ABSTRACT
Purpose
Sedentary time, in particular, prolonged unbroken sedentary time, is detrimental to health and displaces time spent in either light or moderate intensity physical activity. This cross-sectional study aimed to identify the potential impact of reallocating time from sedentary behaviors to more active behaviors on measures of body composition and metabolic health in people with type 2 diabetes.
Methods
Participants were 519 adults with newly diagnosed type 2 diabetes who had been recruited to the Early Activity in Diabetes (Early ACTID) randomized controlled trial. Waist-worn accelerometers were used to obtain objective measurement of sedentary time, light physical activity (LPA), and moderate-to-vigorous physical activity (MVPA) at baseline alongside clinical measurements and fasting blood samples to determine cholesterol, triglycerides, HOMA-IR, and glucose. Isotemporal substitution modeling was performed to determine the potential impact of reallocating 30 min of sedentary time accumulated in a single bout (long bout) with 30 min of interrupted sedentary time, LPA, or MVPA.
Results
Sedentary time accounted for 65% of the waking day, of which 45% was accumulated in prolonged (≥30 min) bouts. Reallocation of 30 min of long-bout sedentary time with 30 min of short-bout sedentary time was associated with lower body mass index (BMI) (adjusted β, −0.60; 95% confidence interval [CI], −1.00, −0.21) and waist circumference (WC) (adjusted β, −1.16; 95% CI, −2.08, −0.25). Stronger effects were seen for LPA and MVPA. Reallocation of 30 min of long-bout sedentary time with LPA was associated with higher HDL-cholesterol (adjusted β, 0.02; 95% CI, 0.00–0.03 mmol·L−1).
Conclusions
Encouraging adults with newly diagnosed type 2 diabetes to break up prolonged periods of sedentary time may be an effective strategy for improving body composition and metabolic health.
Key Words: SEDENTARY, TYPE 2 DIABETES, PHYSICAL ACTIVITY, ACCELEROMETER, SEDENTARY BREAKS
Regular physical activity is recommended for the prevention and management of type 2 diabetes owing to its beneficial effects on weight control, glucose metabolism, and lipid profiles (5,18,20). However, people with type 2 diabetes tend to have low levels of activity, with few achieving the recommended 30-min moderate-to-vigorous activity (MVPA) per day (6,19). In addition, lifestyle interventions to increase physical activity often have weak effects, with most people failing to achieve increases in MVPA sufficient to confer health benefits (2). Recently, increasing emphasis has been placed on the role sedentary time may play in the etiology of diabetes development (11,26). Substantial cross-sectional and longitudinal evidence exists to support the association between sedentary time, impaired metabolic health, diabetes, and mortality, associations which occur independently of time spent in MVPA (6,9,14,21,26). Furthermore, there is evidence to suggest that the pattern in which sedentary time is accumulated may be important, with interruptions in sedentary time being beneficial for health (6,7) and prolonged bouts of unbroken sedentary time being particularly detrimental (15). However, the duration and frequency of bouts of continuous sedentary time in people with diabetes is not known.
During waking hours, individuals participate in a range of activities varying in intensity between sedentary (defined as “any waking behavior characterized by an energy expenditure of less than or equal to 1.5 metabolic equivalents while in a sitting or reclining posture” [25]) and those which are more vigorous in nature (23). Total time in a day is finite, and therefore these activities are interdependent; an increase in time spent in one activity displaces time spent in another activity. The benefit of a particular intensity of activity will be dependent not only on the type of activity it is (sedentary, light, or MVPA) but also on the activity intensity it displaces. Few studies to date have considered this interdependency, instead using statistical adjustment to either present the effects of the activity intensities in isolation or estimates of the independent associations of the different activity intensities in turn.
Isotemporal substitution methods, originally developed in nutritional epidemiology, take into account the finite nature of time and the interrelationships between activities, thus giving estimations of the effect of substitution of one activity type for another (23,24). For example, these models allow you to examine the potential impact of reallocating 30 min of sedentary time with 30 min of MVPA while keeping total time constant. Although these substitutions are often cross-sectional and therefore causality cannot be assumed, the interpretation is more readily interpretable to public health compared to a standard regression model. These methods have previously been applied to data from both the Whitehall II and National Health and Nutrition Survey (NHANES) cohorts to show the beneficial health effects of replacing prolonged sedentary time with MVPA or LPA (4,12). The health-enhancing effects of activities depend on the type of activity performed, the type of activity displaced, and the population of interest. Adults with type 2 diabetes commonly spend a large portion of their day sedentary (6,9), some of which is likely accumulated in prolonged bouts, considered to be more detrimental to health. The aims of this study were therefore to use isotemporal methods to examine the substitution effects of the different activity intensity types on metabolic health by artificially displacing a fixed duration of one activity intensity with a fixed duration of another. This type of analysis will allow understanding of the potential health benefits of reallocating sedentary time to alternative, more intense activities in a population with type 2 diabetes. Interventions to date have had limited success in increasing MVPA in people with type 2 diabetes; and therefore, it is important to consider whether there is a potential benefit of replacing sedentary time with light-intensity activity.
METHODS
This paper presents a cross-sectional secondary data analysis from baseline data collected as part of the Early Activity in Diabetes (Early Actid) study, a randomized controlled trial of physical activity and diet in the early management of type 2 diabetes. This study has been described in detail previously (1). Briefly, participants with newly diagnosed type 2 diabetes were recruited through primary care in the South West of England. Eligible participants had been diagnosed with type 2 diabetes in the previous 6 months and were age 30–80 yr at diagnosis. Participants were excluded based on uncontrolled diabetes (HbA1c >10% [85.8 mmol·mol−1]), blood pressure >180/100 mm Hg, LDL-cholesterol >4 mmol·L−1, and body mass index (BMI) <25 kg·m−2 or body weight >180 kg. Telephone screening was performed on 1634 participants, of whom 712 were eligible for face-to-face screening and 593 were enrolled in the study. All participants provided written informed consent before participation, and ethical approval was obtained from the Bath Hospital Research Ethics Committee (05/Q2001/5). This study is registered (number ISRCTN92162869).
Physical activity and sedentary time
Participants wore a uniaxial accelerometer (Actigraph GT1M; Actigraph LLC, Pensacola, FL, USA) set to record data every minute on a waist-worn belt for 7 d during waking hours except when swimming or bathing. Accelerometer data were downloaded using Actilife software (version 1.0.52, Actigraph LLC) and were processed using Kinesoft (version 3.3.62; Kinesoft, Saskatoon, Saskatchewan, Canada) to generate outcome variables (mean daily minutes of LPA, MVPA, total sedentary time, long-bout sedentary time, and short-bout sedentary time). Long-bout sedentary time was defined as sedentary time accumulated in bouts of 30 consecutive minutes or longer, whereas short-bout sedentary time was calculated as sedentary time accumulated in bouts of less than 30 min. From these data, we calculated the average daily minutes in short and long sedentary bouts, LPA, and MVPA. For isotemporal analysis, data are expressed in units of 30 min·d−1.
For comparison with other studies, thresholds of 1952 counts or more per minute (cpm) for MVPA, 100 or more and less than 1952 for LPA and less than 100 cpm for sedentary time were used to compute the average number of minutes spent in each behavior (15,16). The activity cut points applied were developed and validated in a healthy adult population to reflect intensities of activity, which equate to light (<3 metabolic equivalents [METs]), moderate (3–5.99 METs), and vigorous (6.0–8.99 METs) intensity (10). A cut point of less than 100 cpm was selected to classify sedentary time, as this has previously been shown to include activities such as sitting or working quietly (15). Nonwear time was defined as a period of 60 min or longer with continuous zero values, and days with at least 10 h of measurement were considered valid. For inclusion in the analyses, the participants were required to record at least three valid days of accelerometer data (6).
Metabolic, anthropometric, and demographic outcomes
Body weight and height were measured to the nearest 0.1 kg and 0.5 cm, respectively, with participants wearing light indoor clothing and without shoes. Body mass index (BMI) was calculated as weight divided by height in meters squared (kg·m−2). Waist circumference was measured at the midpoint between the lowest rib and the anterior iliac. Blood pressure was measured in a seated position using an automated blood pressure monitor (Omron, Healthcare, Henfield, UK). Venous blood samples were obtained after an overnight fast for the measurement of HDL-cholesterol, triglycerides (TG), glucose and insulin levels, and homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the HOMA-2 computer model (22). All metabolic and anthropometric measurements were performed after a fast, during a morning visit to the clinic.
Social deprivation was measured using the Index of Multiple Deprivation (IMD) score, a measure of local area deprivation based on residents’ postcode. Information on ethnicity and medication use was obtained by the research nurse.
Statistical analysis
Descriptive characteristics are presented as mean and standard deviation (SD) unless otherwise stated. Data were checked for normality using visual inspection of histograms.
Standard linear regression analyses were used to explore cross-sectional associations between long-bout sedentary time (≥30 min continuous sedentary time) and short-bout sedentary time, LPA, and MVPA with markers of metabolic health. Unstandardized regression coefficients are presented. No significant interactions by sex were present, and therefore results from pooled analysis are presented.
The isotemporal substitution regression approach is described in detail by Mekary et al. (23). First, each intensity of physical activity was fitted in isolation into a single model to give an estimation of the total association for each activity. Then, isotemporal substitution models were fitted for metabolic markers, which had demonstrated an association with activity in the single-activity models. A model is fitted, which includes all activity intensities and a variable for total time. By eliminating one activity component from the model (e.g., long-bout sedentary time) at a time, the coefficient can be interpreted as the effect of substituting a specific duration of activity per day in a specific intensity with the same duration of another intensity. By holding total time constant and expressing the behaviors as a function of 30-min time periods, the models estimate the effect of reallocating 30 min·d−1 in a less intense activity (e.g., sedentary time) with 30 min·d−1 in a more intense activity (e.g., MVPA) on metabolic markers. In this study, these models fit artificial cross-sectional associations and do not estimate causal associations of individuals replacing time at one intensity with another, instead of providing estimates of the mean shift in the outcome that would be observed cross-sectionally when time spent in an active behavior is artificially increased. All regression models were adjusted for age, sex, deprivation score, ethnicity, accelerometer wear-time, BMI (where appropriate), and relevant lipid, blood pressure, or diabetes-lowering medication (dichotomized as medication yes/no). All analyses were conducted using STATA 13 (StataCorp, College Station, TX).
RESULTS
A total of 593 participants were randomized to the Early-ACTID study. Of these, 519 (88%) fulfilled the accelerometer inclusion criteria and were included in the current analyses.
The baseline demographic, metabolic, and physical activity characteristics of the participants are shown in Table 1 (n = 519). On average, the participants spent 25.4 ± 18.9 min of the day in MVPA and 272.5 ± 75.4 min in LPA. Sedentary time accounted for 65% of the day, of which 46% was accrued in bouts longer than 30 min in length.
TABLE 1.
Demographic, metabolic, and physical activity characteristics of participants (n = 519).
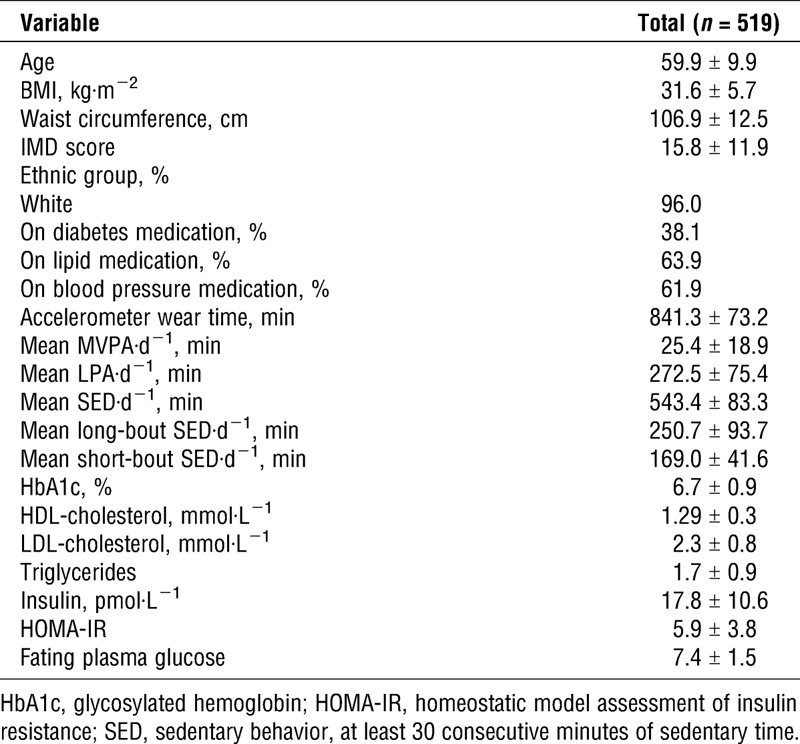
The results of the regression analyses for long-bout sedentary time, short-bout sedentary time, LPA, and MVPA are displayed in Table 2. After adjustment for confounders, long-bout sedentary time was associated with a higher BMI (adjusted β, 0.41; 95% confidence interval [CI], 0.26, 0.56), higher waist circumference (adjusted β, 1.03; 95% CI, 0.69, 1.37), and a lower HDL-cholesterol (adjusted β, −0.02; 95% CI, −0.03, −0.01). No associations with other biomarkers were observed. Short-bout sedentary time, LPA, and MVPA were all associated with a lower BMI with the strongest effect seen for MVPA (adjusted β, −2.15; 95% CI, −2.87, −1.44). Associations were also seen between short-bout sedentary time, LPA, and MVPA and a lower waist circumference. There were suggestions of an association between LPA and HDL-cholesterol (adjusted β, 0.21; 95% CI, 0.01, 0.03). No other associations between LPA, MVPA, and metabolic markers were observed.
TABLE 2.
Associations of each 30 min·d−1 of long sedentary bouts (≥30 min in length), short-bout sedentary time, light-intensity activity, and MVPA with cardiometabolic biomarkers in adults with newly diagnosed type 2 diabetes (n = 519).
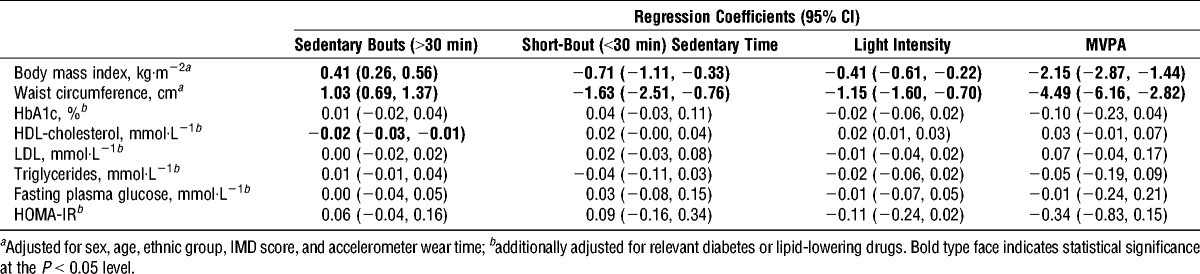
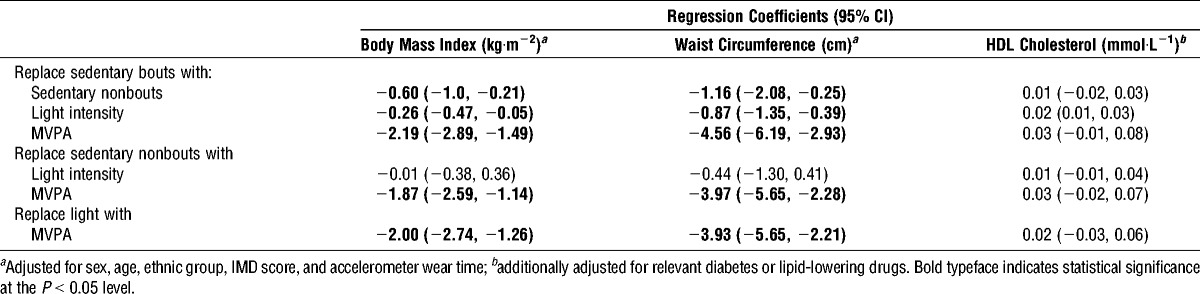
The results of the isotemporal substitution analyses are displayed in Table 3. Results are shown for BMI, waist circumference, and HDL-cholesterol, as these biomarkers were associated with the activity spectrum in simple regression analyses. In cross-sectional analyses, reallocating 30 min·d−1 in long bouts of sedentary time to 30 min of short bout of sedentary time, LPA, or MVPA was associated with a lower BMI and waist circumference. The associations were stronger than those seen in the single-activity models. Reallocation of 30 min of long-bout sedentary time with 30 min of short-bout sedentary time was associated with a lower BMI (adjusted β, −0.60; 95% CI, −1.00, −0.21) and waist circumference (adjusted β, −1.16; 95% CI, −2.08, −0.25). Reallocating 30 min of long-bout sedentary time with LPA was also associated with a higher HDL-cholesterol (adjusted β, 0.02; 95% CI, 0.01, 0.03). Reallocating 30 min of short-bout sedentary time and LPA with 30 min of MVPA were associated with a lower BMI and waist circumference.
TABLE 3.
Estimated impact of reallocating 30 min·d−1 of less active behaviors for 30 min·d−1 of more active behaviors in adults with newly diagnosed type 2 diabetes (n = 519).
DISCUSSION
The results of this study demonstrate that adults with newly diagnosed type 2 diabetes spend 65% of the day sedentary, of which 46% of sedentary time is accumulated in prolonged bouts of more than 30 min. Moderate-to-vigorous physical activity accounted for less than 4% of the waking day. Time spent in long-bout sedentary time (≥30 min) was shown to be detrimentally associated with BMI and waist circumference (WC), whereas short-bout sedentary time, LPA, and MVPA had health-enhancing effects. The greatest effects were shown for MVPA. Light-intensity physical activity was shown to be beneficial for HDL-cholesterol.
Cross-sectional isotemporal substitution analysis suggested that reallocation of 30 min of long-bout sedentary time with 30 min of short-bout sedentary time, LPA, or MVPA could have a beneficial effect on BMI and WC. The strongest associations were shown for MVPA; however, isotemporal regression suggests that reallocation of just 30-min·d−1 long-bout sedentary time with 30 min of short-bout sedentary time, achieved through frequent breaks, may have a favorable inverse relationship with BMI and WC. This finding, although surprising, is in agreement with previous research, which found breaks in sedentary time to be beneficially associated with metabolic risk, independently of total time sedentary and MVPA (15). From a public health perspective, this is an important message to people who perhaps struggle to increase levels of MVPA but would be able to break up prolonged periods of sitting with standing or light walking. Furthermore, replacing 30 min long-bout sedentary time with LPA was associated with a lower HDL-cholesterol, an effect that was not observed for MVPA.
Previous research using these methods in a sample of healthy older adults from the Whitehall II study has shown that replacing 10 min of sedentary time with the equivalent amount of MVPA was associated with favorable effects on markers of metabolic health such as HbA1C and BMI (12). A further study in the National Health and Nutrition Survey (NHANES) cohort demonstrated that reallocation of sedentary time to sleep, LPA, or MVPA was associated with improved health outcomes including reduced WC and improved TG (4). The strongest effects were with MVPA, suggesting that MVPA may be the most potent health-enhancing activity (4). In contrast to this, we did not observe any associations between LPA, MVPA, and TG, HOMA, LDL, or fasting plasma glucose. One potential explanation for the lack of effect observed for metabolic markers in the present study is that the previous research has been conducted on healthy populations free from diabetes and with normal cardiovascular risk profiles (4,12). In comparison, participants in the current study were more obese and had poorer metabolic risk profiles. Furthermore, the levels of physical activity observed in the current study were very low, and it may be that the apparent lack of effect of MVPA demonstrated may be a reflection of the low levels of MVPA exhibited by participants.
The beneficial effects of LPA have previously been demonstrated in an older population with improvements in health-related quality of life observed after reallocation of sedentary time with LPA (3). These results suggest that for some populations such as older people and people with type 2 diabetes, LPA might be sufficiently intense, if displacing prolonged sedentary time, to see health benefits. This supports experimental evidence obtained in a sample of healthy adults, which suggested displacement of sitting time with regular light-intensity exercise had a greater positive effect on insulin level and plasma lipids than a single 1-h bout of more intense physical activity (8). The mechanisms by which sedentary behavior exerts its detrimental effect on metabolic health are still disputed, but possibilities include changes in lipoprotein lipase (LPL) activity, reduced fatty acid clearance, and a loss of adenosine monophosphate activate protein kinase (AMPK) activity (8,13) as a result of reduced muscle activity. Although further experimental work is needed to fully understand the mechanisms involved, findings from the current study that breaking up prolonged sedentary time with frequent breaks and LPA is sufficient to confer metabolic benefits would support the mechanisms suggested.
This study has several strengths. The sample includes a relatively large number of adults with newly diagnosed type 2 diabetes with a large range of outcome measures including objectively measured physical activity and time spent in sedentary behavior. However, objective measurement techniques such as accelerometers are still prone to measurement error, especially in the measurement of sedentary behaviors. Waist-worn accelerometers are limited by their inability to detect differences between sitting and standing; and therefore, the measurement of sedentary time and specifically sedentary bout time may be overestimated in this sample. Furthermore, there is some discrepancy in the literature about the accelerometer thresholds used to define sedentary time (6,12) and the data reduction techniques used to discard continuous periods of zero values, generally interpreted as time when the accelerometer has been removed. Sedentary behavior refers to low-intensity activities and rest; therefore, zero counts on the accelerometer could in fact be a “real” value. Decisions about the data reduction techniques are therefore particularly important when considering sedentary bout time to ensure that continuous zero counts discarded are in fact nonwear time and not continuous sedentary time.
This study is also limited by its cross-sectional design and inability to infer causality. For the single-activity models in particular, there is a risk of reverse causality between BMI and activity behaviors. The cross-sectional isotemporal substitution methods control for total time in the models. However, the substitution that is performed is an artificial replacement of one activity type with another; and therefore, the results should be interpreted accordingly. We did not record time asleep, another discretionary behavior. However, we assume that the accelerometer is only worn during waking hours, and continuous periods of zero counts are removed from the analysis; and therefore, this is unlikely to affect the current study. In addition, we did not include measures of dietary intake in the models. Sedentary behavior may mediate some of its effect on body composition through dietary intake. There is some suggestion that sedentary behavior is associated with increased consumption of high-fat, energy-dense foods (17). The measure of social deprivation provides a measure of multiple deprivation experienced by people living within an area; therefore, it is a population level measure and may have limitations when applied at the individual level.
In conclusion, this study demonstrates an association between long-bout sedentary time and markers of metabolic health. Participants spent more than 9 h of the waking day sedentary, and there is accumulating evidence from this study and others (6,15) that time spent in prolonged sedentary behaviors is particularly harmful. In the present study, artificial reallocation of long-bout sedentary time to either short-bout sedentary time, LPA, or MVPA was associated with improvements in BMI and WC. Stronger associations were seen with MVPA, but these results estimate displacement of long-bout sedentary time with frequently interrupted short-bout sedentary time could be sufficient for beneficial associations with BMI and WC in this population. Therefore, messages aimed at replacing long sedentary bouts with LPA or short-bout sedentary time through frequent breaks may be an alternative public health message for improving health in people with type 2 diabetes. Further work using prospective and experimental study designs is needed to identify the frequency and duration of breaks in sedentary time required for benefits to be seen.
Acknowledgments
C. L. F. performed the statistical analysis, interpreted the data, drafted the manuscript and approved the final version to be published. A. S. P. contributed to drafting the manuscript, and read and approved the final manuscript. A. R. C. and R. C. A. conceived the project and participated in the design and coordination of the project. A. R. C. contributed to the design of the analysis and drafting of the manuscript. All authors read and approved the final manuscript. A. R. C. is the guarantor of this work, and as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The research was supported by the National Institute for Health Research (NIHR) Bristol Nutrition Biomedical Research Unit based at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
The authors declare that there is no conflict of interest associated with this manuscript. Publication of these results of the present study does not constitute an endorsement by the American College of Sports Medicine.
REFERENCES
- 1. Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet. 378(9786): 129– 39. [DOI] [PubMed] [Google Scholar]
- 2. Avery L, Flynn D, van Wersch A, Sniehotta FF, Trenell MI. Changing physical activity behavior in type 2 diabetes: a systematic review and meta-analysis of behavioral interventions. Diabetes Care. 2012; 35(12): 2681– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balboa-Castillo T, Leon-Munoz L, Graciani A, Rodriguez-Artalejo F, Guallar-Castillon P. Longitudinal association of physical activity and sedentary behavior during leisure time with health-related quality of life in community-dwelling older adults. Health and Quality of Life Outcomes. 2011; 9(1): 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buman MP, Winkler EAH, Kurka JM, et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am J Epidemiol. 2014; 179(3): 323– 34. [DOI] [PubMed] [Google Scholar]
- 5. Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010; 33(12): e147– e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper AR, Sebire S, Montgomery AA, et al. Sedentary time, breaks in sedentary time and metabolic variables in people with newly diagnosed type 2 diabetes. Diabetologia. 2012; 55(3): 589– 99. [DOI] [PubMed] [Google Scholar]
- 7. Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012; 35(5): 976– 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duvivier BMFM, Schaper NC, Bremers MA, et al. Minimal intensity physical activity (standing and walking) of longer duration improves insulin action and plasma lipids more than shorter periods of moderate to vigorous exercise (cycling) in sedentary subjects when energy expenditure is comparable. PLoS One. 2013; 8(2): e55542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falconer CL, Cooper AR, Walhin JP, et al. Sedentary time and markers of inflammation in people with newly diagnosed type 2 diabetes. Nutrition Metabolism and Cardiovascular Diseases. 2014; 24(9): 956– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998; 30(5): 777– 81. [DOI] [PubMed] [Google Scholar]
- 11. Grøntved A HFB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011; 305(23): 2448– 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamer M, Stamatakis E, Steptoe A. Effects of substituting sedentary time with physical activity on metabolic risk. Med Sci Sports Exerc. 2014; 46(10): 1946– 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007; 56(11): 2655– 67. [DOI] [PubMed] [Google Scholar]
- 14. Healy G, Wijndaele K, Dunstan D, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care. 2008; 31: 369– 71. [DOI] [PubMed] [Google Scholar]
- 15. Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008; 31(4): 661– 6. [DOI] [PubMed] [Google Scholar]
- 16. Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J. 2011; 32(5): 590– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heinonen I, Helajärvi H, Pahkala K, et al. Sedentary behaviours and obesity in adults: the Cardiovascular Risk in Young Finns Study. BMJ Open. 2013; 3: e002901 doi:10.1136/bmjopen-2013-002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu FB, Stampfer MJ, Solomon C, et al. Physical activity and risk for cardiovascular events in diabetic women. Ann Int Med. 2001; 134(2): 96– 105. [DOI] [PubMed] [Google Scholar]
- 19. Jakicic JM, Gregg E, Knowler W, et al. Activity patterns of obese adults with type 2 diabetes in the look AHEAD study. Med Sci Sports Exerc. 2010; 42(11): 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeon C, Lokken R, Hu F, van Dam R. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007; 30: 744– 52. [DOI] [PubMed] [Google Scholar]
- 21. Katzmarzyk P, Church T, Craig C, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sport Exerc. 2009; 41(5): 998– 1005. [DOI] [PubMed] [Google Scholar]
- 22. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998; 21(12): 2191– 2. [DOI] [PubMed] [Google Scholar]
- 23. Mekary R, Willett W, Hu F, Ding E. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009; 170: 519– 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mekary RA, Lucas M, Pan A, et al. Isotemporal substitution analysis for physical activity, television watching, and risk of depression. Am J Epidemiol. 2013; 178(3): 474– 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedentary Behaviour Research Network. Standardized use of the terms ’sedentary’ and ’sedentary behaivours’ Applied Physiology Nutrition and Metabolism. 2012; 37: 540– 2. [DOI] [PubMed] [Google Scholar]
- 26. Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012; 55(11): 2895– 905. [DOI] [PubMed] [Google Scholar]


