Abstract
Objective:
To investigate the prevalence and determinants of virologic failure and acquired drug resistance-associated mutations (DRMs) in HIV-infected children and adolescents in rural Tanzania.
Design:
Prospective cohort study with cross-sectional analysis.
Methods:
All children 18 years or less attending the paediatric HIV Clinic of Ifakara and on antiretroviral therapy (ART) for at least 12 months were enrolled. Participants with virologic failure were tested for HIV-DRM. Pre-ART samples were used to discriminate acquired and transmitted resistances. Multivariate logistic regression analysis identified factors associated with virologic failure and the acquisition of HIV-DRM.
Results:
Among 213 children on ART for a median of 4.3 years, 25.4% failed virologically. ART-associated DRM were identified in 90%, with multiclass resistances in 79%. Pre-ART data suggested that more than 85% had acquired key mutations during treatment. Suboptimal adherence [odds ratio (OR) = 3.90; 95% confidence interval (CI) 1.11–13.68], female sex (aOR = 2.57; 95% CI 1.03–6.45), and current nonnucleoside reverse transcriptase inhibitor-based ART (aOR = 7.32; 95% CI 1.51–35.46 compared with protease inhibitor-based) independently increased the odds of virologic failure. CD4+ T-cell percentage (aOR = 0.20; 0.10–0.40 per additional 10%) and older age at ART initiation (aOR = 0.84 per additional year of age; 95% CI 0.73–0.97) were protective (also in predicting acquired HIV-DRM). At the time of virologic failure, less than 5% of the children fulfilled the WHO criteria for immunologic failure.
Conclusion:
Virologic failure rates in children and adolescents were high, with the majority of ART-failing children harbouring HIV-DRM. The WHO criteria for immunologic treatment failure yielded an unacceptably low sensitivity. Viral load monitoring is urgently needed to maintain future treatment options for the millions of African children living with HIV.
Keywords: adolescents, Africa, antiretroviral therapy, children, HIV, resistance, resource-limited settings, viral failure
Introduction
In 2015, 1.8 million children were living with HIV worldwide, the vast majority in sub-Saharan Africa (SSA) [1,2]. The roll-out of antiretroviral therapy (ART) in resource-limited countries has resulted in a reduction of HIV-related morbidities and mortality and an increased life expectancy of infected adults and children [3,4]. However, globally only 49% of the children in need have access to treatment [5]. In addition to the low ART coverage in children, long-term treatment success and virologic suppression are harder to achieve in this population [6], mostly because of high pre-ART viral loads and the risk of subtherapeutic drug concentrations caused by limited paediatric drug formulations, variable pharmacokinetics, and continuous bodyweight changes [7–9].
The lack of reliable HIV rapid tests for infants and the limited treatment monitoring in most resource-limited settings, often combined with advanced immunosuppression at ART initiation, further aggravate treatment outcomes [10,11]. Together with the challenge of adherence during childhood and adolescence, these factors promote the emergence of HIV drug resistance mutations (HIV-DRMs) [9,12]. Moreover, although multiple studies confirm that immunological and clinical criteria fail to timely detect treatment failure among children and adolescents [13–15], most resource-limited settings do not have plasma HIV RNA viral load monitoring available [16]. Given the limited ART options in SSA, the emergence of newly acquired HIV-DRM in children is likely to lead to poor clinical outcomes including a reduced survival.
Two recent systematic reviews on the effectiveness of ART among children found virologic success rates of 40–81% after 12 months on treatment [10,17]. Recently, not yet published data collected by the Tanzanian Chronic Disease Clinic (CDC) comparing 15 different settings across the country demonstrated a high average virologic failure rate of 38.8% among children, with HIV-DRM found in 84.4% of the failing children.
We assessed the prevalence and determinants of virologic failure and acquired HIV-DRM after long-term ART exposure within a large paediatric HIV cohort in rural Tanzania.
Patients and methods
Study site and population
All data were prospectively collected from participants enrolled in the Kilombero and Ulanga Antiretroviral Cohort (KIULARCO) after getting informed consent from the patient or caregiver if younger than 19 years. This ongoing, open, prospective cohort is comprises all patients enrolled at the Chronic Diseases Clinic of Ifakara, which serves as a care and treatment centre for HIV/AIDS patients within the Saint Francis Referral Hospital. This is the largest healthcare facility in the Kilombero district, in southern Tanzania, providing treatment and care for a population of approximately 600 000 inhabitants and estimated 38 000 people living with HIV. Established in 2004, this was the first rural clinic accredited to be a Care and Treatment Centre of the National AIDS Control Programme in the country, and over 9000 patients have been enrolled into care. Since 2013, the Chronic Diseases Clinic has a family-centred unit named the ‘One Stop Clinic of Ifakara’, where care to HIV-infected children, mothers, and their families is provided by a specially trained team [18,19].
At each clinical visit, comprehensive clinical data were systematically collected through electronic medical records. Blood samples were drawn at routine clinic visits before ART initiation, 2 weeks, 3 months, and every 6 months thereafter. Plasma was cryopreserved at −80°C [20–22].
All HIV-infected children and adolescents aged 18 years or less enrolled in KIULARCO and who had been on ART for at least 12 months were included in this study.
Viral load testing and HIV genotyping
Blood samples were collected in 8 ml BD Vacutainer EDTA collection tubes (BD, Franklin Lakes, New Jersey, USA). Cell-free plasma was collected by centrifugation at 956g for 5 min and frozen at −80°C until testing for HIV RNA viral load or viral drug resistance. Assays for viral load and sequencing for HIV drug resistance were performed at the Ifakara Health Institute laboratory in Ifakara. HIV RNA from 400 μl plasma was extracted using the NucleoSpin Virus kit (Macherey-Nagel, Oensingen, Switzerland) according to the manufacturer's protocol. Viral RNA quantification was performed using a validated in-house protocol [23] with the Brilliant III Ultra-Fast QRT-PCR Master Mix (Agilent Technologies, La Jolla, California, USA) using the StepOne Real-Time PCR System (Applied Biosystems, Foster City, California, USA), with a detection limit of 200 viral RNA copies/ml of plasma. HIV drug resistance genotyping was performed by Sanger sequencing on a 3130 Genetic Analyser 4-capillary model (Applied Biosystems, Foster City, California, USA) using a validated in-house PCR protocol [23].
Statistical analysis
The primary outcomes were virologic failure, defined as a viral RNA level of at least 1000 copies/ml after at least 12 months on ART, and the acquisition of major HIV-1 DRM in failing patients. For data description, the numeric variables were displayed with medians and interquartile ranges (IQRs), whereas the categorical variables were presented in proportions. Associations between considered variables and virologic failure and HIV-DRM were assessed using multivariate logistic regression models. All analyses were performed using STATA, version 14 (Stata Corporation, College Station, Texas, USA).
Ethical approval
The KIULARCO study received ethical approval from the Ifakara Health Institute Institutional Review Board, the National Institute for Medical Research of Tanzania, the Tanzanian Commission of Science and Technology, and the Ethics Committee of the University and State of Basel.
Results
Characteristics of study population
At the time of analysis, 241 children and adolescents had been on ART for longer than 12 months. Twenty-eight patients were excluded because of several causes (Fig. 1). The remaining 213 children contributed 902.2 person-years of follow-up. The characteristics of the study participants are described in Table 1. The median age was 11 years (IQR 7.5–14.4) and 43% were female. Fifty-five percent were classified as WHO clinical stage 3 or 4, the median CD4+ percentage was 12.2% (IQR 6.3–19.3), and 12.4% reported prior antiretroviral exposure at the time of enrolment in the cohort, excluding exposure to the mother's antiretroviral in utero or through breast milk.
Fig. 1.
Profile of the paediatric study cohort at the Chronic Disease Clinic Ifakara in Ifakara, Morogoro, Tanzania, with virologic outcomes and the presence of drug resistance mutations.
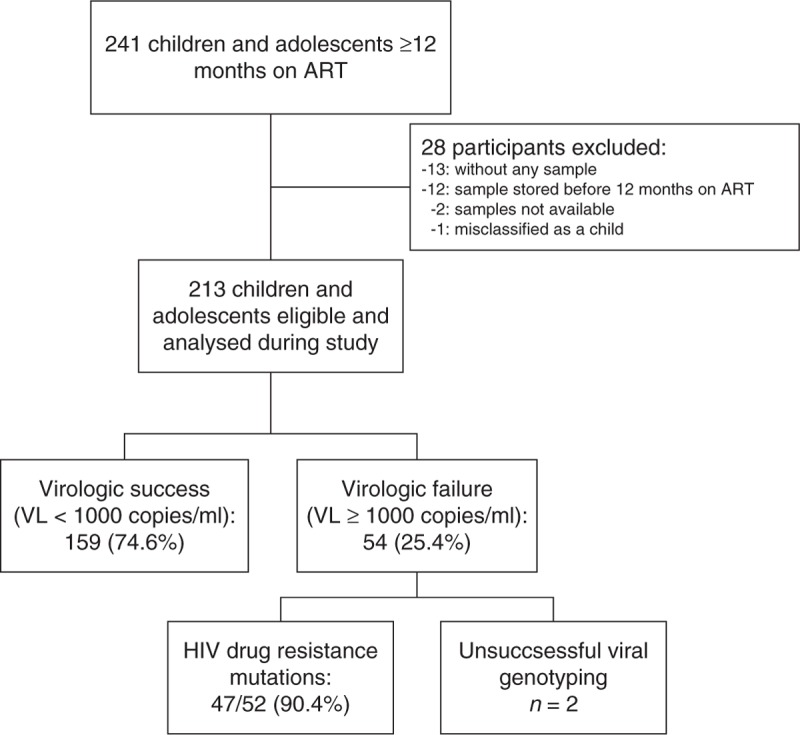
ART, antiretroviral treatment; VL, viral load.
Table 1.
Characteristics of children and adolescents enrolled in the Kilombero and Ulanga Antiretroviral Cohort that had been on antiretroviral treatment for at least 12 months.
| Characteristics | n* | Overall cohort (N = 213) | Virologic success (n = 159) | Virologic failure (n = 54) |
| Female (%) | 213 | 92 (43.2) | 65 (40.9) | 27 (50) |
| Age (years, IQR) | 213 | 11.0 (7.5–14.4) | 11.2 (7.6–13.8) | 10.3 (7.2–15.2) |
| Age at ART initiation (years, IQR) | 206 | 6.4 (3.1–9.5) | 6.3 (3.9–9.3) | 7.2 (2.5–9.7) |
| Time on ART (years, IQR) | 209 | 4.45 (2.4–6.0) | 4.5 (2.4–6.1) | 4.1 (2.5–5.6) |
| Any prior ART exposure (%) | 193 | 24 (12.4) | 19 (13.1) | 5 (10.4) |
| Current CD4+ cell count in cells/μl, median (IQR) | 176 | 636 (440–901) | 680 (477–911) | 485 (364–881) |
| CD4+ cell count in cells/μl at pre-ART, median (IQR) | 139 | 295 (123–561) | 292 (134–523) | 318 (78–643) |
| Current CD4+ percentage, median (IQR) | 171 | 29 (23–34.1) | 30.2 (24.8–35.9) | 24.4 (15–29) |
| CD4+ percentage at pre-ART, median (IQR) | 137 | 12.2 (6.3–19.3) | 13 (7–18.6) | 9.8 (2.7–21.5) |
| Current WHO clinical stage 3–4 (%) | 209 | 141 (67.5) | 104 (67.1) | 37 (68.5) |
| WHO clinical stage 3–4 at pre-ART (%) | 186 | 102 (54.8) | 79 (57.3) | 23 (57.9) |
| Distance to CTC (km) | 183 | 1 (1–25) | 1 (1–25) | 1 (1–33) |
| Good adherencea (%) | 213 | 184 (86.4) | 142 (89.3) | 42 (77.8) |
| Lost to follow-up since last visitb (%) | 213 | 10 (4.7) | 7 (4.4) | 3 (5.6) |
| Initial ART regimen (%) | 195 | |||
| d4T + 3TC + NVP | 76 (39) | 58 (39.5) | 18 (37.5) | |
| ZDV + 3TC + NVP | 37 (19) | 24 (16.3) | 13 (27.1) | |
| ZDV + 3TC + EFV | 70 (36) | 56 (38.1) | 14 (29.2) | |
| Others | 12 (6) | 9 (6.1) | 3 (6.2) | |
| Current ART regimen (%) | 213 | |||
| PI-based regimen | 32 (15.0) | 24 (15.1) | 8 (14.8) | |
| NNRTI-based regimen | 179 (84.0) | 135 (84.9) | 44 (81.5) | |
| Others | 2 (1.0) | 0 (0) | 2 (3.7) | |
| Number of regimen switches (IQR) | 213 | 3 (2–5) | 3 (2–5) | 4 (2–5) |
| ART change because of stock-out (%) | 213 | 50 (23.5) | 36 (22.6) | 14 (25.9) |
| BMI for age z score (IQR) | 209 | −0,74 (−1,42 to −0,03) | −0,82 (−1,5 to −0,12) | −0,57 (−1,39 to 0,16) |
| Weight for height z scorec (IQR) | 14 | 0,21 (0,01 to 1,08) | 0,14 (0,1 to 0,76) (n = 9) | 0,71 (0,08 to 1,1) (n = 5) |
3TC, lamivudine; ART, antiretroviral treatment; CTC, care and treatment centre; d4T, stavudine; EFV, efavirenz; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; ZDV, zidovudine.
aDefined as any missed dose during the last 4 weeks, reported by the patient or their caregiver.
bLast visit = any visit in the past 6 months + 60 days.
cIncludes only children less than 5 years (n = 14).
*Number of patients with nonmissing values.
Initial regimen consisted mostly of coformulated zidovudine (ZDV)/lamivudine (3TC) with a nonnucleoside reverse transcriptase inhibitor (NNRTI) in 54.9% children, whereas stavudine (d4T)/3TC with nevirapine (NVP) was used in 39% of the participants. At the time of investigation, the median time on ART was 4.45 years (IQR 2.4–6.0), 84% were on an NNRTI-based regimen and 15% (32/213) on a boosted protease inhibitor-based regimen, 28 of them as a second-line treatment. The decision to switch to a second-line regimen had been based on clinical and immunological criteria, as viral load monitoring had not been available for routine monitoring of treatment success. Eighty-six percent reported good adherence to ART (defined as no missed doses during the last 4 weeks) and 23.5% had experienced ART regimen changes because of drugs unavailability or shortage, with a median of three regimen switches (range 0–16). Ten children (4.7%) were reported to be lost to follow-up since the last clinical visit.
Virologic outcome
At the time of investigation, 159 of 213 (74.6%) participants had viral plasma levels below the WHO threshold for virologic failure (<1000 copies/ml). In total, 92 of 213 children and adolescents [43.2%, 95% confidence interval (CI) 36.5–49.8] had detectable HIV-1 in plasma. Fifty-four children (25.4%, 95% CI 19.5–31.2) had virologic failure with a median viral load of 20 615 copies/ml, and 38 (17.8%, 95% CI 12.7–23.0) had detectable viral plasma levels below the WHO threshold: 26 (12.2%) had less than 500 copies/ml and 12 (5.6%) between 500 and 1000 copies/ml. Forty-six of the 54 patients with virologic failure were on a first-line ART regimen and eight on second-line treatment. Patients presenting virologic failure were switched to a new ART regimen according to the genotype produced during this study.
Immunologic response after antiretroviral therapy initiation and virologic outcome
The average number of CD4+ T-cell percentage (CD4+%) measurements from the 213 participants was six (range 1–16). The median CD4+% rose from 12.2% at ART initiation to 26% after 12 months on treatment, reaching a maximum of 30.5% after 60–66 months on ART. By comparing the CD4+% recovery of patients with and without virologic failure (Fig. 2), the immune cell recovery in those with virologic failure tended to be diminished, reaching only 22% after 12 months, compared with 27.5% among patients without virologic failure. This difference, however, did not reach statistical significance. Although there was a trend for lower CD4+% among participants with virologic failure, they also showed a median CD4+% recovery after ART initiation, which remained stable at or above 20%. In this group, the median CD4+% after treatment initiation never dropped below the threshold of 10%, a criterion of immunological failure in children below 5 years [24], and the median CD4+ T-cell count stabilized above 500 cells/μl.
Fig. 2.
CD4+ T-cell percentage recovery among the 213 children analysed within this study.
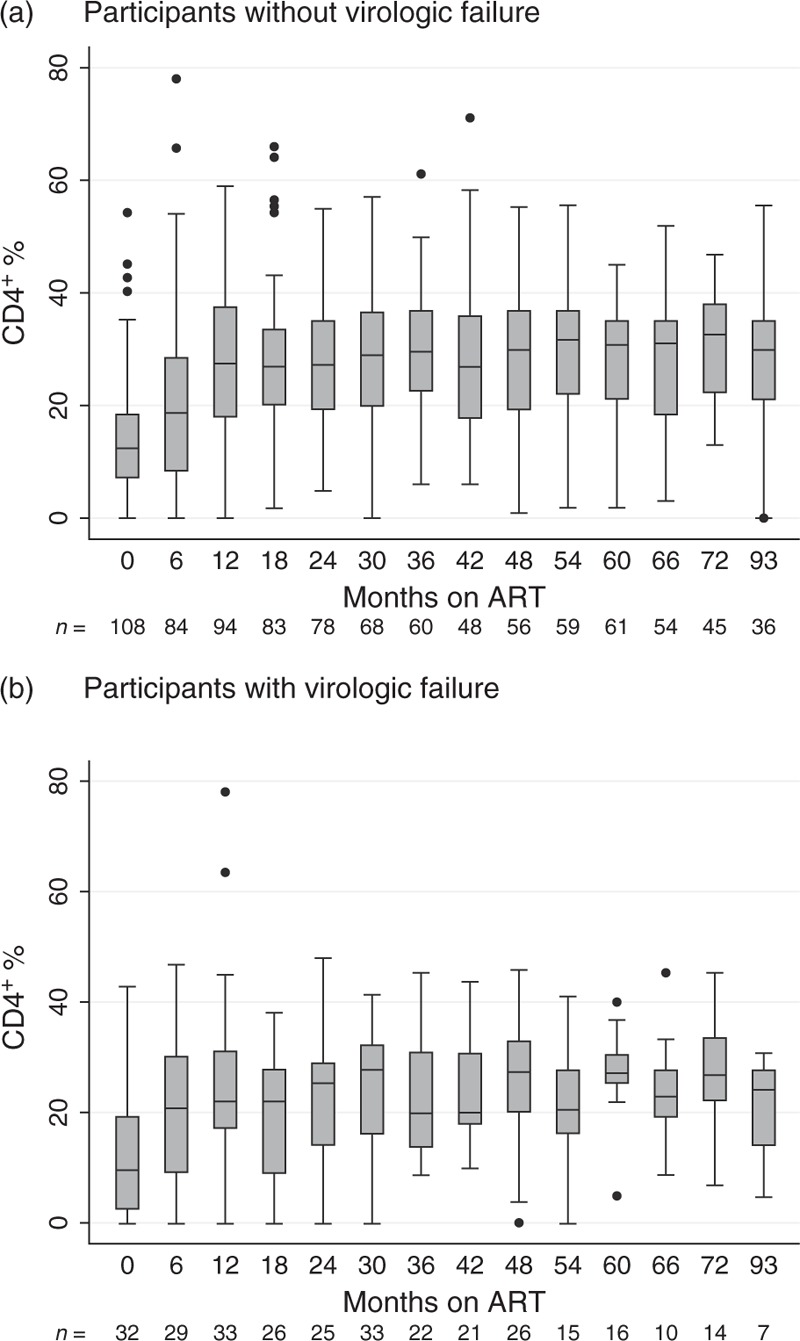
The horizontal line within each box represents the median CD4+ cell percentage, the top and bottom of the box mark the 75th and the 25th percentiles, respectively. The upper and lower bound of the whiskers represent the largest and lowest values within the 75th percentile + 1.5 × IQR and the 25th percentile − 1.5 × IQR, respectively. Data points beyond these intervals are shown as filled circles and represent outliers. (a) All participants with virologic success are represented in the plot, showing a stable CD4+ percentage recovery after ART initiation. (b) Participants with virologic failure are illustrated within this plot, showing an initial CD4+ percentage recovery, which is less stable than the one from patients without virologic failure, though. The time on ART always includes the interval from the previous month up to the one indicated below the axis (e.g. 6≥ 0 and ≤6). ART, antiretroviral therapy.
Predictors of virologic failure
NNRTI-based ART at the time of analysis [adjusted odds ratio (aOR) = 7.32; 95% CI 1.51–35.46; P = 0.013), suboptimal adherence (aOR = 3.90; 95% CI 1.11–13.68; P = 0.034), and female sex (aOR = 2.57; 95% CI 1.03–6.45; P = 0.044) were independently associated with virologic failure. Higher CD4+ cell counts (aOR = 0.20 per additional 10%; 95% CI 0.10–0.41; P < 0.001) and older age at ART initiation (aOR = 0.84 per additional year of age at treatment initiation; 95% CI 0.73–0.97; P = 0.017) were protective of virologic failure (Table 2).
Table 2.
Predictors of virologic failure and acquired HIV drug resistance mutations among children and adolescents attending the Chronic Disease Clinic in Ifakara using logistic regression analysis.
| Virologic failure | Acquisition of HIV-DRM | |||||||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| OR | 95% CI | P | aOR | 95% CI | P | OR | 95% CI | P | aOR | 95% CI | P | |
| Female | 1.45 | 0.78–2.69 | 0.244 | 2.57 | 1.03–6.45 | 0.044 | 1.82 | 0.89–3.70 | 0.101 | 3.99 | 1.40–11.41 | 0.010 |
| Current CD4+ percentage per additional 10 percentage | 0.40 | 0.26–0.62 | <0.001 | 0.20 | 0.10–0.40 | <0.001 | 0.48 | 0.31–0.75 | 0.001 | 0.18 | 0.09–0.40 | <0.001 |
| CD4+ cell percentage at ART initiation per additional 10 percentage | 0.97 | 0.71–1.33 | 0.870 | NA | NA | NA | 1.15 | 0.83–1.58 | 0.404 | NA | NA | NA |
| WHO clinical stage 3–4 at ART initiation | 0.69 | 0.36–1.33 | 0.264 | 0.73 | 0.29–1.86 | 0.513 | 0.75 | 0.36–1.55 | 0.430 | 0.78 | 0.27–2.21 | 0.640 |
| Age at ART initiation per each additional year | 1.02 | 0.94–1.10 | 0.671 | 0.84 | 0.73–0.97 | 0.017 | 0.96 | 0.87–1.05 | 0.354 | 0.81 | 0.68–0.95 | 0.009 |
| Poor adherencea | 2.39 | 1.06–5.39 | 0.037 | 3.90 | 1.11–13.68 | 0.034 | 2.15 | 0.85–5.40 | 0.104 | 3.72 | 0.90–15.34 | 0.069 |
| BMI-for-age z scoreb | 1.11 | 0.86–1.42 | 0.433 | 0.97 | 0.70–1.35 | 0.875 | 1.11 | 0.84–1.47 | 0.465 | 0.99 | 0.69–1.41 | 0.954 |
| Initial ART regimen compared with d4T + 3TC + NVP | ||||||||||||
| ZDV + 3TC + NVP | 1.75 | 0.74–4.11 | 0.203 | 2.13 | 0.56–8.10 | 0.269 | 1.82 | 0.65–5.08 | 0.254 | 2.96 | 0.69–12.79 | 0.145 |
| ZDV + 3TC + EFV | 0.81 | 0.37–1.77 | 0.591 | 1.09 | 0.36–3.30 | 0.884 | 1.24 | 0.52–3.00 | 0.627 | 1.79 | 0.50–6.41 | 0.371 |
| Others | 1.07 | 0.26–4.40 | 0.921 | 1.72 | 0.23–12.58 | 0.596 | 1.82 | 0.42–7.80 | 0.421 | 3.76 | 0.45–31.30 | 0.220 |
| NNRTI-based ART regimen compared with PI-based | 0.78 | 0.35–1.76 | 0.553 | 7.32 | 1.51–35.46 | 0.013 | 1.54 | 0.50–4.72 | 0.450 | 10.73 | 1.75–65.70 | 0.010 |
| Orphan (single or double) | 0.67 | 0.32–1.41 | 0.287 | 0.63 | 0.21–1.86 | 0.398 | 0.65 | 0.29–1.49 | 0.310 | 0.89 | 0.26–3.04 | 0.846 |
| ART switch by stock-out | 1.20 | 0.59–2.44 | 0.623 | 1.39 | 0.45–4.33 | 0.571 | 0.99 | 0.43–2.26 | 0.975 | 1.38 | 0.38–4.98 | 0.624 |
| Number of ART switchesc | 1.04 | 0.94–1.16 | 0.400 | 0.99 | 0.82–1.20 | 0.944 | 1.01 | 0.90–1.14 | 0.867 | 0.93 | 0.75–1.16 | 0.535 |
| Distance to clinicd | 1.01 | 0.91–1.12 | 0.854 | NA | NA | NA | 1.00 | 0.99–1.01 | 0.825 | NA | NA | NA |
| Transferred to CDCI after treatment initiation | 0.77 | 0.27–2.19 | 0.626 | NA | NA | NA | 0.83 | 0.26–2.59 | 0.743 | NA | NA | NA |
3TC, lamivudine; ART, antiretroviral treatment; CDCI, Chronic Disease Clinic Ifakara; CI, confidence interval; CTC, care and treatment centre; d4T, stavudine; DRM, drug resistance mutation; EFV, efavirenz; NA, not applicable; NNRTI, nonnucleoside reverse transcriptase inhibitor; NVP, nevirapine; OR, odds ratio; aOR, adjusted odds ratio; PI, protease inhibitor; ZDV, zidovudine.
aDefined as any missed dose during the last 4 weeks, reported by the patient or their caregiver.
bEach additional unit increase of the z score.
cEach additional number of regimen switch.
dEach additional kilometre distance to the hospital.
HIV drug resistance mutations
The viral genome was successfully sequenced from 52 of 54 children and adolescents with virologic failure. Forty-seven (90.4%; 95% CI 82.4–98.4) harboured at least one major HIV-DRM at the time of virologic failure. Among patients with HIV-DRM, resistance to nucleoside/nucleotide reverse transcriptase inhibitors and NNRTI was found in 80.8% (95% CI 70.1–91.5) and 90.2% (95% CI 82.0–98.4), respectively. Seventy-nine percent (95% CI 67.7–89.9) had major drug resistance against both drug classes. No major protease inhibitor-associated drug resistance mutations were found. Thirteen patients presented with minor protease inhibitor-associated drug resistance mutations, which did not limit the activity of any protease inhibitor.
The most common resistance mutations were M184V/MV, triggering high-level resistance to 3TC and emtricitabine, found in 40 of 52 (77%) patients; K103N/KN, causing high-level resistance to efavirenz and NVP in 25 of 52 (49%) patients; and Y181C/YC/V, leading to high-level resistance against NVP in 16 of 52 (31%) patients. Eighteen patients (34.6%) had detectable thymidine analogue resistance mutations, including M41L, D67N, K70R, T215F/Y, and K219Q/E, with T215F/Y being the most common and found in 13 (25%) children. Notably, the K65R mutation was present in three adolescents, who had no history of tenofovir disoproxil fumarate (TDF) exposure (Table 3). The minor protease inhibitor mutations detected were L10 polymorphisms in eight patients, T74S in four, and L76I in two. Four of the 13 patients with minor protease inhibitor mutations were on a protease inhibitor-based regimen (all with LPV/r), whereas the other nine had never been exposed to protease inhibitors. From the 52 available viral genomes from HIV-1-infected children and adolescents, 44.2% (23/52) were subtype C, 32.7% (17/52) subtype A, 21.2% (11/52) subtype D, and the pol gene of one child (1.9%) consisted of a subtype A protease and subtype D reverse transcriptase. This subtype distribution was consistent with the reported viral subtype circulation in treatment-naive patients in Ifakara from 2009 [23].
Table 3.
Detected drug resistance mutations and respective number of children and adolescents after experienced virologic failure, by drug class.
| NRTI-associated DRM | |
| HIV drug resistance mutations | Number of patients with mutations |
| M184V* | 40 (77%) |
| T215F/Y | 13 (25%) |
| K219Q/E | 11 (21%) |
| K70R | 10 (19%) |
| D67N | 9 (17%) |
| M41L | 5 (10%) |
| T69N/D/G | 4 (8%) |
| K65R, L210W | 3 (6%) |
| A62V | 2 (4%) |
| V75M | 1 (2%) |
| NNRTI-associated DRM | |
| HIV drug resistance mutations | Number of patients with mutations |
| K103N* | 25 (49%) |
| Y181C*/V | 16 (31%) |
| G190A* | 12 (24%) |
| E138A/G/Q | 10 (20%) |
| K101E/P | 9 (18%) |
| V108I | 7 (14%) |
| A98G | 5 (10%) |
| V90I*, L100I/V, Y188L, P225H* | 4 (8%) |
| K238T | 3 (6%) |
| V106M, V179D, H221Y, M230L | 2 (4%) |
| F227L | 1 (2%) |
From the 54 patients with virologic failure, 52 were successfully genotyped. Mutations occurring also as mixtures with the wild-type sequence are indicated by an asterisk.
DRM, drug resistance mutation; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor.
Acquisition of drug resistance mutations after treatment initiation
Additional data were available from 44 of 47 participants with detected HIV-DRM to verify the acquisition of the mutations after ART initiation. Twenty-one (47%) had an available pre-ART plasma sample, which was used to genotype the virus prior to ART exposure. Five of 21 patients harboured DRM, but only one child was on an inactive treatment regimen at ART initiation. For another 10 patients, we analysed stored samples from their mothers, who were still on a first-line ART. All were virologically suppressed, arguing against a vertical transmission of the respective drug-resistant viruses and suggesting that HIV-DRM emerged only after treatment initiation. Of the remaining 13 children, only CD4+ T-cell count and percentage data were available for assessing treatment efficacy. No pre-ART sample of these children had been stored, and their mothers either had been switched to second-line ART or were dead. Eight out of these 13 patients demonstrated stable or increasing CD4+ T-cell counts and percentages after treatment initiation, suggesting initial drug activity, thereby further decreasing the likelihood of vertical transmission of a resistant virus. The remaining five children experienced no elevation of CD4+ T-cell counts or percentages or even showed declining values, which suggest either poor adherence to ART or transmission of a resistant virus from their mothers. Taken together, these data suggest that at least 86.4% of participants (38/44, 95% CI 76.2–96.5) acquired treatment-specific DRM after initiation of ART.
Risk factors for the acquisition of HIV drug resistance mutation
Table 2 summarizes the predictors of acquired DRM. NNRTI-based compared with protease inhibitor-based current regimens (aOR = 10.73; 95% CI 1.75–65.70; P = 0.01) and female sex (aOR = 3.99; 95% CI 1.40–11.41; P = 0.01) increased the odds of HIV-DRM. Additionally, poor adherence showed a trend towards increased odds in acquiring HIV-DRM (aOR = 3.72; 95% CI 0.90–15.34; P = 0.069). Older age (aOR = 0.81 per each additional year; 95% CI 0.68–0.95; P = 0.009) and higher CD4+ percentages (aOR = 0.18 per additional 10%; 95% CI 0.09–0.40; P < 0.001) showed a protective effect.
Sensitivity and specificity of WHO immunological criteria to detect treatment failure
The current WHO immunological criteria of treatment failure for children (CD4+ T-cell count below 200 cells/μl or CD4+% below 10% for children under 5 years of age and CD4+ T-cell count below 100 cells/μl for children aged 5–15 years) correctly classified two of 54 (3.7%) children with virologic failure. The sensitivity of the immunological criteria after including adolescents (CD4+ T-cell count dropping to the pre-ART level or below) to detect virologic failure rose to 14%. As only one patient would have been mistakenly classified with virologic failure, the specificity of these WHO criteria reached 99.3%.
Discussion
To our knowledge, this is one of the first studies to comprehensively assess virologic failure and the acquisition of HIV-DRM among a large paediatric population in SSA. Key findings are a high rate of both virologic failure and acquired HIV-DRM after a median of over 4 years on ART and an increased risk of both virologic failure and HIV-DRM among participants receiving NNRTI, those with younger age at ART initiation and female patients.
The virologic failure rate of 25.4% exceeded by far the 9.1% failure rate observed for the adult population in the same cohort [25], emphasizing the great challenge to successfully suppress HIV in paediatric patients. However, the high virologic failure rate found in our study is comparable with previous reports from similar East and West African settings [9,15,26–30]. Of note, it is significantly lower than the actual national Tanzanian average of 38.8% elaborated by the Tanzanian Chronic Disease Clinic (Ward J, unpublished data), which could be partially attributed to the specialized counsellors and clinicians in our paediatric unit.
The prevalence of HIV-DRM of 90.4% in children and adolescents with virologic failure in our cohort is comparable with similar settings [9,29–30] and the adult population in our clinic [31]. The multiclass resistances, present in almost 80% of all failing patients dramatically limits future treatment options and represents an important public health concern. The presence of the K65R mutation in three patients is also concerning, as this triggers resistance to TDF and abacavir (but not ZDV) again limiting future treatment options. The observed prevalence of TDF resistances in our setting was, however, much lower than in a recently published multicentre cohort study of patients failing to first-line ART [32]. Interestingly, the three patients with the multiclass resistance triggering K65R mutation had not been exposed to TDF. As already highlighted in other studies, d4T (compared with ZDV) may also select this multiclass resistance mutation [33]. Although d4T is currently hardly used, this should be taken into account in patients previously treated with this drug. The absence of major protease inhibitor mutations is reassuring. As protease inhibitors have a short half-life and a higher barrier to resistance, it has been suggested that they are less likely to allow the emergence of drug resistance mutations in early virologic failure [34–36]. Of note, patients on protease inhibitor-based second-line treatment also received additional counselling. Overall, these results indicate that protease inhibitor-based second-line regimens potently suppress HIV in children and adolescents. However, new drug classes such as integrase inhibitors and new paediatric drug combinations are strongly needed in this setting to be able to treat individuals with multiclass resistant virus. Even children with active second-line regimen will eventually depend on new drug classes as they rely on ART for decades, with growing risk to fail on the second line. Although recycled nucleoside/nucleotide reverse transcriptase inhibitors show residual activity in adult populations [37,38], these results cannot be extrapolated to paediatric populations.
In 86.4% of the patients with HIV-DRM, the resistant viral variants likely emerged after ART initiation. However, as imperfect adherence of the mother during pregnancy would lead to the possible archiving of drug resistant proviruses, it cannot be excluded that in these cases viral minorities carrying resistance-associated mutations were also transmitted to the offspring. Such virus would then only emerge once drug pressure with the respective drug was applied. As stated above, although this possibility cannot completely be ruled out, our analysis renders this explanation unlikely for most of the observed resistance cases: no recorded failure of the mother, initial period of treatment success in the child, and paediatric regimen different from the mother's therapy.
Poor adherence predicted virologic failure and showed a trend to predicting the acquisition of HIV-DRM. Adherence is dependent on drug, social, health system, and health workers’ factors [10,39]. The development of new child-friendly drug formulations is needed. In addition, a functioning procurement and distribution system is crucial and tools to facilitate the prescription of paediatric drugs need to be widely disseminated and routinely used. In our cohort, the number of treatment switches significantly increased the odds for poor adherence (by 58%) and for being underdosed for each additional change of treatment (by 50%) (Table S1). It is essential to minimize treatment switches in this context to improve adherence.
Younger age at ART initiation, NNRTI-based regimens, and female sex were identified as risk factors for virologic failure and the acquisition of HIV-DRM. Subtherapeutic drug levels in younger children because of difficulties to administer the drugs, faster metabolism, differences in pharmacokinetics, and dose-prescribing errors could explain this finding [6,8,27,40]. Additional subgroup analysis revealed that 35.2% (19/54) of the study participants with virologic failure were prescribed a drug dose below the recommended for the patient's body weight at least once since ART was initiated, as previously highlighted by a study among this same paediatric population [41]. Of note, after a dedicated paediatric unit had been established in our clinic, such dosing errors were eliminated [41]. Children and adolescents on NNRTI-based regimens had much higher odds to experience virologic failure and to acquire DRM than participants on protease inhibitor-based regimens, again suggesting the good performance of protease inhibitor regimens in suppressing HIV and preventing DRM. As mentioned above, the additional counselling in patients on protease inhibitor-based second-line treatment might have slightly biased this finding. Girls were more vulnerable to virologic failure and the development of HIV-DRM for reasons that could not be explained in the framework of this study, but indeed sex inequalities were also found in other recent studies from East Africa [42].
The WHO criteria for immunological failure showed an alarmingly low sensitivity of 5% in children and 14% in adolescents. For most of the study participants with virologic failure, the CD4+ T-cell counts stayed above 20% and 500 cells/μl after initial immune cell recovery, values considerably higher than the WHO-recommended criteria for immunological failure [24]. A similar study from Rwanda revealed that even a threshold of less than 350 CD4+ T cells/μl to detect treatment failure had a very low sensitivity ranging from 19 to 32% [15]. The late detection of patients with treatment failure leads to the accumulation of drug resistances and dramatically limits treatment options [9,43]. The implementation and upscaling of viral load monitoring is essential to maintain treatment options and optimize health outcomes in resource-limited settings with restricted treatment possibilities [9,10,29].
A limitation of this study was the definition of virologic failure as a single viral load at least 1000 copies/ml, which has a lower sensitivity than the official WHO definition for virologic failure. It is possible that children with lower viral load also carry DRM. Furthermore, the use of population sequencing for genotyping might have led to an underestimation of drug resistance mutations also in pre-ART samples. Our assessment for acquisition of DRM compared with transmitted DRM may have allowed some transmitted DRM to remain unnoticed by sequencing pre-ART samples. In addition, a suppressed viral load of the mother on first-line treatment does not ultimately exclude transmission of DRM.
In conclusion, our study found high rates of virologic failure and emerging HIV-DRM in this paediatric population on long-term ART in rural Tanzania. Both virologic failure and the emergence of HIV-DRM were associated with NNRTI use, younger age at ART initiation, poor adherence, and female sex. Moreover, our results reinforce the current knowledge about the low sensibility of the WHO criteria for immunological treatment failure in children and adolescents. These findings provide relevant information for clinicians and health policymakers and raise concerns about the effectiveness of current paediatric ART programmes in SSA, calling for a critical review of current guidelines. In particular, awareness needs to be raised to advocate for the strengthening of adherence strategies tailored to this vulnerable population, the development and widespread availability of new paediatric ART formulations, and the universal roll-out of routine viral load monitoring for the millions of children and adolescents living with HIV in SSA.
Acknowledgements
The authors are grateful to all children and families attended in the One Stop Clinic and the staff from the Chronic Diseases Clinic of Ifakara.
Their special thanks is extended to all members of the KIULARCO Study Group: Aschola Asantiel, M.B., Adolphina Chale, Diana Faini, I.F., Gideon Francis, H.F., A.G., T.R.G., C.H., Speciosa Hwaya, Aneth Vedastus Kalinjuma, Bryson Kasuga, Namvua Kimera, Yassin Kisunga, T.K., E.L., Antonia Luhombero, L.B.L., Herry Mapesi, Leticia Mbwile, Mengi Mkulila, Julius Mkumbo, Margareth Mkusa, Dorcas K. Mnzava, Germana Mossad, Dolores Mpundunga, Daimon Msami, Athumani Mtandanguo, Kim D. Mwamelo, Selerine Myeya, Sanula Nahota, Regina Ndaki, Agatha Ngulukila, A.J.N., Leila Samson, George Sikalengo, M.T., Fiona Vanobberghen, and Maja Weisser.
The work was supported by the funders of the Chronic Diseases Clinic of Ifakara and its paediatric and PMTCT unit, the One Stop Clinic: the Ministry of Health and Social Welfare of Tanzania; the Swiss Tropical and Public Health Institute; the Ifakara Health Institute; the Government of the Canton of Basel; USAID through its local implementer TUNAJALI-Deloitte; and the Merck for Mothers Global Giving Program.
L.M., A.G., I.F., T.K., and E.L. conceived and designed the study. L.M. and A.J.N. performed the laboratory analysis. A.G. and L.B.L. provided clinical care to the children and adolescents. L.M., A.G., I.F., T.K., and E.L. drafted the manuscript. T.R.G., M.B., H.F., M.T., I.F., T.K., and E.L. reviewed the manuscript.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Thomas Klimkait and Emilio Letang contributed equally to this work.
References
- 1.UN Joint Programme on HIV/AIDS (UNAIDS), ‘The Gap Report, 2014’, http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf [Accessed 22 July 2016] [Google Scholar]
- 2.UN Joint Programme on HIV/AIDS (UNAIDS), ‘Children and HIV, Fact sheet, July 2016’, available at: http://www.unaids.org/sites/default/files/media_asset/FactSheet_Children_en.pdf [Accessed 22 July 2016] [Google Scholar]
- 3.Patel K, Hernán MA, Williams PL, Seeger JD, McIntosh K, Dyke RBV, Seage GR. Pediatric AIDS Clinical Trials Group 219/219C Study Team Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis 2008; 46:1751–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puthanakit T, Aurpibul L, Oberdorfer P, Akarathum N, Kanjananit S, Wannarit P, et al. Hospitalization and mortality among HIV-infected children after receiving highly active antiretroviral therapy. Clin Infect Dis 2007; 44:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UN Joint Programme on HIV/AIDS (UNAIDS), ‘Fact Sheet 2016’, http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf [Accessed 22 July 2016] [Google Scholar]
- 6.van Rossum AMC, Fraaij PLA, de Groot R. Efficacy of highly active antiretroviral therapy in HIV-1 infected children. Lancet Infect Dis 2002; 2:93–102. [DOI] [PubMed] [Google Scholar]
- 7.Abrams EJ, Weedon J, Steketee RW, Lambert G, Bamji M, Brown T, et al. Association of human immunodeficiency virus (HIV) load early in life with disease progression among HIV-infected infants. New York City Perinatal HIV Transmission Collaborative Study Group. J Infect Dis 1998; 178:101–108. [DOI] [PubMed] [Google Scholar]
- 8.Menson EN, Walker AS, Sharland M, Wells C, Tudor-Williams G, Riordan FAI, et al. Collaborative HIV Paediatric Study Steering Committee Underdosing of antiretrovirals in UK and Irish children with HIV as an example of problems in prescribing medicines to children, 1997-2005: cohort study. BMJ 2006; 332:1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigaloff KC, Calis JC, Geelen SP, van Vugt M, de Wit TFR. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: a systematic review. Lancet Infect Dis 2011; 11:769–779. [DOI] [PubMed] [Google Scholar]
- 10.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis 2008; 8:477–489. [DOI] [PubMed] [Google Scholar]
- 11.Bratholm C, Johannessen A, Naman E, Gundersen SG, Kivuyo SL, Holberg-Petersen M, et al. Drug resistance is widespread among children who receive long-term antiretroviral treatment at a rural Tanzanian hospital. J Antimicrob Chemother 2010; 65:1996–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simoni JM, Montgomery A, Martin E, New M, Demas PA, Rana S. Adherence to antiretroviral therapy for pediatric HIV infection: a qualitative systematic review with recommendations for research and clinical management. Pediatrics 2007; 119:e1371–e1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantor R, Diero L, DeLong A, Kamle L, Muyonga S, Mambo F, et al. Misclassification of first-line antiretroviral treatment failure based on immunological monitoring of HIV infection in resource-limited settings. Clin Infect Dis 2009; 49:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Oosterhout JJG, Brown L, Weigel R, Kumwenda JJ, Mzinganjira D, Saukila N, et al. Diagnosis of antiretroviral therapy failure in Malawi: poor performance of clinical and immunological WHO criteria. Trop Med Int Health 2009; 14:856–861. [DOI] [PubMed] [Google Scholar]
- 15.Mutwa PR, Boer KR, Rusine J, Muganga N, Tuyishimire D, Schuurman R, et al. Long-term effectiveness of combination antiretroviral therapy and prevalence of HIV drug resistance in HIV-1-infected children and adolescents in Rwanda. Pediatr Infect Dis J 2014; 33:63–69. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization, World Health Organization., and Department of HIV/AIDS. Antiretroviral therapy for HIV infection in infants and children: towards universal access: recommendations for a public health approach. Geneva, Switzerland: WHO; 2010. [PubMed] [Google Scholar]
- 17.Ciaranello AL, Chang Y, Margulis AV, Bernstein A, Bassett IV, Losina E, Walensky RP. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis 2009; 49:1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamell A, Glass TR, Mapesi H, Lwanda LB, Samson L, Muri L, et al. The One-Stop Clinic of Ifakara: Implementing a bundle of measures for paediatric HIV services in rural Africa to reach the UNAIDS’ Target ‘90-90-90’, 15th European AIDS Conference, European AIDS Clinical Society, Barcelona, Spain 2015. [Google Scholar]
- 19.Gamell A, Lwanda LB, Glass TR, Mpundunga D, Chale A, Samson L, et al. The One-Stop Clinic of Ifakara: a model for integration of prevention of mother-to-child transmission and pediatric HIV services. 9th Conference of Tropical Medicine and International Health, Basel, Switzerland 2015. [Google Scholar]
- 20.Mossdorf E, Stoeckle M, Mwaigomole EG, Chiweka E, Kibatala PL, Geubbels E, et al. Improved antiretroviral treatment outcome in a rural African setting is associated with cART initiation at higher CD4 cell counts and better general health condition. BMC Infect Dis 2011; 11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanobberghen F, Letang E, Gamell A, John A, Faini D, Bonaventure L, et al. ; on behalf of the KIULARCO Study Group. Challenges in estimating death and retention rates in a longitudinal cohort of HIV-infected persons in rural Tanzania. European Congress on Tropical Medicine and International Health (ECTMIH), Basel, Switzerland, Oral Communication 2015. [Google Scholar]
- 22.On behalf of the KIULARCO Study Group. A decade of HIV care and treatment in rural Tanzania: Trends in treatment, opportunistic infections and laboratory abnormalities among HIV-positive adults. European AIDS Clinical Society (EACS), Abstract PE21/5, Barcelona, Spain 2015. [Google Scholar]
- 23.Masimba P, Kituma E, Klimkait T, Horvath E, Stoeckle M, Hatz C, et al. Prevalence of drug resistance mutations and HIV type 1 subtypes in an HIV type 1-infected cohort in rural Tanzania. AIDS Res Hum Retroviruses 2013; 29:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, recommendations for a public health approach. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 25.Erb S, Letang E, Glass TR, Haschke M, Duthalder U, Muri L, et al. Healthcare provider communication training program improves adherence assessment in HIV-infected patients treated with antiretroviral therapy (ART) in rural Tanzania. European AIDS Clinical Society (EACS), Abstract BPD1/3, Barcelona, Spain. 2015. [Google Scholar]
- 26.Emmett SD, Cunningham CK, Mmbaga BT, Kinabo GD, Schimana W, Swai ME, et al. Predicting virologic failure among HIV-1-infected children receiving antiretroviral therapy in Tanzania: a cross-sectional study. J Acquir Immune Defic Syndr 2010; 54:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wamalwa DC, Lehman DA, Benki-Nugent S, Gasper MA, Gichohi R, Maleche-Obimbo E, et al. Long-term virologic response and genotypic resistance mutations in HIV-1 infected Kenyan children on combination antiretroviral therapy. J Acquir Immune Defic Syndr 2013; 62:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamya MR, Mayanja-Kizza H, Kambugu A, Bakeera-Kitaka S, Semitala F, Mwebaze-Songa P, et al. Academic Alliance for AIDS Care and Prevention in Africa Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr 2007; 46:187–193. [DOI] [PubMed] [Google Scholar]
- 29.Salou M, Dagnra AY, Butel C, Vidal N, Serrano L, Takassi E, et al. High rates of virological failure and drug resistance in perinatally HIV-1-infected children and adolescents receiving lifelong antiretroviral therapy in routine clinics in Togo. J Int AIDS Soc 2016; 19:20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kebe K, Thiam M, Diagne Gueye NR, Diop H, Dia A, Signate Sy H, et al. High rate of antiretroviral drug resistance mutations in HIV type 1-infected Senegalese children in virological failure on first-line treatment according to the World Health Organization Guidelines. AIDS Res Hum Retroviruses 2012; 29:242–249. [DOI] [PubMed] [Google Scholar]
- 31.Ntamatungiro AJ, Muri L, Glass TR, Erb S, Battegay M, et al. Strengthening HIV Therapy and Care in Rural Tanzania may Affect Rates of Viral Suppression. In: International HIV Drug Resistance Workshop Boston: Global Antiviral Journal 2016; 58.22.2. [Google Scholar]
- 32.TenoRes Study Group Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis 2016; 16:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nouhin J, Madec Y, Ngo-Giang-Huong N, Ferradini L, Nerrienet E. Increased risk of Q151M and K65R mutations in patients failing stavudine-containing first-line antiretroviral therapy in Cambodia. PLos One 2013; 8:e73744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bangsberg DR. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J Antimicrob Chemother 2004; 53:696–699. [DOI] [PubMed] [Google Scholar]
- 35.R. Harrigan, W. Dong, C. Alexander, et al. The association between drug resistance and adherence determined by two independent methods in a large cohort of drug naive individuals starting triple therapy. Second International Conference on HIV Treatment and Pathogenesis, Paris, 2003. Abstract LB12. IAS International AIDS Society, Stockholm, Sweden: 2003. [Google Scholar]
- 36.Walmsley S, Bernstein B, King M, Arribas J, Beall G, Ruane P, et al. M98-863 Study Team Lopinavir–ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med 2002; 346:2039–2046. [DOI] [PubMed] [Google Scholar]
- 37.Paton NI, Kityo C, Hoppe A, Reid A, Kambugu A, Lugemwa A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med 2014; 371:234–247. [DOI] [PubMed] [Google Scholar]
- 38.Boyd MA, Kumarasamy N, Moore CL, Nwizu C, Losso MH, Mohapi L, et al. SECOND-LINE Study Group Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, noninferiority study. Lancet Lond Engl 2013; 381:2091–2099. [DOI] [PubMed] [Google Scholar]
- 39.Bikaako-Kajura W, Luyirika E, Purcell DW, Downing J, Kaharuza F, Mermin J, et al. Disclosure of HIV status and adherence to daily drug regimens among HIV-infected children in Uganda. AIDS Behav 2006; 10 (4 Suppl):S85–S93. [DOI] [PubMed] [Google Scholar]
- 40.Ellis JC, L’homme RFA, Ewings FM, Mulenga V, Bell F, Chileshe R, et al. Nevirapine concentrations in HIV-infected children treated with divided fixed-dose combination antiretroviral tablets in Malawi and Zambia. Antivir Ther 2007; 12:253–260. [PubMed] [Google Scholar]
- 41.Gamell A, Muri L, Ntamatungiro A, Nyogea D, Luwanda LB, Hatz C, et al. A case series of acquired drug resistance-associated mutations in human immunodeficiency virus-infected children: an emerging public health concern in rural Africa. Open Forum Infect Dis 2016; 3:ofv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazzerini M, Seward N, Lufesi N, Banda R, Sinyeka S, Masache G, et al. Mortality and its risk factors in Malawian children admitted to hospital with clinical pneumonia, 2001-12: a retrospective observational study. Lancet Glob Health 2016; 4:e57–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigaloff KCE, Ramatsebe T, Viana R, de Wit TFR, Wallis CL, Stevens WS. Accumulation of HIV drug resistance mutations in patients failing first-line antiretroviral treatment in South Africa. AIDS Res Hum Retroviruses 2012; 28:171–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


