Abstract
To date, the ivermectin resistance in nematode parasites has been reported and many studies are carried out to determine the causes of this problem. A free-living Caenorhabditis elegans is used as a model system for this study to investigate the response of C. elegans to ivermectin exposure by using larval development assay. Worms were exposed to ivermectin at concentration from 1 ng/mL to 10 ng/mL and dimethyl sulphoxide (DMSO) as a control. The developments of the worms were monitored for 24, 48, 72, and 96 hours until the worms become adults. Results indicated that worms’ growth began to be affected by ivermectin at a concentration of 5 ng/mL, while at the concentration of 6, 7, 8, 9, and 10 ng/mL, the growth of worms were inhibited compared to control worms. Further study of the protein expression in C. elegans should be done to investigate the up-regulated and down-regulated proteins involve in ivermectin resistance.
Keywords: Macrocyclic Lactone, Ivermectin, Nematode Parasite, Caenorhabditis elegans, Ivermectin Resistance
INTRODUCTION
The macrocyclic lactone ivermectin (IVM) is a complex compound, derivative of soil microorganisms belonging to the genus Streptomyces with high parasiticidal efficacy for the control of human and animal parasites. IVM also known as endectocides because of its ability to treat infections of endo- and ectoparasites in a wide range of hosts. It has been the mainstay of livestock parasite control since the early 1980s and has been increasingly used in community wide treatment programs over the last decade (Laing et al. 2012).
After over 30 years of intensive use, ivermectin resistance is now widely spread around the world. It was detected when the previously effective drug is no longer killing the worms at the therapeutically recommended dosages (Jabar et al. 2006). However, the mechanism of ivermectin resistance in nematode parasites at the moment remains unsolved. The lack of suitable functional assay limiting the study of interest genes in nematode parasites (Britton & Murray 2006).
Introduction of Caenorhabditis elegans as a model organism by Sydney Brenner in 1965 gave a new life to the study of nematode parasite (Riddle et al. 1997). C. elegans has been chosen as a model system for parasite nematodes due to it being an important model system for biological research in many fields including genomics, cell biology, and neuroscience (Ankeny 2001). The characteristics of this worm contributed to the success of nematode parasite study as its ability to be genetically manipulated, invariant and fully described developmental program, well-characterised genome, ease of maintenance, short and prolific life-cycle, and small in body size (Ankeny 2001). Besides that, C. elegans has been categorised in Clade V of nematodes which is the same group with nematode parasite (Blaxter et al.1998). Until there is an appropriate method to study parasitic nematodes, C. elegans will remain as the suitable model system where putative homologous exist in the free-living nematode (Britton & Murray 2006).
In this study, C. elegans was used to investigate the response of the worms on egg-hatching and larval development when exposed to ivermectin.
MATERIALS AND METHODS
Caenorhabditis elegans strains, DA1316 and N2, were obtained from the Caenorhabditis Genetics Centre (CGC), USA. Worms were cultured at 20°C on Nematode Growth Medium (NGM) agar (3 g NaCl, 17 g agar, 2.5 g peptone in 975 mL deionised water, with 1 mL 1 M CaCl2, 1 mL 5 mg/mL cholesterol in ethanol, 1 mL 1 M MgSO4 and 25 mL 1M KH2PO4 added after autoclaving) seeded with Escherichia coli strain OP50 as a food source (Stiernagle 2006). Ivermectin (IVM) plates were prepared with concentration ranging from 1 ng/mL to 10 ng/mL and dimethyl sulphoxide (DMSO) was added into the control plates. For larval development assay, synchronised eggs of both strains were isolated by hypochlorite treatment of gravid adults followed by 4× washing off with M9 buffer (Stiernagle 2006). A total of 25 eggs were spot onto IVM plates for each strain and the plates were incubated at 20°C for 4 days. Worm development was observed at 24 hours (L1 larvae), 48 hours (L3 larvae), 72 hours (L4 larvae), and 96 hours (adult) throughout the incubation period. The experiment was repeated three times.
RESULTS AND DISSCUSSION
Results in Figures 1–3 showed the percentage of worm growth for DA1316 and N2 at a ivermectin concentration of 1 ng/mL to 10 ng/mL. DA1316 is a mutant strain which is highly resistance to ivermectin and was used as control, whereas N2 is a wild-type strain and is sensitive to ivermectin.
Figure 1:
Percentage of worm growth of C. elegans DA1316 and Bristol N2 after 48 hours exposed to ivermectin at different concentrations from 1 ng/mL to 10 ng/mL. After 48 hours, the larvae should be in stage L3. All larvae of DA1316 have grown to L3 larvae. For N2, the larval development was distracted at 5 ng/Ml; (N=25).
Figure 2:
Percentage of worm growth of C. elegans DA1316 and Bristol N2 after 72 hours exposed to ivermectin at different concentrations from 1 ng/mL to 10 ng/mL. After 72 hours, the larvae should be in stage L4. All larvae of DA1316 have grown to L4 larvae. For N2, 70% of worms at a ivermectin concentration of 5 ng/mL have grown to L4 larvae. However, worms were slowly growing at 6 to 10 ng/mL, and no L4 larvae were observed; (N=25).
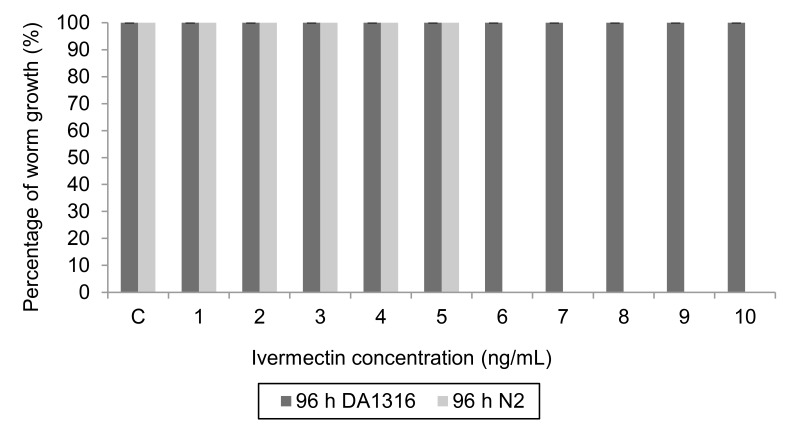
Figure 3:
Percentage of worm growth of C. elegans DA1316 and Bristol N2 after 96 hours exposed to ivermectin at different concentrations from 1 ng/mL to 10 ng/mL. After 96 hours, the larvae should become an adult and be able to lay the eggs. Worms of DA1316 were grown to adults completely. For N2, no adult was observed at a ivermectin concentration of 6 ng/mL and above; (N=25).
After 24 hours of incubation on IVM plates, the synchronised eggs of DA1316 and N2 were hatched to L1 larvae for all ivermectin concentrations. These results showed that ivermectin does not affect the embryo growth during the egg stages. Ivermectin was prevented from passing through the eggshell layer and thus the embryo growth was not interfered by ivermectin. This is because the presence of impermeable eggshell gives mechanical supports to the embryo and prevent the entry of foreign molecule from the outside (Olson et al. 2012). When the embryo was maturing, the eggs hatched and L1 larvae were released.
Without the eggshell, worm began to be exposed directly to ivermectin. After 48 hours of incubation, the L1 larvae of DA1316 had developed into L3 larvae at all ivermectin concentrations. On the other hand, the L1 larvae growth of N2 was inhibited by ivermectin at 5 ng/mL as compared to control (Fig. 1). After 72 hours, worms showed slow growth at 6, 7, 8, 9, and 10 ng/mL as shown in Figure 2. This could be due to the response of the worms toward ivermectin. According to Laing et al. (2012), ivermectin has caused paralysis of the worm body and pharyngeal muscles that affect the rate of food intake. These may cause of stunted growth on the worms.
Figure 3 showed that after 96 hours of incubation, all DA1316 worms have become adult worms as the worm’s growth was completed 100%. For N2 strain, the worm’s growth was completely inhibited by ivermectin at the concentration of 6, 7, 8, 9, and 10 ng/mL. The worm’s growth ceased at the larval stage and not become an adult compared to control. However, suprising result was observed at 5 ng/mL ivermectin concentration, where at 48 hours, the worms were growing slowly but managed to develop into the adult stage in 96 hours. This indicates that the worms were able to acclimatize to this concentration and continue to grow into adults. On the contrary, a study by Dent et al. (1997) showed that, worms that are sensitive to ivermectin will remain as larvae and do not become adults.
CONCLUSION
The growth of N2 strain was significantly reduced due to the increase of ivermectin concentration. However, there is evidence that N2 strain can survive and proceed to adult worms at 5 ng/mL of ivermectin. Further study in protein expression should be conducted to investigate the existence of ivermectin resistance in C. elegans.
Acknowledgments
I would like to express my deepest appreciation to my supervisor, Dr Nik Ahmad Irwan Izzauddin Bin Nik Him for his great support and guiding me throughout this study. I would also like to show my gratitude to MyBrain15 for the scholarship of MyMaster. I am also wanted to show my appreciation to Universiti Sains Malaysia for giving me an opportunity to do my study here and also USM Press’s Tropical Life Sciences Research journal for accepting this paper. This study was funded by Minister of Education, Fundamental Research Grant Scheme (FRGS) (203.PBIOLOGI.6711221).
REFERENCES
- Ankeny RA. The natural history of Caenorhabditis elegans research. Nature Reviews Genetics. 2001;2:474–479. doi: 10.1038/35076538. http://dx.doi.org/10.1038/35076538. [DOI] [PubMed] [Google Scholar]
- Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, et al. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. http://dx.doi.org/10.1038/32160. [DOI] [PubMed] [Google Scholar]
- Britton C, Murray L. Using Caenorhabditis elegans for functional analysis of genes of parasitic nematodes. International Journal for Parasitology. 2006;36:651–659. doi: 10.1016/j.ijpara.2006.02.010. http://dx.doi.org/10.1016/j.ijpara.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Dent JA, Davis M, Avery L. avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. The EMBO Journal. 1997;16(19):5867–5879. doi: 10.1093/emboj/16.19.5867. http://dx.doi.org/10.1093/emboj/16.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbar A, Iqbal Z, Kerboeuf D, Muhammad G, Khan MN, Afaq M. Anthelmintic resistance: The state of play revisited. Life Sciences. 2006;79(26):2413–2431. doi: 10.1016/j.lfs.2006.08.010. http://dx.doi.org/10.1016/j.lfs.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Laing ST, Ivens A, Butler V, Ravikumar SP, Laing R, Woods DJ, Gilleard JS. The transcriptional response of Caenorhabditis elegans to ivermectin exposure identifies novel genes involved in the response to resuced food intake. PLoS ONE. 2012;7(2):1–11. doi: 10.1371/journal.pone.0031367. http://dx.doi.org/10.1371/journal.pone.0031367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SK, Greenan G, Desai A, Muller-Reichert T, Oegema K. Hierarchical assembly of the eggshell and permeability barrier in C. elegans. The Journal of Cell Biology. 2012;198(4):731. doi: 10.1083/jcb.201206008. http://dx.doi.org/10.1083/jcb.201206008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Blumenthal T, Meyer BJ, Priess JR. C. elegans II. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- Stiernagle T. WormBook, editor. Maintenance of C. elegans. 2006. pp. 1–11. http://www.wormbook.org/chapters/www_strainmaintain/strainmaintain.html, http://dx.doi/10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed]