISOLATED FRONTAL GANGLION. Comparative Biochemistry and Physiology a-Physiology. 1983;75:579–587. [Google Scholar]
- 7.Chang JC, Yang RB, Adams ME, Lu KH. Receptor guanylyl cyclases in Inka cells targeted by eclosion hormone. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13371–13376. doi: 10.1073/pnas.0812593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark AC, Del Campo M, Ewer J. Neuroendocrine control of larval ecdysis behavior in Drosophila: complex regulation by partially redundant neuropeptides. J. Neuroscience. 2004;24:4283–4292. doi: 10.1523/JNEUROSCI.4938-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottrell CB. General Observations on the imaginal ecdysis of blowflies. Trans R Entomol Soc London. 1962;114:317–333. [Google Scholar]
- 10.Cottrell CB. Insect Ecdysis with Particular Emphasis on Cuticular Hardening and Darkening. New York: Academic Press; 1964. pp. 175–218. [Google Scholar]
- 11.Dai L, Dewey EM, Zitnan D, Luo CW, Honegger HW, Adams ME. Identification, developmental expression, and functions of bursicon in the tobacco hawkmoth, Manduca sexta . J Comp Neurol. 2008;506:759–774. doi: 10.1002/cne.21575. [DOI] [PubMed] [Google Scholar]
- 12.Davis NT, Homberg U, Dircksen H, Levine RB, Hildebrand JG. CRUSTACEAN CARDIOACTIVE PEPTIDE-IMMUNOREACTIVE NEURONS IN THE HAWKMOTH MANDUCA-SEXTA AND CHANGES IN THEIR IMMUNOREACTIVITY DURING POSTEMBRYONIC DEVELOPMENT. Journal of Comparative Neurology. 1993;338:612–627. doi: 10.1002/cne.903380410. [DOI] [PubMed] [Google Scholar]
- 13.Delachambre J, Delbecque JP, Provansal A, Dereggi ML, Cailla H. INDUCTION OF EPIDERMAL CYCLIC-AMP BY BURSICON IN MEALWORM, TENEBRIO-MOLITOR. Experientia. 1979;35:701–702. [Google Scholar]
- 14. Denlinger DL, Zaarek J. METAMORPHOSIS BEHAVIOR OF FLIES. Annual Review of Entomology. 1994;39:243–266. doi: 10.1146/annurev.en.39.010194.001331. Comprehensive review summarizing earlier work done on pupal and adult ecdysis in flies
- 15.Dewey EM, McNabb SL, Ewer J, Kuo GR, Takanishi CL, et al. Identification of the gene encoding bursicon, an insect neuropeptide responsible for cuticle sclerotization and wing spreading. Curr. Biol. 2004;14:1208–1213. doi: 10.1016/j.cub.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Diao FQ, White BH. A Novel Approach for Directing Transgene Expression in Drosophila: T2A–Gal4 In-Frame Fusion. Genetics. 2012;190:1139-U356. doi: 10.1534/genetics.111.136291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eidmann H. Untersuchungen über Wachstum und Häutung der Insekten. Zeitschr. wiss. Biol. Morph. u. Oekol. 1924;2:567–610. [Google Scholar]
- 18.Ewer J, De Vente J, Truman JW. Neuropeptide induction of cyclic GMP increases in the insect CNS: resolution at the level of single identifiable neurons. J Neurosci. 1994;14:7704–7712. doi: 10.1523/JNEUROSCI.14-12-07704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ewer J, Reynolds S. Neuropeptide control of molting in insects. In: Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM, Rubin RT, editors. Hormones, brain and behavior. San Diego, CA: Academic Press; 2002. pp. 1–92. Authoritative review of ecdysis research up to 2002
- 20.Ewer J, Truman JW. Increases in cyclic 3’,5’-guanosine monophosphate (cGMP) occur at ecdysis in an evolutionarily conserved crustacean cardioactive peptide-immunoreactive insect neuronal network. J. Comp. Neurol. 1996;370:330–341. doi: 10.1002/(SICI)1096-9861(19960701)370:3<330::AID-CNE4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Fraenkel G. Observations and Experiments on the Blow-fly (Calliphora erythrocephala) during the First Day after Emergence. Proc. zool. Soc. Lond. 1935:893–904. [Google Scholar]
- 22.Fraenkel G, Hsiao C. Bursicon, a hormone which mediates tanning of the cuticle in the adult fly and other insects. J. Insect Physiol. 1965;11:513–556. [Google Scholar]
- 23.Fuse M, Truman JW. Modulation of ecdysis in the moth Manduca sexta: the roles of the suboesophageal and thoracic ganglia. J Exp Biol. 2002;205:1047–1058. doi: 10.1242/jeb.205.8.1047. [DOI] [PubMed] [Google Scholar]
- 24.Gammie SC, Truman JW. Neuropeptide hierarchies and the activation of sequential motor behaviors in the hawkmoth, Manduca sexta . J. Neurosci. 1997;17:4389–4397. doi: 10.1523/JNEUROSCI.17-11-04389.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honegger HW, Dewey EM, Ewer J. Bursicon, the tanning hormone of insects: recent advances following the discovery of its molecular identity. J Comp Physiol A. 2008;194:989–1005. doi: 10.1007/s00359-008-0386-3. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Zhang Y, Li M, Wang S, Liu W, et al. RNA interference-mediated silencing of the bursicon gene induces defects in wing expansion of silkworm. FEBS Lett. 2007;581:697–701. doi: 10.1016/j.febslet.2007.01.034. Epub 2007 Jan 24. [DOI] [PubMed] [Google Scholar]
- 27. Hughes TD. Imaginal Ecdysis Of The Desert Locust, Schistocerca-Gregaria .1. Description Of The Behavior. Physiological Entomology. 1980;5:47–54. First of several papers by Hughes comprehensively analyzing the adult ecdysis sequence in the locust
- 28.Hughes TD. Imaginal Ecdysis Of The Desert Locust, Schistocerca-Gregaria .2. Motor-Activity Underlying The Pre-Emergence And Emergence Behavior. Physiological Entomology. 1980;5:55–71. [Google Scholar]
- 29.Kammer AE, Kinnamon SC. Patterned muscle activity during eclosion in the hawkmoth Manduca sexta . J. Comp. Physiol. 1977:114. [Google Scholar]
- 30.Kim YJ, Spalovska-Valachova I, Cho KH, Zitnanova I, Park Y, et al. Corazonin receptor signaling in ecdysis initiation. Proc Natl Acad Sci U S A. 2004;101:6704–6709. doi: 10.1073/pnas.0305291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YJ, Zitnan D, Cho KH, Schooley DA, Mizoguchi A, Adams ME. Central peptidergic ensembles associated with organization of innate behavior. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14211–14216. doi: 10.1073/pnas.0603459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr Biol. 2006;16:1395–1407. doi: 10.1016/j.cub.2006.06.027. Identifies ETH targets in the Drosophila CNS and documents their sequential activation following ETH challenge
- 33. Lahr E, Dean D, Ewer J. Genetic analysis of ecdysis behavior in Drosophila reveals partially overlapping functions of two unrelated neuropeptides. J. Neurosci. 2012;32:6819–6829. doi: 10.1523/JNEUROSCI.5301-11.2012. Shows genetically that CCAP’s role in Drosophila ecdysis is only evident when bursicon is eliminated
- 34. Luan H, Diao F, Peabody NC, White BH. Command and compensation in a neuromodulatory decision network. J Neurosci. 2012;32:880–889. doi: 10.1523/JNEUROSCI.3707-11.2012. Uses targeted neuronal suppression and activation to characterize function of bursicon neurons in Drosophila postedysis
- 35.Luan H, Lemon WC, Peabody NC, Pohl JB, Zelensky PK, et al. Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J Neurosci. 2006;26:573–584. doi: 10.1523/JNEUROSCI.3916-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo C-W, Dewey EM, Sudo S, Ewer J, Hsu SY, et al. Bursicon, the insect cuticle hardening hormone, is a heterodimeric cystine knot protein that activates G protein-coupled receptor LGR2. Proc. Natl. Acad. Sci. USA. 2005;102:2820–2825. doi: 10.1073/pnas.0409916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNabb SL, Baker JD, Agapite J, Steller H, Riddiford LM, Truman JW. Disruption of behavioral sequence by targeted death of peptidergic neurons in Drosophila . Neuron. 1997;19:813–823. doi: 10.1016/s0896-6273(00)80963-0. [DOI] [PubMed] [Google Scholar]
- 38.Mendive FM, Van Loy T, Claeysen S, Poels J, Williamson M, et al. Drosophila molting neurohormone bursicon is a heterodimer and the natural agonist of the orphan receptor DLGR2 . FEBS Lett. 2005;579:2171–2176. doi: 10.1016/j.febslet.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Miles CI, Booker R. The role of the frontal ganglion in the feeding and eclosion behavior of the moth Manduca sexta. Journal Of Experimental Biology. 1998;201:1785–1798. doi: 10.1242/jeb.201.11.1785. [DOI] [PubMed] [Google Scholar]
- 40.Mills RR, Mathur RB, Guerra AA. Studies on the hormonal control of tanning in the American cockroach--I. Release of an activation factor from the terminal abdominal ganglion. Journal of Insect Physiology. 1965;11:1047. doi: 10.1016/0022-1910(65)90120-4. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, Schroeder AJ, Helfrich-Förster C, Jackson FR, Ewer J. Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of ecdysis behavior. Development. 2003;130:2645–2656. doi: 10.1242/dev.00503. [DOI] [PubMed] [Google Scholar]
- 42.Peabody NC, Diao F, Luan H, Wang H, Dewey EM, et al. Bursicon functions within the Drosophila CNS to modulate wing expansion behavior, hormone secretion, and cell death. J Neurosci. 2008;28:14379–14391. doi: 10.1523/JNEUROSCI.2842-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peabody NC, Pohl JB, Diao F, Vreede AP, Sandstrom DJ, et al. Characterization of the decision network for wing expansion in Drosophila using targeted expression of the TRPM8 channel. J Neurosci. 2009;29:3343–3353. doi: 10.1523/JNEUROSCI.4241-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reid SNM, Fraenkel G, Friedman S. EXTRICATION, THE PRIMARY EVENT IN ECLOSION, AND ITS RELATIONSHIP TO DIGGING, PUMPING AND TANNING IN SARCOPHAGA-BULLATA. Journal of Insect Physiology. 1987;33:339. [Google Scholar]
- 45.Reynolds S. Bursicon. In: Downer GH, Laufer H, editors. Endocrinology of Insects. New York: Alan R. Liss, Inc; 1983. pp. 235–248. [Google Scholar]
- 46.Reynolds SE. Hormonal regulation of cuticle extensibility in newly emerged adult blowflies. J Insect Physiol. 1976;22:529–534. doi: 10.1016/0022-1910(76)90172-4. [DOI] [PubMed] [Google Scholar]
- 47. Reynolds SE. Integration of Behavior and Physiology in Ecdysis. In: Berridge M, Treherne JE, Wigglesworth VB, editors. Advances in Insect Physiology. London: Academic Press; 1980. pp. 476–595. Comprehensive review of ecdysis up to 1980, including aspects of natural history
- 48.Simpson JH. Mapping and manipulating neural circuits in the fly brain. Adv Genet. 2009;65:79–143. doi: 10.1016/S0065-2660(09)65003-3. [DOI] [PubMed] [Google Scholar]
- 49.Steel CG, Vafopoulou X. Physiology of Insect Clocks. In: Saunders DS, editor. Insect clocks. Elsevier; 2002. pp. 115–188. [Google Scholar]
- 50.Tinbergen N. On aims and methods of Ethology (Reprinted from Zeitschrift fur Tierpsychologie, vol 20, pg 410, 1963) Animal Biology. 2005;55:297–321. [Google Scholar]
- 51.Truman JW. Physiology Of Insect Ecdysis .3. Relationship Between Hormonal-Control Of Eclosion And Of Tanning In Tobacco Hornworm, Manduca-Sexta. J Exp Biol. 1973;58:821–829. [Google Scholar]
- 52.Truman JW. Hormonal control of insect ecdysis: endocrine cascades for coordinating behavior with physiology. Vitam Horm. 2005;73:1–30. doi: 10.1016/S0083-6729(05)73001-6. [DOI] [PubMed] [Google Scholar]
- 53.Truman JW, Endo PT. Physiology of insect ecdysis: neural and hormonal factors involved in wing-spreading behavior of moths. J. exp. Biol. 1974;61:47–55. doi: 10.1242/jeb.61.1.47. [DOI] [PubMed] [Google Scholar]
- 54.Truman JW, Truman Lm. NEUROENDOCRINE CONTROL OF ECDYSIS IN SILKMOTHS. Science. 1970;167:1624. doi: 10.1126/science.167.3925.1624. [DOI] [PubMed] [Google Scholar]
- 55.Veenstra JA, Rombauts S, Grbic M. In silico cloning of genes encoding neuropeptides, neurohormones and their putative G-protein coupled receptors in a spider mite. Insect Biochem Mol Biol. 2012;42:277–295. doi: 10.1016/j.ibmb.2011.12.009. Epub 11 Dec 28. [DOI] [PubMed] [Google Scholar]
- 56.Venken KJ, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–230. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veverytsa L, Allan DW. Temporally tuned neuronal differentiation supports the functional remodeling of a neuronal network in Drosophila . Proc Natl Acad Sci U S A. 2012;109:E748–E756. doi: 10.1073/pnas.1114710109. Epub 2012 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster SG, Wilcockson DC, Mrinalini, Sharp JH. Bursicon and neuropeptide cascades during the ecdysis program of the shore crab, Carcinus maenas. Gen Comp Endocrinol. 2013;182:54–64. doi: 10.1016/j.ygcen.2012.11.018. Epub 12 Dec 14. [DOI] [PubMed] [Google Scholar]
- 59.White BH, Peabody NC. Neurotrapping: cellular screens to identify the neural substrates of behavior in Drosophila. Front Mol Neurosci. 2009;2:20. doi: 10.3389/neuro.02.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zdarek J, Denlinger DL. POSTECLOSION BEHAVIOR OF THE FLESH FLY, SARCOPHAGA-CRASSIPALPIS - A COMPARISON OF WILD-TYPE AND UNICORN MUTANTS. Archives Of Insect Biochemistry And Physiology. 1987;4:101–106. [Google Scholar]
- 61.Zilberstein Y, Ewer J, Ayali A. Neuromodulation of the locust frontal ganglion during the moult: a novel role for insect ecdysis peptides. Journal Of Experimental Biology. 2006;209:2911–2919. doi: 10.1242/jeb.02339. [DOI] [PubMed] [Google Scholar]
- 62.Zitnan D, Adams M. Neuroendocrine regulation of insect ecdysis. In: Gilbert L, Kostas I, Gill S, editors. Comprehensive Molecular Insect Science. Amsterdam, Boston: Elsevier; 2004. pp. 1–60. [Google Scholar]
- 63.Zitnan D, Adams ME. Excitatory and inhibitory roles of central ganglia in initiation of the insect ecdysis behavioural sequence. J Exp Biol. 2000;203:1329–1340. doi: 10.1242/jeb.203.8.1329. [DOI] [PubMed] [Google Scholar]
- 64. Zitnan D, Adams ME. Neuroendocrine regulation of ecdysis. In: Gilbert LI, editor. Insect Endocrinology. Elsevier; 2012. pp. 253–309. Up-to-date summary of research on the hormonal and cellular control of ecdysis sequences in insects
- 65.Zitnan D, Hollar L, Spalovska II, Takac P, Zitnanova II, et al. Molecular cloning and function of ecdysis-triggering hormones in the silkworm Bombyx mori . J Exp Biol. 2002;205:3459–3473. doi: 10.1242/jeb.205.22.3459. [DOI] [PubMed] [Google Scholar]