Abstract
Purpose
Adrenocortical carcinoma (ACC) is a rare tumor in children with important distinctions from the adult disease. We reviewed the National Cancer Data Base (NCDB) to determine factors associated with long-term survival.
Methods
The NCDB was queried for patients less than 18 years of age who were diagnosed with ACC between 1998 and 2011. Kaplan–Meier analysis was utilized to determine factors significantly associated with overall survival.
Results
A total of 111 patients were included (median age: 4 years, 69% female). ACC was more common in the youngest cohort, with 48% of cases occurring in children younger than the age of 3. Median tumor size was 9.5 cm (IQR: 6.5–13.0), and 87% of patients underwent some form of surgical resection. Among children with available data, 19 of 62 presented with metastases. Overall 1- and 3-year survival was 70% and 64%, respectively. Age, tumor size, extension of tumor into surrounding tissue, and metastatic disease were all found to be significantly associated with survival. Among patients who underwent a surgical procedure, margin status was also found to be significantly associated with survival.
Conclusion
Age, tumor size, extension of tumor, metastatic disease, and margin status are significantly associated with long-term survival in children with adrenocortical carcinoma.
Keywords: Adrenocortical carcinoma, Prognostic factors, Pediatric adrenal tumors, Pediatric cancer staging
Adrenocortical tumors (ACTs) are rare but aggressive endocrine neoplasms responsible for roughly 0.2% of all childhood cancers [1,2]. Patients typically present with a bimodal age distribution, the first period of which is between 1 and 10 years of age [3]. In contrast to adult ACT, the majority of pediatric ACTs are functional, often presenting with excessive androgen production [1,3,4]. While the histologic differentiation between adenomas and carcinomas is difficult, 80–90% of pediatric ACTs are carcinomas [5,6]. Although complete resection of adrenocortical carcinoma (ACC) provides the best chance for cure, long-term survival is limited [5,7]. Most recent reviews report 5-year survival for adults with ACC after curative surgery around 20–50% [8–10]. In children, 5-year survival has been reported to range from 30% to 70% with substantial variation based on disease presentation [5,6,11]. For example, children with completely resected tumors have a 5-year survival around 80–90% while those with metastatic disease have a 5-year survival closer to 0–10% [5,6,12–14].
Staging in adults typically follows either the American Joint Committee on Cancer (AJCC) Tumor, Node, and Metastases (TNM) staging system, or a modified staging system proposed by the European Network for The Study of Adrenal Tumors (ENSAT) [15]. This staging system has not been extended to children. Instead, the traditional staging system for pediatric ACC, based on the work of Sandrini and colleagues, has been modified by the Children's Oncology Group (COG) based on clinical data from the International Pediatric Adrenocortical Tumor Registry (IPACTR) [11]. For staging purposes, children are divided into four groups: stage I: completely resected tumors <100 g and 200 cm3 with normal postoperative hormone levels, stage 2: completely resected tumors ≥100 g or ≥200 cm3 with normal postoperative hormone levels, stage 3: residual disease or inoperable tumors, and stage IV: distant metastatic disease [16].
However, the modified staging system does not account for factors such as age and distant disease status which have previously been associated with survival [5,13,17]. In this study we reviewed a large, national database to further delineate prognostic factors associated with this rare disease in order to help guide clinical decision making.
1. Materials and methods
1.1. National Cancer Data Base
The National Cancer Data Base (NCDB) is a national cancer registry which contains clinical information from more than 1500 hospitals which are accredited by the American College of Surgeons and the American Cancer Society [18]. The database includes patient demographics and comorbidities, treatment regimens, postoperative length of stay and readmission, and long-term overall survival.
1.2. Patient population
The NCDB was queried for pediatric patients (age <18) diagnosed with adrenocortical carcinoma (International Classification of Diseases for Oncology, 3rd Edition codes 8370 and 8373) of the adrenal gland (primary site codes: C740, C741, C749) from 1998 to 2011. Patients with unknown long-term vital status were excluded.
1.3. Statistical analysis
Overall patient characteristics, tumor characteristics, treatment strategies, and outcomes were compiled using standard summary statistics. Kaplan–Meier analysis and the log-rank test were performed to determine the unadjusted association between age, sex, socioeconomic status (defined as private insurance status), tumor extension (confined to adrenal gland vs extension into adjacent connective tissue vs extension into adjacent organs), and metastatic disease status and overall survival among the entire cohort. The same methods were used to determine the association of positive margin status and overall survival among patients who received a surgical resection. Surgical resections were defined by Facility Oncology Registry Data System [FORDS] codes 20–27 (partial adrenalectomy), 30–40 (adrenalectomy), and 60 (radical resection). Because of the impact of surgical resection on survival, Cox proportional hazards regression modeling was also utilized to determine the association of each of the above variables with overall survival after adjustment for surgical resection.
Since prior studies have suggested the importance of tumor size in prognosis of pediatric ACC, we also analyzed the association between overall survival and tumor size not only as a continuous variable but also as a categorical variable. In the NCDB, tumor size is defined by the greatest cross-sectional tumor diameter according to pathologic examination when available, and based on imaging determination when pathologic examination is unavailable. The association of tumor size and the log of the odds of one year mortality was used to determine categorical groupings. This led to four distinct groups (<5.0 cm, 5.0–9.9 cm, 10.0–14.9 cm, 15.0+ cm). Kaplan–Meiermethods and Cox proportional hazards regression modeling were performed to determine the association between tumor size as both a continuous and categorical variable with overall survival both before and after adjustment for surgical approach. A p-value of <0.05 was used to indicate statistical significance for all comparisons. All statistical analyses were performed using R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
2. Results
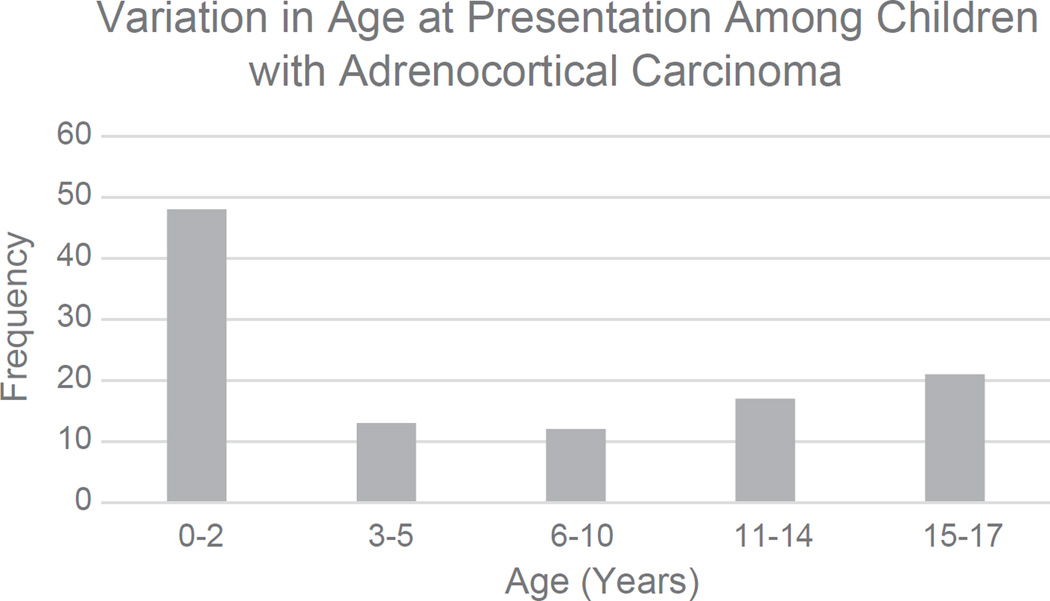
A total of 111 patients met study criteria, with a median age of 4 years (interquartile range [IQR]: 1–13 years, Fig. 1, Table 1), although 48% of patients were younger than the age of 3. Female patients made up 69% (n = 77) of the cohort. The majority of patients underwent surgical resection by a partial adrenalectomy (11%), a simple adrenalectomy (67%), or a radical adrenalectomy (14%). Roughly 31% (n = 19/62) of patients presented with metastatic disease at the time of diagnosis.
Fig. 1.
The variation in age at presentation in patients with adrenocortical carcinoma.
Table 1.
Baseline patient characteristics, tumor characteristics, treatment strategies, and outcomes.
| Variable | Overall |
|---|---|
| N | 111 |
| Age (y) | 4 (1–13) |
| Female sex | 77 (69%) |
| Private insurance status | 63 (57%) |
| Tumor size (cm) | 9.5 (6.5–13.0) |
| Tumor weight (g) | 579 (118–872) |
| Tumor extension | |
| Localized to adrenal gland | 38 (60%) |
| Extending into adjacent tissue | 12 (19%) |
| Extending into adjacent organ | 13 (21%) |
| Nodes examined | 46 (45%) |
| Metastatic disease | 19 (31%) |
| Surgical procedure | |
| Partial adrenalectomy | 11 (11%) |
| Adrenalectomy | 70 (67%) |
| Radical resection | 15 (14%) |
| Positive surgical margins | 16 (21%) |
| Adjuvant systemic therapy | 30 (27%) |
| 30-day survival | 111 (100%) |
| Hospital length of stay (days) | 5.5 (4–10) |
Surgical margin status is presented only among patients who received a surgical procedure. Continuous variables are recorded as median (interquartile range) while categorical variables are recorded as frequency (percentage).
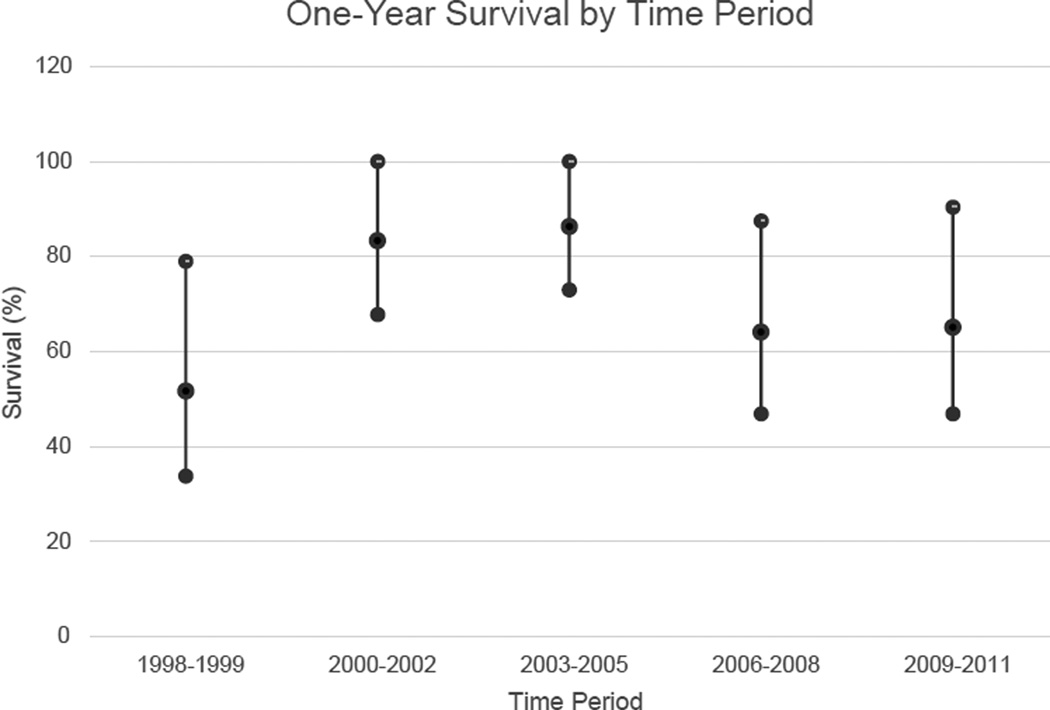
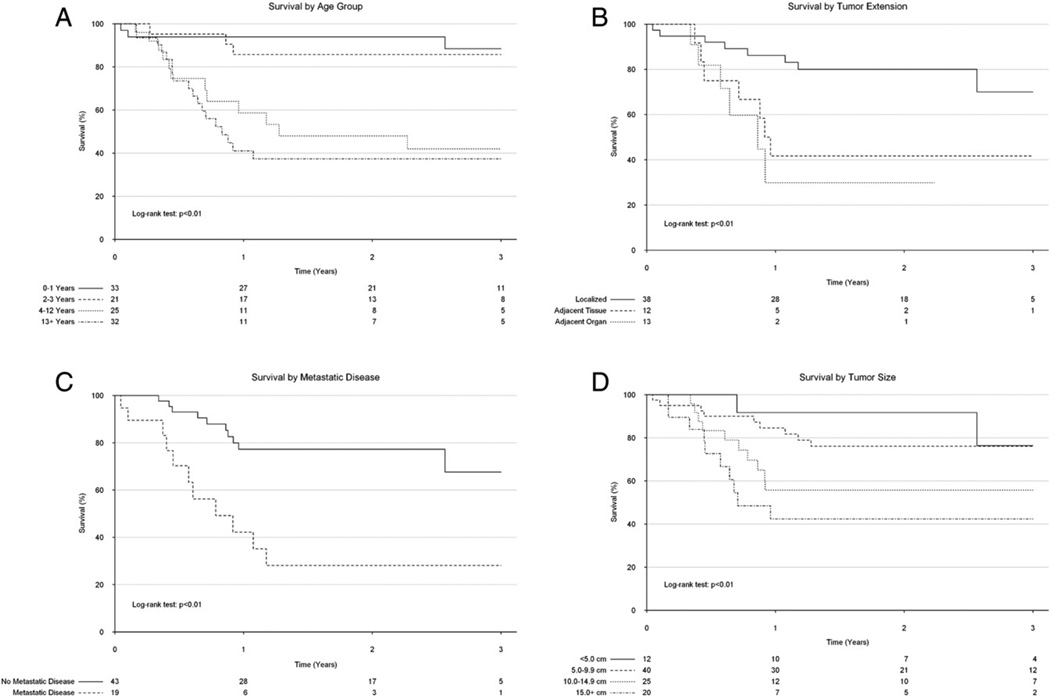
Overall 1- and 3-year survival were 70% (95% confidence interval [CI]: 62–80%) and 64% (95% CI: 54–74%), respectively (Table 2). One-year survival remained relatively constant throughout the study period (Fig. 2). Upon unadjusted analysis, age, tumor extension into adjacent tissue, and metastatic disease were all found to be significantly associated with overall survival (Fig. 3A–C). There were no significant differences in survival by sex or private insurance status. After adjustment for procedure type, age (adjusted hazard ratio [HR]: 1.10, 95% confidence interval [CI]: 1.04–1.18), tumor extension into an adjacent organ (adjusted HR: 4.29, 95% CI: 1.32–14.0), and presence of metastatic disease (adjusted HR: 4.48, 95% CI: 1.84–10.9) remained associated with significantly reduced survival.
Table 2.
Overall survival and survival by select patient characteristics.
| Variable | 1-year survival | 3-year survival | p-value |
|---|---|---|---|
| Overall | 70% (62–78%) | 64% (54–74%) | |
| Age at diagnosis (years) | <0.01 | ||
| 0–1 | 94% (86–100%) | 88% (76–100%) | |
| 2–3 | 86% (72–100%) | 86% (72–100%) | |
| 4–12 | 59% (41–84%) | 42% (25–71%) | |
| 13+ | 41% (26–64%) | 37% (23–60%) | |
| Tumor size | <0.01 | ||
| <5.0 cm | 92% (77–100%) | 76% (51–100%) | |
| 5.0–9.9 cm | 85% (74–97%) | 76% (64–91%) | |
| 10.0–14.9 cm | 56% (39–81%) | 56% (39–81%) | |
| 15.0+ cm | 42% (24–74%) | 42% (24–74%) | |
| Extension of disease | <0.01 | ||
| Limited to adrenal gland | 86% (76–98%) | 70% (51–96%) | |
| Into surrounding connective tissue | 42% (21–81%) | 42% (21–81%) | |
| Into adjacent organ | 30% (10–91%) | X | |
| Metastatic disease | <0.01 | ||
| No | 77% (65–92%) | 68% (50–92%) | |
| Yes | 42% (23–77%) | 28% (12–64%) | |
| Surgical margin (among those who underwent surgery) | <0.01 | ||
| Negative margins | 82% (73–92%) | 74% (63–88%) | |
| Positive margins | 34% (16–73%) | 34% (16–73%) |
Data are presented as survival (95% confidence interval). P-values were determined by the log-rank test. “X” denotes a value that could not be determined based on available data.
Fig. 2.
Trend in one-year survival over the study period (years were grouped owing to the low number of patient events per year). Error bars denote 95% confidence interval.
Fig. 3.
Kaplan–Meier analysis demonstrating differences in long-term survival by age (A), primary tumor extension (B), metastatic disease status (C), and tumor size (D) in pediatric patients with adrenocortical carcinoma.
There was a significant association between tumor size as a continuous variable and long-term survival, with larger tumors having an increased hazard for mortality (HR per cm: 1.08, 95% CI: 1.02–1.14). After grouping patients by tumor size, the association between tumor size and outcome remained significant (Fig. 3D). However, after adjusting for resection type, tumor size was no longer significantly associated with long-term survival (adjusted HR: 1.01, 95% CI: 0.99–1.01).
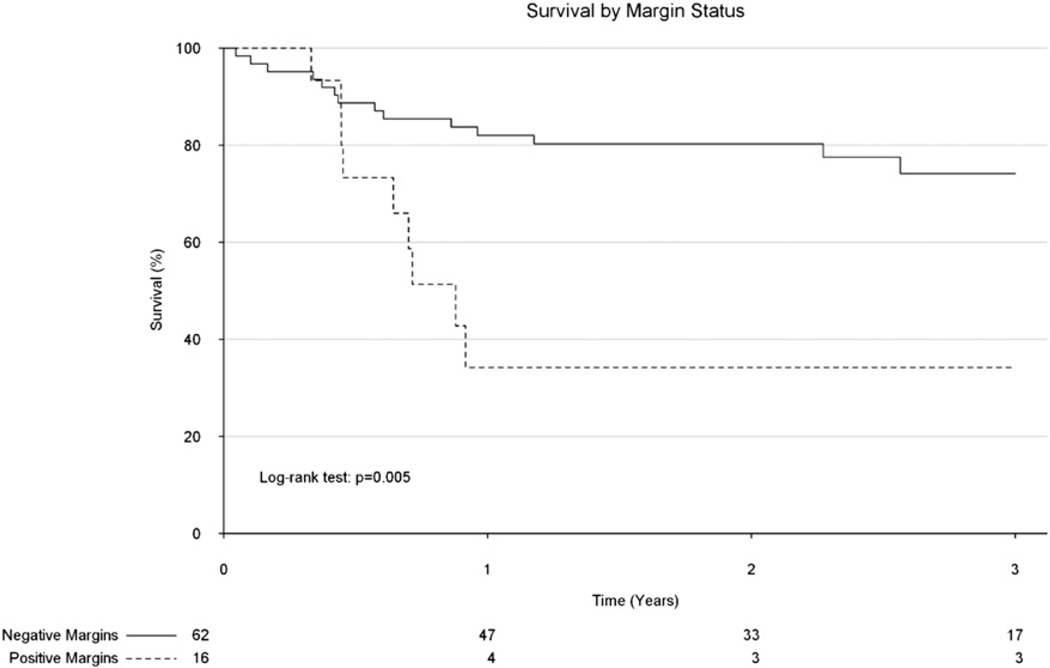
Of the children undergoing surgical resection, 34% had positive margins, which, on unadjusted analysis was significantly associated with poorer survival (Fig. 4). Patients who had a positive margin following surgery were substantially older (median age 9.5 years vs 2.0 years) and were much more likely to have tumors which extended into adjacent tissue (78% vs 32%).
Fig. 4.
Kaplan–Meier analysis demonstrating survival by margin among pediatric patients who underwent some form of surgical resection for adrenocortical carcinoma.
Adjuvant systemic therapy was utilized in 30% of children who underwent surgical resection. Patients who received adjuvant systemic therapy tended to be older (median age 8 years vs 2 years), had larger tumors (median tumor size 12.0 cm vs 8.2 cm), and were more likely to have tumors which extended into adjacent tissue (45% vs 30%). There was no significant difference by group with regard to surgical margin status (25% vs 19% for the systemic and no systemic treatment groups, respectively) or extent of surgical procedure (20% vs 14% of patients received radical surgery in the systemic and no systemic treatment groups, respectively).
3. Discussion
Although ACC has been historically associated with poor outcomes, especially in adult patients, there is substantial variation in long term survival among children diagnosed with ACC. The current staging system for childhood ACTs attempts to stratify patients by prognosis to determine which patients should receive more intensive therapy [5]. However, despite recent modifications to the staging system, our study found that there are additional important prognostic factors in pediatric ACT that are not included in the current staging system. These include age, tumor extension into adjacent organs, and the presence of metastatic disease. We did demonstrate a significant association between the presence of residual disease (positive margins) and long-term survival; however this must be taken in light of the substantial differences between groups with regard to age and tumor extension, both of which have also been shown to be significantly associated with long-term survival.
While smaller studies have demonstrated that age is an important prognostic factor, the age at which survival is substantially reduced has been thought to be closer to adolescence. Dall'Igna and colleagues found that survival did not substantially decrease until after the age of 12 [19]. However, we found that age ≥4 is the point at which survival decreases considerably. Although we could not delineate why age is an important prognostic factor in this study (e.g. it may be owing to a difference in stage at presentation, or a difference in tumor biology among other possibilities), its association with long-term survival proves its usefulness as a prognostic tool. As age is already used in the staging of other more common pediatric cancers, including neuroblastoma and acute lymphocytic leukemia, its use in staging pediatric ACC would not be particularly unusual [20,21].
In addition, we found that tumor extension and margin status are important prognostic factors in pediatric ACC. Extension into adjacent organs reduced 1-year survival from 86% to 30%, a substantial decrease, and even after adjustment for surgical approach, this finding was significantly associated with long-term survival. This factor is currently in use in the adult ENSAT staging system, and as we have shown here, may also be a very useful addition for staging pediatric patients [15].
While we acknowledge that this is not the first study to investigate predictors of outcomes in pediatric ACC, it is one of the largest national studies to date. In an analysis of the Surveillance, Epidemiology, and End Results (SEER) cancer registry of 85 pediatric patients, McAteer and colleagues demonstrated that older age and distant disease were important predictors of poor long-term survival [13]. Furthermore they demonstrated that both size and volume were important prognostic indicators on univariable analysis. Klein and colleagues also performed an extensive single institution review, and found that in 29 pediatric patients, age, tumor size, necrosis, and higher mitotic rate were all predictive of poor outcomes [17].
In our study, we demonstrated that a tumor ≥10.0 cm in diameter tends to have much worse prognosis than smaller tumors. This contradicts the findings of Klein and colleagues who found that tumor size, when modeled as a continuous variable, was not significantly associated with tumor-related death, although it was associated with tumor recurrence. However in that study, the authors performed an adjusted analysis, which has a high risk of succumbing to a type II statistical error owing to small sample size [17]. The 10 cm cut-off is also the tumor size used by McAteer and colleagues, who also found that tumor size ≥10 cm was associated with significantly increased mortality. A tumor diameter of 10.0 cm corresponds to a tumor volume of 524 cm3 (based on the volume of a sphere) which demonstrates that the 200 cm3 cut-off currently used in the COG modification of the Sanrini et al. staging system may not be the most appropriate. Of note, since age is correlated with tumor size, it may be the determining factor leading previous studies to find an association between tumor size and survival.
In this study we also demonstrated the importance of negative margins following surgical resection, in accordance with previous studies. However, patients with positive margins following surgical resection were much more likely to be older and have more extensive disease upon presentation, both factors also associated with poor survival. Unfortunately owing to our limited sample size, we could not properly adjust for these differences and therefore clinicians should be careful about interpreting these results. There has been a large debate in the literature over the appropriateness of minimally invasive approaches for ACC in pediatric patients. Some authors have concluded that laparoscopic resection leads to inferior outcomes because of a higher rate of positive margins [22,23]. Unfortunately the NCDB does record operative approach during the entire time period investigated in this study (it only does so for 2010 and later), and therefore there were not enough patients in the current database for us to investigate the association between surgical approach (laparoscopic vs open) and long-term survival.
Our study has several limitations inherent to using large national datasets to study pediatric solid tumors [24]. The NCDB is a national registry administered by the Commission on Cancer (CoC) of the American College of Surgeons and American Cancer Society. While the registry is quite large with 1500 participating centers and 30 million patients, it is based on convenience sampling from participating centers. Unfortunately, not all freestanding children's hospitals are members of the CoC. Therefore, it is not a comprehensive registry of every child or even most children with ACC over the time period studied [24]. Also, the dataset includes data from centers which are not Children's Oncology Group affiliated centers, thus including children who are not treated on a COG protocol. The finding that some children underwent “partial adrenalectomy” which would not be the proper surgical approach for ACC suggests that either the dataset is inaccurate or that some children treated off COG protocols are receiving suboptimal oncologic treatment, or both. This is a problematic for determining the effects on survival trends with changes in COG protocol-based therapies. Additionally, the dataset does not include many disease-specific variables critical to drawing more nuanced conclusions that could inform the care of children. Data regarding specific adjuvant therapies used, time to recurrence, and specific histopathology are lacking. The NCDB also does not contain data on other tumor characteristics which may be important, such as presenting symptoms, or mitotic rate, and therefore these could not be investigated in our study [12,17]. Also, the lack of central review of pathology from the resected specimens is a significant limitation of the dataset. It should be noted that other national cancer registries, including the SEER dataset, have the same limitations. Despite these clear and significant limitations, the NCDB dataset does provide a large dataset that includes children who may be treated off COG protocols. That a significant number of children with ACC may receive treatment that differs from current or recent COG protocols is itself an important finding that may merit further study or consideration.
3.1. Conclusions
In summary, using a large national cancer registry, we have demonstrated that age ≥4 years, extension of the primary disease into adjacent structures, and the presence of metastatic disease are all associated with poor long term survival in pediatric ACC. We also found that positive margins after resection were associated with poor long-term survival, however the baseline differences in patient characteristics should be taken into account when interpreting these results. Our study therefore supports the continued modification of the staging system described by Sandrini et al. for childhood ACT, with an increased emphasis on the prognostic factors above. The continued development and validation of a more robust staging system will help to better determine which pediatric patients may benefit from more intensive treatment.
Acknowledgments
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in this study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Footnotes
Meeting Information: This work was presented as a poster presentation at the 46th annual meeting of the American Pediatric Surgical Association (Ft Lauderdale, FL).
Funding: Institutional funds.
Conflicts of Interest: None of the authors have any relevant conflicts of interest to declare.
Institution Address: Duke University Medical Center, DUMC Box #3443, Durham, NC 27710.
Level of Evidence: Level II.
Author Contributions: Brian Gulack: Dr Gulack contributed to the study design, data analysis, data interpretation, manuscript drafting, and critical revision of the manuscript. Kristy Rialon: Dr Rialon contributed to the study design, manuscript drafting, and critical revision of the manuscript. Jina Kim: Dr Kim contributed to the data interpretation, manuscript drafting, and critical revision of the manuscript. Lindsay Talbot: Dr Talbot contributed to the data interpretation, manuscript drafting, and critical revision of the manuscript. Obinna Adibe: Dr Adibe contributed to the study design, data interpretation, manuscript drafting, and critical revision of the manuscript. Henry Rice: Dr Rice contributed to the study design, data interpretation, manuscript drafting, and critical revision of the manuscript. Elisabeth Tracy: Dr Tracy contributed to the study design, data interpretation, manuscript drafting, and critical revision of the manuscript.
Contributor Information
Brian C. Gulack, Email: brian.gulack@duke.edu.
Kristy L. Rialon, Email: Kristy.rialon@duke.edu.
Jina Kim, Email: jina.kim1@duke.edu.
Lindsay J. Talbot, Email: Lindsay.jones@duke.edu.
Obinna O. Adibe, Email: Obinna.adibe@duke.edu.
Henry E. Rice, Email: henry.rice@duke.edu.
Elisabeth T. Tracy, Email: Elisabeth.tracy@duke.edu.
References
- 1.Rescorla FJ. Malignant adrenal tumors. Semin Pediatr Surg. 2006;15:48–56. doi: 10.1053/j.sempedsurg.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Miller RW, Young JL, Jr, Novakovic B. Childhood cancer. Cancer. 1995;75:395–405. doi: 10.1002/1097-0142(19950101)75:1+<395::aid-cncr2820751321>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Ciftci AO, Senocak ME, Tanyel FC, et al. Adrenocortical tumors in children. J Pediatr Surg. 2001;36:549–554. doi: 10.1053/jpsu.2001.22280. [DOI] [PubMed] [Google Scholar]
- 4.Sabbaga CC, Avilla SG, Schulz C, et al. Adrenocortical carcinoma in children: clinical aspects and prognosis. J Pediatr Surg. 1993;28:841–843. doi: 10.1016/0022-3468(93)90341-h. [DOI] [PubMed] [Google Scholar]
- 5.Michalkiewicz E, Sandrini R, Figueiredo B, et al. Clinical and outcome characteristics of children with adrenocortical tumors: a report fromthe International Pediatric Adrenocortical Tumor Registry. J Clin Oncol. 2004;22:838–845. doi: 10.1200/JCO.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 6.Wieneke JA, Thompson LD, Heffess CS. Adrenal cortical neoplasms in the pediatric population: a clinicopathologic and immunophenotypic analysis of 83 patients. Am J Surg Pathol. 2003;27:867–881. doi: 10.1097/00000478-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JN, Flageole H, Kavan P. A surgical approach to adrenocortical tumors in children: the mainstay of treatment. J Pediatr Surg. 2004;39:759–763. doi: 10.1016/j.jpedsurg.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Meyer A, Niemann U, Behrend M. Experience with the surgical treatment of adrenal cortical carcinoma. Eur J Surg Oncol. 2004;30:444–449. doi: 10.1016/j.ejso.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Erdogan I, Deutschbein T, Jurowich C, et al. The role of surgery in themanagement of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:181–191. doi: 10.1210/jc.2012-2559. [DOI] [PubMed] [Google Scholar]
- 10.Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6:719–726. doi: 10.1007/s10434-999-0719-7. [DOI] [PubMed] [Google Scholar]
- 11.Sandrini R, Ribeiro RC, DeLacerda L. Childhood adrenocortical tumors. J Clin Endocrinol Metab. 1997;82:2027–2031. doi: 10.1210/jcem.82.7.4057. [DOI] [PubMed] [Google Scholar]
- 12.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 13.McAteer JP, Huaco JA, Gow KW. Predictors of survival in pediatric adrenocortical carcinoma: a Surveillance, Epidemiology, and End Results (SEER) program study. J Pediatr Surg. 2013;48:1025–1031. doi: 10.1016/j.jpedsurg.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Hanna AM, Pham TH, Askegard-Giesmann JR, et al. Outcome of adrenocortical tumors in children. J Pediatr Surg. 2008;43:843–849. doi: 10.1016/j.jpedsurg.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115:243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 16.Children's Oncology Group. 2010. ARAR0332. Treatment of Adrenocortical Tumors with Surgery plus Lymph Node Dissection and Multiagent Chemotherapy: A Groupwide Phase III Study. [Google Scholar]
- 17.Klein JD, Turner CG, Gray FL, et al. Adrenal cortical tumors in children: factors associated with poor outcome. J Pediatr Surg. 2011;46:1201–1207. doi: 10.1016/j.jpedsurg.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 18.American College of Surgeons. National Cancer Data Base. 2013 http://www.facs.org/cancer/ncdb.
- 19.Dall'Igna P, Virgone C, De Salvo GL, et al. Adrenocortical tumors in Italian children: analysis of clinical characteristics and P53 status. Data from the national registries. J Pediatr Surg. 2014;49:1367–1371. doi: 10.1016/j.jpedsurg.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 21.Moricke A, Zimmermann M, Reiter A, et al. Prognostic impact of age in children and adolescentswith acute lymphoblastic leukemia: data fromthe trials ALL-BFM 86, 90, and 95. Klin Padiatr. 2005;217:310–320. doi: 10.1055/s-2005-872515. [DOI] [PubMed] [Google Scholar]
- 22.Miller BS, Ammori JB, Gauger PG, et al. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg. 2010;34:1380–1385. doi: 10.1007/s00268-010-0532-2. [DOI] [PubMed] [Google Scholar]
- 23.Miller BS, Gauger PG, Hammer GD, et al. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery. 2012;152:1150–1157. doi: 10.1016/j.surg.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Rice HE, Englum BR, Gulack BC, Adibe OO, Tracy ET, Kressman SG, Routh JC. Use of patient registries and administrative datasets for the study of pediatric cancer. Pediatr Blood Cancer. 2015 doi: 10.1002/pbc.25506. [DOI] [PMC free article] [PubMed] [Google Scholar]