Abstract
Malaria remains a significant public health burden with 214 million new infections and over 400,000 deaths in 2015. Elucidating relevant Plasmodium parasite biology can lead to the identification of novel ways to control and ultimately eliminate the parasite within geographic areas. Particularly, the development of an effective vaccine that targets the clinically silent pre-erythrocytic stages of infection would significantly augment existing malaria elimination tools by preventing both the onset of blood-stage infection/disease as well as spread of the parasite through mosquito transmission. In this Perspective, we discuss the role of small animal models in pre-erythrocytic stage vaccine development, highlighting how human liver-chimeric and human immune system mice are emerging as valuable components of these efforts.
KEYWORDS : antigen identification, cellular and humoral immunity, human immune system mice, human liver-chimeric mice, in vitro assays, malaria, pre-erythrocytic vaccine, Plasmodium, whole-sporozoite immunization
Malaria continues to cause significant morbidity and mortality despite renewed and concerted efforts to eliminate the causative agents, parasites of the genus Plasmodium. These efforts have largely focused on control of the Anopheles mosquito vectors, and antimalarial drug treatment of symptomatic infected individuals. The 214 million new malaria infections and 438,000 deaths due to malaria in 2015 were disproportionately borne by low income countries where malaria is endemic, and declines in malaria infections since 2000 have been slowest in countries with low resource availability and high malaria burden [1]. In the fight against malaria, effective vaccines preventing both disease and transmission will be important tools to achieve WHO goals of a 90% reduction in disease burden by 2030 [1,2]. In humans, malaria is caused predominantly by the parasite species Plasmodium falciparum and Plasmodium vivax, with the remainder of infections caused by three additional parasite species, Plasmodium malariae, Plasmodium ovale and Plasmodium knowlesi [1].
Malaria parasite transmission occurs when a Plasmodium-infected Anopheles mosquito bites and by probing the skin for a blood meal, deposits motile sporozoite stages into the host. Sporozoites pass through the dermis using active motility, enter a capillary and then rapidly home to the liver. Once in the liver sporozoites enter the parenchyma and infect hepatocytes, wherein they then transmogrify into liver stages, which grow, replicate and ultimately differentiate into tens of thousands of first-generation merozoites. Merozoites of human malaria parasites emerge from the liver into the blood stream 7–10 days after transmission, where they undergo cyclic erythrocytic infection and intraerythrocytic replication. This blood-stage infection is responsible for all mortality and clinical symptoms of malaria, as well as infection of a new mosquito vector by sexual stage parasites for onward transmission [3,4]. Stages prior to blood-stage infection (i.e., the sporozoite and liver stages) are collectively known as pre-erythrocytic (PE) stages of infection and vaccine candidates targeting these stages are collectively termed PE vaccines. By preventing progression to blood-stage infection, PE vaccines have the tremendous potential to prevent disease and death, as well as onward transmission, and have the potential to be a powerful tool in malaria eradication [5,6]. With complex genomes encoding thousands of proteins, many of which are stage-specifically expressed, Plasmodiumspp. are one of the most challenging pathogens to be targeted by vaccination [7,8].
The most clinically advanced malaria vaccine candidate named ‘RTS,S,’ is a PE subunit vaccine for P. falciparum that targets CSP, the most abundant sporozoite surface protein. RTS,S immunization engenders humoral responses with high anti-CSP antibody titers, which wane over time, as well as more limited T-cell responses [5,9,10]. Vaccination with RTS,S has been shown to achieve modest protection in malaria endemic regions with limited duration [11,12], and requires considerable improvement to reach WHO malaria vaccine efficacy goals [1,2]. Thus, the identification of new protective antigens that can be used in combination with CSP to create multivalent subunit vaccines or alternative vaccine designs built on whole attenuated sporozoites is a priority in PE vaccine development [13].
New PE antigens should elicit robust humoral responses that can block sporozoite infection and/or cellular responses that can eliminate infected hepatocytes, thereby preventing onset of blood-stage infection [5]. PE vaccine research has been encumbered by the formidable challenges associated with studying PE infection of human malaria parasites P. falciparum and P. vivax in the laboratory. In consequence, rodent malaria parasite species such as P. yoelii and P. berghei, for which PE infection is easily studied in rats and mice are used as surrogate models. As such, these malaria species serve as critical models for malaria vaccine research. In this perspective, we will explore how traditional animal models of malaria infection, in combination with in vitro assays, have contributed to the advancement of PE vaccine candidates to human clinical trials. While we acknowledge the critical contribution made by non-human primate (NHP) models of malaria [4,14], we focus on the role of small rodent models given their greater accessibility and cost–effectiveness early in vaccine discovery. Furthermore, malaria vaccine research has the advantage of a human challenge model known as controlled human malaria infection (CHMI). In this model, volunteers that are vaccinated and appropriate unvaccinated control volunteers are exposed to the bite of P. falciparum-infected mosquitoes, resulting in reproducible infection that can be rapidly and safely treated upon development of blood-stage parasitemia [15]. Thus, while malaria vaccine research is often encumbered by a lack of sophisticated immunological tools and the challenge to work directly with human parasite PE stages [4], it is buoyed by accessible rodent malaria models and by the availability of the CHMI model. A critical area for future malaria vaccine research and development will be bridging rodent malaria laboratory studies and CHMI trials [16], a challenge that is increasingly being addressed using humanized mouse models.
Lessons learned from rodent malaria models
Malaria vaccine research has greatly benefited from the availability of multiple species of parasites that infect and replicate within a rodent host. Investigation of PE-stage biology and immunity to PE stages would have been nearly impossible without rodent malaria models since, unlike the blood stages, the liver stages of P. falciparum and P. vivax cannot be effectively cultured in vitro. Furthermore, the ability to analyze the parasite infection in its first target organ in rodent malaria models is a significant advantage. Consequently, P. yoelii and P. berghei are the universal starting point for PE vaccine development, and these models have led to the generation and testing of several novel vaccine strategies and candidates, including RTS,S. Another important example is radiation-attenuated sporozoites (RAS). First published in 1967, immunization of mice with P. berghei RAS engendered complete protection from infectious sporozoite challenge [17]. This established a gold standard for PE vaccines, which was subsequently replicated with P. falciparum RAS immunizations using CHMI in humans. Today, P. falciparum RAS are being developed as a purified, cryopreserved, injectable vaccine which has shown up to 100% protection after intravenous immunization [18]. Another successful immunization strategy first identified in mice [19], and later used in humans, has been inoculation with fully infectious sporozoites under chloroquine prophylaxis. This strategy (known as ‘chemoprophylaxis with sporozoites’, CPS) allows full progression of parasite development in the liver but prevents blood-stage replication [20]. After three ‘doses’ of 15 P. falciparum-infectious mosquito bites under chloroquine cover, 100% of volunteers were protected against a 1-month CHMI and 66% were protected following a 28 months CHMI [21].
A more designed and biologically informed strategy for whole parasite vaccines is the development of genetically attenuated parasites (GAPs). While RAS are attenuated by random DNA damage, GAPs have targeted deletion of genes essential for distinct phases of liver-stage development. These genes were originally identified in gene expression screens of rodent malaria salivary gland sporozoite and liver stages [22]. From these studies the first rodent GAP was developed and successfully used in immunization-challenge studies in mice which showed protection at par or superior to RAS [23].
The first human clinical trial with a P. falciparum GAP lacking two genes thought to be essential for liver development (GAP2KO) was conducted in 2012 to assess safety, attenuation and immunogenicity. This study, however, revealed incomplete attenuation of the parasite with one out of six volunteers becoming positive for blood-stage infection [24], highlighting potential limitations in translating preclinical results generated using rodent models to humans. Despite incomplete attenuation this GAP2KO was found to be immunogenic, eliciting both T- and B-cell responses as in rodent studies [24,25]. However, the possibility of blood-stage infection is unacceptable for an attenuated parasite vaccine and a second-generation GAP incorporating a third gene deletion (GAP3KO) was developed. This second-generation GAP3KO showed a much better safety profile in more stringent preclinical tests [26], and has recently completed successful Phase I safety trials in humans in which it appears safe and immunogenic [james g kublin et al. plasmodium falciparum parasite in humans (2016)].
In addition to the development of novel vaccine strategies, the access to liver stages afforded by rodent models allows for the interrogation of innate immune responses. Once thought to be immunologically inert, the liver stages of the parasite have recently been found to indeed induce innate immune responses that are capable of limiting parasite growth [27–29]. How exactly the parasite is sensed is still under investigation [30], and it is also unknown how these early innate events could influence the ensuing adaptive response. This innate–adaptive immunity link could also be informed by measuring innate immune responses during human vaccination to identify innate markers that can predict a protective response after vaccination.
There are, however, limitations to using rodent malaria models for PE vaccine development. These include large genome (and thus potentially antigen) differences between human and rodent malaria parasite species [7]. Furthermore, there are large differences in pathology at the blood stage, with none of the rodent species fully replicating all of the nuances of blood-stage malaria in humans such as semi-immunity after years of exposure, severe malaria, cerebral malaria and placental malaria. The rodent species of malaria also differ significantly from human species during liver stage development. The human malaria parasites P. falciparum and P. vivax emerge from the liver on approximately day 7 and 10 after infection, respectively, while rodent species emerge between day 2 and 3 after infection. Furthermore, rodent malaria parasites do not form dormant liver stages, which are a hallmark of P. vivax [4]. Of course the most relevant model for early testing of PE vaccine candidates is human clinical trials, usually with CHMI as an end point.
Challenges for PE vaccine development
Following the modestly encouraging results with the first subunit vaccine for malaria, RTS,S [11,12], it is evident that next-generation PE vaccines will need to incorporate new targets other than CSP, and will need to engage the immune system in different ways. However discovery of new, effective vaccine targets requires further interrogation of basic parasite biology as well as the interaction of the parasite with the host immune system (Figure 1).
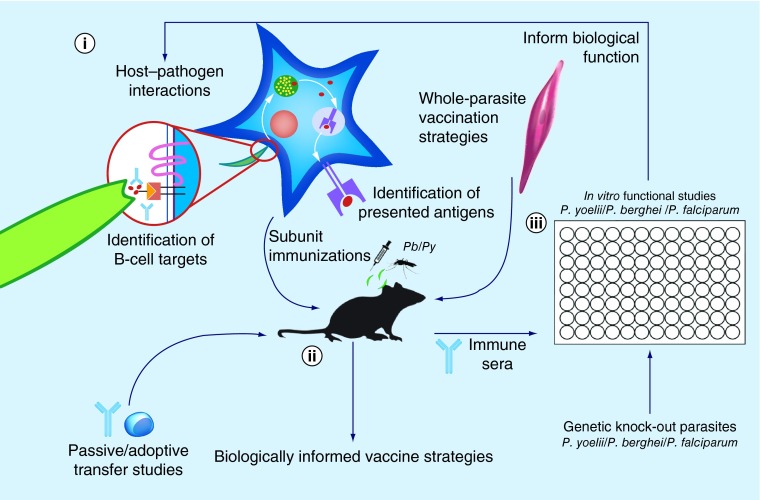
Figure 1. . Informing vaccination strategies through the study of malaria host–pathogen interactions and vaccinology.
(i) Critical surface and secreted sporozoite proteins facilitate the parasites interaction with the host during infection. Better identification and characterization of these components will yield a pool of candidate antigens suitable for humoral-based immunity in the minutes to hours between sporozoite inoculation and hepatocyte infection. Similarly, an improved understanding of antigens presented on MHC Class I during liver-stage infection will guide selection of candidate antigens suitable for engagement of cellular immunity. (ii) Studies of malaria host–pathogen interaction and vaccinology benefit tremendously from the availability of immunocompetent small animal models susceptible to rodent Plasmodium berghei and Plasmodium yoelii malaria species. Active immunization and challenge of these animals, as well as passive or adoptive transfer of antibodies or T cells, available genetic knock outs and access to tissue-resident immune cells are facilitating an increasingly detailed knowledge of the components required for an effective malaria vaccine. Additionally, whole-parasite vaccination strategies such as genetically attenuated and radiation-attenuated parasites are also developed and validated in rodent malaria models. (iii) In vitro assays, such as the sporozoite gliding motility assay and inhibition of sporozoite traversal and invasion assay, contribute to studies of host–pathogen interactions and vaccinology. Antibodies derived from immunization performed with Plasmodium falciparum proteins, as well as improving genetic tools for generating gene knock-outs, are allowing human-relevant vaccine candidates to be interrogated alongside their rodent malaria orthologs. The information collected on protein function during these studies improves our understanding of the biology of the malaria parasite and informs vaccine development.
Pb: Plasmodium berghei; Py: Plasmodium yoelii.
Identification of PE antibody targets typically focuses on the extracellular sporozoite. Antibodies binding to sporozoite surface and secreted proteins are thought to neutralize sporozoites in the skin, blood stream or liver, preventing PE infection [31–34]. Other potentially protective mechanisms of antibodies during PE infection, such as complement fixation to, and opsonic phagocytosis of sporozoites, or antibody-dependent cellular cytotoxicity that can eliminate infected hepatocytes, remain understudied in PE vaccine development.
Development of vaccines based on T-cell responses has been much more difficult, relying heavily on mouse models and, to some extent, NHP models. While antibodies and antibody specificities are now easily probed in human clinical trials, studying T-cell responses present numerous challenges. In order to protect against liver infection T cells must recognize and kill infected hepatocytes, requiring MHC-I presentation of parasite antigen on the surface of an infected hepatocyte to an antigen-specific, MHC-matched CD8+ T cell [35]. While identification of antigen-specific peripheral blood mononuclear cells (PBMCs) from humans immunized with live parasites is possible, functional validation of the ability of these T cells to kill infected hepatocytes cannot currently be tested in vitro (discussed later in this article). Adoptive transfer and evaluation of these human T cells to mice is not possible as mice do not support development of P. falciparum and P. vivax liver stages [4]. The emergence of human liver-chimeric mice, that can be infected with human malaria species (discussed later in this article) [36], somewhat overcomes this hurdle. However, a model suitable for adoptive transfer of T cells would still require additional modifications such as HLA (human version of MHC) matching between hepatocytes and donor cells and has not yet been achieved. The limitations of studying T-cell immunity to human malaria PE parasites positions rodent malaria models at the forefront of preclinical T-cell vaccine development.
Despite the advantages of unparalleled tissue access and refined immunological tools afforded by rodent malaria models, significant questions remain as to how best design a T-cell vaccine for PE malaria infection. Studies in mice have elucidated many potential mechanisms of T-cell protection against PE malaria, including identification of important T-cell subsets [37]. However, we still do not fully understand which of the many parasite antigens expressed during liver-stage development constitute good T-cell targets. For example, eliciting large numbers of T cells is only the first step. These T cells must then home to the liver and establish a liver-resident memory population for effective immunity [38]. Once in the liver, all T-cell targets may not be created equally as the T cell-hepatocyte physical interaction required for killing may be restricted by the liver architecture. Furthermore, not all parasite antigens may be presented by infected hepatocytes, which are restricted to MHC-I antigen presentation and are inefficient at cross-presentation of noncytoplasmic proteins [39]. Thus, just because a T-cell response against a particular antigen presented by a professional APC has been generated, does not imply that an infected hepatocyte will be able to present this antigen so that it can be recognized by the cognate liver-resident CD8 T cell.
Taken together, the hurdles for a T cell-based vaccine remain high as we do not know how to generate a protective liver-resident memory T-cell population with a subunit vaccine. However, a growing list of candidate antigens tested in rodent models should provide the field with P. falciparum orthologous antigens worthy of investigation as vaccine platforms progress. Simultaneously, the rapid advancement of humanized mouse models will help bridge the gap between lessons learned in standard mice and translation to human clinical trials (discussed in the 'The use of humanized mice in malaria parasite PE vaccine research' section below).
Lessons learned from CHMI trials
Efficacy of novel interventions for malaria can be assessed by CHMI, involving infection of volunteers by the bite of five P. falciparum infected mosquitos. These trials have excellent statistical power (all unvaccinated controls will become infected after CHMI), a proven safety profile (in which blood-stage infection is rapidly detected using sensitive assays and treated) and thus represent a powerful advantage for malaria vaccine research. This advantage is not afforded to many other global infectious disease vaccine development efforts. However, most CHMI trials to date are conducted with genetically identical parasites of the same strain used for immunization (homologous challenge) over time periods of 3–24 weeks, which might not accurately predict durable efficacy against antigenicaly diverse heterologous parasites in malaria-endemic area populations. CHMI trials of the subunit vaccine RTS,S showed that 3 weeks after the last dose, approximately 50% of volunteers were sterilely protected (defined as absence of detectable blood-stage infection) against homologous CHMI [40]. However over 16 weeks there was no significant sterile protection in field trials in Kenya, even though there was some protection from symptomatic malaria in approximately 30% of vaccines [41] and in Phase III studies protection was limited and not durable [11,12]. Thus, the threshold that PE vaccine candidates need to meet in CHMI trials should be complete, durable protection against homologous parasite challenge and >50% protection against heterologous challenge.
However, before PE vaccine candidates even enter CHMI, the field must develop more stringent preclinical animal models that better predict protection from infection in endemic areas. These efforts should focus on achieving high levels of protection in outbred or difficult-to-protect mouse strains, assessing greater durability of protection by extending the time of challenge to >6 months, and by using heterologous species and strains to better recapitulate the parasite diversity in areas of high transmission. In addition, while parasite and host heterogeneity may be to blame for poor translation of early-phase studies, a remaining confounding factor is that previous blood-stage infection negatively impacts the development of immunity after malaria vaccination [42]. This could be another reason for the limited efficacy of current PE vaccine candidates in malaria endemic regions, where individuals have almost inevitably suffered previous malaria infections. More recent PE vaccine trials underway in endemic areas will provide a unique opportunity to explore whether mouse models can accurately inform the immunosuppression seen in pre-exposed individuals, and if we can develop effective strategies to reverse this. Characterization of a mouse model that accurately reflects the malaria-endemic resident immune milieu would significantly aid the development of a highly efficacious PE malaria vaccine.
The current state of clinical PE vaccine development: CHMI to correlates & back
Despite the limitations discussed above, relatively small CHMI trials can mitigate the risk of pursuing large, expensive and logistically challenging trials in malaria-endemic areas with ineffective vaccine candidates. Furthermore small CHMI studies with partial efficacy, either by design or as a consequence of a suboptimal vaccine candidate, can offer the opportunity to identify correlates of protection. These correlates can then be incorporated into animal models aiming to develop optimized vaccines which harness the most potent immunological effector mechanisms. For example, anti-CSP antibodies [43], antibodies capable of inhibiting sporozoite invasion in vitro [44], IFNγ+ CD8 T cells [44], CD107a CD8 T cells [45], granzyme B-secreting CD8 T cells [45], γδ T cells [44] and CSP-specific CD4 T cells [43] have all been associated with protection in CHMI trials. However, these correlations are only as valuable as the assays used to generate them, which can be limited in scope.
The most common clinical correlate of PE protection that is easiest to assess is anti-sporozoite antibodies, which are typically measured by ELISA to sporozoite antigens such as CSP. Anti-CSP antibody titers have correlated with protection in some clinical trials [43], but they have also been negatively correlated in one other trial [46]. This is likely because the presence of large amounts of antibodies against a single sporozoite antigen does not necessarily imply the ability of these antibodies to functionally block sporozoites. To assess functionality of antibodies, in vitro assays can be performed by infecting cultured hepatocytes in the presence of immune sera and quantifying inhibition of infection [25,44,47]. However, these assays involve directly infecting hepatoma cells or primary hepatocytes in monoculture with sporozoites isolated from mosquito salivary glands. This is not an accurate model of the complex journey that sporozoites take in traversing multiple cell types in the skin, transiting through the circulation and again traversing multiple cell types in the complex 3D structure of the liver before establishing infection in a hepatocyte. While one trial has correlated inhibition of invasion in vitro to protection following RAS immunization in vivo [44], it remains to be seen if this is true for other vaccination methods.
Studies in mice have shown that a liver-resident memory T-cell population is essential for PE immunity [38]. However, T-cell assays to identify human clinical correlates of protection are limited, relying exclusively on sampling PBMCs and assessing phenotypes with or without antigenic stimulation (e.g., intracellular cytokine staining). It has also been shown in mice and NHP models that the peripheral blood compartment may not accurately reflect engagement of tissue-resident T-cell populations [48], mainly because peripheral sampling relies on capturing rare PBMCs circulating between tissues and secondary lymphoid organs leading to unreliable detection of desired T-cell subsets. This may explain results with adenoviral based vectors delivered intramuscularly, which elicits large peripheral T-cell responses against the parasite as measured from PBMCs but fails to confer significant protection, perhaps due to improper liver localization of effector and memory T cells [49]. Still, while the exact mechanisms of how T cells kill parasite-infected hepatocytes is unclear in humans, animal models have been instrumental in identifying certain effector mechanisms that can eliminate parasites such as IFN-γ and iNOS [37,50]. Mice have also afforded the opportunity to image not only the killing of infected hepatocytes by effector T cells in real-time, but also to recruit other effector lymphocytes capable of controlling infection [51]. These studies provide guidance as to what phenotypes to interrogate in human samples, which will increase our ability to identify rare immune cell populations in the periphery by focusing assays only on certain subsets. However, a critical limitation remaining is that there is currently no assay for CD8+ T-cell killing of infected hepatocytes, or for CD4 T-cell augmentation of T- or B-cell responses ex vivo.
Even with these limitations, clinical progress with PE vaccines continues to be made. This is especially true for whole sporozoite immunizations, with numerous clinical trials in progress both in malaria-naive and endemic populations [18]. These trials are investigating various dosing strategies to improve immunogenicity and delivery, and will undoubtedly significantly improve our understanding of human vaccination. Progress is also being made with antigen-based vaccinations using viral vectors or adjuvants directing T- and B-cell responses against antigens other than CSP, such as TRAP and CelTOS (these and other malaria vaccines are excellently reviewed elsewhere [52]). However, these trials at best can only reveal whether known mechanisms are present in protected individuals immunized with current-generation vaccine candidates. To best inform the development of the next generation of vaccines we will need to continue to directly interrogate these effector mechanisms in animal models, including understanding how early innate responses can impact the effectiveness of protective adaptive immune responses.
Current strategies to identify & validate novel PE vaccine targets
The parasites' infection journey from the site of inoculation in the dermis to the liver presents both opportunities and challenges for malaria vaccine development. While it provides a relatively prolonged period (minutes to hours) within which liver infection can be prevented, our incomplete understanding of this complex process has precluded identification of a large number of involved antigens that could be the target for blocking antibodies [5,53].
Each step of the infection path is mediated by multiple sporozoite proteins facilitating motility, cell traversal and invasion of hepatocytes [3,8]. Blocking of these proteins by antibodies is an attractive concept given the relative ease in producing large quantities of an antibody-based vaccine using current protein-in-adjuvant formulation strategies. However, the challenge to identify and validate the most promising targets worthy of being advanced into CHMI studies is formidable, since the molecular mechanisms governing each of these steps are only partially understood. Candidate proteins have previously been selected on the basis of their known function derived from detailed in vitro and in vivo studies. For example, TRAP has been pursued as a potential vaccine target because of its essential role in gliding motility and hepatocyte infection [54]. These and other parasite proteins with known critical functions have been the leading candidates for subunit vaccination, and have been extensively studied through genetic manipulation of the parasite. While CSP has shown some clinical success in the form of RTS,S [11], TRAP has failed to show more than modest efficacy in CHMI trials [49]. Clearly, better immunogens for existing targets, better novel targets and/or better immune responses are needed to improve existing PE vaccine strategies.
In an effort to accelerate vaccine development, groups of in vitro assays investigating antibody function against P. falciparum sporozoite proteins are increasingly being used as a precursor to animal models and CHMI trials. These antibodies are incubated with P. falciparum sporozoites and their effects measured using the gliding motility assay [55], inhibition of sporozoite traversal and invasion assay [25,44,47] and inhibition of liver-stage development assay [56]. These assays model different components of the sporozoites biological activities such as motility in the skin and traversal of multiple cell types as they seek to gain access to the liver. The significant advantage of using in vitro assays with P. falciparum sporozoites in place of simply relying on active animal immunization-challenge models with rodent malaria parasites is a much larger throughput, the ability to readily combine multiple antibodies in vitro and the advantage of directly testing with the relevant human malaria parasite. This allows candidate proteins and combinations to be rapidly down-selected on the basis of their individual and combined performance, leading to assessment of the most promising antigen combinations. These combinations can then be interrogated in a targeted fashion in rodent malaria parasites in vivo using orthologous proteins, rather than performing potentially hundreds of antigen immunization combinations in animal models. While a number of studies have suggested that antigens other than CSP are capable of conferring protection in vivo [49], their identities remain enigmatic. Increasingly, the generation and integration of multiple ‘omics’ datasets are guiding candidate antigen selection. These are being informed by transcriptomic studies, the recent identification of the sporozoite surface proteome [8], identification of antibody responses to whole sporozoite human vaccination by protein array [57] and efforts to probe antibody responses following vaccination [58].
A significant limitation of in vitro assays is that they do not fully recapitulate the entirety of the in vivo response to immunization and infection. For antibody-based responses this issue could have significant consequences, as Fc mediated functions such as sporozoite lysis by complement fixation, and sporozoite opsonization for phagocytosis by antigen-presenting cells, are not measured. Our understanding of the Fc-dependent mechanisms involved in antibody-mediated inhibition of sporozoites is poor, with limited studies providing evidence both for and against a significant role of Fc functions [59,60]. Indeed, while opsonization of recombinant P. falciparum CSP has been correlated with protection in RTS,S clinical trials [61], these studies were not performed with whole sporozoites and as such, this assay is unsuitable to investigate other candidate targets. Opsonization could be expected to have a significant role in the protective immunity of a subunit vaccine in vivo because antigen presenting cells engulfing opsonized sporozoites could augment both CD4+ and CD8+ responses. Thus, a medium- to high-throughput assay for sporozoite opsonization and APC uptake could contribute significantly to malaria vaccine development, although such an assay has yet to be developed.
Rodent models of malaria vaccinology, involving either passive transfer of immune sera to mice or active immunization strategies using candidate antigen combinations, are a critical component of vaccine development. These models can validate in vitro-selected antibody targets in an immunocompetent context, and can assess T-cell targets which cannot be screened in vitro. Antibody and T-cell responses can be probed independently by immunizations in animals either lacking or transiently depleted of relevant effector molecules or cells (e.g., Aid-/- mice which lack B cells, depleting T cells using monoclonal antibodies (mAbs) or depleting complement via cobra venom factor). Several studies have pointed to a critical role for liver-resident T cells in an effective immune response elicited by liver-stage antigens [38,44,48], while antibody responses appear to mainly function in preventing access of sporozoites to hepatocytes [62,63]. Small animal models can thus facilitate a more thorough and mechanistic investigation of PE vaccine-induced immunity by assessing the ability of candidate vaccines to activate distinct arms of the immune system and the relative contribution of each to protection.
Analysis of vaccine efficacy in vivo can be achieved using qPCR for parasite 18s RNA, an extremely precise and sensitive method for comparing parasite burden in the liver [64,65], or measurement of time to onset of blood-stage patency, a stringent method that however suffers from a lack of sensitivity in measuring partial protection. These approaches are mutually exclusive in the same animal, though, as qPCR analysis requires animal euthanasia and liver extraction prior to blood-stage infection – resulting in a doubling of animal numbers if both liver burden and time to patency are to be measured. Recently, measurement of liver-stage burden without animal sacrifice has become possible through live bioluminescent imaging techniques and the generation of parasites expressing the firefly luciferase gene [34,66–70]. This approach strikes a balance between sensitivity of measuring liver-stage burden while keeping the animal alive to measure parasite progression to blood-stage patency, thus reducing animal use and also enabling recurrent challenge for investigation of duration of protection and immunological memory.
While much of our understanding of malaria pathogenesis and the immune response to infection is derived from rodent malaria models, we cannot completely rely on them for informing clinical development decisions for human vaccine testing given the fundamental differences between man and mice and human and rodent malaria parasites. As mentioned previously, there are significant differences in the genomes and encoded antigen repertories of rodent and human malaria species, including the absence of numerous P. falciparum genes in the rodent Plasmodium spp [71]. Furthermore, the differences in the life cycle and pathogenesis between human and rodent malaria are of concern as is the narrow genetic diversity of inbred host mouse strains [72,73]. As such, candidate vaccines must ultimately be tested using more human-relevant systems.
While some aspects of P. falciparum PE infection can be modeled in vitro, such as sporozoite gliding, traversal and invasion of hepatocytes as mentioned above, intrahepatic development rates of less than 0.1% are observed in the most permissive of immortalized human cell lines [4]. These development rates can be improved through the use of primary human hepatocytes and specialized culture systems [74]; however, not all primary hepatocyte lots are susceptible to infection with P. falciparum nor do they replicate in culture. Combined with the fact that human malaria parasites do not infect mice, vaccine development using human malaria parasites has been left without an accessible small animal model.
Large animals such as NHPs are uniquely positioned as immunocompetent models that can support infection by multiple Plasmodium spp. relevant to humans. Vaccination studies using human malaria proteins with no rodent malaria ortholog are possible, with immune systems more similar to humans than that of mice [75]. Importantly, NHPs are the only model in which tissue-resident immune responses to human malaria parasite infection and vaccination can be assessed through tissue sampling, and NHP models have proven critical in P. vivax research [76]. NHP studies perhaps remain underutilized because of their significantly higher logistical, ethical and financial barriers, and while their immune systems more closely resemble that of humans, their more diverse histocompatibility complex loci could potentially misinform vaccine efficacy [75–77]. Furthermore, P. falciparum and P. vivax malaria parasites must be adapted to NHP models, a process that has been shown to result in altered parasite biology [72,78]. Because of these difficulties, researchers have focused more on pursuing methods to enhance the relevance of readily available rodent models of malaria infection.
One path to harness the power of rodent malaria models is through generation of transgenic P. yoelii and P. berghei parasites that express a P. falciparum antigen of interest. This allows sporozoite infection, complete liver-stage development and blood-stage infection within rodents, providing the opportunity to study the protective capacity of a relevant P. falciparum antigen [79]. The utility of these transgenic parasites is influenced by several factors, most notably the ability of the P. falciparum gene of interest to complement the function of its rodent parasite ortholog and generate a viable parasite. A recombinant P. berghei parasite expressing the P. falciparum CSP central repeat region has been successfully used for interrogation of antibody responses to CSP [80,81], although the absence of N-terminal and C-terminal domains of P. falciparum CSP render this parasite ill-suited to studying T-cell responses [79]. While this is a predictable outcome in the context of the well characterized protein CSP, the potential for unknown alterations in the immune profile of less well characterized antigens needs to be acknowledged when interpreting such studies. Generation of transgenic parasites can be difficult, and becomes more complex when multiple antigens (requiring multiple genetic manipulations) are to be considered simultaneously. Multi-epitope transgenic parasites have however recently been generated, and the advent of CRISPR/Cas9 usage in Plasmodium spp, may accelerate their creation by allowing direct replacement of essential genes without selection [73,82,83]. The predictive efficacy of the transgenic parasite screening approach remains unknown, and only once CHMI studies have been performed with antigens selected in this way will the utility of this model be determined definitively.
The use of humanized mice in human malaria parasite PE vaccine research
P. falciparum and P. vivax PE stages exhibit a narrow host–cell tropism in which productive infection of host cells and complete liver-stage development is largely restricted to human and great ape hepatocytes [14]. This has been a significant hurdle for studying relevant liver-stage biology, immunology and vaccinology of these pathogens. NHPs are a useful tool as they can support primate-adapted strains of both P. falciparum and P. vivax liver infection in an immunocompetent model. However, these animals are expensive, require highly specialized housing and research environments and do not support blood-stage infection unless splenectomized. The integration of findings from CHMI, NHP and readily accessible rodent models of malaria infection early in the vaccine development process has been identified as an urgent need by the malaria vaccine development community [16]. Newly available humanized mouse models to study human malaria parasite infection are emerging as bridging models that address this requirement, and could considerably improve PE preclinical vaccine evaluation in small animal models before moving to costly human trials [36].
Recently, we and others have incorporated human liver-chimeric mouse models into preclinical PE vaccine research. Given their high susceptibility to P. falciparum sporozoite infection and support of complete liver-stage development [36], they can be used both to validate complete attenuation of whole parasite P. falciparum vaccine strains [26,84] and enable the testing of antibody efficacy in protecting against sporozoite challenge by passive immunization. These analyses were previously only possible using NHPs [34,85]. Mouse models which allow xeno-engrafting of primary human hepatocytes carry genetic backgrounds that render them immunodeficient with congenital hepatotoxicity. This generates a niche within which human hepatocyte xenografts are able to replace dying murine hepatocytes, resulting in human hepatocyte repopulation that can exceed 90% of the liver parenchyma [36]. Liver humanized mice support complete development of both P. falciparum [84] and P. vivax liver stages [86], enabling for the first time in vivo pre-clinical testing of interventions against human malaria parasite PE stages in a small animal model. These studies are conducted by passive transfer of antibodies against human parasite antigens, establishing a robust link between rodent malaria models and CHMI trials. This has been demonstrated using mAbs targeting CSP of P. falciparum [85,87] and IgG from whole sporozoite-immunized human volunteers [55]. While liver humanized mice are still costly, they are far less so than NHPs both in terms of animal purchase price and housing. Thus, liver-humanized mice are best positioned as a final down-selection tool for antibody-based vaccine antigens prior to moving them forward to clinical trials, and for assessing humoral immunity from samples collected in human vaccination trials.
Because these immunocompromized mice lack an adaptive immune system, active immunizations are not possible and this constitutes a severe limitation for their use as preclinical vaccine development models [16]. Nevertheless, their ability to support in vivo development of human malaria parasites is sufficient for studies assessing the efficacy of passively transferred human-derived antibodies, and many new targets may be assessed using antibodies derived from active immunizations of other animals. These responses however may not predict the specificity or diversity of the response mounted by the human immune system (HIS). This limitation becomes apparent as we investigate novel sporozoite antibody targets where immunization of humans or NHPs with a large number of candidate subunit vaccines, to generate antibodies for detailed in vivo studies, is neither financially viable nor practical. Thus, alternative approaches are required to interrogate novel malaria vaccine candidates. Here, HIS mice are bridging the gap between traditional animal and human vaccine development platforms.
At least eight mouse models that generate human immunoglobulins have been created. Most advanced are fully human immunoglobulin transgenic animals harboring completely human V(D)J regions and with endogenous murine immunoglobulin sequences inactivated. Immunization of these animals generates a diverse antibody response against a number of pathogens [88]. Although these animals have not yet been used in the context of malaria, isolation of mAbs or polyclonal sera derived from these animals will better represent the diversity of human immunoglobulins raised against the relevant human pathogen. By collection and deep sequencing of the B cells derived from these animals, it may be possible to identify rare B-cell subsets with high protective capacity as has been done for HIV [89]. Expression of antibodies with human V(D)J sequences would generate a large number of mAbs that can be tested using in vitro and in vivo analyses. In combination with antigen mapping, this approach could significantly advance difficult antibody vaccine targets such as TRAP, that have failed to show protective efficacy despite having known essential function in sporozoite motility and hepatocyte invasion, by identifying the most protective epitopes.
While humanized B-cell mice are a significant leap forward in better representing antibodies produced by humans, it is well established that CD8 T cells are extremely effective in eliminating liver-stage infection [37]. Furthermore, CD4 T cells can greatly enhance both the CD8 and antibody responses and thus are key in the context of protective immunity to PE infection. While mice humanized in both the T- and B-cell compartment do not yet completely replicate T-cell priming in humans, mice which are capable of generating human CD8 T-cell responses have been generated and recently tested with adjuvant-based CSP immunization. These mice were able to expand CSP-specific CD8 T cells, which could mediate antigen-specific killing of liver cells transduced with CSP-expressing DNA [81]. The same group also generated a mouse reconstituted with human CD4 and B cells, which was able to generate antibodies capable of protecting against liver infection with a transgenic rodent malaria sporozoite expressing P. falciparum CSP [90]. The need for challenge with a transgenic rodent parasite in the last example highlights the major challenge of humanized mouse models for PE malaria vaccine research: the need for dual humanization of the immune compartment and the target organ of the pathogen, such as the liver. A mouse model has recently been created which does contain a humanized liver and bone marrow, but this has not been tested for functionality of the immune compartment [91]. The progress and limitations of developing this mouse model has recently been excellently reviewed elsewhere [92].
At this point, HIS mice or even dual-chimeric mice still do not and probably cannot completely replicate human immunobiology. Even though liver-chimeric mice have a high percentage of human hepatocytes, their liver sinusoidal endothelial cells, Kupffer cells, stellate cells and remaining innate immune cells are all of mouse origin. Some of these cells may be important for immune responses to immunization with live-attenuated sporozoites, or even for controlling infection. HIS mice also have several shortcomings as current models may not be able to recapitulate tissue-trafficking and tissue residence of immune cells, proper germinal center formation or education of both T and B cells in a manner that replicates a human thymus with human MHC molecules. However, attempts to overcome these challenges are underway, and in the meantime researchers are combining the best clinical, in vitro and in vivo models to improve our understanding of how to effectively target malaria infection with the immune system.
Humanized mouse models are thus facilitating a critical step in the PE malaria vaccine development pipeline, linking human CHMI studies with established rodent malaria models early in the PE vaccine development process (Figure 2). P. falciparum candidate antigens can now be tested directly rather than relying on results from immunizations with P. yoelii and P. berghei orthologues, and this testing is strengthened by the use of HIS mice to generate human relevant immunoglobulins. As such, these humanized mice are informing the development of novel vaccine strategies, although they are also important for the re-engineering of existing vaccines. Passive transfer of immunoglobulin isolated from volunteers in CHMI studies to human liver-chimeric mice challenged by mosquito bite now offers a physiologically relevant manner in which to dissect the contribution of antibodies in protection with novel vaccine candidates. Where a significant role for antibodies is observed, P. falciparum antigen arrays can be used to identify novel vaccine candidate proteins. These candidates can then enter the vaccine development pipeline in standard rodent malaria models and humanized mice to reverse-engineer effective multisubunit vaccine components. However, humanized mice are not suitable for all PE vaccine development processes. Traditional rodent models are still required for functional characterization of new vaccine targets, because of the greater ease of genetic manipulation of these parasites, availability of immunocompetent mouse models as well has models lacking specific parts of the immune system (Figure 1). Standard rodent malaria models will be required for the development of next-generation whole parasite vaccines, such GAPs, although human liver-chimeric mice are useful for verifying the attenuation phenotypes of the next generation of GAPs generated in P. falciparum, before CHMI. HIS mice are now the preferred animal host for transgenic parasite challenge studies, because their active immunization partially reflects what can be expected in human subjects.
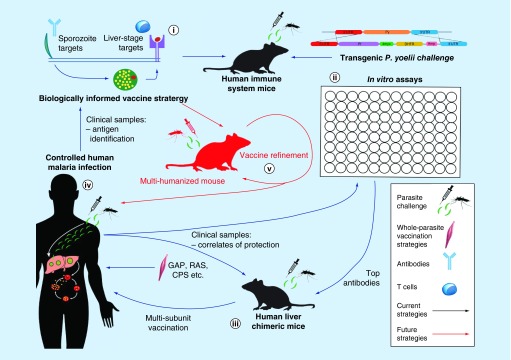
Figure 2. . Pre-erythrocytic vaccine research with human malaria parasites.
(i) Candidate vaccine antigens are identified from host–pathogen interaction studies, vaccinology with rodent parasites and systems biology approaches from rodent and human malaria parasite species. These candidates may include sporozoite proteins (significant antibody targets) and liver-stage antigens (significant T-cell targets). Immunization of human immune system mice enables these antigens to generate a B- or T-cell response that could be expected to better predict efficacy in human subjects. (ii) Immunizing and challenging HIS mice with Plasmodium yoelii or Plasmodium berghei transgenic parasites enables the in vivo protective capacity of human malaria proteins to be measured with partially humanized immune responses. Using in vitro analyses, antigen capacity to generate antigen-specific effector T cells (intracellular cytokine staining) and effective antibody responses (sporozoite gliding motility assay, inhibition of sporozoite traversal and invasion assay, inhibition of liver-stage development assay) can be used to rank candidate antigens for further testing. Antibodies, alone or in combination, can then be tested for in vivo protective efficacy against P. falciparum in human liver-chimeric mice (iii). This pipeline, involving both in vivo and in vitro model systems, recapitulates multiple aspects of human pre-erythrocytic (PE) infection and immunity. The resulting candidate vaccination strategies are expected to more accurately predict the outcome of future controlled human malaria infection trials (iv). Clinical samples from human trials can then be used to probe for correlates of protection using available assays, including the identification of novel candidate antigens using emerging antibody and T-cell array technologies. (v) The ultimate goal for human PE vaccine development is to achieve cross-stage protection, where a single strategy protects against both PE and blood-stage infection. Humanization of multiple compartments will be required to measure cross-stage protection, including liver, hematopoietic and immune compartments. Such an animal would enable direct immunization with human parasite immunogens and challenge with human parasites, facilitating an accessible immunocompetent model in which human vaccines can be refined before moving directly to controlled human malaria infection.
CPS: Chemoprophylaxis with sporozoites; GAP: Genetically-attenuated parasite; RAS: Radiation-attenuated sporozoite.
Future perspective
While conventional mouse models have been the workhorses of infectious disease research for many decades, humanized mice have recently revolutionized the direct study of human pathogens such as HCV, HIV and human malaria parasites. In malaria research, the availability of human liver-chimeric mice has allowed interrogation of human malaria parasite PE stage infection at a level that was previously impossible. This enables discovery of liver stage-expressed proteins as vaccine targets and for interrogation of antibodies acting on the sporozoite stage during its journey from the skin to the liver. The liver stages of P. vivax including the dormant hypnozoite stage are also accessible as never before and are under intense study in these mice [86]. Human liver-chimeric mice have also revolutionized the study of human parasite genetics as they have greatly increased the ease of genetic crosses of P. falciparum strains. This has the potential to expand our knowledge of everything from drug resistance to the underpinnings of malaria sexual reproduction and genetic diversity [93]. Mice with humanized immune systems are also a boon to vaccine development as novel vaccination regimens can be tested in a manner that more closely mimics what we would expect in humans. This type of strategy is already beginning to be used and will also form a bridge between conventional mouse studies and human clinical studies [90].
An ideal malaria vaccine candidate would target multiple lifecycle stages of the parasite, with some combination of protection against sporozoites, liver stages, blood stages or transmission to the mosquito vector. To model such vaccines will require a combination of multitissue humanized mouse models including the liver, bone marrow, peripheral lymphoid system and the erythrocytic compartment. However, even if this type of model is achievable, significant hurdles remain such as the need for HLA matching of the solid tissue and the immune compartment, as well as overcoming the complexity of producing human immune cells that are educated in the periphery by mouse thymic cells. These hurdles are however being tackled, for instance with dual-chimeric mice and the expression of human MHC in the periphery [90,91]. While such a highly advanced humanized mouse model can and should be pursued, existing tools can already be used in iterative cycles of identifying vaccine targets through basic parasitology conducted in rodent models and in vitro models of P. falciparum, testing of vaccine candidates in human CHMI clinical trials and testing of the resulting samples in human liver-chimeric mice. Leads generated in these studies can be further honed in rodent models and finally in HIS mice before further testing in humans (Figures 1 & 2). The malaria vaccine candidates of the future will thus be composed of rationally selected targets that engage components of the immune system deemed most relevant. These will be informed as a result of data collected harmoniously across multiple model systems and ultimately human clinical studies, thereby increasing the likelihood of success for candidates in costly, later phase endemic area clinical trials.
EXECUTIVE SUMMARY.
Background
Pre-erythrocytic (PE) antigens of the clinically silent stages of the Plasmodium lifecycle (from sporozoite infection of the skin to liver-stage development) are excellent vaccine targets, capable of preventing disease and transmission.
Lessons learned from rodent malaria models
Today’s leading vaccination strategies were developed in mice using rodent malaria models and have demonstrated considerable protection when transitioned to controlled human malaria infection (CHMI) studies in the USA and Europe.
Rodent malaria models afford access to developing liver stages in vivo, facilitating the study of biology, as well as innate and adaptive responses to infection at a level not possible with human vaccination and infection.
Rodent malaria parasites present shortcomings in that they differ in genome, biology of life cycle stages and pathology limiting their power in predicting outcomes in humans.
Challenges for PE vaccine development
Human malaria parasites exhibit a narrow host cell tropism and are unable to infect rodent hepatocytes.
Model systems to determine T-cell antigen specificity from CHMI studies would significantly inform vaccine development.
Lessons learned from CHMI trials
Vaccine protection observed in malaria endemic populations is often significantly lower than what is observed with CHMI. This demonstrates a requirement to improve vaccine development models and protection studies.
The current state of clinical PE vaccine development: CHMI to correlates & back
CHMI studies resulting in protection of only some individuals have resulted in the identification of correlates of protection. These correlates can now further guide vaccine development efforts.
In vitro studies used to determine correlates may not accurately reflect the immune status of the individual sampled, nor can they always mimic the interaction between the parasite and the immune system.
Current strategies to identify & validate novel PE vaccine targets
The complex motility and invasion apparatus of the sporozoite presents a large number of proteins against which antibodies may be able to prevent PE infection.
Identification of sporozoite proteins able to generate protective antibody responses is an active area of research. Animal models with rodent malaria species, transgenic rodent parasites expressing Plasmodium falciparum antigens of interest and in vitro assays with P. falciparum sporozoites are increasingly being used in combination to identify and validate these targets.
Rodent and non-human primate models of malaria infection remain critical for the study of protective T-cell responses.
The use of humanized mice in human malaria parasite PE vaccine research
Human liver-chimeric mice support both P. falciparum and P. vivax liver-stage development, facilitating study of liver-stage biology as well as providing an in vivo model in which protection by antibodies can be assessed.
Human immune system mice allow a more accurate modeling of the human response to immunization to be incorporated into pre-clinical testing of vaccine candidates.
Future perspective
Development of mice humanized in multiple tissue compartments that are able to support thecomplete life cycle of human malaria parasites and mount an adaptive human-like immune response will enable the study of vaccines eliciting protection against multiple stages of the malaria life cycle.
In lieu of a multiple-tissue-humanized mouse, the current combination of rodent malaria models, clinical trials with novel vaccine candidates, in vitro assays for the human malarias and newly developed humanized mouse models provide a framework for designing the next generation of biologically and immunologically-informed malaria vaccine candidates.
Acknowledgements
We thank A Pellicciotti for assistance with graphic design, and apologize to all colleagues whose work could not be cited due to space constraints.
Footnotes
Financial & competing interests disclosure
Work by the authors is funded by the National Institute of Allergy and Infectious Diseases (NIAID) (grant numbers NIH 5 F32 AI114113-03 and R01AI117234), the Malaria Vaccine Initiative (MVI), the Bill & Melinda Gates Foundation (BMGF) and the US Department of Defense (DoD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutions. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest, •• of considerable interest
- 1.Organization WH. World Malaria Report 2015. http://apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf?ua=1
- 2.Organization WH. Global Technical Strategy for Malaria 2016–2030. http://apps.who.int/iris/bitstream/10665/176712/1/9789241564991_eng.pdf?ua=1&ua=1
- 3.Kaushansky A, Kappe SHI. Selection and refinement: the malaria parasite’s infection and exploitation of host hepatocytes. Curr. Opin. Microbiol. 2015;26:71–78. doi: 10.1016/j.mib.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prudencio M, Mota MM, Mendes AM. A toolbox to study liver stage malaria. Trends Parasitol. 2011;27(12):565–574. doi: 10.1016/j.pt.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman SL, Vekemans J, Richie TL, et al. The march toward malaria vaccines. Am. J. Prev. Med. 2015;49(6):S319–333. doi: 10.1016/j.amepre.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kappe SHI, Vaughan AM, Boddey JA, et al. That was then but this is now: malaria research in the time of an eradication agenda. Science. 2010;328(5980):862–866. doi: 10.1126/science.1184785. [DOI] [PubMed] [Google Scholar]
- 7.Carlton JM, Angiuoli SV, Suh BB, et al. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii . Nature. 2002;419(6906):512–519. doi: 10.1038/nature01099. [DOI] [PubMed] [Google Scholar]
- 8.Lindner SE, Swearingen KE, Harupa A, et al. Total and putative surface proteomics of malaria parasite salivary gland sporozoites. Mol. Cell Proteomics. 2013;12(5):1127–1143. doi: 10.1074/mcp.M112.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoute JA, Slaoui M, Heppner DG, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N. Engl. J. Med. 1997;336(2):86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 10.Coppi A, Natarajan R, Pradel G, et al. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J. Exp. Med. 2011;208(2):341–356. doi: 10.1084/jem.20101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RTS S CTP. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a Phase 3, individually randomised, controlled trial. Lancet. 2015;386(9988):31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olotu A, Fegan G, Wambua J, et al. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N. Engl. J. Med. 2016;374(26):2519–2529. doi: 10.1056/NEJMoa1515257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler NS, Vaughan AM, Harty JT, et al. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 2012;33(5):247–254. doi: 10.1016/j.it.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Beignon AS, Le Grand R, Chapon C. In vivo imaging in NHP models of malaria: challenges, progress and outlooks. Parasitol. Int. 2014;63(1):206–215. doi: 10.1016/j.parint.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spring M, Polhemus M, Ockenhouse C. Controlled human malaria infection. J. Infect. Dis. 2014;209(Suppl. 2):S40–S45. doi: 10.1093/infdis/jiu063. [DOI] [PubMed] [Google Scholar]
- 16.Langhorne J, Buffet P, Galinski M, et al. The relevance of non-human primate and rodent malaria models for humans. Malar. J. 2011;10:23. doi: 10.1186/1475-2875-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nussenzweig RS, Vanderberg J, Most H, et al. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei . Nature. 1967;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]; •• The first example of using radiation-attenuated malaria sporozoites as a vaccine in mice, which is now the most efficacious pre-erythrocytic vaccine strategy in clinical development.
- 18.Hoffman SL, Vekemans J, Richie TL, et al. The march toward malaria vaccines. Am. J. Prev. Med. 2015;49(6 Suppl. 4):S319–S333. doi: 10.1016/j.amepre.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaudoin RL, Strome CP, Mitchell F, et al. Plasmodium berghei: immunization of mice against the ANKA strain using the unaltered sporozoite as an antigen. Exp. Parasitol. 1977;42(1):1–5. doi: 10.1016/0014-4894(77)90054-6. [DOI] [PubMed] [Google Scholar]
- 20.Keitany GJ, Vignali M, Wang R. Live attenuated pre-erythrocytic malaria vaccines. Hum. Vaccin. Immunother. 2014;10(10):2903–2909. doi: 10.4161/21645515.2014.972764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roestenberg M, Teirlinck AC, Mccall MB, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet. 2011;377(9779):1770–1776. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser K, Matuschewski K, Camargo N, et al. Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol. Microbiol. 2004;51(5):1221–1232. doi: 10.1046/j.1365-2958.2003.03909.x. [DOI] [PubMed] [Google Scholar]; • An example of probing the rodent transcriptome to identify genes that could be deleted to create a genetically attenuated parasite. This would later form the rationale to develop Plasmodium falciparum genetically attenuated parasites currently in much-anticipated clinical studies.
- 23.Mueller AK, Labaied M, Kappe SH, et al. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433(7022):164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 24.Spring M, Murphy J, Nielsen R, et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine. 2013;31(43):4975–4983. doi: 10.1016/j.vaccine.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Finney OC, Keitany GJ, Smithers H, et al. Immunization with genetically attenuated P. falciparum parasites induces long-lived antibodies that efficiently block hepatocyte invasion by sporozoites. Vaccine. 2014;32(19):2135–2138. doi: 10.1016/j.vaccine.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikolajczak SA, Lakshmanan V, Fishbaugher M, et al. A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol. Ther. 2014;22(9):1707–1715. doi: 10.1038/mt.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liehl P, Zuzarte-Luis V, Chan J, et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat. Med. 2014;20(1):47–53. doi: 10.1038/nm.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller JL, Sack BK, Baldwin M, et al. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell. Rep. 2014;7(2):436–447. doi: 10.1016/j.celrep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Liehl P, Meireles P, Albuquerque IS, et al. Innate immunity induced by Plasmodium liver infection inhibits malaria reinfections. Infect. Immun. 2015;83(3):1172–1180. doi: 10.1128/IAI.02796-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng H, Tan Z, Zhou T, et al. The TLR2 is activated by sporozoites and suppresses intrahepatic rodent malaria parasite development. Sci. Rep. 2015;5:8239. doi: 10.1038/srep18239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart MJ, Nawrot RJ, Schulman S, et al. Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect. Immun. 1986;51(3):859–864. doi: 10.1128/iai.51.3.859-864.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 2004;34(9):991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Kebaier C, Voza T, Vanderberg J. Kinetics of mosquito-injected Plasmodium sporozoites in mice: fewer sporozoites are injected into sporozoite-immunized mice. PLoS Pathog. 2009;5(4):e1000399. doi: 10.1371/journal.ppat.1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sack BK, Miller JL, Vaughan AM, et al. Model for in vivo assessment of humoral protection against malaria sporozoite challenge by passive transfer of monoclonal antibodies and immune serum. Infect. Immun. 2014;82(2):808–817. doi: 10.1128/IAI.01249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trimnell A, Takagi A, Gupta M, et al. Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes. J. Immunol. 2009;183(9):5870–5878. doi: 10.4049/jimmunol.0900302. [DOI] [PubMed] [Google Scholar]
- 36.Vaughan AM, Kappe SHI, Ploss A, et al. Development of humanized mouse models to study human malaria parasite infection. Future Microbiol. 2012;7(5):657–665. doi: 10.2217/fmb.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Braeckel-Budimir N, Harty JT. CD8 T-cell-mediated protection against liver-stage malaria: lessons from a mouse model. Front. Microbiol. 2014;5:272. doi: 10.3389/fmicb.2014.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tse SW, Radtke AJ, Espinosa DA, et al. The chemokine receptor CXCR6 is required for the maintenance of liver memory CD8(+) T cells specific for infectious pathogens. J. Infect. Dis. 2014;210(9):1508–1516. doi: 10.1093/infdis/jiu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grakoui A, Crispe IN. Presentation of hepatocellular antigens. Cell. Mol. Immunol. 2016;13(3):293–300. doi: 10.1038/cmi.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kester KE, Cummings JF, Ofori-Anyinam O, et al. Randomized, double-blind, Phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 2009;200(3):337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 41.Polhemus ME, Remich SA, Ogutu BR, et al. Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLoS ONE. 2009;4(7):e6465. doi: 10.1371/journal.pone.0006465. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Seminal trial of RTS,S in Sub-Saharan Africa in malaria-exposed individuals demonstrating the difficulty in translating efficacy results from CHMI in Western populations to endemic areas.
- 42.Portugal S, Pierce SK, Crompton PD. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J. Immunol. 2013;190(7):3039–3046. doi: 10.4049/jimmunol.1203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White MT, Bejon P, Olotu A, et al. The Relationship between RTS,S Vaccine-Induced Antibodies, CD4(+) T Cell Responses and Protection against Plasmodium falciparum Infection. PLoS ONE. 2013;8(4):e61395. doi: 10.1371/journal.pone.0061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seder RA, Chang LJ, Enama ME, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 45.Bijker EM, Teirlinck AC, Schats R, et al. Cytotoxic markers associate with protection against malaria in human volunteers immunized with Plasmodium falciparum sporozoites. J. Infect. Dis. 2014;210(10):1605–1615. doi: 10.1093/infdis/jiu293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nahrendorf W, Scholzen A, Bijker EM, et al. Memory B-cell and antibody responses induced by Plasmodium falciparum sporozoite immunization. J. Infect. Dis. 2014;210(12):1981–1990. doi: 10.1093/infdis/jiu354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaushansky A, Rezakhani N, Mann H, et al. Development of a quantitative flow cytometry-based assay to assess infection by Plasmodium falciparum sporozoites. Mol. Biochem. Parasitol. 2012;183(1):100–103. doi: 10.1016/j.molbiopara.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Epstein JE, Tewari K, Lyke KE, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science. 2011;334(6055):475–480. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 49.Ewer KJ, Sierra-Davidson K, Salman AM, et al. Progress with viral vectored malaria vaccines: a multi-stage approach involving “unnatural immunity”. Vaccine. 2015;33(52):7444–7451. doi: 10.1016/j.vaccine.2015.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frevert U, Krzych U. Plasmodium cellular effector mechanisms and the hepatic microenvironment. Front. Microbiol. 2015;6:482. doi: 10.3389/fmicb.2015.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cockburn IA, Amino R, Kelemen RK, et al. In vivo imaging of CD8(+) T cell-mediated elimination of malaria liver stages. Proc. Natl Acad. Sci. USA. 2013;110(22):9090–9095. doi: 10.1073/pnas.1303858110. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Elegant in vivo demonstration of direct CD8 T-cell killing infected hepatocytes using intravital imaging.
- 52.Ouattara A, Laurens MB. Vaccines against malaria. Clin. Infect. Dis. 2015;60(6):930–936. doi: 10.1093/cid/ciu954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mokgethi-Morule T, N’da DD. Cell based assays for anti-Plasmodium activity evaluation. Eur. J. Pharm. Sci. 2016;84:26–36. doi: 10.1016/j.ejps.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Dunachie SJ, Walther M, Epstein JE, et al. A DNA prime-modified vaccinia virus Ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect. Immun. 2006;74(10):5933–5942. doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Behet MC, Foquet L, Van Gemert GJ, et al. Sporozoite immunization of human volunteers under chemoprophylaxis induces functional antibodies against pre-erythrocytic stages of Plasmodium falciparum . Malar. J. 2014;13:136. doi: 10.1186/1475-2875-13-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou X, House BL, Zyzak MD, et al. Towards an optimized inhibition of liver stage development assay (ILSDA) for Plasmodium falciparum . Malar. J. 2013;12:394. doi: 10.1186/1475-2875-12-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felgner PL, Roestenberg M, Liang L, et al. Pre-erythrocytic antibody profiles induced by controlled human malaria infections in healthy volunteers under chloroquine prophylaxis. Sci. Rep. 2013;3:3549. doi: 10.1038/srep03549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aguiar JC, Bolton J, Wanga J, et al. Discovery of novel Plasmodium falciparum pre-erythrocytic antigens for vaccine development. PLoS ONE. 2015;10(8):e0136109. doi: 10.1371/journal.pone.0136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yilmaz B, Portugal S, Tran TM, et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 2014;159(6):1277–1289. doi: 10.1016/j.cell.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Potocnjak P, Yoshida N, Nussenzweig RS, et al. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J. Exp. Med. 1980;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwenk R, Asher LV, Chalom I, et al. Opsonization by antigen-specific antibodies as a mechanism of protective immunity induced by Plasmodium falciparum circumsporozoite protein-based vaccine. Parasite Immunol. 2003;25(1):17–25. doi: 10.1046/j.1365-3024.2003.00495.x. [DOI] [PubMed] [Google Scholar]
- 62.Keitany GJ, Sack B, Smithers H, et al. Immunization of mice with live-attenuated late liver stage-arresting Plasmodium yoelii parasites generates protective antibody responses to preerythrocytic stages of malaria. Infect. Immunol. 2014;82(12):5143–5153. doi: 10.1128/IAI.02320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vanderberg J, Mueller AK, Heiss K, et al. Assessment of antibody protection against malaria sporozoites must be done by mosquito injection of sporozoites. Am. J. Pathol. 2007;171(4):1405–1406. doi: 10.2353/ajpath.2007.070661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bruna-Romero O, Hafalla JC, Gonzalez-Aseguinolaza G, et al. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 2001;31(13):1499–1502. doi: 10.1016/s0020-7519(01)00265-x. [DOI] [PubMed] [Google Scholar]
- 65.Foquet L, Meuleman P, Hermsen CC, et al. Assessment of parasite liver-stage burden in human-liver chimeric mice. Methods Mol. Biol. 2015;1325:59–68. doi: 10.1007/978-1-4939-2815-6_5. [DOI] [PubMed] [Google Scholar]
- 66.Mwakingwe A, Ting LM, Hochman S, et al. Noninvasive real-time monitoring of liver-stage development of bioluminescent Plasmodium parasites. J. Infect. Dis. 2009;200(9):1470–1478. doi: 10.1086/606115. [DOI] [PubMed] [Google Scholar]
- 67.Ploemen I, Behet M, Nganou-Makamdop K, et al. Evaluation of immunity against malaria using luciferase-expressing Plasmodium berghei parasites. Malar. J. 2011;10:350. doi: 10.1186/1475-2875-10-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaughan AM, Mikolajczak SA, Camargo N, et al. A transgenic Plasmodium falciparum NF54 strain that expresses GFP-luciferase throughout the parasite life cycle. Mol. Biochem. Parasitol. 2012;186(2):143–147. doi: 10.1016/j.molbiopara.2012.10.004. [DOI] [PubMed] [Google Scholar]; • Demonstrates complete P. falciparum development in the livers of humanized liver mice. This significantly expanded the ability to study the liver stages of human malaria including Plasmodium vivax.
- 69.Miller JL, Murray S, Vaughan AM, et al. Quantitative bioluminescent imaging of pre-erythrocytic malaria parasite infection using luciferase-expressing Plasmodium yoelii . PLoS ONE. 2013;8(4):e60820. doi: 10.1371/journal.pone.0060820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Annoura T, Chevalley S, Janse CJ, et al. Quantitative analysis of Plasmodium berghei liver stages by bioluminescence imaging. Methods Mol. Biol. 2013;923:429–443. doi: 10.1007/978-1-62703-026-7_30. [DOI] [PubMed] [Google Scholar]
- 71.Fidock DA, Gras-Masse H, Lepers JP, et al. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J. Immunol. 1994;153(1):190–204. [PubMed] [Google Scholar]
- 72.Teixeira C, Gomes R. Experimental models in vaccine research: malaria and leishmaniasis. Braz. J. Med. Biol. Res. 2013;46(2):109–116. doi: 10.1590/1414-431X20122460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ewer KJ, Sierra-Davidson K, Salman AM, et al. Progress with viral vectored malaria vaccines: a multi-stage approach involving “unnatural immunity”. Vaccine. 2015;33(52):7444–7451. doi: 10.1016/j.vaccine.2015.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.March S, Ng S, Velmurugan S, et al. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe. 2013;14(1):104–115. doi: 10.1016/j.chom.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joyner C, Barnwell JW, Galinski MR. No more monkeying around: primate malaria model systems are key to understanding Plasmodium vivax liver-stage biology, hypnozoites, and relapses. Front. Microbiol. 2015;6:145. doi: 10.3389/fmicb.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campo B, Vandal O, Wesche DL, et al. Killing the hypnozoite – drug discovery approaches to prevent relapse in Plasmodium vivax . Pathog. Glob. Health. 2015;109(3):107–122. doi: 10.1179/2047773215Y.0000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beignon A-S, Le Grand R, Chapon C. In vivo imaging in NHP models of malaria: challenges, progress and outlooks. Parasitology Int. 2014;63(1):206–215. doi: 10.1016/j.parint.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Obaldia N, Iii, Dow GS, Gerena L, et al. Altered drug susceptibility during host adaptation of a Plasmodium falciparum strain in a non-human primate model. Sci. Rep. 2016;6:21216. doi: 10.1038/srep21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang M, Kaneko I, Tsao T, et al. A highly infectious Plasmodium yoelii parasite, bearing Plasmodium falciparum circumsporozoite protein. Malar. J. 2016;15:8. doi: 10.1186/s12936-016-1248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deal C, Balazs AB, Espinosa DA, et al. Vectored antibody gene delivery protects against Plasmodium falciparum sporozoite challenge in mice. Proc. Natl Acad. Sci. USA. 2014;111(34):12528–12532. doi: 10.1073/pnas.1407362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang J, Li X, Coelho-Dos-Reis JG, et al. An AAV vector-mediated gene delivery approach facilitates reconstitution of functional human CD8+ T cells in mice. PLoS ONE. 2014;9(2):e88205. doi: 10.1371/journal.pone.0088205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Koning-Ward TF, Gilson PR, Crabb BS. Advances in molecular genetic systems in malaria. Nat. Rev. Microbiol. 2015;13(6):373–387. doi: 10.1038/nrmicro3450. [DOI] [PubMed] [Google Scholar]
- 83.Longley RJ, Hill AVS, Spencer AJ. Malaria vaccines: identifying Plasmodium falciparum liver-stage targets. Front. Microbiol. 2015;6:965. doi: 10.3389/fmicb.2015.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vaughan AM, Mikolajczak SA, Wilson EM, et al. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J. Clin. Invest. 2012;122(10):3618–3628. doi: 10.1172/JCI62684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foquet L, Hermsen CC, Van Gemert GJ, et al. Vaccine-induced monoclonal antibodies targeting circumsporozoite protein prevent Plasmodium falciparum infection. J. Clin. Invest. 2013;122(10):3618–3628. doi: 10.1172/JCI70349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mikolajczak SA, Vaughan AM, Kangwanrangsan N, et al. Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host Microbe. 2015;17(4):526–535. doi: 10.1016/j.chom.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sack BK, Miller JL, Vaughan AM, et al. A model for in vivo assessment of humoral protection against malaria sporozoite challenge by passive transfer of monoclonal antibodies and immune serum. Infect. Immun. 2013;82(2):808–817. doi: 10.1128/IAI.01249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brueggemann M, Osborn MJ, Ma B, et al. Human antibody production in transgenic animals. Arch. Immunol. Ther. Exp. 2015;63(2):101–108. doi: 10.1007/s00005-014-0322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sattentau QJ. Immunogen design to focus the B-cell repertoire. Curr. Opin. HIV AIDS. 2014;9(3):217–223. doi: 10.1097/COH.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 90.Huang J, Li X, Coelho-Dos-Reis JG, et al. Human immune system mice immunized with Plasmodium falciparum circumsporozoite protein induce protective human humoral immunity against malaria. J. Immunol. Methods. 2015;427:42–50. doi: 10.1016/j.jim.2015.09.005. [DOI] [PubMed] [Google Scholar]; •• Use of human immune system (HIS) mice to elicit functional antibody responses to circumsporozoite protein which were then tested for functionality with transgenic rodent parasites. A unique combination of two cutting-edge technologies in pre-erythrocytic vaccine development.
- 91.Wilson EM, Bial J, Tarlow B, et al. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res. 2014;13(Pt.3A):404–412. doi: 10.1016/j.scr.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Good MF, Hawkes MT, Yanow SK. Humanized mouse models to study cell-mediated immune responses to liver-stage malaria vaccines. Trends Parasitol. 2015;31(11):583–594. doi: 10.1016/j.pt.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 93.Vaughan AM, Pinapati RS, Cheeseman IH, et al. Plasmodium falciparum genetic crosses in a humanized mouse model. Nat. Methods. 2015;12(7):631. doi: 10.1038/nmeth.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]