Abstract
Aim:
Ascorbate can inhibit growth and even decrease viability of various microbial species including Candida albicans. However the optimum conditions and the mechanism of action are unclear.
Materials/methodology:
Candida albicans shaken for 90 min in a buffered solution of ascorbate (90 mM) gave a 5-log reduction of cell viability, while there was no killing without shaking, in growth media with different carbon sources or at 4°C. Killing was inhibited by the iron chelator 2,2′-bipyridyl. Hydroxyphenyl fluorescein probe showed the intracellular generation of hydroxyl radicals.
Results/conclusion:
Ascorbate-mediated killing of C. albicans depends on oxygenation and metabolism, involves iron-catalyzed generation of hydroxyl radicals via Fenton reaction and depletion of intracellular NADH. Ascorbate could serve as a component of a topical antifungal therapy.
KEYWORDS : ascorbate, Candida albicans, hydroxyl radicals, oxidative stress, vitamin C
Candida albicans is a major opportunistic fungal pathogen that is associated with a range of clinical conditions. The organism can lead to superficial infections of genital, oral and cutaneous sites as well as systemic infections, especially in hospitalized and/or immunocompromised patients, such as those suffering from AIDS or undergoing chemotherapy [1–4]. Although most superficial infections caused by C. albicans are not life threatening, these infections can have debilitating effects on the patient’s quality of life. Several classes of antifungal agents (e.g., azoles, echinocandins) have been developed which are active against Candida infections; however, possible drug interactions and adverse side-effects, emergence of drug-resistant isolates, as well as the high costs of some of these agents underscore the necessity for development of additional treatment alternatives [3,5].
Ascorbate (vitamin C, ascorbic acid) is an essential vitamin and is considered to be a potent antioxidant owing to its ability to act as an electron-donating reducing agent, and a quencher of reactive oxygen species (ROS) [6]. However, there is also increasing evidence that at pharmacologic concentrations, ascorbate can exert pro-oxidant effects via the reduction of transition metal ions, such as iron and copper [7]. Previous studies have shown that high-dose ascorbate exerts selective cytotoxic effects on cancer cells both in vitro and in vivo [6,8]. There have also been several clinical trials in cancer patients, testing the effects of high-dose ascorbate either alone or in combination with chemotherapy, but the results are still controversial [9]. Although the mechanism of action is still not well understood it has been proposed to depend on oxidation of ascorbate. The Fenton reaction and the coupled Haber–Weiss reaction together comprise the following steps: (Equation 1) ferrous ion reacts with oxygen to produce superoxide (Equation 2), which in turn generates H2O2 via dismutation (Equation 3), H2O2 then reacts with ferrous ion to form HO. (Equation 4) ascorbate can reduce Fe3+ back to Fe2+ to allow the cycle to continue [10].
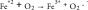 |
(1) |
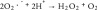 |
(2) |
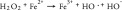 |
(3) |
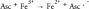 |
(4) |
A few studies also show that ascorbate could function as a potential anti-infective agent, but the literature is scarce and sometimes conflicting [11–15]. Vilcheze and colleagues in 2013, reported that 4 mM ascorbate could sterilize both drug-susceptible and drug-resistant Mycobacterium tuberculosis, however several weeks of incubation was required for ascorbate to exert its effects in vitro. On the other hand, when cultures were treated with ascorbate in an anaerobic chamber, ascorbate had no effect on M. tuberculosis viability [11]. In another study by Tabak et al. it was shown that 100 mM ascorbate incubated for 48 h was able to decrease viability of Helicobacter pylori by four orders of magnitude in liquid medium under microaerophilic conditions [12]. Ojha et al. studied the effect of ascorbate on growth and pathogenicity markers of ascorbate on C. albicans [16]. The group demonstrated that upon 24 h of incubation with 0.85 M ascorbate, 80% cytotoxicity could be achieved. Brajtburg et al. reported that ascorbic acid enhanced the lethal effects of amphotericin B on C. albicans and Cryptococcus neoformans cells [13]. A recent in vivo study demonstrated that pharmacologic ascorbate concentrations and low-dose amphotericin B was effective in an animal model of recurrent candidemia sepsis [17]. Topically applied ascorbic acid, was not effective in treating acute vaginal candidiasis, but was effective in preventing fungal reinfection following completion of a successful antifungal treatment [18].
Our laboratory has studied photodynamic therapy (PDT) for many years [19,20]. In PDT a nontoxic dye called a photosensitizer (PS) is excited with otherwise harmless visible light, and produces ROS which can kill a broad-spectrum of pathogenic microorganisms and cancer cells [19]. In one of our previous studies, we have reported that addition of ascorbate as an external electron donor could potentiate PDT of HeLa cancer cells [20]. However this effect was concentration, PS and wavelength dependent, such that inhibition of PDT was also observed when the ROS quenching effect of ascorbate predominated [20]. In an attempt to observe whether ascorbate could also potentiate or inhibit antimicrobial PDT (aPDI) of microbial cells, we added sodium ascorbate (ascorbate) and a polycationic functionalized fullerene PS, to a suspension of bacteria or yeast cells in phosphate-buffered saline (PBS), which is the normal medium to carry out aPDT experiments. Taking previous studies into consideration, we used a relatively high concentration of ascorbate and used significantly shorter incubation times expecting that ascorbate alone would have no effect on cell viability. On analyzing the control data we realized that ascorbate did not in fact potentiate the aPDT killing effect, but rather rapidly killed the microbial cells on its own, depending on concentration, incubation time and the level of oxygenation.
This intriguing observation encouraged us to systematically study the ability of ascorbate to kill C. albicans In an attempt to validate previous studies, define the conditions under which C. albicans is either sensitive or resistant to ascorbate and gain more insight into its mechanism of action, we evaluated and compared the effects of PBS and growth media, shaking to allow oxygenation, temperature to regulate level of metabolism, growth phase and addition of a mitochondrial inhibitor or an iron chelator on the killing of C. albicans with ascorbate.
Materials & methods
• Materials
All chemicals were purchased from Sigma-Aldrich (MO, USA) unless otherwise stated. L-ascorbic acid was dissolved in PBS (without Ca2+/Mg2+) (PBS), PBS with D-(+)-glucose (20 g/l), YPD (BD-DifcoTM [NJ, USA], yeast extract 10 g/l; peptone 20 g/l; dextrose 20 g/l) or YPG (yeast extract-peptone [30 g/l; ForMedium Ltd. (Norfolk, UK)], glycerol [38 g/l]) media. Stock solutions of ascorbate were prepared on the same day of experiment and buffered with NaHCO3 (Fisher Scientific, PA, USA) to bring pH to approximately 7. The final concentration of ascorbate used in all experiments was 90 mM. 2,2′-Bipyridyl (bipyridyl) was dissolved in dimethylacetamide and diluted to 500 μM. Antimycin A (from Streptomyces spp.) was dissolved in ethanol and diluted to 10 μM. 3′(4-Hydroxyphenyl)-fluorescein (HPF; Molecular Probes, OR, USA) was used at 5 μM.
• Cell culture
The C. albicans strain CEC 749 was used in this study [21]. Candida albicans was routinely grown at 30°C on YPD agar and subcultured in liquid YPD medium in an shaking incubator at rpm 120 (New Brunswick Scientific, NJ, USA), at 30°C. Log phase cultures obtained by reculturing stationary overnight cultures were used for all experiments except as follows. In one set of experiments, stationary cultures grown overnight or 4 days and a stationary culture grown for 4 days and refreshed for 4 h were used. The reason for selecting two different time points for stationary phase cultures was because in some studies, C. albicans culture grown overnight or for 48 h was considered to be in stationary phase, while other studies reported the stationary phase to start much later, for example, between 3 and 8 days [22]. All broth cultures were centrifuged at 3200 rpm for 10 min (centrifuge 5417 C; Eppendorf, Hamburg, Germany) and resuspended in PBS. The concentrations were then adjusted by measuring optical density (OD570 of 0.65) to give an approximate cell density of 107 CFU/ml. In one experiment, YPG media and in another PBS with glucose, was used as described above.
• Experimental design
The experiments were performed in 35 × 10 mm diameter Petri dishes (BD Falcon NJ, USA) containing approximately 3 × 107 cells in 3 ml growth media, PBS with glucose or PBS. To examine the effects of different media and glucose on ascorbate sensitivity, cells were compared in different growth media; YPD or YPG, or in PBS with glucose. YPD or YPG was used both for initial growth and also for ascorbate treatment. Cells were shaken at 157 rpm at 37°C. At each time point two aliquots (10 μl each) were withdrawn. One was plated on YPD or YPG, the second was added to 90 μl PBS and four additional tenfold dilutions were carried out and plated on agar plates via drop plate method. Plates were incubated between 24 and 48 h in an incubator at 30°C and cell viability was assessed by colony counting. In the next experiment cells were treated with ascorbate in PBS (PBS ascorbate) in a shaker incubator (157 rpm, 4 and 37°C); left at room temperature (25 and 37°C) without shaking, or PBS in a shaker incubator (157 rpm, 4 and 37°C) with no ascorbate. Samples were withdrawn at time points (0, 10, 20, 30, 60 and 90 min).
To determine whether the effect of ascorbate is dependent on the presence of iron, washed cells were resuspended in PBS solution with ascorbate (PBS ascorbate), and bipyridyl (500 μM), an iron chelator that predominantly binds Fe2+ [23], was added subsequently, incubated in a shaker (157 rpm, 37°C) and samples withdrawn at time points (0, 10, 20, 30, 60 and 90 min).
For studies using pretreatment with antimycin A (a metabolic inhibitor of the electron transport chain) C. albicans cells were refreshed for 4 h in YPD, with antimycin A (10 μM) added or not after 1 h [24]. Then washed cells were resuspended and kept in a shaker incubator (157 rpm, 37°C) in either PBS or YPD with or without ascorbate and samples withdrawn at time points (0, 10, 20, 30, 60 and 90 min).
Microscopy
Detection of hydroxyl radical generation
HPF fluorescent probe was added to washed cells and kept in a shaker incubator with or without ascorbate in YPD or PBS or kept without shaking at 25°C with ascorbate PBS. Subsequently, cells were spun down (3200 rpm, 3 min) immediately after treatment, supernatant was removed and cells were resuspended in PBS. Images were acquired using a 35 mm 4-chamber glass-bottom petri dish (in vitro scientific).
Fluorescence of HPF was imaged by Olympus FV1000-MPE system with 40× NA0.8 water immersion lens. The HPF was imaged with single photon excitation at 488 nm using multiline argon laser and the fluorescence emission were collected with laser scanning spectral detector at bandwidth 500–545 nm. Brightfield images were acquired simultaneously.
Measurement of intracellular redox status
Intracellular redox status was measured by confocal imaging of the autofluorescence of flavoproteins and NADH using an Olympus FV1000-MPE system with 40× NA0.8 water immersion lens. The flavoprotien autofluorescence was imaged with single-photon excitation at 488 nm using multiline argon laser and the autofluorescence was collected with laser scanning spectral detector at bandwidth 500–600 nm. The autofluorescence of NADH was measured with two-photon excitation at 710 nm using MaiTai Deep See Ti:Sapphire laser (femtosecond) (Spectra-Physics, MaiTai HP DS-OL). The autofluorescence from two-photon excitation was collected with external two-channel photomultiplier detector for NADH (band-pass filter 420–460 nm). Brightfield images were acquired simultaneously.
Transmission electron microscopy
Candida albicans cells were spun down (3200 rpm, 3 min) immediately after treatment, supernatant was removed, cells were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde, and stored overnight at 4°C. After spinning down (1200 rpm, 10 min) and decanting the fixative, 0.1 M sodium cacodylate buffer (pH 7.2) was added to the pellets. After fixation, hot agar was added to each pellet. The solidified cell pellets were then processed routinely for transmission electron microscopy (TEM). The cell pellets were postfixed in 2% OsO4 in sodium cacodylate, dehydrated in graded alcohol, embedded in Epont812 (Tousimis, MD, USA). Ultrathin sections were cut on a Reichert-Jung Ultracut E microtome (Vienna, Austria), collected on uncoated 200 mesh copper grids, stained with uranyl acetate and lead citrate, and examined on a Philips CM-10TEM (Eindhoven, The Netherlands) at 80 kV.
Statistics
Experiments were repeated at least three times. Data points are means and error bars are standard deviations. Means were compared for significance (p < 0.05) by one-way ANOVA and Bonferroni post hoc test.
Results
• Ascorbate kills C. albicans in PBS but not in growth media, & presence of glucose in PBS decreases killing
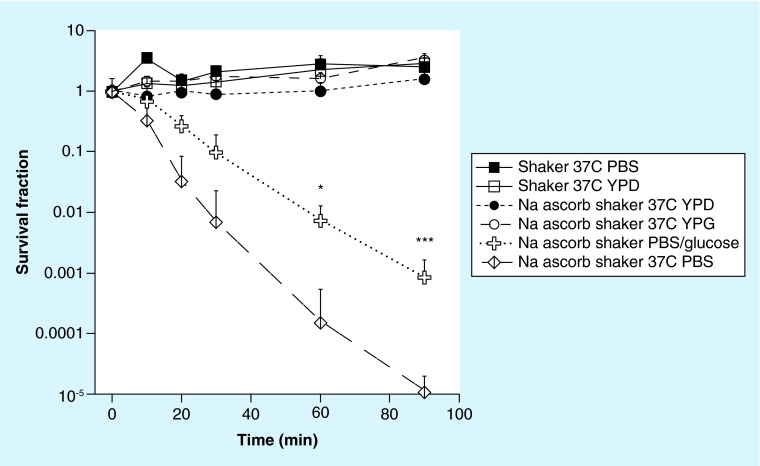
As preliminary studies had shown that the ascorbate-mediated killing of C. albicans was pronounced when the cells were incubated in PBS, we compared the time-dependent killing when the cells were shaken at 37°C, suspended either in growth medium (yeast-peptone-dextrose [YPD]) or in PBS. Moreover to test whether the antifungal activity of ascorbate against Candida is altered in different growth media, we compared media containing fermentable carbon source dextrose (YPD) or a nonfermentable carbon source glycerol (yeast-peptone-glycerol [YPG]) [25]. Figure 1 shows that incubation with shaking in PBS ascorbate at 37°C almost eradicated the cells (>5 logs killing) after 90 min, while in growth media there was no killing, but rather modest proliferation (up to fivefold). Growth media containing fermentable or nonfermentable carbon sources made no difference with no killing observed in either case. To further investigate whether presence of glucose in PBS would alter the metabolism or antioxidant capacity of Candida, thus alter killing, we compared incubation with shaking in PBS ascorbate with PBS-glucose-ascorbate at 37°C. Almost two logs of inhibition of killing was observed with added glucose at 60 and 90 min (Figure 1).
Figure 1. . Time-dependent killing of Candida albicans with 90 mM sodium ascorbate shaken in phosphate-buffered saline, YPD, YPG or phosphate-buffered saline with 20 g/l glucose at 37°C.
Controls included shaking in PBS or YPD without ascorbate.
*p < 0.05; ***p < 0.001.
PBS: Phosphate-buffered saline; YPD: Yeast-peptone-dextrose; YPG: Yeast-peptone-glycerol.
• Ascorbate requires active metabolism & oxygen to kill Candida in PBS
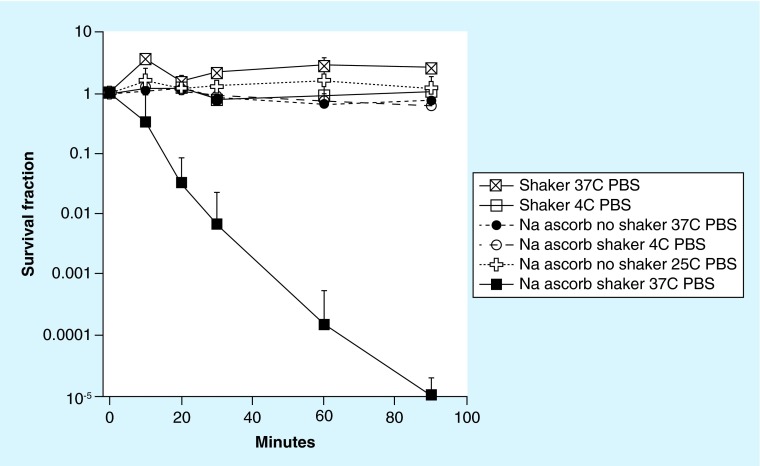
Since there was a large difference in viability between Candida cells shaken with ascorbate in PBS and shaken with ascorbate in growth media, we asked whether active metabolism could explain this striking difference. Therefore we compared cells that were either shaken with PBS ascorbate at 4°C, or kept in PBS ascorbate without shaking at 25 and 37°C, with cells shaken with PBS ascorbate at 37°C. Control cells were shaken in PBS with no ascorbate at both 4 and 37°C. Figure 2 shows that all three conditions, completely abrogated the killing with PBS ascorbate shaken at 37°C. However, cells exposed to ascorbate without shaking formed colonies at a much later time when compared with nonexposed cells. These findings show the importance of both temperature and sufficient oxygenation levels, implying that active respiration is necessary to achieve killing.
Figure 2. . Time-dependent killing of Candida albicans with 90 mM sodium ascorbate shaken in phosphate-buffered saline at 37 or 4°C, and not shaken at 37 or 25°C.
Controls included shaken in PBS alone at 37 or 4°C.
PBS: Phosphate-buffered saline.
• Ascorbate killing is dependent on free iron concentration & is increased with mitochondrial inhibition
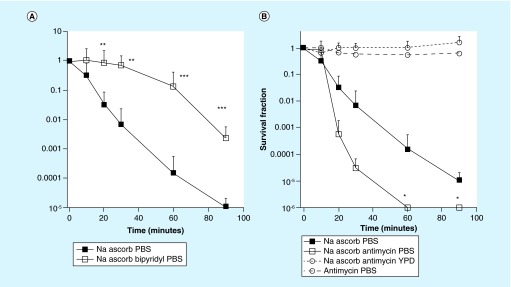
The literature suggests that iron-catalyzed Fenton reaction producing HO. is involved in the toxicity of ascorbate, thus we tested the effect of a cell-permeable iron chelator, bipyridyl (500 μM) on the PBS ascorbate killing of Candida [26]. There was substantial inhibition of killing (>2 logs) at time-points between 30 and 90 min of incubation in shaken PBS ascorbate (Figure 3A). We hypothesized that mitochondrial respiration played a role in the killing effect, and therefore tested the effect of an inhibitor of Complex III, antimycin A. We reasoned that mitochondrial inhibition could either reduce killing by inhibiting respiration, or alternatively it could increase killing by producing more H2O2 [24]. Initial control experiments showed that cells pre-incubated with antimycin A alone were not killed at the concentration employed. Figure 3B shows that the pre-incubation of Candida cells with antimycin A (10 μM) for 3 h increased the subsequent cytotoxic effect of PBS ascorbate.
Figure 3. . Effects of iron chelator and mitochondrial inhibitor on ascorbate-mediated killing of Candida albicans.
(A) Time-dependent killing of Candida albicans shaken in PBS at 37°C with 90 mM sodium ascorbate with or without addition of the iron chelator 2,2-bipyridyl (500 μM). **p < 0.01; **p < 0.001. (B) Time-dependent killing of C. albicans shaken in PBS at 37°C with 90 mM ascorbate, or in PBS or YPD with 90 mM ascorbate following treatment with complex III inhibitor antimycin A (10 μM) or cells pretreated with antimycin and shaken in PBS at 37°C in PBS as control.
PBS: Phosphate-buffered saline; YPD: Yeast-peptone-dextrose.
• Ascorbate killing is dependent on growth phase
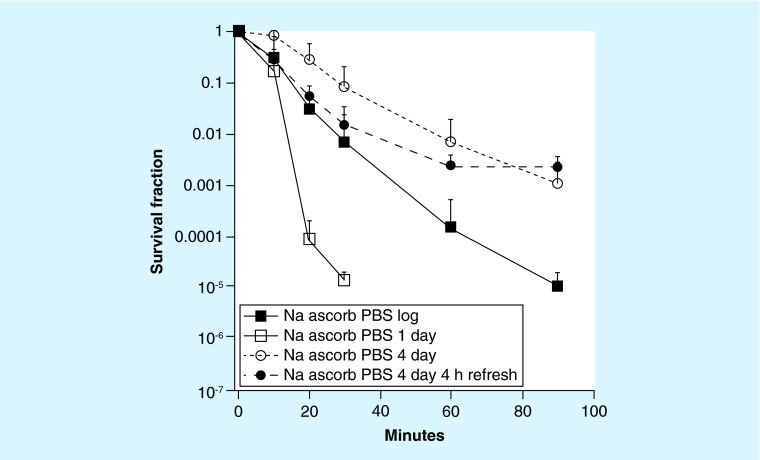
It is known that stationary phase cells of most microorganisms have higher resistance to many stresses including ROS, however, exact timing that cells go into stationary phase is still not clearly defined in the literature [22]. We compared the susceptibility of log phase, 1 day (early) and 4 days (late) stationary phase, and in the latter case, compared refreshed and not refreshed cultures to PBS ascorbate shaken at 37°C. Early stationary phase cells (1 day) were the most sensitive, being killed 2–3 logs more than log phase cells (Figure 4). Late stationary phase cells (4 days) were most resistant, and 4 h refreshing provided more killing during the first part of the treatment (<60 min) but not during the later part (>60 min). Therefore 4 h of refreshing had opposite effects, giving more killing with 4 days cultures, but less killing with 1 day cultures.
Figure 4. . Time-dependent killing of Candida albicans taken from cultures at different growth phases shaken in phosphate-buffered saline at 37°C with 90 mM sodium ascorbate.
PBS: Phosphate-buffered saline.
• Imaging studies show hydroxyl radical formation, changes in cellular redox state & cell morphology
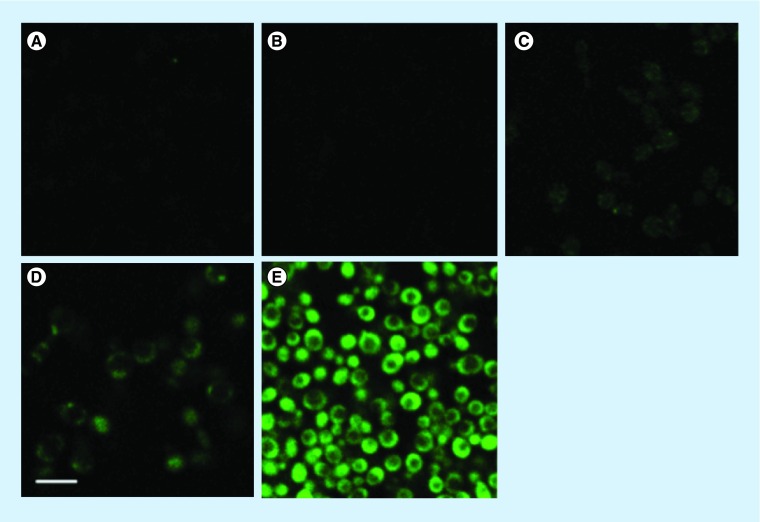
The literature suggests that HO. formation could explain the toxicity of ascorbate [11]. Thus, we used the relatively specific fluorescence probe HPF for imaging of intracellular HO. generation [27]. Figure 5A & B showed no HO. production (green fluorescence) when cells were shaken at 37°C either in YPD alone or in YPD-ascobate. A slight green fluorescence was observed in Figure 5C when Candida was shaken in PBS without ascorbate at 37°C. More pronounced (but still modest) green fluorescence was observed in Figure 5D when Candida was incubated with PBS ascorbate without shaking at 25°C. However when Candida was shaken in PBS ascorbate at 37°C for 30 min there was an intense green fluorescence visible in every cell indicating a marked increase in HO..
Figure 5. . Representative confocal imaging of hydroxyl radical (HO.) generation in Candida albicans after 20 min of incubation in phosphate-buffered saline or phosphate-buffered saline-ascorbate with or without shaker at 37 and 25°C, respectively (cells were stained with 3′(4-hydroxyphenyl)-fluorescein and images were acquired at 40X-zm-6), scale bar = 5 μM.
Cells incubated in: (A) yeast-peptone-dextrose in shaker 37°C; (B) yeast-peptone-dextrose ascorbate in shaker 37°C; (C) phosphate-buffered saline (PBS) in shaker 37°C; (D) PBS ascorbate without shaker 25°C; (E) PBS ascorbate shaker 37°C.
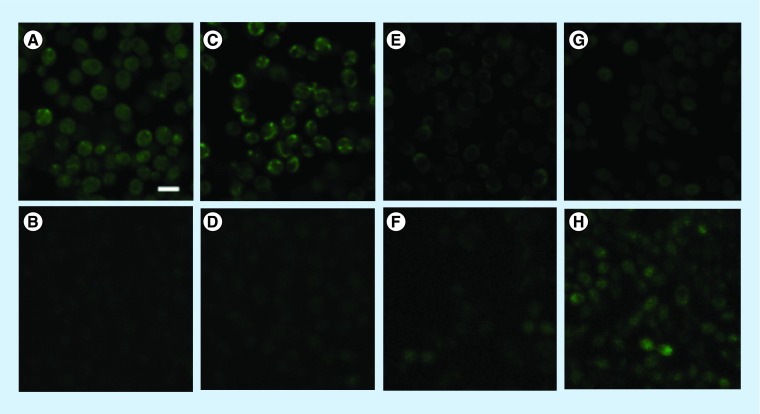
The cellular redox state can be observed by detecting the blue (420–460 nm) autofluorescence from NADH, using 2-photon excitation and the green autofluorescence (495–540 nm) from flavoproteins excited by 1-photon [28–30]. Healthy cells without oxidative stress have high NADH fluorescence and low flavinoprotein fluorescence, whereas cells that have been subjected to oxidative stress have lower NADH and higher flavoprotein fluorescence.
Figure 6 shows that cells stirred in YPD (Figure 6A & B) for 30 min without ascorbate had significant amounts of NADH fluorescence and no visible flavoprotein fluorescence. Cells stirred in PBS for 30 min with no ascorbate (Figure 6C & D) had slightly reduced NADH and slightly increased flavoprotein fluorescence. Cells kept in PBS ascorbate without stirring at 25°C for 30 min (Figure 6E & F) showed a prominent reduction in NADH fluorescence and a small amount of flavoprotein fluorescence appeared. Cells stirred in PBS ascorbate for 20 min (Figure 6G & H) also showed a prominent loss of NADH fluorescence, while a substantial amount of flavoprotein fluorescence was visible. We have determined that a slight component of NADH fluorescence may correspond to flavoprotein fluorescence due to the minor overlap in their two-photon excitation emission wavelengths [29]. Thus the NADH signal observed in 20 min is likely to be due to the increased autofluorescence of flavoprotein.
Figure 6. . Representative confocal imaging of cell redox changes in Candida albicans cells after various treatments.
Simultaneous imaging of autofluorescence of NADH (A, C, E & G) and flavoproteins (B, D, F & H). All images were acquired in phosphate-buffered saline (PBS) at 40×. Scale bar = 5 μM. Cells incubated in: (A–B) YPD in shaker at 37°C for 30 min; (C-D) PBS in shaker at 37°C for 30 min; (E–F) PBS ascorbate, without shaker, at 25°C for 30 min; (G–H) PBS ascorbate in shaker at 37°C for 20 min.
• TEM & bright field microscopy
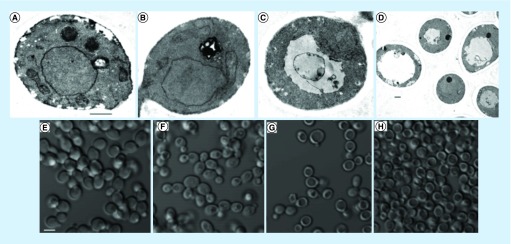
Fungal vacuoles, resembling mammalian lysosomes are the most acidic compartment of the cell with roles in protein degradation, ionic transport, metabolite storage as well as detoxification [31]. Changes in vacuolar morphology have been reported in response to stress ranging from acute osmotic and ionic shock to long-term nutrient deprivation [31]. TEM images of C. albicans treated with PBS ascorbate for 30 min in a shaker incubator revealed vacuole overgrowth, nuclear condensation as well as loss of organelle identity (Figure 7C–D). Consistent with these findings, bright field microscopy images showed a similar pattern resembling a peripheral ring with a central hole (Figure 7H). Same morphology was also observed in bright field images of PBS ascorbate-treated cells without shaking (Figure 7G).
Figure 7. . Transmission electron microscopy images of Candida albicans.
(A–D) Transmission electron microscopy images of Candida albicans cells following 30 min incubation at 37°C in: (A) yeast-peptone-dextrose in shaker; (B) phosphate-buffered saline (PBS) in shaker; (C–D) PBS ascorbate in shaker. (A–C) were imaged at 9800× direct magnification, (D) was imaged at 3800×. Scale bar = 500 nm. (E–H) Brightfield microscopy images of C. albicans cells following 30 min incubation in: (E) yeast-peptone-dextrose in shaker at 37°C (F) PBS in shaker at 37°C; (G) PBS ascorbate without shaker at 25°C; (H) PBS ascorbate in shaker at 37°C. Scale bar 5 μM.
Discussion
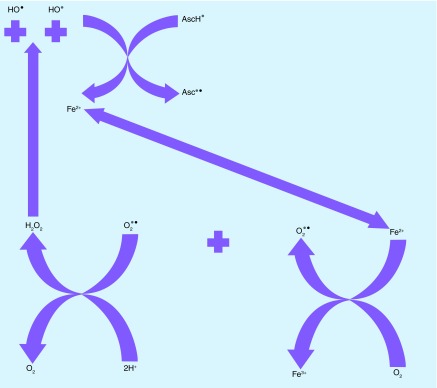
Candida albicans is a dimorphic yeast that is both a commensal organism and an opportunistic pathogen of humans. It can cause serious infections in immunocompromised patients [2]. Ascorbate, owing to its pro-oxidant properties at high concentrations, has been proposed to be used as an antifungal agent [6]. Ojha et al. demonstrated the concentration-dependent effects of ascorbate on growth and pathogenicity markers (e.g., proteinase secretion, yeast to hyphal transition) of C. albicans [16]. In their study, 250 mg/ml (1.42 M) ascorbate reduced proteinase secretion and at 85 mM there was an arrest of hyphal transition. Moreover, when varying concentrations of ascorbate were added to C. albicans cells in growth media and incubated for 24 h, cytotoxicity was found to be 33% at 57 mM and 80% at 0.85 M. In a subsequent study, ascorbate exerted pro-oxidant effects on catalase, decreased glutathione and enzyme activities of glutathione peroxidase, glutathione reductase and glutathione-S-transferase [32]. We tried to further elucidate the mechanism of action and investigated the conditions under which ascorbate is cytotoxic toward C. albicans. We propose that the antifungal effect of ascorbate on C. albicans depends on active metabolism, oxygenation, depletion of reduced NADH, liberation of free iron and Fenton-mediated production of HO., that act together as the key elements in its pro-oxidant activity [33]. Ascorbate, as a reducing agent can recycle Fe3 to Fe2+, and facilitate subsequent Fenton cycles (Figure 8).
Figure 8. . Ascorbate monoanion (AscH−) is a one-electron reducing agent that can reduce ferric iron (Fe3+) to ferrous (Fe2+) iron producing ascorbate radical (Asc-.).
Fe2+ generated via this reaction, together with free Fe2+ that has been possibly released by disruption of iron sulfur clusters in the mitochondria and ferrous polyphosphate stores in the vacuole, Fe2+ reacts with O2 reducing it to superoxide radical (O2 -.) and producing Fe3+ [6,34]. O2 -. undergoes dismutation leading to generation of H2O2 and O2 [6]. Then H2O2 reacts with the Fe2+ and generates Fe3+ as well as hydroxyl radical (HO.) by the Fenton reaction. Depending on the concentration of the elements, HO. could also be produced by a partly different pathway, via the Haber–Weiss reaction. The presence of ascorbate allows the recycling of Fe3+ back to Fe2+, which in turn allows the cycle to continue.
Oxygen and intracellular redox-active iron seem to play a significant role in ascorbate-mediated cell death. Verrax et al. [35] demonstrated that pre-incubation with deferoxamine mesylate, a cell-permeable iron chelator, had a protective effect against ascorbate toxicity in cancer cells, which is also in agreement with our results. In contrast, two different cell-impermeable iron chelators failed to protect against ascorbate toxicity, emphasizing the importance of intracellular iron as compared with extracellular iron [36]. The Collins laboratory concluded that cellular respiration is necessary for bactericidal antibiotics to exert their full effects [37]. O2 has only limited solubility in liquid media, thus it must be constantly shaken (aerated) to provide enough dissolved O2. The explanation of the high level of HO. generation and killing observed in cells treated with ascorbate in PBS with shaking (aeration), and the lack of killing` without shaking may be attributed to this requirement for oxygenation.
In microbiology, dormancy often refers to a state in which cells are not able to form a colony when plated on an agar medium. However, when conditions are more favorable they can revert to a state of colony-forming competence [38]. It is possible that C. albicans cells exposed to ascorbate in PBS without shaking may have moved toward a state of ‘early apoptosis’ as there was no overt loss of viability by CFU determination, but only a delayed formation of colonies [39].
In an attempt to determine whether in vitro antifungal activity of ascorbate depends on cellular metabolism, temperature was altered. Cells exposed to ascorbate in PBS with shaking were killed at 37°C, whereas no killing was detected at 4°C. To date, C. albicans stress responses have mainly been investigated on cells cultured on rich, glucose-containing media and this environment differs significantly from mammalian host microenvironments that are often glucose limited [40]. To examine the effects of different media, ascorbate sensitivity was tested in YPD, YPG and PBS media. Metabolism contributes to stress adaptation by generating antioxidants such as glutathione for which the cells need both carbon and nitrogen. Therefore both YPD and YPG could exert dramatic effects upon the stress resistance of C. albicans and protect against ascorbate [40,41]. Subsequently, when glucose as a carbon source was introduced into PBS in the absence of nitrogen, ascorbate killing was partially inhibited.
Based on previous reports cells in stationary phase are generally more tolerant to stress conditions than are cells in the logarithmic-growth phase [42]. In our study, cells in PBS were found to be more sensitive to ascorbate when they were in early stationary phase or in log phase than when they had come from late stationary phase. These findings are in agreement with Uppuliru et al.’s study which reported higher levels of expression of genes involved in resistance against oxidative stress, DNA repair, repair and maintenance of the mitochondrial genome at >3 days [22].
Intracellular autofluorescence is usually dominated by the reduced NADH, and the oxidized flavoprotein, and mitochondrial NADH autofluorescence can be used as an indicator of cellular respiration [28,43]. Elevations in oxidized flavoprotein autofluorescence were shown to be correlated with markers of apoptosis [44]. Increased oxidative stress is most likely responsible for decreasing the redox potential of Candida, thus the intense autofluorescence of NADH in control samples was attenuated by addition of ascorbate. NADH-cytochrome b5 reductase enzyme may reduce the ascorbate radical to ascorbate monoanion, which would in turn draw electrons from NADH [6]. H2O2 and HO. generated via ascorbate cycle may also decrease the intracellular concentration of NADH because ROS-scavenging enzymes require NADH [28,45]. Fungal vacuoles, containing metal ions, such as iron [31], have a significant role in the response to various stresses, and the vacuoles subsequently change their morphology. Upon exposure to ascorbate in PBS, both with or without shaking, we observed a large central depression with a peripheral ring representing the large electron lucent vacuole. This is likely to be caused by the stress generated by ascorbate, and is consistent with the reduced NADH levels.
Inhibition of cytochrome bc1 (Complex III) with antimycin A increases mitochondrial levels of ROS [24]. Our findings demonstrated that, pretreatment with antimycin A increased the sensitivity of cells to ascorbate treatment in PBS with shaking. This is in agreement with Ojha et al.’s study, in which ascorbic acid reduced C. albicans tolerance to oxidative stress that had been induced by incubation of cells with H2O2 [16].
Future perspective
Taken together, our results demonstrate that with oxygenation C. albicans can be rapidly eradicated by pharmacologic concentrations of ascorbate. However environmental distinctions, such as variations in the level of tissue perfusion and oxygenation in different organ systems will probably affect its toxicity toward Candida. Alterations in concentration of metallic ions as well as accessibility to nutrition will also enhance or reduce the potential killing effect of ascorbate. Understanding these environmental distinctions may provide unambiguous conditions to compare ascorbate’s effects on C. albicans. Although NADH levels are altered due to the pro-oxidant effects of ascorbate, Fenton reaction mediated HO. generation plays a major role in its cytotoxicity as an active participant in oxidative damage to cells. Unlike commonly used antifungals, ascorbate acts via generation of ROS, therefore can be used in combination with these agents to potentiate their effects. These points should be taken into consideration; when ascorbate is employed as a component of antifungal agents such as antifungal creams for Candida infections and dermatophytes. Further studies are needed to examine whether this approach applies to other microbial species.
EXECUTIVE SUMMARY.
Ascorbate at pharmacological concentrations can kill Candida albicans.
Ascorbate sensitivity of C. albicans depends on temperature, oxygenation, availability of energy sources, growth media and growth phase.
Killing is potentiated by a mitochondrial inhibitor and inhibited by an iron chelator.
Alterations are observed in the morphology of the vacuole, and the NADH concentration.
Intracellular hydroxyl radicals are produced.
Ascorbate exerts its pro-oxidant effects on C. albicans via Fenton reaction mediated reactive oxygen species generation.
Environmental conditions and the mechanism of action should be taken into consideration in both the evaluation of in vitro and in vivo studies and future drug development.
Footnotes
Financial & competing interests disclosure
Funded by US NIH grant R01AI050875. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Sardi JC, Scorzoni L, Bernardi T, et al. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013;62(Pt 1):10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 2007;20(1):133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kullberg BJ, Arendrup MC. Invasive Candidiasis. N. Engl. J. Med. 2015;373(15):1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Pappas PG, Wingard JR. Invasive fungal pathogens: current epidemiological trends. Clin. Infect. Dis. 2006;43(Suppl. 1):S3–S14. [Google Scholar]
- 5.Vandeputte P, Ferrari S, Coste AT. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012;2012:713687. doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochem. Biophys. Acta. 2012;1826(2):443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shatzer AN, Espey MG, Chavez M, et al. Ascorbic acid kills Epstein–Barr virus positive Burkitt lymphoma cells and Epstein–Barr virus transformed B-cells in vitro but not in vivo . Leuk. Lymphoma. 2013;54(5):1069–1078. doi: 10.3109/10428194.2012.739686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yun J, Mullarky E, Lu C, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs C, Hutton B, Ng T, et al. Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. Oncologist. 2015;20(2):210–223. doi: 10.1634/theoncologist.2014-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du J, Martin SM, Levine M, et al. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin. Cancer Res. 2010;16(2):509–520. doi: 10.1158/1078-0432.CCR-09-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vilcheze C, Hartman T, Weinrick B, et al. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat. Commun. 2013;4:1881. doi: 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabak M, Armon R, Rosenblat G, et al. Diverse effects of ascorbic acid and palmitoyl ascorbate on Helicobacter pylori survival and growth. FEMS Microbiol. Lett. 2003;224(2):247–253. doi: 10.1016/S0378-1097(03)00439-7. [DOI] [PubMed] [Google Scholar]
- 13.Brajtburg J, Elberg S, Kobayashi GS, et al. Effects of ascorbic acid on the antifungal action of amphotericin B. J. Antimicrob. Chemother. 1989;24(3):333–337. doi: 10.1093/jac/24.3.333. [DOI] [PubMed] [Google Scholar]
- 14.Pichat P, Reveilleau A. Comparison between the in vivo and in vitro bactericidal action of vitamin C and its metabolite, and ascorbic acid level. Ann. Inst. Pasteur. 1951;80(2):212–213. [PubMed] [Google Scholar]
- 15.Pichat P, Reveilleau A. Bactericidal action for Koch’s bacilli of massive doses of vitamin C; comparison of its action on a certain number of other microbes. Ann. Inst. Pasteur. 1950;79(3):342–344. [PubMed] [Google Scholar]
- 16.Ojha R, Manzoor N, Khan LA. Ascorbic acid modulates pathogenicity markers of Candida albicans . Int. J. Microbiol. Res. 2009;1(1):19–24. [Google Scholar]
- 17.Leelahavanichkul A, Somparn P, Bootprapan T, et al. High-dose ascorbate with low-dose amphotericin B attenuates severity of disease in a model of re-appearance of candidemia during sepsis in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309(3):R223–R234. doi: 10.1152/ajpregu.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mailland F. Pharmaceutical compositions comprising ascorbic acid or the treatment of fungal superinfections and fungal recurrence. www.google.com/patents/CA2528879A1?cl=en
- 19.St Denis TG, Dai T, Izikson L, et al. All you need is light: antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence. 2011;2(6):509–520. doi: 10.4161/viru.2.6.17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperandio FF, Sharma SK, Wang M, et al. Photoinduced electron-transfer mechanisms for radical-enhanced photodynamic therapy mediated by water-soluble decacationic C(7)(0) and C(8)(4)O(2) Nanomedicine. 2013;9(4):570–579. doi: 10.1016/j.nano.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enjalbert B, Rachini A, Vediyappan G, et al. A multifunctional, synthetic Gaussia princeps luciferase reporter for live imaging of Candida albicans infections. Infect. Immun. 2009;77(11):4847–4858. doi: 10.1128/IAI.00223-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uppuluri P, Chaffin WL. Defining Candida albicans stationary phase by cellular and DNA replication, gene expression and regulation. Mol. Microbiol. 2007;64(6):1572–1586. doi: 10.1111/j.1365-2958.2007.05760.x. [DOI] [PubMed] [Google Scholar]
- 23.Elandalloussi LM, Afonso R, Nunes PA, et al. Effect of desferrioxamine and 2,2′-bipyridyl on the proliferation of Perkinsus atlanticus . Biomol. Eng. 2003;20(4–6):349–354. doi: 10.1016/s1389-0344(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 24.Chabrier-Rosello Y, Giesselman BR, De Jesus-Andino FJ, et al. Inhibition of electron transport chain assembly and function promotes photodynamic killing of Candida . J. Photochem. Photobiol. 2010;99(3):117–125. doi: 10.1016/j.jphotobiol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata T, Takahashi T, Yamada E, et al. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob. Agents Chemother. 2012;56(11):5892–5897. doi: 10.1128/AAC.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staubli A, Boelsterli UA. The labile iron pool in hepatocytes: prooxidant-induced increase in free iron precedes oxidative cell injury. Am. J. Physiol. 1998;274(6 Pt 1):G1031–G1037. doi: 10.1152/ajpgi.1998.274.6.G1031. [DOI] [PubMed] [Google Scholar]
- 27.Hwang IS, Lee J, Hwang JH, et al. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012;279(7):1327–1338. doi: 10.1111/j.1742-4658.2012.08527.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuznetsov AV, Margreiter R, Amberger A, et al. Changes in mitochondrial redox state, membrane potential and calcium precede mitochondrial dysfunction in doxorubicin-induced cell death. Biochim. Biophys. Acta. 2011;1813(6):1144–1152. doi: 10.1016/j.bbamcr.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Vergen J, Hecht C, Zholudeva LV, et al. Metabolic imaging using two-photon excited NADH intensity and fluorescence lifetime imaging. Microsc. Microanal. 2012;18(4):761–770. doi: 10.1017/S1431927612000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemar KM, Aon MA, Cortassa S, et al. Diallyl disulphide depletes glutathione in Candida albicans: oxidative stress-mediated cell death studied by two-photon microscopy. Yeast. 2007;24(8):695–706. doi: 10.1002/yea.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li SC, Kane PM. The yeast lysosome-like vacuole: endpoint and crossroads. Biochim. Biophys. Acta. 2009;1793(4):650–663. doi: 10.1016/j.bbamcr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ojha R, Prasad R, Manzoor N, et al. Vitamin C modulates oxidative stress related enzyme activities in Candida albicans . Turkish J. Biochem. 2010;1(35):35–40. [Google Scholar]
- 33.Koppenol WH. The Haber-Weiss cycle – 70 years later. Redox Rep. 2001;6(4):229–234. doi: 10.1179/135100001101536373. [DOI] [PubMed] [Google Scholar]
- 34.Kohanski MA, Dwyer DJ, Hayete B, et al. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130(5):797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 35.Verrax J, Calderon PB. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic. Biol. Med. 2009;47(1):32–40. doi: 10.1016/j.freeradbiomed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Duarte TL, Almeida GM, Jones GD. Investigation of the role of extracellular H2O2 and transition metal ions in the genotoxic action of ascorbic acid in cell culture models. Toxicol. Lett. 2007;170(1):57–65. doi: 10.1016/j.toxlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Lobritz MA, Belenky P, Porter CB, et al. Antibiotic efficacy is linked to bacterial cellular respiration. Proc. Natl Acad. Sci. USA. 2015;112(27):8173–8180. doi: 10.1073/pnas.1509743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boucherit Z, Seksek O, Bolard J. Dormancy of Candida albicans cells in the presence of the polyene antibiotic amphotericin B: simple demonstration by flow cytometry. Med. Mycol. 2007;45(6):525–533. doi: 10.1080/13693780701487821. [DOI] [PubMed] [Google Scholar]
- 39.Hao B, Cheng S, Clancy CJ, et al. Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob. Agents Chemother. 2013;57(1):326–332. doi: 10.1128/AAC.01366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown AJ, Brown GD, Netea MG, et al. Metabolism impacts upon Candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014;22(11):614–622. doi: 10.1016/j.tim.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodaki A, Bohovych IM, Enjalbert B, et al. Glucose promotes stress resistance in the fungal pathogen Candida albicans . Mol. Biol. Cell. 2009;20(22):4845–4855. doi: 10.1091/mbc.E09-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blomberg A, Larsson C, Gustafsson L. Microcalorimetric monitoring of growth of Saccharomyces cerevisiae: osmotolerance in relation to physiological state. J. Bacteriol. 1988;170(10):4562–4568. doi: 10.1128/jb.170.10.4562-4568.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piston DW, Masters BR, Webb WW. Three-dimensionally resolved NAD(P)H cellular metabolic redox imaging of the in situ cornea with two-photon excitation laser scanning microscopy. J. Microsc. 1995;178(Pt 1):20–27. doi: 10.1111/j.1365-2818.1995.tb03576.x. [DOI] [PubMed] [Google Scholar]
- 44.Rajpurohit R, Mansfield K, Ohyama K, et al. Chondrocyte death is linked to development of a mitochondrial membrane permeability transition in the growth plate. J. Cell Physiol. 1999;179(3):287–296. doi: 10.1002/(SICI)1097-4652(199906)179:3<287::AID-JCP6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 45.Hyslop PA, Hinshaw DB, Halsey WA, et al. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J. Biol. Chem. 1988;263(4):1665–1675. [PubMed] [Google Scholar]