Abstract
To understand the effects of fish predator’s kairomones on Aedes mosquitoes’ oviposition, we established an experiment using gravid Aedes females. Kairomones concentrations were established using Hampala macrolepidota. One individual fish was placed inside containers with varying water levels (1 L, 5 L, and 10 L of water). The fish were kept in the containers for 24 hours and were removed immediately at the start of each trial in order to have the kairomones remnants. Twenty gravid adult females of Aedes aegypti and Aedes albopictus were allowed to lay eggs on oviposition site with various treatments: (1) control without any kairomones; (2) kairomone remnant in 1 L of water; (3) kairomone remnant in 5 L of water; and (4) kairomone remnant in 10 L of water. There are significant differences between the numbers of eggs laid by both Aedes species for each different treatment (F = 9.131, df = 16, p<0.001). However, fewer eggs were laid by Ae. albopictus compared to Ae. aegypti in the presence of kairomone remnants. This suggested that Ae. albopictus are significantly affected by the kairomones itself and have ability to detect the residual kairomone presence from H. macrolepidota.
Keywords: Aedes albopictus, Hampala macrolepidota, Kairomone, Mosquito, Predator
INTRODUCTION
Regardless of the control efforts done by various parties, mosquitoes (Diptera: Culicidae) still plays a major role in transmitting vector borne diseases in many parts of the world, with over 1 million cases of death in children annually involving malaria (World Health Organization 1999; Breman 2001) and the outbreak of dengue fever and dengue haemorrhagic fever worldwide due to spreading of vector Aedes aegypti and Aedes albopictus with recently estimate of 390 million dengue infections per year (Bhatt et al. 2013). Reckless and unmanaged practice of chemical insecticide in pest management sector has given rise to resistance problems in insect (Rafikov et al. 2009; Wijesinghea et al. 2009; Nyamah et al. 2011). Studies showed that in Malaysia, Ae. aegypti has developed temephos resistance (Lee 1991), while Ae. albopictus was found to be resistant towards DDT and permethrin (Wesson 1990). Since chemical insecticidal approach causes much worry over increasing resistant traits in mosquitoes, not to mention the impact they posed on the environment, biological control has received much attention and interest as an alternative management action on mosquito borne diseases’ vector (Collins & Blackwell 2000; Focks 2007; Wijesinghea et al. 2009; Nyamah et al. 2011). Fish predator especially Gambusia species has been one of the interest in biological of mosquitoes and identified as significant predator with wide range of habitat (Griffin 2014). Here, we tested the effectiveness of kairomones remnant from Malaysia endemic predatory fish, Hampala macrolepidota on oviposition site choose by two species of dengue and dengue hemorrhagic fever vector; Ae. albopictus and Ae. aegypti.
MATERIAL AND METHODS
A series of experiments to investigate the effects of H. macrolepidota predatory fish kairomone on Aedes’ oviposition was conducted under field condition at School of Biological Sciences, Universiti Sains Malaysia with temperature of 30±2ºC, 65±10% RH and 12L:12D. A total of 20 full gravid females of Ae. albopictus and Ae. aegypti, aged 2–3 days old were placed separately in mosquito cages sized 30 × 30 × 30 cm. Two days after emergence, these mosquitoes were provided blood meal from white mice prior to dusk. Oviposition medium which was made of a cone-shaped filter paper fixed in Petri dish was utilised in this experiment as oviposition sites for mosquitoes. Treatments were established as follows with discrepant kairomones concentration: (1) 1 L; (2) 5 L; (3) 10 L; (4) without kairomones (only seasoned water) served as control. Kairomone were established by accommodating one predatory Hampala fish into different water levels of 1 L, 5 L, 10 L, and 10 L for 24 h prior to experiment in order to have different kairomones concentrations. Approximately of 10 mL of kairomones remnant from Hampala fish was used to moisten the oviposition medium for everyday throughout this study. A total 5 mL of kairomones was added in the morning (0900) and evening (1800). The oviposition medium was observed daily for the presence of eggs deposited and replaced with a new one until no more eggs were laid by Aedes mosquitoes. The number of eggs deposited will be counted using light microscope and recorded. These studies were replicated three times for each replicate. Data were tested for normality using a one-sample Kolmogorov-Smirnov test. All data were log transformed prior to analysis to satisfy the assumptions of ANOVA. Data were then analysed using Repeated Measure General Linear Model analysis, which within subject factors types of treatment (1 L, 5 L, 10 L, and control), mosquito species (Ae. albopictus and Ae. aegypti) and oviposition day (day 1 and day 2).
RESULTS
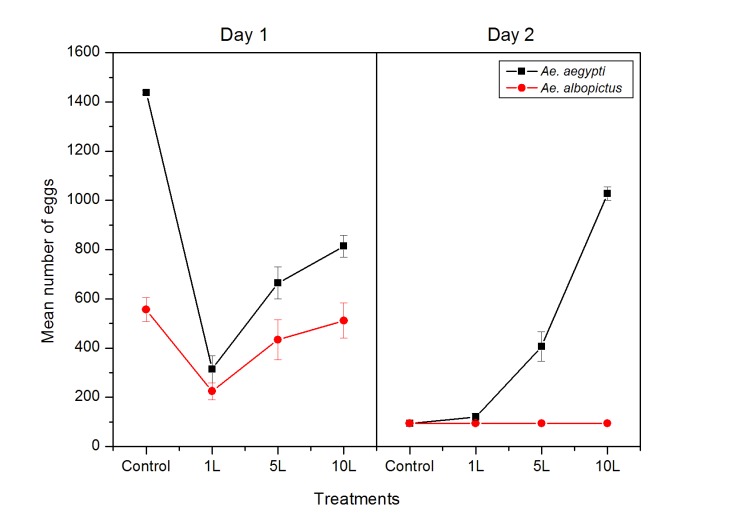
There were significant differences on the number of eggs oviposited by both adult females Ae. albopictus and Ae. aegypti (F = 78.35, df = 1, p = 0.000; Table 1). Similarly, significant differences were also detected in all treatment tested for both species (F = 9.13, df = 3, p = 0.000). Thus, female Ae. albopictus and Ae. aegypti appeared to choose non-treated oviposition site when laid their eggs and avoided oviposition sites with H. macrolepidota’s kairomones in it due to sense of predator. Other than that, it seemed that Ae. aegypti delayed to oviposit their eggs until day 2 in the presence of kairomone remnant from Hampala fish (Fig. 1), whereas Ae. albopictus completed their oviposition on day 1. In 10 L of kairomone, Ae. aegypti continued to lay significantly more eggs on day 2 under single bloodmeal as compared to other treatments of 1 L and 5 L kairomones (Tukey post-hoc; p<0.05). This maybe due to Ae. eagypti sense is less threatening from fish predator after the second day as the kairomones become less strong by the day which allowed mosquitoes to fully oviposit their eggs. Significant differences were also recorded between days of oviposition by female Aedes mosquitoes (F= 416.79, df = 1, p = 0.000). These results suggested that both Ae. albopictus and Ae. aegypti oviposition behaviour was altered in the presence of H. macrolepidota fish, either reduced the number of eggs oviposited or delayed their oviposition.
Table 1:
Results of repeated measure ANOVA are examining the cumulative number of Ae. aegypti and Ae. albopictus in response to different types of H. macrolepidota kairomones treatments and days of oviposition.
| Source | F | df | MS | Significance |
|---|---|---|---|---|
| Species (S) | 78.35 | 1 | 8.75 | 0.000 |
| Treatment (T) | 11.88 | 3 | 1.181 | 0.000 |
| Days (D) | 416.79 | 1 | 41.42 | 0.000 |
| S × T | 9.13 | 3 | 1.02 | 0.001 |
| S × D | 59.73 | 1 | 6.64 | 0.000 |
| S × T × D | 13.72 | 3 | 1.53 | 0.000 |
| Species × subject within groups | 16 | 0.11 | ||
| Species × subject between groups | 16 | 0.09 |
Notes: df = degree of freedom, MS = mean squared value. Significant values are in bold. Data were log transformed prior to analysis.
Figure 1:
The effects of fish predator (H. macrolepidota) on the number of eggs deposited ±SE by Ae. aegypti and Ae. albopictus.
DISCUSSION
This study has predicted that both Ae. albopictus and Ae. aegypti females will alter their oviposition behaviour in response to H. macrolepidota predatory fish’s kairomones. This prediction is based on a few studies that found evidence that alteration on mosquito oviposition may happen when facing with the presence of predators or its kairomones (Blaustein et al. 1995; Blaustein 1998; Blaustein et al. 2004; Kiflawi et al. 2003; Eitam & Blaustein 2004; Blaustein et al. 2005). A study by Barendonk (1999) also found that not only mosquito, but adult aquatic insects, Chauborus as well avoided to oviposit in waterbodies that contains fish kairomones. However, there is some anomaly to this theory sometimes. For instance, Culex pervigilans mosquitoes do not modify their oviposition pattern though the existence of a predator due to kairomones release by Anisops predator is not strong enough to mark their presence in water bodies (Zuharah & Lester 2010).
Most of the terrestrial adult insects will turn on the water to oviposit their eggs and it is important to find a suitable and safer place to oviposit their next progeny (Berendock 1999). In our recent study, we found evidence that Ae. albopictus and Ae. aegypti oviposition pattern was influenced by the presence of H. macrolepidota fish kairomones. Even 10 mL of kairomones from the lowest kairomones concentration of 10 L can reduce the number of eggs oviposited by both Aedes species. Only a small amount of kairomones may alert and give a strong alarm to the female mosquitoes in the presence of predator and avoid to oviposit in this particular oviposition site. This selection should favour Aedes mosquito female adults on alteration of oviposition behaviour to maximise the survival of their juvenile.
In regards to the kairomones, mosquitoes such as Culiseta longireolata can detect chemical from Notonecta for up to 8 days after their removal from the pools (Blaustein et al. 2004). Thus, it seems reasonable that Ae. aegypti had delayed their oviposition on day 2 for all concentration tested, but it did not happen to Ae. albopictus. The way Ae. albopictus dealt with this kairomones was different from Ae. aegypti, with less number of eggs were deposited by Ae. albopictus due to pressure from this fish predator. Therefore, modification of behaviour regarding to oviposition is very essential for mosquitoes to ensure there is a continuous flow of generation without being extinct.
In conclusion, with the presence of H. macrolepidota fish predator, the number of mosquitoes in water bodies will be decreased. Other than become a predator, the kairomones remnant from the fish is enough to reduce the number of Aedes mosquito larvae in water bodies. Therefore, this fish predator can act as one of the potential biological controls for controlling the rising of dengue fever cases in the future.
Acknowledgments
The authors are grateful to the staff of the Vector Control Research Unit and School of Biological Sciences, Universiti Sains Malaysia for assisting in sample collections and their continuous support during the course of the experiment. This project was funded by Incentive Grant USM (304/JPNP/600004).
REFERENCES
- Berendonk TU. Influence of fish kairomones on the ovipositing behavior of Chaoborus imagines. Limnology and Oceanography. 1999;44(2):454–458. http://dx.doi.org/10.4319/lo.1999.44.2.0454. [Google Scholar]
- Blaustein L, Blaustein J, Chase J. Chemical detection of predator Notonecta irrorata by ovipositiong Culex mosquitoes. Journal of Vector Ecology. 2005;30(2):299–301. [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. http://dx.doi.org/10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein L, Kiflawi M, Eitam A, Mangel M, Cohen JE. Oviposition habitat selection in response to risk of predation in temporary pools: Mode of detection and consistency across experimental venue. Oecologia. 2004;138(2):300–305. doi: 10.1007/s00442-003-1398-x. http://dx.doi.org/10.1007/s00442-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Blaustein L, Kotler BP, Ward D. Direct and indirect effects of the predatory backswimmer (Notonecta maculata) on community structure of desert temporary pools. Ecological Entomology. 1995;20(4):311–318. http://dx.doi.org/10.1111/j.1365-2311.1995.tb00462.x. [Google Scholar]
- Blaustein L. Influence of predatory backswimmer, Notonecta maculata, on invertebrate community structure. Ecological Entomology. 1998;23(3):246–252. http://dx.doi.org/10.1046/j.1365-2311.1998.00138.x. [Google Scholar]
- Breman JG. The ears of the hippopotamus: Manifestations, determinants, and estimates of the malaria burden. American Journal of Tropical Medicine and Hygiene. 2001;64(1):1–11. doi: 10.4269/ajtmh.2001.64.1. [DOI] [PubMed] [Google Scholar]
- Collins LE, Blackwell A. The biology of Toxorhynchites mosquitoes and their potential as biocontrol agents. Biocontrol. 2000;21(4):105–116. [Google Scholar]
- Eitam A, Blaustein L. Oviposition habitat selection by mosquitoes in response to predator (Notonecta maculata) density. Physiological Entomology. 2004;29(2):188–191. http://dx.doi.org/10.1111/j.0307-6962.2004.0372.x. [Google Scholar]
- Focks DA. Toxorhynchites as biocontrol agents. The American Mosquito Control Association Bulletin. 2007;23:118–127. doi: 10.2987/8756-971X(2007)23[118:TABA]2.0.CO;2. http://dx.doi.org/10.2987/8756-971X(2007)23[118:TABA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Griffin L. Laboratory evaluation of predation on mosquito larvae by Australian mangrove fish. Journal of Vector Ecology. 2014;39(1):197–203. doi: 10.1111/j.1948-7134.2014.12087.x. http://dx.doi.org/10.1111/j.1948-7134.2014.12087.x. [DOI] [PubMed] [Google Scholar]
- Kiflawi M, Blaustein L, Mangel M. Oviposition habitat selection by the mosquito Culiseta longiareolata in response to risk of predation and conspecific larval density. Oecologia. 2003;28(2):168–173. http://dx.doi.org/10.1046/j.1365-2311.2003.00505.x. [Google Scholar]
- Lee HL. Esterases activity and temephos susceptibility in Aedes aegypti (L.) larvae. Mosquito-Borne Disease Bulletin. 1991;8(3):91–94. [Google Scholar]
- Nyamah MA, Sulaiman S, Omar B. Field observation on the efficacy of Toxorhynchites splendens (Wiedemann) as a biocontrol agent against Aedes albopictus (Skuse) larvae in a cemetery. Tropical Biomedicine. 2011;28(2):312–319. [PubMed] [Google Scholar]
- Rafikov M, Bevilacqua L, Wyse APP. Optimal control strategy of malaria vector using genetically modified mosquitoes. Journal of Theoretical Biology. 2009;258(3):418–425. doi: 10.1016/j.jtbi.2008.08.006. http://dx.doi.org/10.1016/j.jtbi.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Wesson DM. Susceptibility to organophosphate insecticides in larval Aedes albopictus. Journal of American Mosquito Control Association. 1990;6(2):258–264. [PubMed] [Google Scholar]
- Wijesinghea WMGS, Wickramasingheb MB, Kusumawathiec PHD, Jayasooriyac GAJSK, De Silva BGDNK. Studies on the efficacy of Toxorhynchites larvae and three larvivorous fish species for the control of Aedes larval populations in water-storage tanks in the Matale district of Sri Lanka. Dengue Bulletin. 2009;3(1):140–147. [Google Scholar]
- World Health Organization . Removing obstacles to healthy department Report on infectious diseases. Geneva: World Health Organization; 1999. [Google Scholar]
- Zuharah WF, Lester PJ. Can adults of the New Zealand mosquito Culex pervigilans (Bergorth) detect the presence of a key predator in larval habitat? Journal of Vector Ecology. 2010;35(1):100–105. doi: 10.1111/j.1948-7134.2010.00035.x. http://dx.doi.org/10.1111/j.1948-7134.2010.00065.x. [DOI] [PubMed] [Google Scholar]